Abstract
Background
In small studies and cases series, a history of tuberculosis has been associated with both airflow obstruction, which is characteristic of chronic obstructive pulmonary disease, and restrictive patterns on spirometry.
Objective
To assess the association between a history of tuberculosis and airflow obstruction and spirometric abnormalities in adults.
Methods
The study was performed in adults, aged 40 and above, who took part in the multicentre cross-sectional, general population-based, Burden of Obstructive Lung Disease study, had provided acceptable post-bronchodilator spirometry measurements and information on a history of tuberculosis.
The associations between a history of tuberculosis and airflow obstruction and spirometric restriction were assessed within each participating centre, and estimates combined using meta-analysis. These estimates were stratified by high and low/middle income countries, according to gross national income.
Results
A self-reported history of tuberculosis was associated with airflow obstruction (adjusted odds ratio = 2.51, 95% confidence interval 1.83-3.42) and spirometric restriction (adjusted odds ratio = 2.13, 95% confidence interval 1.42-3.19).
Conclusion
A history of tuberculosis was associated with both airflow obstruction and spirometric restriction, and should be considered as a potentially important cause of obstructive disease and low lung function, particularly where tuberculosis is common.
Keywords: Tuberculosis, airflow obstruction, chronic obstructive pulmonary disease, spirometric restriction
Introduction
In 2012, there were an estimated 8.6 million new cases of tuberculosis worldwide (1), with South-East Asia, Western Pacific Regions, and Africa accounting for more than 75% of the toll. More than 30% of the world population may have latent tuberculosis, but only 5-20% of them develop active tuberculosis at some point in their lifetime (1, 2). Those who survive it usually show post-treatment sequelae in the lung that may contribute to reduced quality of life and disability (3-5).
Airflow obstruction is characteristic of chronic obstructive pulmonary disease (COPD), and its main risk factor is tobacco smoking (6, 7). However, more than 20% of patients satisfying the criteria for COPD do not have a history of tobacco smoking (8, 9). Among other potential risk factors, a history of tuberculosis has been suggested by several studies as a strong predictor of chronic airflow obstruction that could explain COPD among non-smokers (9-11). With some exceptions, most of these studies were small (n < 1000), not population-based (i.e. participants not randomly selected from general population) and limited to a single centre or country, and several used pre-bronchodilator instead of post-bronchodilator spirometric measurements (11).
Spirometric restriction is characteristic of restrictive lung diseases and has been reported as a consequence of tuberculosis since the late 1910s (12, 13). More recent epidemiological studies with South African miners and hospital-based cases have suggested that a history of tuberculosis and increasing number of events of this disease may lead to a deficit in lung function (14-18). However, population data to support the association between a history of tuberculosis and spirometric restriction is lacking.
The aim of the present analysis was to assess the association of airflow obstruction and spirometric restriction with a history of tuberculosis in the large international, population-based, Burden of Obstructive Lung Disease (BOLD) study.
Methods
Participants
The design and rationale for the BOLD study have been reported elsewhere (19). Non-institutionalised adults aged 40 years and older were sampled and invited to take part in the study. Sampling plans designed to randomly recruit a representative sample of the population at all study sites were used.
Of the 21,962 participants who responded to the core questionnaire, 18,669 had acceptable post-bronchodilator spirometry, and of these 18,664 answered a question on history of tuberculosis. Data were available from 27 sites, but Australia (Sydney), India (Mumbai, Srinagar), Malaysia (Penang), Nigeria (Ife), Norway (Bergen), Tunisia (Sousse), Turkey (Adana), which each contained less than five participants with history of tuberculosis, were excluded; therefore the present study population consists of 14,050 participants from 19 sites. The countries and sites represented in this analysis are: Albania (Tirana), Algeria (Annaba), Austria (Salzburg), Canada (Vancouver), China (Guangzhou), England (London), Estonia (Tartu), Germany (Hannover), Iceland (Reykjavik), India (Pune), Morocco (Fes), Netherlands (Maastricht), Philippines (Manila, Nampicuan & Talugtug), Poland (Krakow), Portugal (Lisbon), South Africa (Cape Town), Sweden (Uppsala), and USA (Lexington). All sites received approval from their local ethics committee, and participants provided written informed consent.
History of tuberculosis
Face-to-face interviews were conducted by trained and certified staff in the native language of the participant in order to collect information on respiratory symptoms, health status, and exposure to risk factors. A history of tuberculosis was defined as a positive answer to the question: “Has a doctor or other health care provider ever told you that you have tuberculosis?” Participants who were on treatment for tuberculosis at the time of the study were excluded from participation.
Outcome measures
Lung function, including forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), was measured using the ndd EasyOne Spirometer (ndd Medizintechnik AG, Zurich, Switzerland), before and 15 minutes after administration of salbutamol (200 μg) from a metered dose inhaler through a spacer. The BOLD Pulmonary Function Reading Centre reviewed each spirogram and assigned them a quality score based on acceptability and reproducibility criteria from the American Thoracic Society (ATS) and European Respiratory Society (ERS) (20). Spirometry technicians at BOLD sites were certified before data collection, received regular feedback on quality, and were required to maintain a pre-specified quality standard.
Outcome measures were: i) airflow obstruction, defined as a post-bronchodilator FEV1/FVC ratio below the low limit of normal (LLN) for age and sex (21), based on reference equations for Caucasians derived from the third US National Health and Nutrition Examination Survey (NHANES) (22); and ii) spirometric restriction, defined as a post-bronchodilator FVC below the LLN for height, age and sex, based on the same reference population.
Statistical analysis
To assess the association of airflow obstruction and spirometric restriction with history of tuberculosis, multivariable logistic regression models were fitted and adjusted for age (years), sex, body mass index (underweight: <18.5, normal: 18.5 to <24, overweight: 24 to <30, obese: 30+ kg/m2), and pack-years of smoking. Additional variables were considered as potential confounders: education (years of schooling complete), passive smoking (yes, no), and cumulative exposure to dust in the workplace (years). The association with a history of tuberculosis was estimated for each site using probability weights to allow for the sampling design at each site and then combined in a random effects meta-analysis. The meta-analyses were stratified by gross national income, i.e. high vs low/middle income countries. The level of heterogeneity was summarised using the I2 statistic. We also regressed FEV1/FVC and FVC (L) as continuous variables against the same independent variables as above. Sensitivity analyses were conducted excluding participants presenting with both airflow obstruction and spirometric restriction. In another set of sensitivity analyses, the association of a history of tuberculosis with airflow obstruction, spirometric restriction, FEV1/FVC, and FVC was assessed omitting all sites with a cooperation rate below 60%. All analyses were conducted using Stata/IC V.12.1 (StataCorp LP, College Station, TX, USA).
Results
The characteristics of the 14,050 participants with acceptable post-bronchodilator spirometry, who responded to the core questionnaire and answered the question on history of tuberculosis are presented, by site, in table 1. There were slightly more females than males, and the overall age ranged from 52.3 to 59.6 across sites. Cumulative smoking exposure, i.e. pack-years, and passive smoking varied widely across sites. The prevalence of a history of tuberculosis [0.7% in Albania (Tirana) to 15.4% in South Africa (Cape Town)] as well as the prevalence of airflow obstruction [6.1% in Estonia (Tartu) and India (Pune) to 19.5% in South Africa (Cape Town)] and spirometric restriction [8.5% in Canada (Vancouver) and Estonia (Tartu) to 66.1% in India (Pune)] also varied across sites (Tables 1 and 2).
Table 1.
Characteristics of participants from 19 sites of the Burden of Obstructive Lung Disease (BOLD) study with good quality spirometry and data on history of tuberculosis (at least 5 cases).
| Albania (Tirana) | Algeria (Annaba) | Austria (Salzburg) | Canada (Vancouver) | China (Guangzhou) | England (London) | Estonia (Tartu) | Germany (Hannover) | Iceland (Reykjavik) | India (Pune) | Morocco (Fes) | Netherlands (Maastricht) | Philippines (Manila) | Philippines (Nampicuan & Talugtug) | Poland (Krakow) | Portugal (Lisbon) | South Africa (Cape Town) | Sweden (Uppsala) | USA (Lexington) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 939 | 890 | 1253 | 827 | 461 | 675 | 613 | 680 | 757 | 845 | 768 | 590 | 892 | 722 | 526 | 711 | 846 | 547 | 508 |
| Age (yrs), mean (SD) | 55.4 (11.7) | 53.5 (10.9) | 59.2 (12.2) | 56.8 (12.7) | 54.0 (10.6) | 58.0 (12.4) | 59.6 (12.2) | 57.7 (11.1) | 57.0 (12.0) | 52.3 (10.0) | 54.2 (11.9) | 58.5 (12.0) | 52.7 (11.1) | 54.2 (10.6) | 56.2 (11.8) | 58.5 (12.0) | 53.5 (10.5) | 58.9 (11.4) | 57.0 (11.6) |
| Males (%) | 48.9 | 49.9 | 46.1 | 47.3 | 52.0 | 46.2 | 39.1 | 45.4 | 51.3 | 59.8 | 52.2 | 46.8 | 47.5 | 49.4 | 49.6 | 45.3 | 43.8 | 47.1 | 46.4 |
| BMI (kg/m2), mean (SD) * | 28.0 (4.6) | 28.2 (5.6) | 26.4 (4.3) | 26.7 (5.1) | 23.3 (3.2) | 27.3 (5.2) | 28.4 (5.4) | 27.1 (4.6) | 27.9 (5.0) | 22.1 (3.9) | 27.5 (5.2) | 27.5 (4.6) | 24.4 (4.7) | 21.6 (4.1) | 27.8 (4.7) | 27.9 (4.7) | 27.5 (7.3) | 26.9 (4.4) | 30.6 (6.5) |
| Pack-years, mean (SD) ** | 11.5 (19.3) | 10.4 (18.3) | 12.7 (20.7) | 12.1 (21.4) | 11.8 (17.7) | 17.5 (27.4) | 7.4 (13.0) | 14.6 (20.4) | 12.9 (24.9) | 0.7 (3.6) | 6.6 (14.9) | 14.5 (19.0) | 10.6 (18.6) | 12.9 (18.4) | 15.8 (25.5) | 13.1 (25.0) | 11.9 (16.1) | 10.4 (16.2) | 24.4 (34.7) |
| Passive smoking (%) | 37.4 | 10.9 | 21.8 | 5.7 | 23.6 | 16.6 | 15.6 | 18.6 | 16.8 | 11.1 | 11.4 | 17.7 | 48.8 | 47.3 | 39.3 | 18.7 | 50.6 | 5.8 | 29.6 |
| Education (yrs), mean (SD) † | 10.0 (4.6) | 7.7 (5.4) | 9.8 (2.2) | 15.4 (3.4) | 8.4 (3.9) | 13.6 (3.6) | 13.5 (3.8) | 10.3 (2.2) | 13.2 (4.4) | 4.3 (4.3) | 4.2 (5.3) | 14.9 (5.1) | 9.4 (3.6) | 7.8 (3.6) | 10.4 (3.4) | 8.5 (4.9) | 7.8 (3.3) | 12.8 (4.0) | 12.8 (3.3) |
| Cumulative exposure to dust in workplace (yrs), mean (SD) ‡ | 15.0 (14.1) | 5.6 (10.3) | 5.2 (11.7) | 3.1 (7.5) | 6.9 (11.6) | 4.0 (9.7) | 5.0 (10.1) | 3.3 (8.7) | 4.2 (9.6) | 1.8 (5.5) | 8.5 (12.8) | 3.3 (8.8) | 7.1 (10.8) | 6.1 (11.8) | 10.5 (13.4) | 10.6 (14.3) | 6.8 (10.3) | 5.5 (11.3) | 8.3 (12.1) |
| PB-FEV1 (l/s), mean (SD) | 2.8 (0.8) | 2.7 (0.8) | 2.9 (0.9) | 3.0 (0.9) | 2.4 (0.7) | 2.7 (0.9) | 2.9 (0.9) | 3.0 (0.8) | 3.0 (0.9) | 2.2 (0.6) | 2.7 (0.7) | 2.9 (0.9) | 2.1 (0.6) | 2.1 (0.7) | 2.9 (0.9) | 2.7 (0.9) | 2.3 (0.7) | 3.0 (0.9) | 2.7 (0.9) |
| PB-FVC (l), mean (SD) | 3.5 (0.9) | 3.4 (0.9) | 3.9 (1.0) | 4.0 (1.1) | 3.1 (0.8) | 3.6 (1.1) | 3.8 (1.1) | 3.9 (1.0) | 4.0 (1.0) | 2.7 (0.7) | 3.4 (0.9) | 3.8 (1.1) | 2.6 (0.7) | 2.7 (0.8) | 3.8 (1.0) | 3.4 (1.1) | 3.0 (0.9) | 3.9 (1.1) | 3.5 (1.1) |
| History of tuberculosis (%) | 0.7 | 2.2 | 2.9 | 3.2 | 3.5 | 2.1 | 7.0 | 3.6 | 4.0 | 0.9 | 1.4 | 1.4 | 10.8 | 3.6 | 2.8 | 4.5 | 15.4 | 1.1 | 1.9 |
SD, standard deviation. BMI, body mass index. PB-FEV1, post-bronchodilator forced expiratory volume in 1 second. PB-FVC, post-bronchodilator forced vital capacity. Education, years of schooling complete.
Missing: 3 in Poland (Krakow); 2 in South Africa (Cape Town); and 1 in USA (Lexington).
Missing: 7 in Philippines (Manila); 3 in Philippines (Nampicuan & Talugtug) and Poland (Krakow); 2 in Canada (Vancouver), South Africa (Cape Town) and Netherlands (Maasstricht); and 1 in Iceland (Reykjavik), Morocco (Fes) and Sweden (Uppsala).
Missing: 7 in Philippines (Nampicuan & Talugtug); 5 in England (London); and 1 in Estonia (Tartu), Morocco (Fes), Portugal (Lisbon) and South Africa (Cape Town).
Missing: 4 in Netherlands (Maasstricht).
Table 2.
Estimated population prevalence of airflow obstruction and spirometric restriction in 19 sites of the Burden of Obstructive Lung Disease (BOLD) study with good quality spirometry and data on history of tuberculosis (at least 5 cases).
| Albania (Tirana) | Algeria (Annaba) | Austria (Salzburg) | Canada (Vancouver) | China (Guangzhou) | England (London) | Estonia (Tartu) | Germany (Hannover) | Iceland (Reykjavik) | India (Pune) | Morocco (Fes) | Netherlands (Maastricht) | Philippines (Manila) | Philippines (Nampicuan & Talugtug) | Poland (Krakow) | Portugal (Lisbon) | South Africa (Cape Town) | Sweden (Uppsala) | USA (Lexington) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 939 | 890 | 1253 | 827 | 461 | 675 | 613 | 680 | 757 | 845 | 768 | 590 | 892 | 722 | 526 | 711 | 846 | 547 | 508 |
| Airflow obstruction (%) | 8.9 | 6.4 | 17.4 | 13.5 | 7.6 | 17.6 | 6.1 | 8.2 | 11.3 | 6.1 | 8.9 | 18.8 | 9.4 | 15.2 | 13.5 | 8.3 | 19.5 | 9.6 | 14.4 |
| Spirometric restriction (%) | 16.1 | 26.5 | 9.3 | 8.5 | 29.9 | 17.8 | 8.5 | 9.0 | 12.5 | 66.1 | 19.3 | 10.1 | 62.7 | 56.7 | 10.1 | 10.7 | 46.7 | 10.2 | 26.2 |
| Response rate (%) * | 82.3 | 94.6 | 65.0 | 26.0 | 87.0 | 17.0 | 49.0 | 59.0 | 81.0 | 97.0 | 98.0 | 48.0 | 58.0 | 85.5 | 78.0 | 10.0 | 63.0 | 61.0 | 14.0 |
| Cooperation rate (%) ** | 84.0 | 94.6 | 67.0 | 51.0 | 87.0 | 37.0 | 70.0 | 61.0 | 84.0 | 97.0 | 98.0 | 55.0 | 58.0 | 86.2 | 79.0 | 27.0 | 68.0 | 63.0 | 27.0 |
Airflow obstruction, FEV1/FVC < LLN. Spirometric restriction, FVC < LLN.
Denominator comprises people of unknown eligibility status who could not be contacted. Only known participants considered ineligible were excluded.
Denominator comprises only participants who were contacted and eligible.
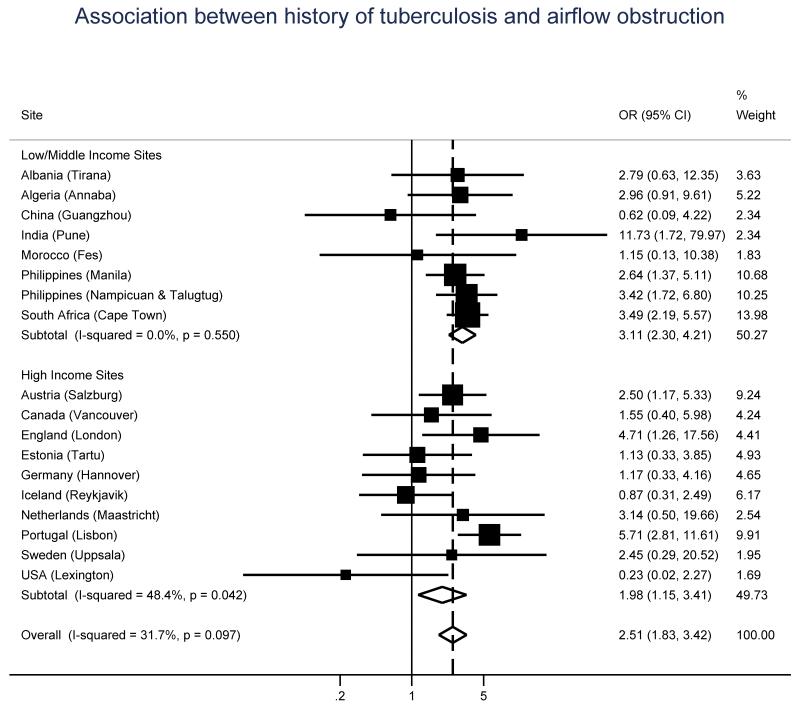
The unadjusted odds ratio (OR), and 95% confidence interval (CI), for the association between airflow obstruction and history of tuberculosis was 3.33 (2.54-4.37). Figure 1 shows adjusted ORs, and 95% CIs, for this association, by gross national income group and site. Overall, the risk of airflow obstruction in people with a history of tuberculosis was more than twice as much as that of people without such a history (aOR = 2.51, 95% CI 1.83-3.42). This association was stronger in low/middle income sites (aOR = 3.11, 95% CI 2.30-4.21) and showed no evidence of heterogeneity (I2 = 0%, P = 0.55).
Figure 1.
Odds ratios of airflow obstruction for a history of tuberculosis, by gross national income group (low/middle vs high) and site. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
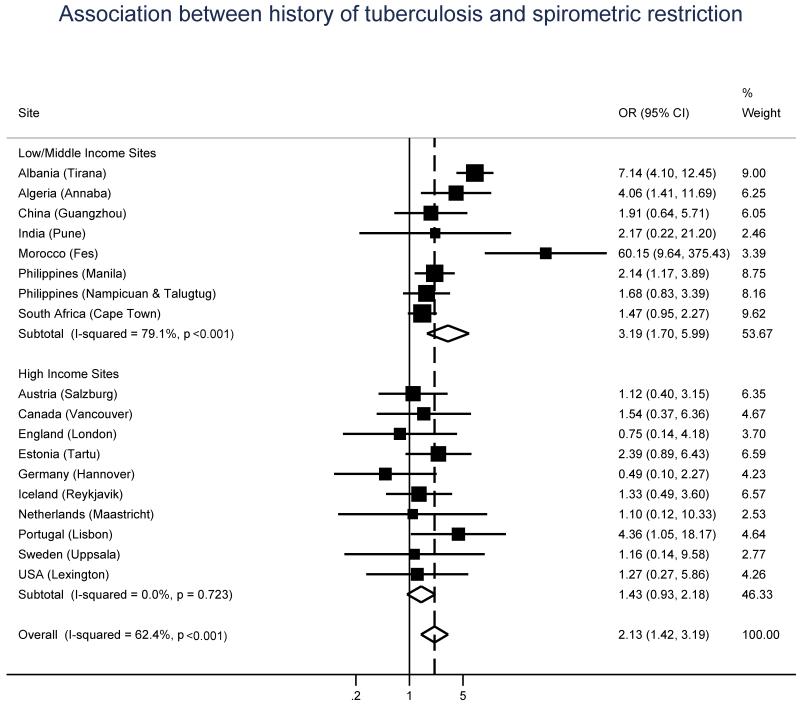
The unadjusted OR, and 95% CI, for the association between spirometric restriction and history of tuberculosis was 2.02 (1.42-2.86). Figure 2 shows adjusted ORs, and 95% CIs, by gross national income group and site for this association. The overall pooled aOR was 2.13 (95% CI 1.42-3.19), and significant heterogeneity across sites was recorded (I2 = 62.4%, P < 0.001). In high income sites there was no evidence of heterogeneity (I2 = 0%; p=0.72) but the risk was low and not significant (aOR = 1.43, 95% CI 0.93, 2.18). In low income countries, although the risk was higher and significant (aOR = 3.19; 95% CI 1.70, 5.99) there was a marked and unexplained heterogeneity in the risk estimates (I2 = 79.1%; p<0.001). In both figures 1 and 2, ORs are adjusted for age, sex, body mass index, and pack-years. Adjustment for education, passive smoking, and cumulative exposure to dust in the workplace did not materially change the estimates for the effect of tuberculosis. Poland (Krakow) was excluded from the analyses due to insufficient number of participants with both history of tuberculosis and either airflow obstruction (n = 0) or spirometric restriction (n = 2).
Figure 2.
Odds ratios of spirometric restriction for a history of tuberculosis, by gross national income group (low/middle vs high) and site. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
A history of tuberculosis was also associated with both decreased FEV1/FVC (beta = −3.43, 95% CI −5.05 to −1.80; I2 = 65.3%, P < 0.001) and decreased FVC (beta = −0.15, 95% CI −0.23 to −0.06; I2 = 48.6%, P = 0.01) (supplementary figures 1 and 2).
Sensitivity analyses, excluding 482 participants who had both FEV1/FVC<LLN and FVC<LLN, showed that the magnitude of the effects of tuberculosis reduced slightly but remained statistically significant (aOR for airflow obstruction = 2.13, 95% CI 1.40-3.23; aOR for spirometric restriction = 2.11, 95% CI 1.31-3.38), suggesting that these effects are largely independent of each other.
The omission of sites with a cooperation rate below 60% did not materially change the results (supplementary figures 3-6).
Discussion
In this population-based study of adults aged 40 years and over, a history of tuberculosis was associated with increased risk of airflow obstruction. A history of tuberculosis was also associated with spirometric restriction, but mainly in sites in low/middle income countries. The strengths of the present study are: i) its large population-based sample and the inclusion of a great number of sites; ii) the use of a standardised questionnaire for collection of data on risk factors and protocol for spirometry across sites; and iii) the use of post- instead of pre-bronchodilator spirometric measurements. The most convincing effect relates to obstruction in low/middle income countries where the odds ratio was high (OR = 3.11) and the results were consistent between sites (I2 = 0%).
Our study also has some limitations. One is its cross-sectional nature, which impedes us from drawing conclusions in terms of temporality and makes us consider the possibility of reverse causation. Tuberculosis is more common in people with some restrictive diseases such as silicosis, but these are relatively very rare. Tuberculosis may also be reactivated in those who take corticosteroids, and particularly inhaled corticosteroid treatment recommended in chronic airway disease. However, their use is rare in this population and even rarer in the low/middle income countries where the association between tuberculosis and airflow obstruction is most pronounced. In some sites response rates were lower than desirable, but when we omitted all sites with a cooperation rate below 60% results did not materially change. Another limitation is the use of data on a self-reported history of tuberculosis, which may suffer from under-reporting due to stigmatisation of the diagnosis. However, differential under-reporting due to stigmatisation between people with and without airflow obstruction or spirometric restriction seems unlikely. It is also possible that several participants have suffered from tuberculosis and healed without any treatment and thus tuberculosis infection may be underestimated. However, in a study in China, the magnitude of the association of airflow obstruction with tuberculosis was similar between self-reports and radiological confirmation (23). According to ATS/ERS (24), pulmonary restriction is defined by a total lung capacity (TLC) less than the fifth percentile of the predicted value. This implies measuring TLC by plethysmography or helium dilution, which is unrealistic in large-scale epidemiological studies, such as this, and especially at centres in low/middle income countries. We are mindful that our choice of FVC as a surrogate of TLC may lead to false positive findings in those with increased residual volumes, but outside the clinical environment the prevalence of this is very low. We are also aware that the use of the NHANES reference equations in our spirometry measurements may overemphasize lung function abnormality in some study sites, but the effect of this is unlikely to be differential as the analyses were done within each site (the sites are ethnically fairly homogeneous) and only then were meta-analysed. In addition, the results from the binary outcomes (FEV1/FVC < LLN, and FVC < LLN) are supported by those of the continuous outcomes (FEV1/FVC, and FVC), which are independent of reference equations.
Our findings add to existing knowledge and support the majority of previous smaller studies that have reported an association between airflow obstruction and a history of tuberculosis (10, 11, 25). We also confirm findings from the few occupational and small hospital-based studies that have observed a decline in lung function associated with both history of and radiographically-confirmed tuberculosis (14-18).
Although it is widely accepted that tuberculosis and the healing process the lung undergoes during and after treatment can cause scarring that leads to loss of parenchymal tissue and restrictive spirometry, it is not clear what mechanisms explain airflow obstruction associated with tuberculosis. The finding that tuberculosis is associated with airflow obstruction, and not only with spirometric restriction, suggests that this is not solely the result of parenchymal scarring. One possibility is that this is caused by bronchiectasis and bronchial stenosis, which can occur as a result of tuberculosis (26). Another possibility is that this is caused by a dysregulation of macrophages arising from latent intracellular infection (27). Macrophages in the lung act primarily to kill bacteria or to facilitate wound healing and resolution (28), and it is widely accepted that they play a central role in the remodelling that causes chronic airflow obstruction. It is possible that latent mycobacteria in lung macrophages could lead to maintenance of inflammation in the lung and more aggressive remodelling of the airways (28, 29).
In summary, a history of tuberculosis was associated with both airflow obstruction and spirometric restriction. Nevertheless, large longitudinal studies with post-bronchodilator spirometry are recommended to confirm or refute these findings. With the continuing spread of tuberculosis in developing countries, an increasing incidence of multi-drug resistant disease, and an aging world population, it is important to improve our understanding of the mechanisms that link tuberculosis to airflow obstruction and COPD, and to devise effective strategies to limit this problem.
Supplementary Material
Supplementary figure 1. Change in FEV1/FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 2. Change in FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 3. Odds ratios of airflow obstruction for a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 4. Odds ratios of spirometric restriction for a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 5. Change in FEV1/FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 6. Change in FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Acknowledgements
We want to thank the participants and field workers of this study for their time and cooperation. We also want to thank Anamika Jithoo and the BOLD Coordinating Centre (UK) members not included in the author list for their technical and scientific support.
Funding
The BOLD Study is funded by a grant from the Wellcome Trust (085790/Z/08/Z). The initial BOLD programme was funded in part by unrestricted educational grants to the Operations Center in Portland, Oregon from ALTANA, Aventis, AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Merck, Novartis, Pfizer, Schering-Plough, Sepracor, and the University of Kentucky. Additional local support for BOLD sites was provided by Boehringer Ingelheim China. (GuangZhou, China); Turkish Thoracic Society, Boehringer-Ingelheim, and Pfizer (Adana, Turkey); Altana, Astra-Zeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck Sharpe & Dohme, Novartis, Salzburger Gebietskrankenkasse and Salzburg Local Government (Salzburg, Austria); Research for International Tobacco Control, the International Development Research Centre, the South African Medical Research Council, the South African Thoracic Society GlaxoSmithKline Pulmonary Research Fellowship, and the University of Cape Town Lung Institute (Cape Town, South Africa); and Landspítali-University Hospital-Scientific Fund, GlaxoSmithKline Iceland, and AstraZeneca Iceland (Reykjavik, Iceland); GlaxoSmithKline Pharmaceuticals, Polpharma, Ivax Pharma Poland, AstraZeneca Pharma Poland, ZF Altana Pharma, Pliva Kraków, Adamed, Novartis Poland, Linde Gaz Polska, Lek Polska, Tarchomińskie Zaklady Farmaceutyczne Polfa, Starostwo Proszowice, Skanska, Zasada, Agencja Mienia Wojskowego w Krakowie, Telekomunikacja Polska, Biernacki, Biogran, Amplus Bucki, Skrzydlewski, Sotwin, and Agroplon (Krakow, Poland); Boehringer-Ingelheim, and Pfizer Germany (Hannover, Germany); the Norwegian Ministry of Health’s Foundation for Clinical Research, and Haukeland University Hospital’s Medical Research Foundation for Thoracic Medicine (Bergen, Norway); AstraZeneca, Boehringer-Ingelheim, Pfizer, and GlaxoSmithKline (Vancouver, Canada); Marty Driesler Cancer Project (Lexington, Kentucky, USA); Altana, Boehringer Ingelheim (Phil), GlaxoSmithKline, Pfizer, Philippine College of Chest Physicians, Philippine College of Physicians, and United Laboratories (Phil) (Manila, Philippines); Air Liquide Healthcare P/L, AstraZeneca P/L, Boehringer Ingelheim P/L, GlaxoSmithKline Australia P/L, Pfizer Australia P/L (Sydney, Australia), Department of Health Policy Research Programme, Clement Clarke International (London, United Kingdom); Boehringer Ingelheim and Pfizer (Lisbon, Portugal), Swedish Heart and Lung Foundation, The Swedish Association against Heart and Lung Diseases, Glaxo Smith Kline (Uppsala, Sweden); GlaxoSmithKline, Astra Zeneca, Eesti Teadusfond (Estonian Science Foundation) (Tartu, Estonia); AstraZeneca, CIRO HORN (Maastricht, The Netherlands); Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K (Srinagar, India); Foundation for Environmental Medicine, Kasturba Hospital, Volkart Foundation (Mumbai, India); Boehringer Ingelheim (Sousse, Tunisia); Boehringer Ingelheim (Fes, Morocco); Philippines College of Physicians, Philippines College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Orient Euro Pharma, Otsuka Pharma, United laboratories Phillipines (Nampicuan&Talugtug, Philippines); National Heart and Lung Institute, Imperial College, London (Pune, India); The Wellcome Trust, National Population Commission, Ile-Ife, Osun State, Nigeria (Ile-Ife, Nigeria), Kyrgyz Thoracic Society (Bishkek, Kyrgyzstan), GlaxoSmithKline (Tirana, Albania), GSK, Liverpool School of Tropical Medicine, the Malawi Liverpool Wellcome Trust (Blantyre, Malawi), The Saudi Thoracic Society, King Abdullah International Medical Research Center KAIMRC (Riyadh, Saudi Arabia), Salmawit Pharmaceuticals & Medical International Company Limited, The Epidemiological Laboratory (Khartoum, Sudan), Boehringer Ingelheim (Annaba, Algérie), GlaxoSmithKline Pharmaceutical Sdn. Bhd. (Penang, Malaysia), BRAC Health Nutrition and Population Programme (Dhaka, Bangladesh).
BOLD collaborators
NanShan Zhong (PI), Shengming Liu, Jiachun Lu, Pixin Ran, Dali Wang, Jingping Zheng, Yumin Zhou (Guangzhou Institute of Respiratory Diseases, Guangzhou Medical College, Guangzhou, China); Ali Kocabaş (PI), Attila Hancioglu, Ismail Hanta, Sedat Kuleci, Ahmet Sinan Turkyilmaz, Sema Umut, Turgay Unalan (Cukurova University School of Medicine, Department of Chest Diseases, Adana, Turkey); Michael Studnicka (PI), Torkil Dawes, Bernd Lamprecht, Lea Schirhofer (Paracelsus Medical University, Department of Pulmonary Medicine, Salzburg Austria); Eric Bateman (PI), Anamika Jithoo (PI), Desiree Adams, Edward Barnes, Jasper Freeman, Anton Hayes, Sipho Hlengwa, Christine Johannisen, Mariana Koopman, Innocentia Louw, Ina Ludick, Alta Olckers, Johanna Ryck, Janita Storbeck, (University of Cape Town Lung Institute, Cape Town, South Africa); Thorarinn Gislason (PI), Bryndis Benedikdtsdottir, Kristin Jörundsdottir, Lovisa Gudmundsdottir, Sigrun Gudmundsdottir, Gunnar Gundmundsson, (Landspitali University Hospital, Dept. of Allergy, Respiratory Medicine and Sleep, Reykjavik, Iceland); Ewa Nizankowska-Mogilnicka (PI), Jakub Frey, Rafal Harat, Filip Mejza, Pawel Nastalek, Andrzej Pajak, Wojciech Skucha, Andrzej Szczeklik,Magda Twardowska, (Division of Pulmonary Diseases, Department of Medicine, Jagiellonian University School of Medicine, Cracow, Poland); Tobias Welte (PI), Isabelle Bodemann, Henning Geldmacher, Alexandra Schweda-Linow (Hannover Medical School, Hannover, Germany); Amund Gulsvik (PI), Tina Endresen, Lene Svendsen (Department of Thoracic Medicine, Institute of Medicine, University of Bergen, Bergen, Norway); Wan C. Tan (PI), Wen Wang (iCapture Center for Cardiovascular and Pulmonary Research, University of British Columbia, Vancouver, BC, Canada); David M. Mannino (PI), John Cain, Rebecca Copeland, Dana Hazen, Jennifer Methvin, (University of Kentucky, Lexington, Kentucky, USA); Renato B. Dantes (PI), Lourdes Amarillo, Lakan U. Berratio, Lenora C. Fernandez, Norberto A. Francisco, Gerard S. Garcia, Teresita S. de Guia, Luisito F. Idolor, Sullian S. Naval, Thessa Reyes, Camilo C. Roa, Jr., Ma. Flordeliza Sanchez, Leander P. Simpao (Philippine College of Chest Physicians, Manila, Philippines); Christine Jenkins (PI), Guy Marks (PI), Tessa Bird, Paola Espinel, Kate Hardaker, Brett Toelle (Woolcock Institute of Medical Research, Sydney, Australia); Peter GJ Burney (PI), Caron Amor, James Potts, Michael Tumilty, Fiona McLean (National Heart and Lung Institute, Imperial College, London); E.F.M. Wouters, G.J. Wesseling (Maastricht University Medical Center, Maastricht, the Netherlands); Cristina Bárbara (PI), Fátima Rodrigues, Hermínia Dias, João Cardoso, João Almeida, Maria João Matos, Paula Simão, Moutinho Santos, Reis Ferreira (The Portuguese Society of Pneumology, Lisbon, Portugal); Christer Janson (PI), Inga Sif Olafsdottir, Katarina Nisser, Ulrike Spetz-Nyström, Gunilla Hägg and Gun-Marie Lund (Department of Medical Sciences: Respiratory Medicine & Allergology, Uppsala University, Sweden); Rain Jõgi (PI), Hendrik Laja, Katrin Ulst, Vappu Zobel, Toomas-Julius Lill (Lung Clinic, Tartu University Hospital); Parvaiz A Koul (PI), Sajjad Malik, Nissar A Hakim, Umar Hafiz Khan (Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K, India); Rohini Chowgule (PI), Vasant Shetye, Jonelle Raphael, Rosel Almeda, Mahesh Tawde, Rafiq Tadvi, Sunil Katkar, Milind Kadam, Rupesh Dhanawade, Umesh Ghurup (Indian Institute of Environmental Medicine, Mumbai, India); Imed Harrabi (PI), Myriam Denguezli, Zouhair Tabka, Hager Daldoul, Zaki Boukheroufa, Firas Chouikha, Wahbi Belhaj Khalifa (Faculté de Médecine, Sousse, Tunisia); Luisito F. Idolor (PI), Teresita S. de Guia, Norberto A. Francisco, Camilo C. Roa, Fernando G. Ayuyao, Cecil Z.Tady, Daniel T. Tan, Sylvia Banal-Yang, Vincent M. Balanag, Jr., Maria Teresita N. Reyes, Renato. B. Dantes (Lung Centre of the Philippines, Philippine General Hospital, Nampicuan&Talugtug, Philippines); Sundeep Salvi (PI), Siddhi Hirve, Bill Brashier, Jyoti Londhe, Sapna Madas, Somnath Sambhudas, Bharat Chaidhary, Meera Tambe, Savita Pingale, Arati Umap, Archana Umap, Nitin Shelar, Sampada Devchakke, Sharda Chaudhary, Suvarna Bondre, Savita Walke, Ashleshsa Gawhane, Anil Sapkal, Rupali Argade, Vijay Gaikwad (Vadu HDSS, KEM Hospital Research Centre Pune, Chest Research Foundation (CRF), Pune India); Mohamed C Benjelloun (PI), Chakib Nejjari, Mohamed Elbiaze, Karima El Rhazi (Laboratoire d’épidémiologie, Recherche Clinique et Santé Communautaire, Fès, Morroco); Daniel Obaseki (PI), Gregory Erhabor, Olayemi Awopeju, Olufemi Adewole (Obafemi Awolowo University, Ile-Ife, Nigeria); Al Ghobain M (PI), Alorainy H (PI), El-Hamad E, Al Hajjaj M, Ayan H, Rowena D, Rofel F, Elizabeth E, Imelda I, Safia H, Lyla (Saudi Thoracic Society, Saudi Arabia); Talant M. Sooronbaev (PI), Bermet M. Estebesova, MeerimAkmatalieva, SaadatUsenbaeva, JyparaKydyrova, Eliza Bostonova, Ulan Sheraliev, NuridinMarajapov, NurgulToktogulova, BerikEmilov, ToktogulAzilova, GulnaraBeishekeeva, NasyikatDononbaeva, AijamalTabyshova (Pulmunology and Allergology Department, National Centre of Cardiology and Internal Medicine, Bishkek, Kyrgyzstan); Kevin Mortimer (PI), Wezzie Nyapigoti, Ernest Mwangoka, Mayamiko Kambwili, Martha Chipeta,Gloria Banda, Suzgo Mkandawire, Justice Banda (The Malawi Liverpool Wellcome Trust, Blantyre, Malawi); Asma Elsony (PI), Hana A. Elsadig, Nada Bakery Osman, Bandar Salah Noory, Monjda Awad Mohamed, Hasab Alrasoul Akasha Ahmed Osman, Namarig Moham ed Elhassan, Abdel Mu’is El Zain, Marwa Mohamed Mohamaden, Suhaiba Khalifa, Mahmoud Elhadi, Mohand Hassan,Dalia Abdelmonam (The Epidemiological Laboratory, Khartoum, Sudan); Hasan Hafizi (PI), Anila Aliko, Donika Bardhi, Holta Tafa, Natasha Thanasi, Arian Mezini, Alma Teferici, Dafina Todri, Jolanda Nikolla, Rezarta Kazasi (Tirana University Hospital “Shefqet Ndroqi, Albania); Hamid Hacene Cherkaski (PI), Amira Bengrait, Tabarek Haddad, Ibtissem Zgaoula, Maamar Ghit, Abdelhamid Roubhia, Soumaya Boudra, Feryal Atoui, Randa Yakoubi, Rachid Benali, Abdelghani Bencheikh, Nadia Ait-Khaled (Faculté de Médecine Annaba, SEMEP Elhadjar, Algérie); Akramul Islam (PI), Syed Masud Ahmed (Co-PI), Shayla Islam, Qazi Shafayetul Islam, Mesbah-Ul-Haque, Tridib Roy Chowdhury, Sukantha Kumar Chatterjee, Dulal Mia, Shyamal Chandra Das, Mizanur Rahman, Nazrul Islam, Shahaz Uddin, Nurul Islam, Luiza Khatun, Monira Parvin, Abdul Awal Khan, Maidul Islam (James P.Grant School of Public Health, BIGH/BRAC University, Bangladesh).
References
- 1.World Health Organization (WHO) Global tuberculosis report 2013. WHO; Geneva: [Google Scholar]
- 2.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A Prospective Study of the Risk of Tuberculosis among Intravenous Drug Users with Human Immunodeficiency Virus Infection. New England Journal of Medicine. 1989;320:545–50. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 3.Ando M, Mori A, Esaki H, Shiraki T, Uemura H, Okazawa M, et al. The effect of pulmonary rehabilitation in patients with post-tuberculosis lung disorder. Chest. 2003;123:1988–95. doi: 10.1378/chest.123.6.1988. [DOI] [PubMed] [Google Scholar]
- 4.Hicks A, Muthukumarasamy S, Maxwell D, Howlett D. Chronic inactive pulmonary tuberculosis and treatment sequelae: chest radiographic features. Int J Tuberc Lung Dis. 2014;18:128–33. doi: 10.5588/ijtld.13.0360. [DOI] [PubMed] [Google Scholar]
- 5.Miller TL, McNabb SJ, Hilsenrath P, Pasipanodya J, Weis SE. Personal and societal health quality lost to tuberculosis. PLoS One. 2009;4:e5080. doi: 10.1371/journal.pone.0005080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 7.de Marco R, Accordini S, Marcon A, Cerveri I, Anto JM, Gislason T, et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183:891–7. doi: 10.1164/rccm.201007-1125OC. [DOI] [PubMed] [Google Scholar]
- 8.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–63. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 10.Menezes AM, Hallal PC, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30:1180–5. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 11.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86:76–85. doi: 10.1159/000350917. [DOI] [PubMed] [Google Scholar]
- 12.Garvin A, Lundsgaard C, Van Slyke DD. Studies of Lung Volume: Ii. Tuberculous Men. J Exp Med. 1918;27:87–94. doi: 10.1084/jem.27.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garvin A, Lundsgaard C, Van Slyke DD. Studies of Lung Volume: Iii. Tuberculous Women. J Exp Med. 1918;27:129–42. doi: 10.1084/jem.27.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–8. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 2007;131:1817–24. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 16.Baig IM, Saeed W, Khalil KF. Post-tuberculous chronic obstructive pulmonary disease. J Coll Physicians Surg Pak. 2010;20:542–4. [PubMed] [Google Scholar]
- 17.Ross J, Ehrlich RI, Hnizdo E, White N, Churchyard GJ. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax. 2010;65:1010–5. doi: 10.1136/thx.2009.129999. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich RI, Myers JE, te Water Naude JM, Thompson ML, Churchyard GJ. Lung function loss in relation to silica dust exposure in South African gold miners. Occup Environ Med. 2011;68:96–101. doi: 10.1136/oem.2009.048827. [DOI] [PubMed] [Google Scholar]
- 19.Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2:277–83. [PubMed] [Google Scholar]
- 20.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 21.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–51. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010;137:593–600. doi: 10.1378/chest.09-1435. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 25.Hwang YI, Kim JH, Lee CY, Park S, Park YB, Jang SH, et al. The association between airflow obstruction and radiologic change by tuberculosis. J Thorac Dis. 2014;6:471–6. doi: 10.3978/j.issn.2072-1439.2014.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001;21:839–58. doi: 10.1148/radiographics.21.4.g01jl06839. discussion 59-60. [DOI] [PubMed] [Google Scholar]
- 27.Alber A, Howie SE, Wallace WA, Hirani N. The role of macrophages in healing the wounded lung. Int J Exp Pathol. 2012;93:243–51. doi: 10.1111/j.1365-2613.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2013;19:95–102. doi: 10.1097/MCP.0b013e32835cfff5. [DOI] [PubMed] [Google Scholar]
- 29.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Change in FEV1/FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 2. Change in FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 3. Odds ratios of airflow obstruction for a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 4. Odds ratios of spirometric restriction for a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 5. Change in FEV1/FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.
Supplementary figure 6. Change in FVC due to a history of tuberculosis, by gross national income group (low/middle vs high) and site, omitting sites with a cooperation rate below 60%. All models were adjusted for age, sex, body mass index, and pack-years of smoking.