Abstract
The study of intact soluble protein assemblies by means of mass spectrometry is providing invaluable contributions to structural biology and biochemistry. A recent breakthrough has enabled similar study of membrane protein complexes, following their release from detergent micelles in the gas phase. Careful optimization of mass spectrometry conditions, particularly with respect to energy regimes, is essential for maintaining compact folded states as detergent is removed. However, many of the saccharide detergents widely employed in structural biology can cause unfolding of membrane proteins in the gas phase. Here, we investigate the potential of charge reduction by introducing three membrane protein complexes from saccharide detergents and show how reducing their overall charge enables generation of compact states, as evidenced by ion mobility mass spectrometry. We find that charge reduction stabilizes the oligomeric state and enhances the stability of lipid-bound complexes. This finding is significant since maintaining native-like membrane proteins enables ligand binding to be assessed from a range of detergents that retain solubility while protecting the overall fold.
Structural characterization of membrane proteins is challenging due to their hydrophobic nature, low expression levels, and requirement for membrane mimics for solubilization. Amphipathic detergent molecules, which form micelles in solution, are widely adopted for solubilization and their ability to mimic the membrane environment.1 In a standard nondenaturing mass spectrometry (MS) experiment detergent micelles are removed by collisional activation in the gas phase.2 Subsequently the folded nature of membrane proteins is assessed using ion-mobility (IM)–MS through measurement of collision cross sections (CCS) and comparison with values calculated from X-ray coordinates.3 Furthermore, CCS is used to compare candidate models of assemblies to gain structural information about different conformational states.4,5 It has also been shown that resistance to unfolding, assessed via measuring CCS, can be parametrized and used to rank lipids for their effects on stability.6
For soluble proteins the folded state is affected by combination of the charge state and the voltages applied during acceleration through the mass spectrometer. High charge states are susceptible to Coulombic repulsion between charged residues promoting local unfolding7 or subunit dissociation.8 As a consequence of these effects lower charge states are often associated with “native-like” structures.9 Removal of detergent from membrane protein ions, however, requires activation, and as such, reduction of acceleration voltages is not viable. An alternative strategy is therefore to reduce the charge state of the membrane protein complex. To access low charge states, and thereby compact states, many strategies have been developed for soluble complexes.10–12 These include addition of crown ethers as competitive proton chelators8 and the presence of organic solvents in the atmosphere surrounding the electrospray (ES) plume in the source housing.13,14
The charging process for membrane proteins in ES is more complicated than for soluble ones since part of the accessible surface area of the protein complex is surrounded by the micelle when charging takes place.15 The regions embedded in the micelle are the least charged hydrophobic transmembrane residues that are unlikely to participate in charge reduction. The presence of detergent in solution, however, at twice the critical micelle concentration, may impair charge reduction. Moreover, given their inherent low charge, further reduction may not be effective in maintaining low energy compact states of membrane proteins.
We investigated charge reduction of three membrane proteins, with different secondary structure content and oligomeric state. We selected two Escherichia coli proteins: the outer membrane protein (OmpF) and the trimeric ammonium channel (AmtB), with β-barrel and α-helical secondary structure, respectively. Both were introduced from octyl glucoside (OG) micelles. P-glycoprotein (P-gp), a monomeric ABC transporter from mouse, is predominantly α-helical and was introduced from dodecylmaltoside (DDM) micelles. We used DDM and OG as these are the two most commonly used detergents for membrane protein solubilization. While the polyethylene glycols (PEG) used in previous studies require low collision energy and preserve the folded state,6 the number of proteins characterized in PEG micelles is relatively few since many are not soluble/functional in these detergents.16
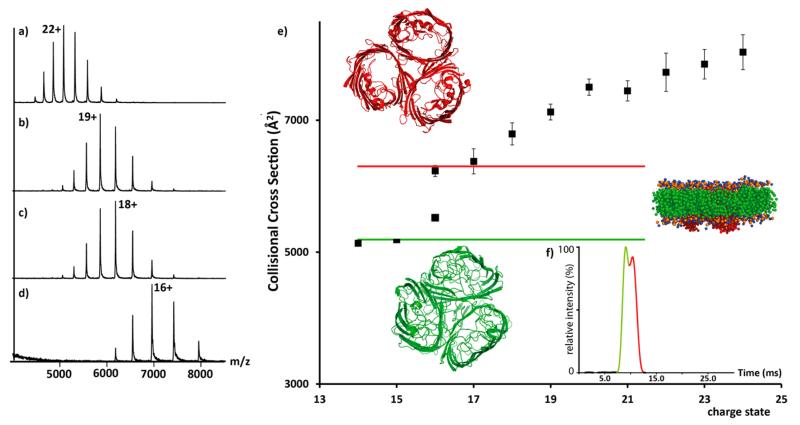
First we used established techniques for membrane protein MS, expelling OmpF from OG micelles in the gas phase (Figure 1a). An average charge state of +22 was observed. We then admitted organic solvent to the source housing to surround the ES plume and observed a reduction in the mean charge state to +19 (Figure 1b). Similarly addition of imidazole to solutions containing OmpF in OG micelles resulted in a decrease to +18 (Figure 1c). We then investigated the effect of adding both charge reducing agents simultaneously. This resulted in further reduction to +16 implying that combination of these charge reducing agents is additive (Figure 1d).
Figure 1.
Charge reduction of the trimeric outer membrane protein OmpF. (a) Mass spectra recorded for a solution containing 200 mM ammonium acetate and 1% OG (standard conditions), (b) exposed to acetonitrile vapor, (c) 10 mM imidazole added in solution, and (d) exposed to acetonitrile with addition of imidazole. (e) Plot of average CCS measured for all charge states of OmpF. Theoretical CCS calculated from the PDB (3O0E)17 and for collapsed states derived from MD (red and green, respectively). (f) Arrival time distribution of the +16 charge state showing peak splitting coincident with CCS of native-like and partially collapsed structures (red and green, respectively). Inset: structure of OmpF highlighting its disc-like structure embedded in the micelle.
Applying IM–MS to measure CCS of the charge states of OmpF from the different solution conditions we found that for the +24 to +25 ions, the CCS was ~7900 Å2, a higher value than estimated from the crystal structure (3O0E)17 (6180 Å2). We attribute this increase to Coulombic unfolding of the complex, which retains its trimeric interactions and demonstrates that standard ES conditions are not ideal for maintaining folded structures.18 The CCS for the +17 (6300 Å2) is closer to the X-ray value (Figure 1e). The +16 ions show two populations coincident with folded and collapsed states (Figure 1f). The +14 and +15 ions have CCS lower than the X-ray value, in close agreement with a collapsed structure generated from MD simulations (Figures 1 and S1a, Supporting Information (SI)).
Turning to AmtB, which in contrast to OmpF is helical, previous MS studies have shown it is challenging to retain compact states in many detergents.6 Peaks devoid of detergent were observed in OG (Figure S2a, SI). The trimeric oligomer (+21) was, however, accompanied by monomeric and dimeric species. The asymmetric nature of the charge states observed is indicative of dissociation in the gas-phase.19 Applying charge reduction we found that exposure to acetonitrile, or addition of imidazole, increased the population of the trimer and decreased dissociation. Combining both charge reduction strategies resulted in +16 ions (Figure S2d, SI) reducing average charge state by approximately five, sufficient to stabilize the trimer. This highlights the importance of charge reduction for determining oligomeric states.
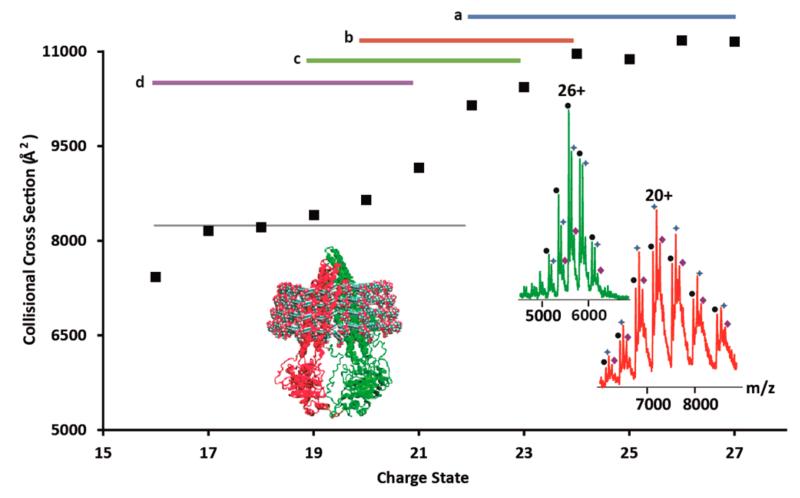
We next applied our approach to P-gp, a monomeric protein shown previously to populate two conformations restricted to maltoside detergents for solubility. Mass spectra of P-gp introduced from DDM micelles reveal a greater difference in charge states than for AmtB or OmpF with a reduction of approximately eight charges (Figure S3, SI). For charge states +22 to +26 the CCS was ~11000 Å2, significantly higher than the estimated CCS (8240 Å2) based on the crystal structure of P-gp (3G5U),21 after modeling the missing loops (supplementary methods and Figure S1b, SI, and Figure 2). Charge states obtained following acetonitrile exposure, or addition of imidazole, showed a significant decrease in CCS with peak broadening indicating that unfolded and partially folded conformations coexist. Combining charge reducing methods resulted in an average charge state of +18 (Figure S3, SI) with calculated CCS in agreement with theoretical CCS values. Interestingly the lowest charge state observed (+16) showed a reduction in CCS potentially attributed to partial collapse of the P-gp structure, as observed for OmpF above. It was thus possible to preserve compact conformations of P-gp selectively by reducing charge states.
Figure 2.
Charge reduction and its effect on the CCS of P-gp. Plot of the average CCS measured for different charge states before and after charge reduction as in Figure 1a–d. Inset spectra for cardiolipin binding to P-gp with (red) and without charge reduction (green). Inset structure of P-gp in a DDM micelle.
To investigate whether charge reducing reagents perturb folded structures of P-gp, we studied the effects of imidazole on the solution conformation of P-gp using circular dichroism spectroscopy. We confirmed that the overall fold was retained (Figure S4, SI). Ligand binding properties were examined by adding solutions of cardiolipin to P-gp. We found enhanced preservation of protein–lipid complexes following charge reduction (Figures 2 (inset) and S5, SI). Together these experiments show that charge reduction of P-gp maintains native-like states and stabilizes lipid binding.
Charge states obtained for all membrane complexes studied here without charge reduction are lower than for folded soluble complex (Figure S6a, SI).22 Comparing their response to charge reduction, OmpF and AmtB are encapsulated within the micelle providing limited access to reagents but were charge reduced (~+23 to ~+15) (Figures 1 and S6b, SI). By contrast P-gp provides greater access to soluble domains and forms the largest range of charge states (+29 to +16) (Figures 2 and S6c, SI). The lowest charge states accessed by both OmpF and P-gp exhibit collapsed states. This phenomenon has been reported for soluble proteins12 and for other β-barrel membrane proteins.3,5 For P-gp this state could form following transition to the outward facing conformer with a lower CCS or by a decrease in the angle at the apex of the hydrophobic cavity, as shown previously.23
Considering possible mechanisms, for soluble complexes it is thought that imidazole forms nonspecific contacts to protein as evaporation occurs.24 This may reduce surface accessibility of the protein to charging, due to its basic nature, either by competing for charge during ES or during evaporation of imidazole from the complex. Solvents likely influence the surface tension of ES droplets, which defines the Rayleigh instability limit, and consequently the charge accommodated without fission.25 Applying both strategies simultaneously results in significant charge reduction and was necessary to maintain folded states of P-gp, both mechanisms being operable; changing surface tension and competing for charge.
In summary, as MS is applied to a growing number of membrane protein complexes, the detergents used for their introduction will expand. Consequently charge reduction strategies that are independent of detergent will become increasingly important for maintaining folded states when detergent choice is limited and saccharide detergents are preferred. Charge reduction of membrane proteins in detergents that mimic closely the membrane bilayer is therefore critical, not only for establishing binding26 but also for quantifying its effects using unfolding strategies in which the starting point is a compact state.6
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge with thanks E. Reading and A. Laganowsky for the AmtB plasmid and N. Housden and C. Kleanthous for OmpF (University of Oxford). We thank G. Chang (University of California, San Diego) for P-gp and funding from ERC IMPRESS (26851) and the Medical Research Council (98101).
Footnotes
Materials and methods, Figures S1–S6, and Table S1. This material is available free of charge via the Internet at http://pubs.acs.org..
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Seddon AM, Curnow P, Booth PJ. Biochim. Biophys. Acta. 2004;1666:105. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- (2).Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Science. 2008;321:243. doi: 10.1126/science.1159292. [DOI] [PubMed] [Google Scholar]
- (3).Borysik AJ, Hewitt DJ, Robinson CV. J. Am. Chem. Soc. 2013;135:6078. doi: 10.1021/ja401736v. [DOI] [PubMed] [Google Scholar]
- (4).Zhou M, Politis A, Davies RB, Liko I, Wu KJ, Stewart AG, Stock D, Robinson CV. Nat. Chem. 2014;6:208. doi: 10.1038/nchem.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Marcoux J, Politis A, Rinehart D, Marshall DP, Wallace MI, Tamm LK, Robinson CV. Structure. 2014;22:781. doi: 10.1016/j.str.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Nature. 2014;510:172. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Clemmer DE, Hudgins RR, Jarrold MF. J. Am. Chem. Soc. 1995;117:10141. [Google Scholar]
- (8).Pagel K, Hyung SJ, Ruotolo BT, Robinson CV. Anal. Chem. 2010;82:5363. doi: 10.1021/ac101121r. [DOI] [PubMed] [Google Scholar]
- (9).Hall Z, Robinson CV. J. Am. Soc. Mass Spectrom. 2012;23:1161. doi: 10.1007/s13361-012-0393-z. [DOI] [PubMed] [Google Scholar]
- (10).Scalf M, Westphall MS, Krause J, Kaufman SL, Smith LM. Science. 1999;283:194. doi: 10.1126/science.283.5399.194. [DOI] [PubMed] [Google Scholar]
- (11).Catalina MI, van den Heuvel RH, van Duijn E, Heck AJ. Chemistry. 2005;11:960. doi: 10.1002/chem.200400395. [DOI] [PubMed] [Google Scholar]
- (12).Hall Z, Politis A, Bush MF, Smith LJ, Robinson CV. J. Am. Chem. Soc. 2012;134:3429. doi: 10.1021/ja2096859. [DOI] [PubMed] [Google Scholar]
- (13).Hopper JT, Oldham NJ. Anal. Chem. 2011;83:7472. doi: 10.1021/ac201686f. [DOI] [PubMed] [Google Scholar]
- (14).Hopper JT, Sokratous K, Oldham NJ. Anal. Biochem. 2012;421:788. doi: 10.1016/j.ab.2011.10.034. [DOI] [PubMed] [Google Scholar]
- (15).Morgner N, Montenegro F, Barrera NP, Robinson CV. J. Mol. Biol. 2012;423:1. doi: 10.1016/j.jmb.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Parker JL, Newstead S. Protein Sci. 2012;21:1358. doi: 10.1002/pro.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Housden NG, Wojdyla JA, Korczynska J, Grishkovskaya I, Kirkpatrick N, Brzozowski AM, Kleanthous C. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21412. doi: 10.1073/pnas.1010780107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Ruotolo BT, Hyung SJ, Robinson PM, Giles K, Bateman RH, Robinson CV. Angew. Chem., Int. Ed. 2007;46:8001. doi: 10.1002/anie.200702161. [DOI] [PubMed] [Google Scholar]
- (19).Benesch JL, Aquilina JA, Ruotolo BT, Sobott F, Robinson CV. Chem. Biol. 2006;13:597. doi: 10.1016/j.chembiol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- (20).Marcoux J, Wang SC, Politis A, Reading E, Ma J, Biggin PC, Zhou M, Tao H, Zhang Q, Chang G, Morgner N, Robinson CV. Proc. Natl. Acad. Sci. U.S.A. 2013;110:9704. doi: 10.1073/pnas.1303888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Science. 2009;323:1718. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Testa L, Brocca S, Grandori R. Anal. Chem. 2011;83:6459. doi: 10.1021/ac201740z. [DOI] [PubMed] [Google Scholar]
- (23).Ward AB, Szewczyk P, Grimard V, Lee CW, Martinez L, Doshi R, Caya A, Villaluz M, Pardon E, Cregger C, Swartz DJ, Falson PG, Urbatsch IL, Govaerts C, Steyaert J, Chang G. Proc. Natl. Acad. Sci. U.S.A. 2013;110:13386. doi: 10.1073/pnas.1309275110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sun D, Rosokha SV, Kochi JK. J. Phys. Chem. B. 2007;111:6655. doi: 10.1021/jp068994o. [DOI] [PubMed] [Google Scholar]
- (25).Konermann L. J. Am. Soc. Mass Spectrom. 2009;20:496. doi: 10.1016/j.jasms.2008.11.007. [DOI] [PubMed] [Google Scholar]
- (26).Hopper JT, Robinson CV. Angew. Chem., Int. Ed. 2014 doi: 10.1002/anie.201403741. DOI: 10.1002/anie.201403741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.