Abstract
Idiosyncratic drug reactions (IDRs) represent a major health problem, as they are unpredictable, often severe and can be life threatening. The low incidence of IDRs makes their detection during drug development stages very difficult causing many post-marketing drug withdrawals and black box warnings. The fact that IDRs are always not predictable based on the drug’s known pharmacology and have no clear dose–effect relationship with the culprit drug renders diagnosis of IDRs very challenging, if not impossible, without the aid of a reliable diagnostic test. The drug provocation test (DPT) is considered the gold standard for diagnosis of IDRs but it is not always safe to perform on patients. In vitro tests have the advantage of bearing no potential harm to patients. However, available in vitro tests are not commonly used clinically because of lack of validation and their complex and expensive procedures. This review discusses the current role of in vitro diagnostic testing for diagnosis of IDRs and gives a brief account of their technical and mechanistic aspects. Advantages, disadvantages and major challenges that prevent these tests from becoming mainstream diagnostic tools are also discussed here.
Keywords: adverse drug events, adverse drug reactions, drug allergy, idiosyncratic drug reactions, in vitro diagnosis
Introduction
Idiosyncratic drug reactions (IDRs), are important health problems that can cause extra patient suffering or death and high healthcare cost. Accurate diagnosis is key to effective management and prevention. Clinical diagnosis of IDRs can be difficult and often is inaccurate, if based only on medical history and physical examination. While drug provocation testing (DPT) (drug challenge or systemic re-exposure) is considered the gold standard for diagnosis of IDRs, it can be ethically problematic to perform due to possible severe consequences and it is contraindicated in patients with suspected severe reactions such as Stevens-Johnson syndrome (SJS), toxic epidermal neclolysis (TEN) and DRESS (Drug Rash with Eosinophilia and Systemic Symptoms) syndrome 1. Other in vivo tests such as the patch test or transdermal applications may cause reaction flare-ups or even systemic reactions 2. Currently, known in vitro tests are not in wide clinical use largely due to their complicated and expensive procedures as well as their undetermined predictive values 3,4. This review evaluates the role of in vitro testing for the diagnosis of IDRs and discusses some technical and mechanistic aspects, and challenges that prevent these tests from becoming a mainstream clinical approach in management of IDRs.
According to the classification proposed by Rawlins & Thompson adverse drug reactions (ADRs) are either type A reactions, which are predictable, dose-dependent and related to the pharmacological action of the drug or type B reactions, which are unpredictable, have delayed onset, typically unrelated to the drug pharmacology (or at least the known pharmacology) and do not have clear dose-dependency 5. Type A ADRs are the most common and account for 75%–80%, while type B represent 20%–25% of all ADRs (Figure 1 ).
Figure 1.
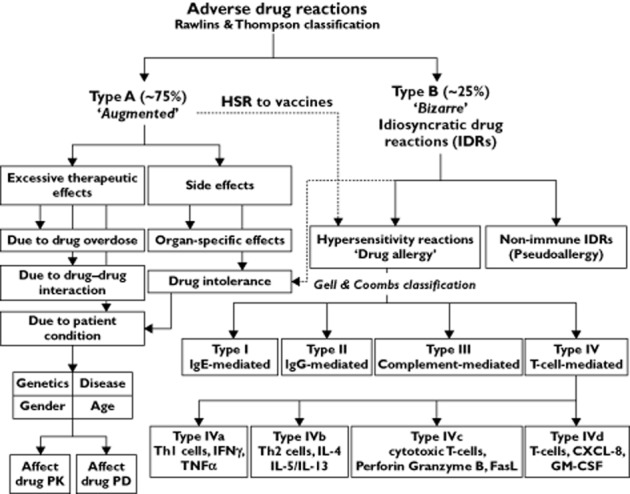
Classification of adverse drug reactions (see text for details)
Type B ADRs (IDRs)
Type B ADRs are also called IDRs. The term means that the reaction is specific to the individual and it is impossible to predict without an identified genetic marker. IDRs include ADRs such as ‘drug hypersensitivity’, which can be either immune-mediated and called allergic hypersensitivity or, if the immunologic mechanism is excluded, is called non-allergic hypersensitivity (pseudoallergy) 6,7. The term ‘hypersensitivity’ does not necessarily imply any immune system involvement and has been defined as ‘objectively reproducible symptoms or signs, initiated by exposure to a defined stimulus at dose tolerated by normal subjects’ 8,9. Drug hypersensitivity reactions have been estimated to represent approximately one-sixth of all ADRs. However, their precise incidence is not known due to under-reporting 10.
Immune-mediated IDRs are classified according to the Combs & Gells classification into four types (I–IV); Type I is mediated by immunoglobulin E (IgE), type II is mediated by IgG and IgM, type III is mediated by formation of an immune complement complex and type IV is T-cell mediated (Table 1) 11. Despite being a useful classification, many recently understood immune-mediated reactions do not fit into the four Combs & Gells classes. A revised and more detailed system has now been introduced 12. It is also of clinical relevance to classify IDRs according to the time required for the symptoms to appear into immediate reactions (≤1 h, e.g. anaphylaxis), intermediate (5–14 days) and delayed (2–7 weeks, Table 1) 13.
Table 1.
Classification of immune-mediated IDRs
| Type | Mediator | Pathogenesis | Clinical picture | Chronology |
|---|---|---|---|---|
| I | IgE | Degranulation of mast cells and basophils | Urticaria, anaphylaxis, allergic rhinitis, bronchospasm, angio-oedema | Immediate (≤1 h) |
| II | IgG/M | FcR dependent cell lysis | Blood cell dyscrasia | Intermediate (5–14 days) |
| III | IgG/M | FcR-dependent immune complexes deposition | Serum sickness, vasculitis, arthus reaction | Intermediate (7–8 days) |
| IVa | TH1 (IFNγ, TNFα) | Monocyte/macrophage mediated inflammatory response | Eczema | Delayed (1–3 weeks) |
| IVb | TH2-IL4, IL5, IL13) | Eosinophils mediated inflammatory response | Maculopapular exanthema, bullous exanthema | Delayed (2–7 weeks) |
| IVc | Cytotoxic T cells (Perforine, Granzym B, FasL) | Cytotoxicity/apoptosis | Maculopapular exanthema, bullous/pustular exanthema | Delayed (1–3 weeks) |
| IVd | T cells (IL8, CXCL8, GM-CSF) | Neutrophils mediated inflammatory response | AGEP, Behςet’s disease | Intermediate (≤2 days) |
Related to their low incidence, IDRs are difficult to detect during drug development clinical trials and there are only few validated animal models to perform any mechanistic studies 14. Also their unpredictability renders prospective studies in humans very difficult, if not impossible. Therefore, our understanding of the underlying pathophysiology of IDRs is still lacking and their classification and nomenclature remains debated. However, IDRs appear to be immune-mediated in many cases 10,15–19. Evidence that supports this hypothesis includes the delayed nature of these types of reactions and that the time between exposure and appearance of the symptoms shortens, if the patient is pre-exposed to the agent, although some exceptions do occur. Drug-specific T-cells have also been detected in the peripheral blood of the affected individuals 20.
Although the skin, liver and blood cells are the most commonly affected, IDRs can affect any organ and patients can present with fever, skin rash (including maculopapular, morbilliform, urticarial, fixed drug eruption or severe bullous reactions such as SJS and TEN), blood dyscrasias (eosinophilia and thrombocytopenia), hepatitis, nephritis, myocarditis, thyroiditis, interstitial pneumonitis and encephalitis. The case can present with any combination of these symptoms. Idiosyncratic drug-induced liver injury (IDILI) is a common ADR to drugs including antibiotics (amoxicillin/clavulan), non-steroidal anti-inflammatory drugs (NSAIDs), isoniazid, sulfamethoxazole, nitrofurantoin, phenytoin and anti-fungals 21. It can be hepatocellular, cholestatic or mixed based on the biochemical pattern of liver function tests. The true incidence of IDILI is difficult to determine due to lack of data on the drug usage although some studies have estimated the annual incidence to be between 15–20 cases per 100 000 inhabitants 22,23. IDILI can also develop as part of DRESS. Evidence exists that implies the involvement of the immune system in the underlying pathophysiology of IDILI 24.
The molecular mechanism(s) underlying IDRs is not fully understood although thought to be immune-mediated in cases where the immune-mechanism is demonstrated 10,25,26. It is noteworthy here that some IDRs exist, which are not mediated through the immune system (e.g. IDRs to NSAIDs). For immune-mediated IDRs, generation of reactive nucleophiles that are able to modify covalently endogenous macromolecules (proteins and DNA) through metabolism is thought to be an important step in the cascade of events leading to activation of the immune system and eliciting the reaction 14,15,21,27. Several hypotheses have been proposed in an attempt to explain the mechanistic pathophysiology of IDRs 10,12,16,28–31. Briefly, accumulated reactive nucleophiles metabolites (the reactive metabolite hypothesis) can modify endogenous macromolecules rendering them antigenic (the hapten hypothesis) and also provide, through causing damage and stress to neighbouring cells, ‘danger signals’ (the danger hypothesis) resulting in maturation of antigen presenting cells (APCs) and T-cells involved in the immune response. The parent molecule and, possibly its reactive metabolite can also interact with the immune receptors directly and non-conelantly producing direct stimulation as per the ‘p-i hypothesis’ (pharmacological interaction of drugs with the immune system) 32.
Discussing the molecular pathophysiology of IDRs in detail is beyond the scope of this review. For further details we suggest these recent references 12,17,18,25,30,33.
Diagnosis of IDRs
Clinicians should bear in mind the possibility of an ADR once a patient has presented with an unexpected event. Patient medical history including a history of drug allergy may give an important clue to the case. Drugs that are known to cause IDRs should be treated as a red flag even if other differential diagnoses exist. Patients on poly-pharmacy represent a major challenge as identifying the culprit drug among multiple drugs has often proved to be difficult and discontinuing important drugs is not always a feasible option. Diagnosis of IDRs is two-fold: i) identifying the reaction as an IDR and ii) determining the culprit drug. Different strategies exist to achieve both goals. Currently the diagnosis of drug hypersensitivity is made on clinical grounds. The process of clinical diagnosis begins with the development of a differential diagnosis factoring in all possible aetiologies. The differential diagnosis is narrowed as the findings of the history and physical examination are factored in, which can include certain entities and exclude others. Classical laboratory tests can also be included in this consideration, although they frequently are not very helpful in the case of the evaluation of possible drug hypersensitivity. When all of the elements of history and physical examination have been considered, the clinician must then decide on the basis of probabilities, which is the most likely diagnosis. The nature of this process highlights the importance of accurate reporting of findings. As an example, SJS is characterized by erythema multiforme associated with mucositis. The mucositis is typically inflammatory and often very painful. In this context, drugs are often the causative agent. In the case of a patient with erythema multiforme and swollen lips with no evidence of mucositis, the diagnosis is more likely erythema multiforme major and not SJS, in which case the aetiology is much more likely to be infectious. The difficulty in making an accurate clinical diagnosis points to the need for a more objective standard for diagnosis.
In vitro approaches to IDRs
A reliable and safe in vitro diagnostic test for IDRs would have a profound effect on the clinical management of IDRs. Although several in vitro tests for IDRs have been recently developed and optimized, their real predictive values are yet to be determined accurately 3,4,34–36. Selection of an in vitro diagnostic test for IDRs depends on the type of reactions and the underlying pathophysiology predicted from the clinical picture and the natural history of the reaction. Immediate, IgE-mediated and delayed T-cell mediated reactions require different sets of in vitro tests for their diagnosis.
In vitro tests for immediate IgE-mediated IDRs
Detection of drug-specific IgE antibody
Measuring drug-specific IgE is the most commonly used diagnostic test for allergic diseases 37. The test is based on quantification of specific IgE antibodies using different laboratory techniques. The radioallergosorbent test (RAST), cellular fluorescent assay-IgE (CAP-IgE) and enzyme-linked immunosorbent assay (ELISA) are commonly used technologies. Although the radioactive technique is no longer used, ‘RAST’ has become a generic name for the technique.
Technical and mechanistic aspects
In the test procedure, suspected antigen (drug) bound to the insoluble phase is incubated with a serum sample from the patient, washed, and then bound IgE is quantified using labelled anti IgE antibody. Direct detection of a drug-specific IgE in patient blood is a strong indicator of an immune reaction but this is not necessarily true in all cases. An individual may have circulating IgE that recognizes a drug molecule without having an immune reaction towards that drug. Nevertheless, the test is known to have high positive predictive value (PPV) when combined with good clinical and medical history. Its negative predictive value (NPV) is inherently low, which is probably due to the low sensitivity of the currently used techniques to detect low titres of circulating immunoglobulins 38,39. Thus, positive results strongly indicate immune mediation of the reaction but negative results do not exclude the reaction. In such cases and if the clinical history is highly suggestive of an allergic reaction, either skin tests or DPT are required to determine safety of future therapy. The low clinical sensitivity of IgE measurement renders the test of limited usefulness as a diagnostic tool. One commercially available test (CAP-FEIA, Phadia®) has a sensitivity and specificity of 0% to 25% and 83.3% to 100%, respectively, in diagnosis of immediate reactions to β-lactam antibiotics 40. Measurement of drug specific IgE antibodies is widely used for diagnosis of immediate reactions to β-lactam antibiotics, muscle relaxants and some NSAIDs 41,42. Levels of specific IgE antibodies tend to decrease over time in patients with immediate allergic reactions, which leads to a decrease in the test sensitivity over time. Therefore, the test must be done as soon as possible after the reaction 43. Another pitfall of in vitro measurement of drug-specific IgE is the high false positive results in patients with high total IgE levels and high false negative in patients with high IgG levels 37.
Basophil activation test (BAT)
The BAT is a useful diagnostic tool for immediate IgE-mediated reactions to both foods and drugs. Its major limitation is the low count of basophil in peripheral blood, but recent flow cytometric techniques have allowed the use of whole blood samples and more accurate determination of the levels of different markers. Its other pitfall is the low sensitivity and this problem is tackled by using different cut off values when evaluating activation markers by flow cytometry 44. However, the timing of the test with regard to the initial reaction is very critical as the test tends to lose its sensitivity with time 45. On the other hand, time during which basophils maintain their activity after blood sampling seems to be short and it has been recommended that samples are processed within 3 h of sampling 46, which limits the availability of the test. The test is quite reproducible but only when used with a limited number of standardized drugs 47.
Basophils are effector cells in immediate-type hypersensitivity reactions and they respond to antigen stimulation in vitro by degranulation (e.g. release of histamine and leukotrienes) and expression of certain surface markers including CD45, CD11b, CD11c, CD62L, CD203c and CD63. Originally, the BAT was performed by measuring the release of histamine. Alternatively, flow cytometry based techniques are now used to measure specific surface markers that are up-regulated during basophil activation. The most commonly used are the antigens CD63, also known as lysosomal-associated membrane glycoprotein-3 (LAMP-3) and CD203c, a glycosylated type II transmembrane molecule 48.
The BAT has been validated for type I reactions to muscle relaxants 49, β-lactam antibiotics 50, pyrazolones and NSAIDs 51,52. Sensitivity of the test for reactions to β-lactam antibiotics, quinolones and rocuonium was reported as 33–67%, 71.1% and 80%, respectively 53–55.
The lymphocyte transformation test (LTT)
The LTT is discussed in details below as part of the in vitro tests for delayed type IDRs. Nevertheless, positive LTT results have been obtained on samples from patients suspected with immediate type I (IgE-mediated) reactions to β-lactam antibiotics 56,57. This observation is attributed to the involvement of activated T-cells in the process of producing drug-specific IgE in immediate type allergic reactions. However, the meaning of detecting drug-specific T-cells in IgE-mediated reactions remains unkown 58.
In vitro tests for delayed (T-cell-mediated) IDRs
Delayed, T-cell-mediated, IDRs are believed to be a result of a complex interplay of many different pathways. Biochemical and genetic approaches have recently begun to shed some light on the pathophysiology of these types of ADRs. Understanding this pathophysiology is a prerequisite for development of evidence-based approaches for better management. Two key players in the in the underlying molecular pathophysiology of immune-mediated IDRs are the drug (or its reactive metabolites) and the immune cells, particularly circulating lymphocytes (when isolated from peripheral blood samples often referred to as peripheral blood monocytes, PBMCs). In addition recent development has led to the use of blood platelets as a surrogate cell model for in vitro toxicity assays. According to the ‘reactive metabolite’ hypothesis, metabolic activation of drugs to reactive metabolite(s) represents the first step in a series of events 28. The ‘reactive metabolite’ hypothesis postulates that IDRs develop as a result of imbalance between metabolic activation and detoxification of drugs in the biological system leading to accumulation of one or more toxic reactive metabolites 59–61. It is important to understand that reactive metabolites may not be the principle direct activator of the immune system as parent, non-reactive drugs can activate isolated T-cells in vitro without need for any bioactivation or processing 32. However, chemically reactive electrophilic metabolites seem to be the major, if perhaps not the only, products capable of supporting two important pathways in the immune system activation process: generation of haptenated endogenous proteins (act as antigens, signal 1) and generation of danger signals from stressed and dying cells (signal 2, Figure 2) 16,29. Signal 2 can also be provided by factors such as trauma, bacterial and viral infections, or co-administered drugs and environmental pollutants. The clinical manifestations of IDRs are probably primarily mediated by the immune system although in some cases a direct toxic effect of the reactive species generated from the drug during metabolism may be manifested clinically 28,59. It has been established for several decades that PBMCs from hypersensitive patients are more susceptible to in vitro toxicity from the reactive metabolite(s) of the suspected drug than are cells from healthy individuals (controls) who have tolerated the drug 60,62–69. T-cells are key mediators of any reaction that involve the immune system and T-cells that specifically recognize culprit drugs and their metabolites have long been cloned from patient blood samples and characterized 70–73. In vitro detection of these drug-specific T-cells is considered indicative of the occurrence of immune-mediated reactions. This is achieved by measuring T-cell proliferation in short term primary cultures as a response to incubation with the suspected drug.
Figure 2.
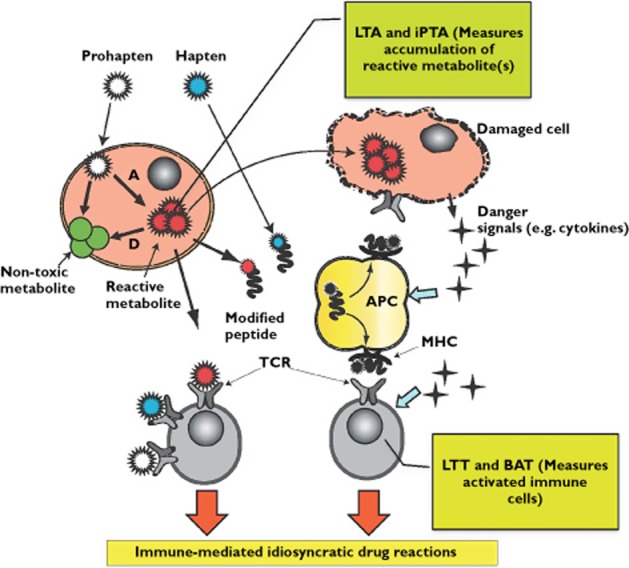
Molecular mechanisms of immune mediated idiosyncratic drug reactions demonstrating the principle of the lymphocyte toxicity assay (LTA), the in vitro platelet toxicity assay (iPTA), the basophil activation test (BAT) and the lymphocyte transformation test (LTT). (A = Activation); (D = detoxication); non-reactive parent drug ( ); reactive parent drug (
); reactive parent drug ( ); reactive metabolite (
); reactive metabolite ( )
)
The lymphocyte transformation test (LTT)
The LTT is the most extensively studied and widely used in vitro diagnostic test for drug, food and environmental allergies. The method was first used back in the early 1960s to evaluate hypersensitivity reactions to phenytoin 74 and sulfa drugs 75. The test involved tedious visual counting of mitotic figures until Vischer 76 adopted measuring radio-labelled thymidine incorporation into cellular DNA as a reflection of cell division rate. Drug specific T-cell clones can be isolated and cloned in vitro and they respond to incubation with the culprit drug with proliferation and expression of certain surface markers 58,71,77.
Technical and mechanistic aspects
First, the anticoagulated blood sample is obtained from the patient and the PBMCs are separated over density gradient (Ficoll®). Cells are then cultured in RPMI 1640 medium supplemented with fetal bovine serum (FBS) for 5 days at 37°C in the presence of the concentration range of the suspected drug (preferably in pure form). Tritium-labelled thymidine (3H-thymidine) is then added to the culture and cells are harvested on day 6 to count incorporated radioactivity as a measure of cell proliferation. The increase in cell proliferation is expressed as a ratio called the stimulation index (SI = 3H-thymidine in the presence of the drug/3H-thymidine uptake in the absence of the drug) 3,18. Other markers of T-cell stimulation have also been used including secretion of mediators (IL-5, IL-10) and expression of specific antigens (e.g. CD 69) 78,79.
Probably one of the most convincing factors for the involvement of the immune system in IDRs is the possibility to isolate drug-specific T-cell clones from blood samples of affected patients. This represents the mechanistic basis of the LTT as a diagnostic tool for IDRs.
The sensitivity of the LTT for the diagnosis of (drug hypersensitivity syndrome) has been estimated to be from 56% to 78% and its specificity to range from 85% to 93% 3,80. Factors that affect the performance of the LTT include (i) timing of the test with respect to the initial reaction, (ii) the clinical picture of the reactions, (iii) the type of drug involved and (iv) the test procedure and read-out systems used 3,80. The use of LTT in the diagnosis of drug hypersensitivity reactions has recently been systematically reviewed 3.
The lymphocyte toxicity assay (LTA)
The LTA is similar to the LTT in using isolated PBMCs, but the principle of the assay is quite different 81. The test is based on the observation that cells from hypersensitive patients express a higher degree of cell death when incubated with the culprit drug metabolite(s) than cells from healthy (drug tolerant) controls. Clinical and practical data and theoretical explanations exist to support this hypothesis but the validity of in vitro cell death as a marker for in vivo drug-induced IDR has long been questioned 67,82. Our three decade clinical and laboratory experience with the use of the LTA in the diagnosis of drug-induced hypersensitivity reactions have proven the test as a very useful diagnostic tool for IDRs to many drug classes including aromatic anticonvulsants, sulfonamides and β-lactam antibiotics 34.
Technical and mechanistic aspects
The test includes incubation of Ficoll gradient isolated PBMCs from patients and controls with the drug in the presence of a metabolic activation system (usually phenobarbital-induced rat liver microsomes, RLM) or with the synthesized drug metabolite (if known and available) 81. Following incubation with different concentrations of the tested drug for 2 h at 37°C, cells are incubated for recovery for 16 h and cell death is then determined using different methods (e.g. trypan blue exclusion, tetrazolium salt 3-(4, 5-dimethylthiazol-2-yl) 2, 5 diohenyl-tetrazolium bromide (MTT)). Degrees of cell death are expressed as percentage of the control (cells incubated with vehicle without drug) and compared with percentage of cell death in cells from healthy individuals who did not experience an ADR with the same drug (controls). A cut off value of the percentage of increase in cell death of incubated patient cells (vs. controls) is considered as an indication of patient susceptibility. The predictive value of the LTA remains difficult to define due to lack of a ‘gold standard’ test for comparison and the technical complexity of the test 3,34.
We have recently performed a population survey on a cohort of pre-tested patients to evaluate the predictive values of the LTA for diagnosis of IDRs to different classes of drugs including β-lactam antibiotics, sulfonamides and aromatic anticonvulsants 34. In this study we included 147 patients who developed an IDR and searched for cases of accidental or purposeful re-exposure of the patient to the suspected drug. Among these patients we identified 26 cases of re-exposure in 22 patients. It is clear from our evaluation that the performance of the LTA test is affected by factors including timing of the test with respect to the initial reaction, type of reaction and class of drugs involved.
On the other hand, the metabolic activation system of the LTA test lacks standardization. There are many pharmacokinetic and pharmacodynamic factors that do not enter the equation including lack of the absence of any functional detoxification pathways. One important observation is that use of a synthetic reactive metabolite (e.g. as in case of sulfonamide drugs) resulted in increased test sensitivity and its positive predictive value 34,62,63. Another factor limiting the more routine use of the LTA is the requirement for careful isolation of PBMCs.
Recent developments
Recent research in our laboratory focused on developing and validating the use of peripheral blood platelets (PBPs) as a surrogate cell model for in vitro toxicity testing 35,83. Due to their small size and low density, PBPs are readily collectable from blood using differential centrifugation 84. In addition to blood homeostasis, the role of platelets in inflammation, allergy and hypersensitivity reactions has recently been recognized 85–88. Platelets are metabolically active and contain a full apoptotic system, which make them a good model to study drug toxicity in vitro. Furthermore, they do not proliferate which adds another advantage to the use of platelets as a cell model to evaluate the degree of cell death. Cell proliferation may mask part of cell death in the PBMCs model. Platelets from hypersensitive patients respond to in vitro chemical insult in a similar fashion to PBMCs and the degree of cell death is higher and easier to detect 83. We also attributed this phenomenon to the lower capacity of platelets for detoxication of reactive metabolites.
In a validation study of the in vitro plasma toxicity assay (iPTA) using rigorous inclusion criteria of identified IDRs cases to sulfa drugs, there was 85% agreement (11 out of 13) between the LTA and the iPTA results in the 13 cases we tested. In the two clinically confirmed cases where the two tests did not agree the LTA was negative and the iPTA was positive. This disagreement between the LTA and the iPTA is probably due to the higher sensitivity of the platelet test to detect patient susceptibility.
In conclusion, the iPTA offers a simplified procedure for in vitro toxicity testing for IDRs with higher sensitivity than the LTA. We believe that the iPTA is more suitable as a diagnostic procedure for IDRs for wider clinical use.
Other in vitro tests for IDRs
In addition to the aforementioned specific tests, these are other in vitro tests, which play a major role in management of IDRs. However, most authors do not list them in their reviews as in vitro tests for IDRs. Because management of such a complex disease requires quick decisions based on evidence based medicine, a global approach is the most successful and the least costly in terms of patient wellbeing and healthcare resources. Valuable data from these tests can guide the treatment journey to a safe port.
Tissue biopsy
Microscopic examination of tissue biopsies can be very valuable in the diagnosis of IDRs involving the skin, the liver, lymph nodes and other tissues 89–91. In severe cases of skin lesions such as exanthematous pustulosis (AGEP), DRESS and SJS/TEN skin biopsy is an important part of disease management and may affect the course of therapy 92. Early detection of severe cutaneous IDRs (CIRDs) can save lives and it is important to differentiate SJS/TEN from erythema multiforme (EM) early on because they have different courses of therapy. Hosaka et al. used frozen skin sections in 35 patients to differentiate TEN from EM in their early stages. From the 35 patients nine had signs of TEN, of them six were later diagnosed with TEN/SJS and three had EM and none of the rest developed TEN/SJS 93. Drug-induced skin rashes have many distinct clinical and histopathological characteristics and features that range in severity from mild self-resolving simple rash to life threatening bullous reactions (SJS/TEN), and in delay time from hours to days. Each has characteristic histopathology features that can be determined in skin biopsy samples.
Measurement of serum markers for IDRs
Analysis of peripheral blood samples of immune-mediated IDRs and studying circulating immune cell subpopulations can help determine the type of reaction and the underlying pathophysiology. Immediate and delayed hypersensitivity reactions have distinct circulating affector cells. Delayed reactions are characterized by Th1 pattern with expression of interferon γ (IFN γ), interleukin-12 (IL-12) and tumour necrosis factor α (TNFα) and down regulation of IL-4 expression. IgE-mediated immediate reactions, on the other hand, are characterized by Th2 pattern with production of IL-4 and downregulation of IFN γ. Fujita et al. developed a rapid immunichromatographic strip test to detect serum granulysin for early prediction of SJS/TEN. Although the sample size was not large enough to validate the test, the test seems very promising as a diagnostic tool for these severe types of reactions 94. Soluble Fas (sFas) levels were also found to increase significantly in sera of SJS/TEN patients before skin detachment develops, which opens the possibility of using this marker for early diagnosis of SJS/TEN 95,96. Caproni et al. have also described that SJS/TEN patients with wide spread skin detachment have a high serum expression of soluble CD40L 97. Serum markers for different types of IDRs is a fast growing field which is useful for both diagnosis and understanding the pathophysiology of the disease 98.
In vitro test for IDILI
In addition to skin, the liver is the organ most affected by IDRs, mainly because it is the main site of drug metabolism. IDILI is a major cause of post marketing drug withdrawal and black box warnings. IDILI are estimated to have an incidence of 1 in 1000 to 1 in 10 000 patients with variable latency periods that ranges from days to months and are estimated to make up 20% of cases of severe liver injury requiring hospitalization 99. It has been found that paracetamol (acetaminophen) overdose and idiosyncratic reactions are the most frequent cause of acute liver failure 100. Prediction and prevention of IDILI have been difficult because of the lack of a reliable screening test and the lack of understanding of the underlying pathophysiology 101. DILI can manifest as a main ADR or present as part of a full drug hypersensitivity syndrome (DRESS) 25. IDILI were responsible for 13% of acute liver failure (ALF) in the USA between the years 1997 and 2001 99. DILI should be considered in any patient with liver dysfunction. A serologic test should be used to rule out any possibility of viral infection. Detailed history of prescription and over the counter medications should be taken as well as alternative and herbal products and foods including alcohol consumption. Time of exposure should be assessed very carefully as DILI most often occur within 6 months of drug exposure but can also occur within days or after a year. Causality assessment can be a challenge especially in cases of multiple medications. In vitro testing for DILI includes evaluation of liver function using liver enzymes as biological markers. Serum concentrations of alkaline phosphatase (ALP), alanine transaminase (ATA) and bilirubin are indicative of the degree of liver injury. Also hepatocellular liver injury can be differentiated from cholestatic liver injury by measuring liver enzymes. The latter is characterized by higher increase in alkaline phosphatase and bilirubin relative to alanine transaminase. DILI is often characterized by the presence of anti-drug antibodies and autoantibodies but tests to measure them are not always available 25. Liver histology studies of biopsies or explant samples can confirm the mechanism of liver injury, as immune cell infiltrates are indicative of immune-mediated reaction.
Genetic testing
Genetic testing for predisposing alleles for IDRs has recently increased exponentially. Genetic analysis has linked a few specific genetic polymorphisms with certain IDRs to some drugs in specific ethnic groups (e.g. HLA B*-1502 for carbamazepine-induced severe bullous reactions in the Han Chinese and HLA B*-5701 and abacavir hypersensitivity) 102. Genetic testing has proven to be very useful in cases such as abacavir hypersensitivity and carbamazepine SJS/TEN reactions in Han Chinese populations. In fact, after implementing mandatory genetic testing for abacavir prescription, abacavir hypersensitivity cases have dropped dramatically in the last few years. Unfortunately, given the incidence of these haplotypes and the fact that the haplotypes are not clearly linked to mechanism, it is likely that using HLA typing to predict risk of adverse drug effects will deny therapy to many patients who would have tolerated the drug. These studies have also made it clear that much more work is required in both basic and clinical science to enable us to predict better, manage and prevent these type of ADRs. Further research is required to elucidate the pathophysiology of drug hypersensitivity syndrome as well as rigorous trials to determine which of the available in vitro evaluations is most suitable for the assessment of patients or research subjects with possible DHRs.
Conclusion
Evaluation and management of IDRs require a great deal of clinical and laboratory experience 103. Incomplete understanding of the underlying pathophysiology of these complex ADRs has always been an obstacle to develop reliable in vitro diagnostic test for this disease. Available tests have proven to be useful tools for IDR management but they are not always available to clinicians and are still confined to well equipped research laboratories (Table 2). Lack of a ‘gold standard test’ for IDRs has made accurate determination of in vitro testing predictive values quite a challenge. Attempts should be made to simplify and standardize in vitro testing procedure, if wider clinical use is to be achieved. Clinicians should be aware that safe alternative in vitro tests for severe IDRs are available in order to avoid unnecessarily risky DPT. With a robust plan and a multidisciplinary approach, in vitro testing can play an important role in IDR management.
Table 2.
Pros and cons of in vitro tests available for idiosyncratic drug reactions
| Detection of drug-specific | ||||||
|---|---|---|---|---|---|---|
| IgEs | LTT | LTA | BAT | iPTA | ||
| Pros |
|
|
|
|
|
|
| Cones |
|
|
|
|
|
BAT, basophil activation test; iPTA, in vitro platelet toxicity assay; LTA, lymphocyte toxicity assay; LTT, lymphocyte transformation test.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work.
References
- Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, Brockow K, Pichler WJ, Demoly P. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–863. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- Torchia D, Capretti C, Pizzo B, Francalanci S. Patch test triggering recurrence of distant dermatitis: the flare-up phenomenon. CMAJ. 2008;179:341. doi: 10.1503/cmaj.080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzagallaai AA, Knowles SR, Rieder MJ, Bend JR, Shear NH, Koren G. In vitro tests for the diagnosis of anticonvulsant hypersensitivity syndrome (AHS): a systematic review. Mol Diagn Ther. 2009;13:313–330. doi: 10.1007/BF03256336. [DOI] [PubMed] [Google Scholar]
- Mayorga C, Sanz ML, Gamboa P, Garcia-Aviles MC, Fernandez J, Torres MJ. In vitro methods for diagnosing nonimmediate hypersensitivity reactions to drugs. J Investig Allergol Clin Immunol. 2013;23:213–225. ; quiz precedeing 225. [PubMed] [Google Scholar]
- Rawlins M, Thompson J. Pathogenesis of adverse drug reactions. In: Davies D, editor. Textbook of Adverse Drug Reactions. Oxford: Oxford University Press; 1977. p. 10. [Google Scholar]
- Issa AM, Phillips KA, Van Bebber S, Nidamarthy HG, Lasser KE, Haas JS, Alldredge BK, Wachter RM, Bates DW. Drug withdrawals in the United States: a systematic review of the evidence and analysis of trends. Curr Drug Saf. 2007;2:177–185. doi: 10.2174/157488607781668855. [DOI] [PubMed] [Google Scholar]
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, Kowalski ML, Mygind N, Ring J, van Cauwenberge P, van Hage-Hamsten M, Wuthrich B. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- Demoly P, Viola M, Rebelo Gomes E, Romano A. Epidemiology and causes of drug hypersensitivity. In: Pichler W, editor. Drug Hypersensitivity. Basel: Karger; 2007. pp. 2–17. [Google Scholar]
- Rieder MJ. Immune mediation of hypersensitivity adverse drug reactions: implications for therapy. Expert Opin Drug Saf. 2009;8:331–343. doi: 10.1517/14740330902933736. [DOI] [PubMed] [Google Scholar]
- Coombs R, Gell P. Classifications of allergi reactions responsible for clinical hypersensitivity and disease. In: Gell P, Coombs R, Lachman P, editors. Clinical Aspects of Immunology. London: Blackwell Scientific Publications; 1975. pp. 761–781. [Google Scholar]
- Pichler WJ, Adam J, Daubner B, Gentinetta T, Keller M, Yerly D. Drug hypersensitivity reactions: pathomechanism and clinical symptoms. Med Clin North Am. 2010;94:645–664. doi: 10.1016/j.mcna.2010.04.003. , xv. [DOI] [PubMed] [Google Scholar]
- Elzagallaai AA, Koren G, Bend JR, Rieder MJ. In vitro testing for hypersensitivity-mediated adverse drug reactions: challenges and future directions. Clin Pharmacol Ther. 2011;90:455–460. doi: 10.1038/clpt.2011.155. [DOI] [PubMed] [Google Scholar]
- Ng W, Lobach AR, Zhu X, Chen X, Liu F, Metushi IG, Sharma A, Li J, Cai P, Ip J, Novalen M, Popovic M, Zhang X, Tanino T, Nakagawa T, Li Y, Uetrecht J. Animal models of idiosyncratic drug reactions. Adv Pharmacol. 2012;63:81–135. doi: 10.1016/B978-0-12-398339-8.00003-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu F, Chen X, Zhu X, Uetrecht J. Involvement of the immune system in idiosyncratic drug reactions. Drug Metab Pharmacokinet. 2011;26:47–59. doi: 10.2133/dmpk.dmpk-10-rv-085. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, Naisbitt DJ, Gordon F, Park BK. The danger hypothesis - potential role in idiosyncratic drug reactions. Toxicology. 2002;181-182:55–63. doi: 10.1016/s0300-483x(02)00255-x. [DOI] [PubMed] [Google Scholar]
- Earnshaw CJ, Pecaric-Petkovic T, Park BK, Naisbitt DJ. T cell responses to drugs and drug metabolites. EXS. 2014;104:137–163. doi: 10.1007/978-3-0348-0726-5_10. [DOI] [PubMed] [Google Scholar]
- Pichler WJ, Naisbitt DJ, Park BK. Immune pathomechanism of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 Suppl):S74–81. doi: 10.1016/j.jaci.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Downey A, Jackson C, Harun N, Cooper A. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66:995–1003. doi: 10.1016/j.jaad.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, Naisbitt DJ. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125:411–418. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95–106. doi: 10.1016/j.mayocp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. . 1934 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, Lemoine A, Hillon P. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS) Semin Cutan Med Surg. 1996;15:250–257. doi: 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- Uetrecht J, Naisbitt DJ. Idiosyncratic adverse drug reactions: current concepts. Pharmacol Rev. 2013;65:779–808. doi: 10.1124/pr.113.007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol. 2007;47:513–539. doi: 10.1146/annurev.pharmtox.47.120505.105150. [DOI] [PubMed] [Google Scholar]
- Smith W. Adverse drug reactions - allergy? side-effect? intolerance? Aust Fam Physician. 2013;42:12–16. [PubMed] [Google Scholar]
- Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587–1591. doi: 10.1016/S0140-6736(00)03137-8. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Park BK, Laverty H, Srivastava A, Antoine DJ, Naisbitt D, Williams D. Drug bioactivation and protein adduct formation in the pathogenesis of drug-induced toxicity. Chem Biol Interact. 2011;192:30–36. doi: 10.1016/j.cbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Naisbitt DJ, Williams D, Pirmohamed M, Kitteringham NR, Park BK. Reactive metabolites and their role in drug reactions. Curr Opin Allergy Clin Immunol. 2001;1:317–325. doi: 10.1097/01.all.0000011033.64625.5a. [DOI] [PubMed] [Google Scholar]
- Pichler WJ. Pharmacological interaction of drugs with antigen-specific immune receptors: the p-i concept. Curr Opin Allergy Clin Immunol. 2002;2:301–305. doi: 10.1097/00130832-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Elzagallaai AA, Garcia-Bournissen F, Finkelstein Y, Bend JR, Rieder MJ, Koren G. Severe bullous hypersensitivity reactions after exposure to carbamazepine in a Han-Chinese child with a positive HLA-B*1502 and negative in vitro toxicity assays: evidence for different pathophysiological mechanisms. J Popul Ther Clin Pharmacol. 2011;18:e1–9. [PubMed] [Google Scholar]
- Elzagallaai AA, Jahedmotlagh Z, Del Pozzo-Magana BR, Knowles SR, Prasad AN, Shear NH, Rieder MJ, Koren G. Predictive value of the lymphocyte toxicity assay in the diagnosis of drug hypersensitivity syndrome. Mol Diagn Ther. 2010;14:317–322. doi: 10.1007/BF03256387. [DOI] [PubMed] [Google Scholar]
- Elzagallaai AA, Koren G, Rieder MJ. The predictive value of the in vitro platelet toxicity assay (iPTA) for the diagnosis of hypersensitivity reactions to sulfonamides. J Clin Pharmacol. 2013;53:626–632. doi: 10.1002/jcph.85. [DOI] [PubMed] [Google Scholar]
- Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 Suppl):S67–73. doi: 10.1016/j.jaci.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Makhija M, O’Gorman MR. Chapter 31: common in vitro tests for allergy and immunology. Allergy Asthma Proc. 2012;33(Suppl. 1):S108–111. doi: 10.2500/aap.2012.33.3564. [DOI] [PubMed] [Google Scholar]
- Akaho E, Miyake A, Yoshii N, Bandai A, Onoue K. Selector system design for the Iowa drug information service microfilm file. Drug Inf J. 1982;16:131–136. doi: 10.1177/009286158201600305. [DOI] [PubMed] [Google Scholar]
- Stone S, Phillips E, Wiese M, Heddle R, Brown S. Immediate-type hypersensitivity reactions. Br J Clin Pharmacol. 2014;78:1–13. doi: 10.1111/bcp.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solensky R, Earl HS, Gruchalla RS. Penicillin allergy: prevalence of vague history in skin test-positive patients. Ann Allergy Asthma Immunol. 2000;85:195–199. doi: 10.1016/S1081-1206(10)62466-0. [DOI] [PubMed] [Google Scholar]
- Fontaine C, Mayorga C, Bousquet PJ, Arnoux B, Torres MJ, Blanca M, Demoly P. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy. 2007;62:47–52. doi: 10.1111/j.1398-9995.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- Assem ES. Anaphylactic anaesthetic reactions. The value of paper radioallergosorbent tests for IgE antibodies to muscle relaxants and thiopentone. Anaesthesia. 1990;45:1032–1038. doi: 10.1111/j.1365-2044.1990.tb14881.x. [DOI] [PubMed] [Google Scholar]
- Fernandez TD, Torres MJ, Blanca-Lopez N, Rodriguez-Bada JL, Gomez E, Canto G, Mayorga C, Blanca M. Negativization rates of IgE radioimmunoassay and basophil activation test in immediate reactions to penicillins. Allergy. 2009;64:242–248. doi: 10.1111/j.1398-9995.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- Chirumbolo S. Major pitfalls in BAT performance may be caused by gating protocols and CD63% cut off evaluation. Cytometry A. 2014;85:382–385. doi: 10.1002/cyto.a.22466. [DOI] [PubMed] [Google Scholar]
- Sanz ML, Gamboa PM, Mayorga C. Basophil activation tests in the evaluation of immediate drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2009;9:298–304. doi: 10.1097/ACI.0b013e32832d5311. [DOI] [PubMed] [Google Scholar]
- Sturm GJ, Kranzelbinder B, Sturm EM, Heinemann A, Groselj-Strele A, Aberer W. The basophil activation test in the diagnosis of allergy: technical issues and critical factors. Allergy. 2009;64:1319–1326. doi: 10.1111/j.1398-9995.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- Sanz ML, Maselli J, Gamboa PM, Oehling A, Dieguez I, de Weck AL. Flow cytometric basophil activation test: a review. J Investig Allergol Clin Immunol. 2002;12:143–154. [PubMed] [Google Scholar]
- McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep. 2013;13:101–109. doi: 10.1007/s11882-012-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Bardagi M, Roura X, Zanna G, Ravera I, Ferrer L. Long term follow-up of dogs diagnosed with leishmaniosis (clinical stage II) and treated with meglumine antimoniate and allopurinol. Vet J. 2011;188:346–351. doi: 10.1016/j.tvjl.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Owens L, Butcher G, Gilmore I, Kolamunnage-Dona R, Oyee J, Perkins L, Walley T, Williamson P, Wilson K, Pirmohamed M. A randomised controlled trial of extended brief intervention for alcohol dependent patients in an acute hospital setting (ADPAC) BMC Public Health. 2011;11:528. doi: 10.1186/1471-2458-11-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler SL, Fung MA. Off-center fold: indurated plaques on the arms of a 52-year-old man. Diagnosis: cutaneous reaction to phytonadione injection. Arch Dermatol. 2001;137:957–962. [PubMed] [Google Scholar]
- Leung PS, Fung ML, Sernia C. Chronic hypoxia induced down-regulation of angiotensinogen expression in rat epididymis. Regul Pept. 2001;96:143–149. doi: 10.1016/s0167-0115(00)00169-5. [DOI] [PubMed] [Google Scholar]
- Abuaf N, Rostane H, Rajoely B, Gaouar H, Autegarden JE, Leynadier F, Girot R. Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy. Clin Exp Allergy. 2008;38:921–928. doi: 10.1111/j.1365-2222.2008.02960.x. [DOI] [PubMed] [Google Scholar]
- Aranda A, Mayorga C, Ariza A, Dona I, Rosado A, Blanca-Lopez N, Andreu I, Torres MJ. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011;66:247–254. doi: 10.1111/j.1398-9995.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- Leysen J, Bridts CH, Clerck LSD, Vercauteren M, Lambert J, Weyler JJ, Stevens WJ, Ebo DG. Allergy to rocuronium: from clinical suspicion to correct diagnosis. Allergy. 2011;66:1014–1019. doi: 10.1111/j.1398-9995.2011.02569.x. [DOI] [PubMed] [Google Scholar]
- Bircher AJ. Lymphocyte transformation test in the diagnosis of immediate type hypersensitivity reactions to penicillins. Curr Probl Dermatol. 1995;22:31–37. doi: 10.1159/000424228. [DOI] [PubMed] [Google Scholar]
- Cederbrant K, Stejskal V, Broman P, Lindkvist L, Sundell K. In vitro lymphocyte proliferation in the diagnosis of allergy to phenoxymethylpenicillin. Allergy. 1998;53:1155–1161. doi: 10.1111/j.1398-9995.1998.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–820. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, Kitteringham NR, Park BK. The role of active metabolites in drug toxicity. Drug Saf. 1994;11:114–144. doi: 10.2165/00002018-199411020-00006. [DOI] [PubMed] [Google Scholar]
- Shear NH, Spielberg S. In vitro evaluation of a toxic metabolite of sulfadiazine. Can J Physiol Pharmacol. 1985;63:1370–1372. doi: 10.1139/y85-225. [DOI] [PubMed] [Google Scholar]
- Shear NH, Spielberg S. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest. 1988;82:1826–1832. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder MJ, Uetrecht J, Shear NH, Cannon M, Miller M, Spielberg S. Diagnosis of sulfonamide hypersensitivity reactions by in-vitro ‘rechallenge’ with hydroxylamine metabolites. Ann Intern Med. 1989;110:286–289. doi: 10.7326/0003-4819-110-4-286. [DOI] [PubMed] [Google Scholar]
- Rieder MJ, Uetrecht J, Shear NH, Spielberg SP. Synthesis and in vitro toxicity of hydroxylamine metabolites of sulfonamides. J Pharmacol Exp Ther. 1988;244:724–728. [PubMed] [Google Scholar]
- Riley RJ, Leeder JS. In vitro analysis of metabolic predisposition to drug hypersensitivity reactions. Clin Exp Immunol. 1995;99:1–6. doi: 10.1111/j.1365-2249.1995.tb03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear NH, Spielberg SP. An in vitro lymphocytotoxicity assay for studying adverse reactions to sulphonamides. Br J Dermatol. 1985;113(Suppl. 28):112–113. doi: 10.1111/j.1365-2133.1985.tb15637.x. [DOI] [PubMed] [Google Scholar]
- Spielberg SP. Acetaminophen toxicity in human lymphocytes in vitro. J Pharmacol Exp Ther. 1980;213:395–398. [PubMed] [Google Scholar]
- Spielberg SP. In vitro assessment of pharmacogenetic susceptibility to toxic drug metabolites in humans. Fed Proc. 1984;43:2308–2313. [PubMed] [Google Scholar]
- Spielberg SP, Gordon GB, Blake DA, Goldstein DA, Herlong HF. Predisposition to phenytoin hepatotoxicity assessed in vitro. N Engl J Med. 1981;305:722–727. doi: 10.1056/NEJM198109243051302. [DOI] [PubMed] [Google Scholar]
- Spielberg SP, Gordon GB, Blake DA, Mellits ED, Bross DS. Anticonvulsant toxicity in vitro: possible role of arene oxides. J Pharmacol Exp Ther. 1981;217:386–389. [PubMed] [Google Scholar]
- Sanderson JP, Naisbitt DJ, Farrell J, Ashby CA, Tucker MJ, Rieder MJ, Pirmohamed M, Clarke SE, Park BK. Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007;178:5533–5542. doi: 10.4049/jimmunol.178.9.5533. [DOI] [PubMed] [Google Scholar]
- Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J Immunol. 2000;164:6647–6654. doi: 10.4049/jimmunol.164.12.6647. [DOI] [PubMed] [Google Scholar]
- Lochmatter P, Zawodniak A, Pichler WJ. In vitro tests in drug hypersensitivity diagnosis. Immunol Allergy Clin North Am. 2009;29:537–554. doi: 10.1016/j.iac.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Pichler WJ. Predicting drug hypersensitivity by in vitro tests. ALTEX. 2007;24(Spec No):49–52. [PubMed] [Google Scholar]
- Holland P, Mauer AM. Drug-induced in-vitro stimulation of peripheral lymphocytes. Lancet. 1964;1:1368–1369. doi: 10.1016/s0140-6736(64)92046-x. [DOI] [PubMed] [Google Scholar]
- Caron GA, Sarkany I. Lymphoblast transformation in sulphonamide sensitivity. Br J Dermatol. 1965;77:556–560. doi: 10.1111/j.1365-2133.1965.tb14575.x. [DOI] [PubMed] [Google Scholar]
- Vischer TL. Lymphocyte cultures in drug hypersensitivity. Lancet. 1966;2:467–469. doi: 10.1016/s0140-6736(66)92773-5. [DOI] [PubMed] [Google Scholar]
- Yawalkar N, Hari Y, Frutig K, Egli F, Wendland T, Braathen LR, Pichler WJ. T cells isolated from positive epicutaneous test reactions to amoxicillin and ceftriaxone are drug specific and cytotoxic. J Invest Dermatol. 2000;115:647–652. doi: 10.1046/j.1523-1747.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- Beeler A, Zaccaria L, Kawabata T, Gerber BO, Pichler WJ. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy. 2008;63:181–188. doi: 10.1111/j.1398-9995.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- Merk HF. Diagnosis of drug hypersensitivity: lymphocyte transformation test and cytokines. Toxicology. 2005;209:217–220. doi: 10.1016/j.tox.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy. 1997;27:175–181. [PubMed] [Google Scholar]
- Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest. 1988;82:1826–1832. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly TP, MacArthur RD, Farrough MJ, Crane LR, Woster PM, Svensson CK. Is hydroxylamine-induced cytotoxicity a valid marker for hypersensitivity reactions to sulfamethoxazole in human immunodeficiency virus-infected individuals? J Pharmacol Exp Ther. 1999;291:1356–1364. [PubMed] [Google Scholar]
- Elzagallaai AA, Rieder MJ, Koren G. The in vitro platelet toxicity assay (iPTA): a novel approach for assessment of drug hypersensitivity syndrome. J Clin Pharmacol. 2011;51:428–435. doi: 10.1177/0091270010365554. [DOI] [PubMed] [Google Scholar]
- McNicol A. Platelet preparation and estimation of functional responses. In: Watson S, Authi K, editors. Platelets. Oxford: Oxford University Press; 1996. pp. 1–26. [Google Scholar]
- Capron A, Joseph M, Ameisen JC, Capron M, Pancre V, Auriault C. Platelets as effectors in immune and hypersensitivity reactions. Int Arch Allergy Appl Immunol. 1987;82:307–312. doi: 10.1159/000234214. [DOI] [PubMed] [Google Scholar]
- Pitchford SC. Defining a role for platelets in allergic inflammation. Biochem Soc Trans. 2007;35(Pt 5):1104–1108. doi: 10.1042/BST0351104. [DOI] [PubMed] [Google Scholar]
- Pitchford SC, Yano H, Lever R, Riffo-Vasquez Y, Ciferri S, Rose MJ, Giannini S, Momi S, Spina D, O’Connor B, Gresele P, Page CP. Platelets are essential for leukocyte recruitment in allergic inflammation. J Allergy Clin Immunol. 2003;112:109–118. doi: 10.1067/mai.2003.1514. [DOI] [PubMed] [Google Scholar]
- Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelets play important roles in the late phase of the immediate hypersensitivity reaction. J Allergy Clin Immunol. 2009;123:581–587. doi: 10.1016/j.jaci.2008.12.1114. , 587 e1-9. [DOI] [PubMed] [Google Scholar]
- Mainra RR, Card SE. Trimethoprim-sulfamethoxazole-associated hepatotoxicity – part of a hypersensitivity syndrome. Can J Clin Pharmacol. 2003;10:175–178. [PubMed] [Google Scholar]
- Altuntas Y, Ozturk B, Erdem L, Gunes G, Karul S, Ucak S, Sengul A. Phenytoin-induced toxic cholestatic hepatitis in a patient with skin lesions: case report. South Med J. 2003;96:201–203. doi: 10.1097/01.SMJ.0000051269.23361.4A. [DOI] [PubMed] [Google Scholar]
- Rim MY, Hong J, Yo I, Park H, Chung DH, Ahn JY, Park S, Park J, Kim YS, Lee JH. Cervical lymphadenopathy mimicking angioimmunoblastic T-cell lymphoma after dapsone-induced hypersensitivity syndrome. Korean J Pathol. 2012;46:606–610. doi: 10.4132/KoreanJPathol.2012.46.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockenhaupt M. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. J Dtsch Dermatol Ges. 2009;7:142–160. doi: 10.1111/j.1610-0387.2008.06878.x. ; quiz 161-2. [DOI] [PubMed] [Google Scholar]
- Hosaka H, Ohtoshi S, Nakada T, Iijima M. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis: frozen-section diagnosis. J Dermatol. 2010;37:407–412. doi: 10.1111/j.1346-8138.2009.00746.x. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yoshioka N, Abe R, Murata J, Hoshina D, Mae H, Shimizu H. Rapid immunochromatographic test for serum granulysin is useful for the prediction of Stevens-Johnson syndrome and toxic epidermal necrolysis. J Am Acad Dermatol. 2011;65:65–68. doi: 10.1016/j.jaad.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Murata J, Abe R, Shimizu H. Increased soluble Fas ligand levels in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis preceding skin detachment. J Allergy Clin Immunol. 2008;122:992–1000. doi: 10.1016/j.jaci.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Abe R, Yoshioka N, Murata J, Fujita Y, Shimizu H. Granulysin as a marker for early diagnosis of the Stevens-Johnson syndrome. Ann Intern Med. 2009;151:514–515. doi: 10.7326/0003-4819-151-7-200910060-00016. [DOI] [PubMed] [Google Scholar]
- Caproni M, Antiga E, Parodi A, Schena D, Marzano A, Quaglino P, De Simone C, Placa ML, Volpi W, Del Biano E, Fabbri P. Elevated circulating CD40 ligand in patients with erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis spectrum. Br J Dermatol. 2006;154:1006–1007. doi: 10.1111/j.1365-2133.2006.07211.x. [DOI] [PubMed] [Google Scholar]
- Zawodniak A, Lochmatter P, Yerly D, Kawabata T, Lerch M, Yawalkar N, Pichler WJ. In vitro detection of cytotoxic T and NK cells in peripheral blood of patients with various drug-induced skin diseases. Allergy. 2010;65:376–384. doi: 10.1111/j.1398-9995.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33:155–164. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M. Pharmacogenetics of idiosyncratic adverse drug reactions. Handb Exp Pharmacol. 2010;196:477–491. doi: 10.1007/978-3-642-00663-0_17. [DOI] [PubMed] [Google Scholar]
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]


