Abstract
Background
The dynamics of binocular rivalry may be a behavioural footprint of excitatory and inhibitory neural transmission in visual cortex. Given the presence of atypical visual features in Autism Spectrum Conditions (ASC), and evidence in support of the idea of an imbalance in excitatory/inhibitory neural transmission in ASC, we hypothesized that binocular rivalry might prove a simple behavioural marker of such a transmission imbalance in the autistic brain. In support of this hypothesis, we previously reported a slower rate of rivalry in ASC, driven by reduced perceptual exclusivity.
Methods
We tested whether atypical dynamics of binocular rivalry in ASC are specific to certain stimulus features. 53 participants (26 with ASC, matched for age, sex and IQ) participated in binocular rivalry experiments in which the dynamics of rivalry were measured at two levels of stimulus complexity, low (grayscale gratings) and high (coloured objects).
Results
Individuals with ASC experienced a slower rate of rivalry, driven by longer transitional states between dominant percepts. These exaggerated transitional states were present at both low and high levels of stimulus complexity, suggesting that atypical rivalry dynamics in autism are robust with respect to stimulus choice. Interactions between stimulus properties and rivalry dynamics in autism indicate that achromatic grating stimuli produce stronger group differences.
Conclusion
These results confirm the finding of atypical dynamics of binocular rivalry in ASC. These dynamics were present for stimuli of both low and high levels of visual complexity, suggesting an imbalance in competitive interactions throughout the visual system of individuals with ASC.
Introduction
The visual system often receives ambiguous information about the external world. Typically, this ambiguity can be resolved through contextual information and prior expectations (Bayerl & Neumann, 2004; Scholl & Nakayama, 2002). However, when two interpretations of the input are equally viable, a phenomenon known as bistable perception occurs: the two percepts compete for perceptual dominance, alternating back and forth in perceptual awareness.
Binocular rivalry is a striking example of bistable perception, occurring when conflicting monocular images are presented to the same retinal location of each eye. During rivalry, observers report a perceptual experience that alternates between the two images. This oscillation is thought to be facilitated by competitive interactions between populations of neurons that code for the two possible percepts at various levels of visual processing (Tong, Meng, & Blake, 2006).
This role of inhibition in rivalry is highlighted in many models of binocular rivalry (Blake, 1989; Hohwy, Roepstorff, & Friston, 2008; Klink, Brascamp, Blake, & Van Wezel, 2010; Moreno-Bote, Rinzel, & Rubin, 2007; Said & Heeger, 2013; Wilson, 2003). While some models posit top-down signals (Hohwy et al., 2008) or neural noise (Moreno-Bote et al., 2007) as the primary triggers of rivalry alternations, these models often still include inhibition between percept-selective neuronal pools as a key element of rivalry dynamics (Hohwy et al., 2008; Moreno-Bote et al., 2007). The role of inhibition in binocular rivalry is supported by the strong relationship between binocular rivalry dynamics and the inhibitory neurotransmitter GABA in the visual cortex (Lunghi, Emir, Morrone, & Bridge, 2015; van Loon et al., 2013). Two recent computational models of binocular rivalry offer specific predictions about how alterations in inhibitory signalling would affect rivalry dynamics, specifically positing a relationship between the inhibitory connection strength and the perceptual exclusivity of the two rivalling percepts (Klink et al., 2010; Said, Egan, Minshew, Behrmann, & Heeger, 2012).
As a result, binocular rivalry can be thought of as a behavioural marker of the balance of excitatory and inhibitory neural transmission in the brain (the E/I ratio). We and others have proposed that binocular rivalry can serve as a tool to study a clinical population in which this ratio might be altered (Robertson, Kravitz, Freyberg, Baron-Cohen, & Baker, 2013; Said et al., 2012), such as Autism Spectrum Conditions (ASC, Rubenstein & Merzenich, 2003). There is converging evidence from animal models (Chao et al., 2010; Gogolla et al., 2009; Tsai et al., 2012; Yizhar et al., 2011), genetic findings (Bundey, Hardy, Vickers, Kilpatrick, & Corbett, 1994; Menold et al., 2001; Buxbaum et al., 2002; Kim et al., 2008; Warrier, Baron-Cohen, & Chakrabarti, 2013) and post-mortem studies (Fatemi, Reutiman, Folsom, & Thuras, 2009b) suggesting an alteration in E/I neurotransmission in the autistic cortex. Such an alteration could explain a wide array of autistic symptoms (Rubenstein & Merzenich, 2003), as well as the elevated co-morbidity between autism and epilepsy (Canitano, 2007). Therefore, a behavioural test of the integrity of E/I dynamics in the autistic brain would significantly help our understanding of the condition.
Two studies have examined binocular rivalry in individuals with ASC (Robertson et al., 2013; Said et al., 2012). One study, from our lab, reported a slower rate of rivalry in ASC with longer mixed percept durations (Robertson et al., 2013); the other did not examine the overall rate of rivalry, and reported only a statistical trend towards a larger proportion of mixed percepts in ASC (Said et al., 2012). This pattern of results warrants further investigation. It is possible that these studies, taken together, point towards a fundamental perturbation in binocular rivalry dynamics in ASC.
The difference in the effect sizes of these two studies might arise from a difference between the stimuli used in each study, which could offer insight into the nature of the putative I/E imbalance in the autistic cortex. The study showing the greatest difference between ASC and controls used complex coloured object stimuli to test binocular rivalry dynamics (Robertson et al., 2013), while the study reporting a trend towards reduced perceptual exclusivity in ASC used simple grayscale gratings (Said et al., 2012). These different stimulus categories are thought to recruit competitive interactions at different levels of the visual hierarchy. Specifically, grayscale grating rivalry is thought to involve mutual inhibition between eye and orientation-selective neuronal populations in early visual cortex (Haynes & Rees, 2005; Menon, Ogawa, Strupp, & Uğurbil, 1997), while coloured objects are thought to recruit additional levels of competitive interactions between object-selective neuronal populations in higher-level visual cortex (Logothetis & Sheinberg, 1996) and colour-selective neuronal populations. The difference between the results of the two previous investigations of binocular rivalry in ASC might therefore indicate that atypical rivalry dynamics are only evident with chromatic object stimuli, which engage relatively more levels of competitive cortical interactions across which an E/I imbalance could accumulate.
The aims of the present study were therefore twofold. First, we tested whether our previous finding of a slower rate of binocular rivalry with longer mixed percepts in ASC would replicate in a new, larger sample of participants with and without ASC. Second, we tested whether this finding was selective for stimuli with the particular visual properties shown to elicit atypical rivalry dynamics in ASC in prior work: we intermixed trials using achromatic gratings and coloured images in order to assess whether stimuli varying on multiple dimensions differentially affect rivalry dynamics in ASC. Our results demonstrate an overall slower rate of rivalry in ASC with longer mixed percept durations and reduced perceptual exclusivity, which cannot be accounted for by group differences in decision criteria or motor latencies. These effects were evident, and stronger, with achromatic grating stimuli. These findings are consistent with the E/I imbalance hypothesis in autism, and indicate that atypical binocular rivalry is a robust behavioural marker in autism with respect to stimulus choice.
Methods
Participants and Psychometric Testing
53 participants took part in the study (26 with ASC). The two groups were matched for mean age (Controls: 28.7±9.8; ASC: 32.0±11.0; p >= 0.26, Table 1) and performance (non-verbal) IQ (Controls: 114.0±12.9; ASC: 118.2±11.2 p >= 0.22, Table 1), assessed using the Wechsler Abbreviated Scale of Intelligence (WASI). Participants were recruited from the Cambridge Autism Research Database (CARD), and online adverts, and there was no overlap between participants recruited for this study and Robertson et al., (2013). Participants with ASC all had clinical diagnoses of an ASD (DSM-IV criteria), as evaluated by a qualified clinical psychologist or psychiatrist in a recognized clinic. To quantify autistic symptoms, participants with ASC were also assessed using the ADOS-II (ASC: 9.6±3.1). Participants also completed the Autism-Spectrum Quotient (AQ, Controls: 16.6±6.7, ASC: 37.5±7.1, Table 1) (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001), the Sensory and Perception Questionnaire (SPQ, Controls: 113.5±27.0, ASC: 87.3±24.2) (Tavassoli, Hoekstra, & Baron-Cohen, 2014), and the Glasgow Sensory Questionnaire (GSQ, Controls: 40.9±17.1, ASC: 74.9±20.9) (Robertson & Simmons, 2012). All participants had normal or corrected-to-normal vision, and were free of epilepsy or Attention-Deficit/Hyperactivity Disorder diagnoses. 6 participants (5 with ASC) were on psychiatric medication (3: antidepressant, 1: antianxiety, 2: antipsychotic). Exclusion of these participants did not qualitatively alter our results: all effects involving Diagnosis remained significant.
Table 1. Descriptive Statistics and tests of equality between the two groups.
Psychometric Data. Means +/− 1 standard deviation, as well as the range of data, are reported for each group. Groups were matched for age, IQ, and gender.
| Age | IQ | Gender | AQ | ADOS (A+B) | GSQ | SPQ | |
|---|---|---|---|---|---|---|---|
| Controls | 28.7 ± 9.8 (21-72) | 114.0±12.9 (87-135) | M:F 17:10 | 16.6±6.7 (6-33) | - | 40.9±17.1 (9-81) | 113.5±27.0 (72-148) |
| ASC | 32.0±11.0, (17-56) | 118.2±11.2 (99-139) | M:F 17:9 | 37.5±7.1 (23-47) | 9.6±3.1 (5-16) | 74.9±20.9 (41-120) | 87.3±24.2 (56-141) |
| p-value | p >= 0.26 | p >= 0.22 | p >= 0.85 | p < 0.001 | - | p < 0.001 | p < 0.001 |
Materials and Procedure
We conducted two experiments: one natural binocular rivalry experiment, and one control experiment in which binocular rivalry was simulated. In both experiments, participants viewed a calibrated Dell LCD monitor (width: 43.5 cm; resolution: 1600×900; refresh rate: 60 Hz) from a distance of 60 cm through a mirror stereoscope. The stereoscope reflected the left/right sides of the screen into the participants’ left/right eyes, respectively.
Before the experiment began, fusion was established for each participant by moving two boxes (white/black, width: 4.95°) towards each other along the screen’s horizontal meridian until the participant first reported their inner edges to touch. The two boxes were then moved by half the box width. Participants were then given practice with the task, performing four 20s binocular rivalry trials (2 for each stimulus condition). Finally, participants began the main experiment, performing 12 40s binocular rivalry trials (6 for each stimulus condition; see Stimuli: Rivalry Experiment) and 24 40s control trials (6 for each transition type and stimulus condition; see Stimuli: Control Experiment). All 36 trials were presented in random order. A 20s pause occurred between trials, and a 15-minute break was taken every 12 trials.
On each trial, participants were instructed to continuously press either the Left, Right, or Up Arrow on the keyboard to report their perceptual state (“the red image, the green image, or a mixture of the two”, respectively). Participants were instructed to define a “mixed image” as a perceptual state in which neither the green nor the red object was perceptually dominant.
Stimuli: Rivalry Experiment
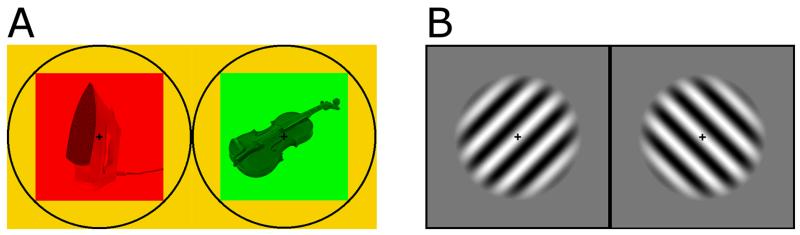
Two sets of stimuli were used, Objects and Gratings. Object stimuli consisted of grayscale images taken from a bank of standard, non-social images (e.g. a baseball and a broccoli) and were identical to those used in our previous study (Robertson et al., 2013). A random, non-repeating sequence of six image pairs was generated for each participant, which was used for both the Rivalry and Control experiments. Each image (average height: 2.31°, width: 2.79°) was presented on a coloured square (width: 3.5°). A black circle surrounded the tinted squares (radius: 4.95°) and a black fixation cross was set in the centre of the circle to provide vergence cues. On each trial, one eye viewed a red square, and one eye viewed a green square.
Grating stimuli consisted of sinusoidal luminance gratings (spatial frequency: 3 cycles/degree; Michelson contrast: 60%), displayed in a circular aperture (diameter: 3.5°). A black box surrounded the gratings (width: 4.95°) and a fixation cross was set in the centre of the box to provide vergence cues. On each trial, one eye viewed gratings tilted +45 degrees, and the other −45 degrees.
Stimuli: Control Experiment
The stimuli used in the control experiment were identical to those used in the rivalry experiment. However, the same image was consistently presented to both eyes throughout the trial, and rivalry was simulated by presenting the two stimuli in alternation on the screen, separated by simulated transitions which were created by blending the two images (OpenGL blending, Brainard, 1997).
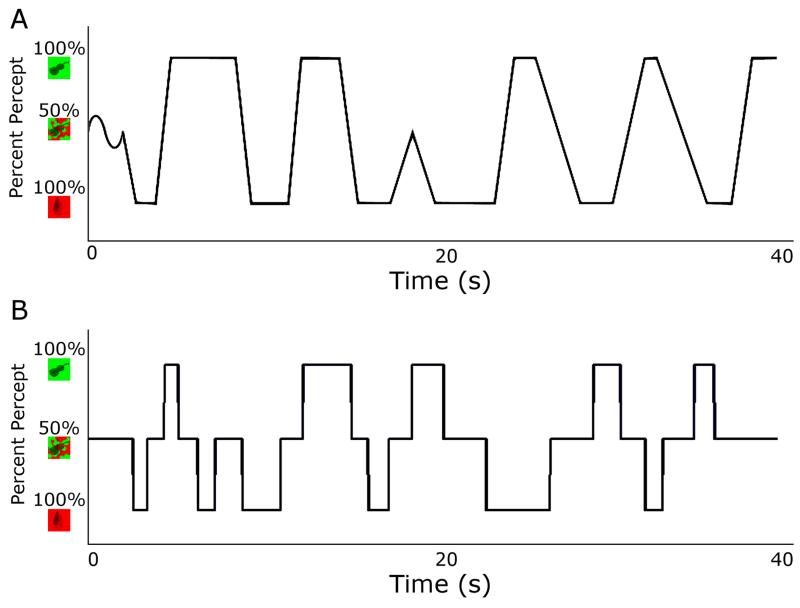
There were two trial types in the control experiment: smooth and sudden (Figure 1). In both trial types, the displayed stimulus alternated between the two dominant images. In the sudden transition trials, alternations were abrupt: either a dominant (e.g. 100% baseball) or a mixed image (e.g. 50% baseball, 50% broccoli) was displayed at any one time. In smooth transition trials, alternations were dynamic: a linear transition was placed between the two dominant images. The proportion of the images displayed at each pixel was determined by placing 15 two-dimensional Gaussian curves (average extent: 0.4°) in random positions in the alpha layer and increasing their amplitude throughout a transition. To simulate onset ambiguity, a mixed image was displayed at the start of all trials, which transitioned sinusoidally in the smooth trials around the 50% mixture point (Figure 2).
Figure 1. Stimuli used in the binocular rivalry experiment.
A. Example stimuli for the object condition. Object stimuli consisted of grayscale images taken from a bank of standard, non-social images (e.g. a baseball and a broccoli). Each image (average height: 2.31°, width: 2.79°) was presented on a coloured square (width: 3.5°). A black circle surrounded the tinted squares (radius: 4.95°) and a black fixation cross was set in the centre of the circle to provide vergence cues. On each trial, one eye viewed a red square, and one eye viewed a green square. B. Example stimuli for the grating condition. Grating stimuli consisted of sinusoidal luminance gratings (spatial frequency: 3 cycles/degree; Michelson contrast: 60%), displayed in a circular aperture (diameter: 3.5°). A black box surrounded the gratings (width: 4.95°) and a fixation cross was set in the centre of the box to provide vergence cues. On each trial, one eye viewed gratings tilted +45 degrees, and the other −45 degrees.
Figure 2. Example time courses of control experiment stimulus presentation.
A. Smooth, linear transitions between images, designed to measure participants’ response criteria to judge the boundary between a mixed and dominant image. Stimuli simulated natural rivalry, starting with a mixed image (Object Condition: 50% green/red; Grating Condition: 50% 45°/−45°) and thereafter smoothly oscillating between the two percepts (Object Condition: 100% green or 100% red; Grating Condition: 100% 45° or 100% −45°). B. Sudden transitions between images, designed to measure participants’ motor latencies to report the onset of a mixed or dominant image. Trials began with a mixed image, after which stimuli abruptly alternated between three states (Object Condition: 100% green, 100% red, and 50% red/green; Grating Condition: 100% 45°, 100% −45°, and 50% 45°/−45°).
Stimulus durations for the Object condition were drawn from a distribution of percept durations obtained in a previous rivalry study (Robertson et al., 2013). In half the trials, durations were drawn from those of the control group means (dominant/mixed: 2.0s/1.5s). In the other half, durations were drawn from those of the ASC group means (dominant/mixed: 2.0s/2.0s). Durations for the Grating condition were drawn from the same distribution, adjusted so that the mean matched the means obtained in a previous study of rivalry using grating stimuli (ASC-matched dominant/mixed: 2.3s/1.73s, Control-matched dominant/mixed: 1.73s/1.3s (Said et al., 2012)). All stimulus durations were a minimum of 0.5s.
Performance Analysis: Rivalry Experiment
Key presses throughout a trial were parsed into a sequence of perceptual transitions. Perceptual transitions during binocular rivalry can be broadly classified into “switches” (when the percept changes from one image to the other, typically via an intermediate mixed percept) and “reversions” (when the percept changes from one image to a mixed percept, but then returns again to the original percept). We excluded responses shorter than 150 ms and periods when no button was pressed. These occurrences were rare, 1.3% (ASC) and 1.5% (Con) of button presses, and were matched for the two groups (p > 0.93).
We calculated the frequency of transitions, switches, and reversions, the average duration of mixed and dominant percepts, and the perceptual exclusivity, defined as the proportion of dominant percepts, for each participant and trial. These measures were analysed in separate 2×2 ANOVAs, using Stimulus Condition (gratings or images) as a within-subject factor, and diagnosis as a between-subject factor. Participants were excluded from all subsequent analyses if their percept durations were more than two standard deviations above or below the mean of both groups combined (n = 5, 2 with ASC). Including these participants in the analysis did not change the outcome of any statistical tests. One further participant (Control) was excluded who continuously reported a mixed percept, indicating that stable binocular viewing was not achieved. All results reported below remained significant when repeated while co-varying for age, gender, and IQ.
Performance Analysis: Control Experiment
Control experiment analyses allowed us to assess whether any differences in rivalry performance between groups were due to slower reactions or different perceptual criterion levels in either group by measuring participants’: 1) task understanding, 2) motor-response latencies, and 3) decision-criteria to judge the boundary between a mixed and dominant percept. To assess reaction time, we calculated the mean RT of a subject in the sudden-onset trials. Finally, to assess perceptual decision-criteria, we calculated the stimulus composition at the time-point at which participants reported a percept in the smooth-transition trials (e.g. 60% baseball, 40% broccoli), corrected for each participant’s mean reaction time in the sudden-onset trials.
Results
We tested whether individuals with ASC evidence atypical dynamics of binocular rivalry, and whether such differences are specific to high or low levels of stimulus complexity. In addition, to explore participants’ response latencies and response criteria, we ran two control rivalry stimulation experiments. We first present the results of the binocular rivalry experiment, followed by the results of the control experiment. In short, these results indicate atypical dynamics of binocular rivalry in ASC with both achromatic gratings and coloured objects, which cannot be accounted for by differences in response latencies or response criteria.
Overall Slower Rate of Binocular Rivalry in ASC
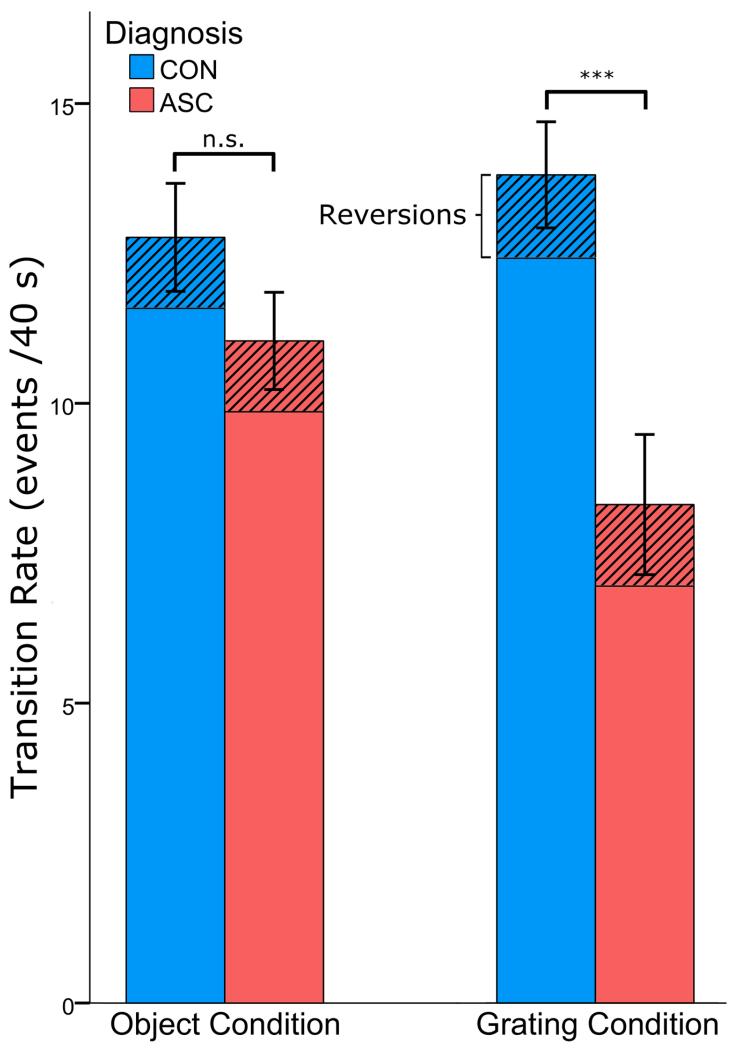
Participants with ASC demonstrated fewer perceptual transitions during binocular rivalry than controls (main effect of Diagnosis: F(1, 45) = 8.715, p < 0.005, ηp2 = 0.178), reporting on average 9.3 transitions per trial, compared to 12.3 in controls, across both stimulus conditions (Figure 3). This replicates our previous result of slower binocular rivalry dynamics in ASC (Robertson et al., 2013), demonstrating that the rate at which two percepts compete for perceptual awareness is reduced in individuals with ASC. To further characterize these dynamics, we next analysed the two possible types of perceptual transitions: switches and reversions separately.
Figure 3. Slower rate of binocular rivalry in ASC.
ASC subjects demonstrated overall fewer perceptual transitions between the images presented to their right and left eyes (main effect of Diagnosis: F(1, 45) = 8.717, p < 0.005) The mean number of these transitions which were switches or reversions is marked (stripes) for each group. Error bars represent one standard error of the mean and *** p < 0.001 difference between the two groups.
Overall Slower Rate of Switches in ASC
Again confirming our previous report (Robertson et al., 2013), participants with ASC switched between percepts significantly less frequently than controls (main effect of Diagnosis: F(1, 45) = 8.717, p < 0.005, ηp2 = 0.176), reporting on average 8.0 switches per trial, compared with 11.1 in controls across both stimulus conditions (Figure 3). Reversions were equally frequent in both groups (ASC: 1.2, CON: 1.2, F(1, 45) = 0.004, p < 0.947), and although the proportion of transitions that resulted in reversions, rather than switches, was numerically higher in the ASC group (ASC: 15.1%, Con: 11.9%), no main effect of Diagnosis was observed (F(1, 45) = 1.795, p < 0.187). These findings confirm slower overall dynamics of binocular rivalry in individuals with ASC.
Overall Longer Mixed Percepts in ASC
In order to test whether the slower rate of rivalry observed in ASC was driven by a disproportionate amount of time spent reporting dominant percepts, mixed percepts, or both, we calculated the mean duration of dominant and mixed percepts. To calculate the duration of dominant percepts, we collapsed across clockwise/counter-clockwise and red/green responses, as we observed no response biases for any percepts for either group or stimulus type (all p > 0.77).
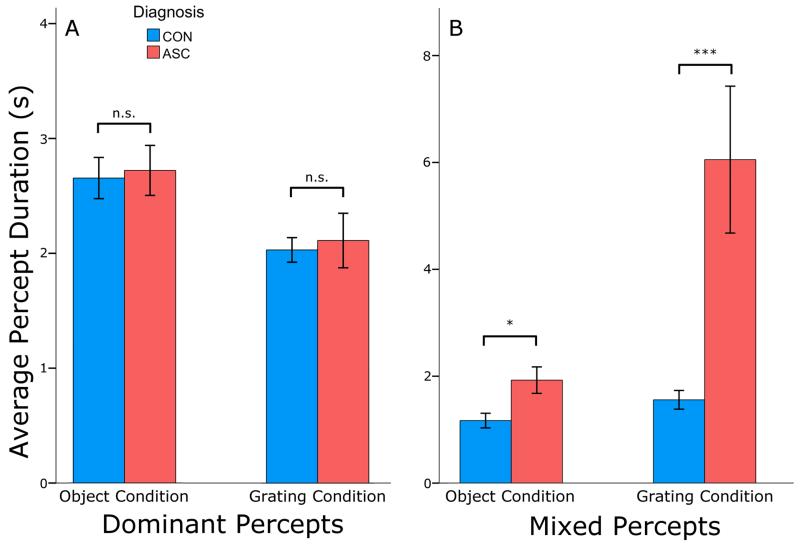
Overall, individuals with ASC experienced significantly longer mixed percepts than controls (ASC: 4.0 s, CON: 1.36 s, main effect of Diagnosis: F(1, 45) = 11.855, p < 0.001, ηp2 = 0.289) (Figure 4). However, the durations of dominant percepts were comparable between the two groups (ASC: 2.34 s, CON: 2.42 s, main effect of Diagnosis: F(1, 45) = 0.099, p < 0.754), attributing the slower rate of rivalry in ASC to a disproportionately long transitional (mixed) state between two dominant percepts. Indeed, the proportion of time participants spent in a mixed state, as opposed to a dominant perceptual state, was significantly larger in ASC as compared to controls (F(1, 45) = 9.674, p < 0.003, ηp2 = 0.231), and this proportion strongly correlated with the rate of perceptual switches in both stimulus conditions (p < 0.002). This replicates our previous finding (Robertson et al., 2013), and confirms a key prediction of how an E/I imbalance would alter the dynamics of binocular rivalry in models of rivalry (Klink et al., 2010; Said et al., 2012).
Figure 4. Lengthened mixed percepts in ASC.
A. The durations of dominant percepts were equivalent between the two groups in both stimulus conditions. Both groups experienced longer dominant percepts in the object condition than in the grating condition. B. The ASC group experienced overall longer mixed percepts than the control group in both stimulus conditions (main effect of Diagnosis: F(1, 45) = 11.855, p < 0.001). Both groups experienced shorter mixed percepts in the object condition than in the grating condition. In both plots, error bars represent one standard error of the mean and * p < 0.05, *** p < 0.001 difference between the two groups.
Effects of Stimulus Type on Rivalry Dynamics in ASC
No effect of Stimulus Type was observed on switch rate (F(45, 1) = 2.795, p < 0.10), indicating that the level of stimulus complexity did not significantly impact rivalry rate overall. However, an interaction between Stimulus Type and Diagnosis was observed (Switches: F(1, 45) = 9.084, p < 0.004, ηp2 = 0.157), driven by a particularly slower rate of switches in ASC as compared to controls in the grating condition (U(23, 24) = 97.5, p < 0.001, 12.46 ± 4.64 (Control), 9.57 ± 4.01 (ASC), Cohen’s d = 0.67), as opposed to the object condition (U(23, 24) = 230.5, p < 0.34, 11.61 ± 4.76 (Control), 6.88 ± 5.28 (ASC), Cohen’s d = 0.94). No interactions or main effects involving Stimulus Type were observed for reversions.
As expected from previous literature (Brascamp, Klink, & Levelt, 2015), both groups demonstrated shift towards longer mixed and shorter dominant percepts in the grating condition, as evidenced by a main effect of Stimulus Type (mixed percepts: F(1, 45) = 11.069, p < 0.002, ηp2 = 0.194; dominant: F(1, 45) = 19.402, p < 0.001 ηp2 = 0.280). Individuals with ASC were disproportionately affected by this shift, resulting in a significant interaction between Stimulus Type and Diagnosis for mixed (F(1, 45) = 4.201, p < 0.046, ηp2 = 0.105) but not dominant (F(1, 45) = 0.003, p < 0.957) percepts. Critically, this exaggerated duration of mixed-percepts in ASC was observed at both levels of stimulus complexity (objects, U(23, 24) = 173, p < 0.028, 1.19±0.71 s (Control), 1.93±1.5 s (ASC), Cohen’s d = 0.63; gratings, U(23, 24) = 110, p < 0.001, 1.57±0.86 s (Control), 4.09±3.14 s (ASC), Cohen’s d = 1.09), suggesting that longer mixed percepts during binocular rivalry are a stable signature of atypical competitive dynamics in the autistic brain which replicates across levels of visual processing.
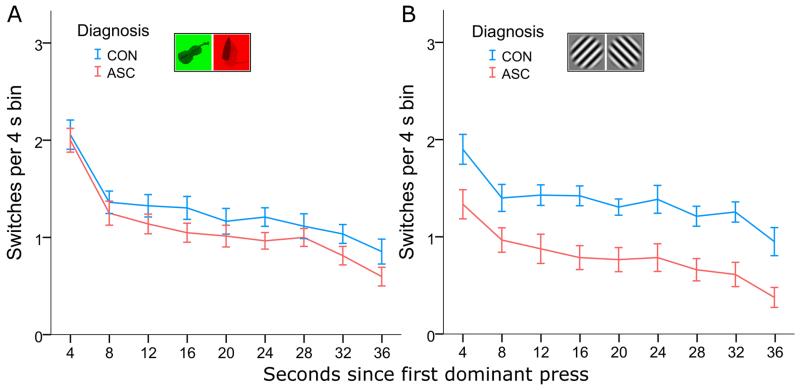
Change of Rivalry Dynamics over Time
As has previously been observed (Hollins & Hudnell, 1980), the rate of perceptual switches declined over the course of a 40s trial. To test whether the rate of this decline differed between individuals with and without ASC, switches were parsed into 4s time-bins, the first of which began with the first dominant button-press in each trial. A 2×2×9 repeated-measures ANOVA of this binned data, using Time Bins and Stimulus Type as a within-subject factors, revealed that switch rate fell significantly during a trial (main effect of Time F(8, 360) = 78.724, p < 0.001, ηp2 = 0.904). We observed no interaction between Time and Diagnosis (F(8, 360) = 0.766, p < 0.633), indicating that this decline was comparable between the two groups. There was, however, an interaction between Time and Stimulus Type (F(8, 360) = 4.040, p < 0.001, ηp2 = 0.383), reflecting a steeper decline of switch rate in the object condition in both groups (Figure 5).
Figure 5. Decline of rivalry rate over time.
For both groups, the frequency of perceptual switches declined throughout the trial. The rate of this decline was comparable between the two groups in both the Object Condition (A) and the Grating Condition (B), with individuals with ASC reporting overall fewer perceptual transitions (main effect of Diagnosis: F(1, 45) = 8.717, p < 0.005).
Comparable Response Latencies and Criteria between ASC and Controls
The results of our control experiment demonstrate that the atypical dynamics of binocular rivalry evidenced in ASC cannot be attributed to any non-perceptual differences between the two groups, such as response latency or response criteria. During the control experiment, when there were physical changes in the stimuli simulating rivalry alternations, individuals with and without ASC reported a similar proportion of image transitions and no group differences in the duration of dominant or mixed-images were observed (all p > 0.53).
Overall, the two groups responded to a comparable proportion of simulated rivalry alternations (Control, 87 ± 15%; ASC, 88 ± 13%, p < 0.71). Critically, individuals with and without ASC also exhibited comparable response latencies to report both single and mixed-image stimuli. During our sudden-onset control experiment, both groups exhibited comparable response latencies to report the onset of single (F(1, 45) = 0.217, p < 0.64) and mixed-image stimuli (F(1, 45) = 0.4, p < 0.53). No other main effects or interactions were observed (all p > 0.64). These results indicate that both groups evidence similar motor latencies to detect sudden stimulus onsets. Likewise, during our smooth-onset control experiment, no differences were observed between the two groups’ response criteria to report the onset of single (F(1, 45) = 3.3, p < 0.076) or mixed-image (F(1, 45) = 1.145, p < 0.29) stimuli, and no other main effects or interactions were observed (all p > 0.64). These results indicate that both groups also exhibit comparable perceptual response criteria to judge the borders between simulated perceptual transitions. In sum, this demonstrates that any differences in the dynamics of binocular rivalry in autism do not arise from simple differences in the speed or criteria of report.
Correlation with Autistic Traits
We tested whether rivalry dynamics predicted two measures of autistic traits: the AQ and ADOS scores. AQ significantly predicted switch rates (Pearson’s r = −0.299, p < 0.031) and mixed percepts (Pearson’s r = 0.387, p < 0.005) in the grating condition. However, these correlations did not hold in each group individually (all p > 0.078), and therefore were likely driven by the group differences in AQ and rivalry dynamics. There was no significant correlation between ADOS scores and any variables.
There was also a significant correlation between the GSQ Visual Subscale and switches (Pearson’s r = −0.334, p < 0.030), mixed-percept durations (Pearson’s r = 0.331, p < 0.037) and overall mixed percept proportion (Pearson’s r = 0.323, p < 0.042) in the grating condition when the two groups were combined. Again, when analysed separately for each group, no correlation was statistically significant in each group individually (all p > 0.09). The GSQ also correlated with the AQ (r = 0.789, p < 0.001), replicating previous reports in the literature of a strong relationship between autistic symptoms measured on perceptual and social processing levels (Robertson & Simmons, 2012).
Discussion
Our findings indicate that the dynamics of binocular rivalry are robustly altered in ASC. Specifically, individuals with high-functioning ASC demonstrate a slower rate of binocular rivalry with disproportionately long periods of transitional states between dominant percepts (mixed percepts). These results replicate our previous findings (Robertson et al., 2013), and lend support to a computational model of how a perturbation in the ratio of excitatory/inhibitory transmission in the autistic brain would alter binocular rivalry dynamics (Said et al., 2012). These findings occur with both coloured object stimuli and achromatic grating stimuli, indicating that they are not specific to a particular type of visual complexity. Importantly, interactions between stimulus properties and group suggest that achromatic gratings, which produce longer mixed percepts overall in typical populations, also produce larger group differences between individuals with and without ASC.
An increase in the E/I ratio has been proposed as a neurophysiological explanation for a wide range of symptoms associated with ASC. First described by Rubenstein and Merzenich (2003), this hypothesis was inspired, in part, by the observation that individuals with classic autism exhibit a high co-morbidity with epilepsy, estimated as high as 20-25% (Canitano, 2007). Since the original proposal of this hypothesis, converging genetic (Bundey et al., 1994; Menold et al., 2001; Kim et al., 2008; Buxbaum et al., 2002; Warrier et al., 2013), animal (Chao et al., 2010; Gogolla et al., 2009; Tsai et al., 2012; Yizhar et al., 2011), computational (Vattikuti & Chow, 2010), and neuroanatomical (Fatemi et al., 2009b; Oblak, Gibbs, & Blatt, 2011; Yip, Soghomonian, & Blatt, 2007) findings have further supported the role of altered E/I signalling in the neurobiology of ASC. In particular, subunits of receptors for GABA, the primary agent of inhibitory neurotransmission in the adult brain, have been reported to be under-expressed in histological studies of autism (Fatemi, Folsom, Reutiman, & Thuras, 2009a; Fatemi et al., 2009b).
An alteration in GABAergic signalling would likely have wide-reaching implications for many neural computations, as GABA plays a formative role during development, particularly during the critical period (Ben-Ari, 2002). Recent reports of architectural alterations of the autistic visual system are consistent with this hypothesis, demonstrating weaker surround suppression (Foss-Feig, Tadin, Schauder, & Cascio, 2013), larger population receptive fields (Schwarzkopf, Anderson, de Haas, White, & Rees, 2014), and atypical responses to motion stimuli in early visual cortex (Robertson et al., 2014). Therefore, a replicable behavioural marker of autistic symptomatology that would be predicted to directly couple with GABAergic signalling would greatly enhance our understanding of autistic neurobiology. Here, we confirm atypical dynamics of binocular rivalry in ASC using two very different sets of stimuli (coloured objects and achromatic gratings). This finding may be a simple behavioural index of a pervasive imbalance in E/I interactions in the autistic visual cortex.
Previous studies have investigated the dynamics of binocular rivalry in other clinical populations. Typical rivalry rates have been reported in individuals with schizophrenia (Miller et al., 2003). However, in bipolar disorder, a slower rate of rivalry is found with drifting (Pettigrew & Miller, 1998) and stationary gratings (Miller et al., 2003; Nagamine, Yoshino, Miyazaki, Takahashi, & Nomura, 2009). Crucially, the atypical rivalry dynamics reported in bipolar disorder were found to be specific to bipolar I, and are driven by longer dominant percepts (Nagamine et al., 2009). This is an important distinction from our findings in autism, where rivalry dynamics are marked by longer mixed percepts. These findings highlight the importance of characterizing the duration of perceptual states in binocular rivalry in clinical populations, rather than just the rate of alternation.
Computational descriptions of binocular rivalry further emphasize this importance of characterizing percept durations during binocular rivalry. Two recent computational models of binocular rivalry specifically predict that an E/I imbalance in the visual system would affect the ratio of mixed and dominant percepts during binocular rivalry (Klink et al., 2010; Said et al., 2012; Said & Heeger, 2013). Specifically, while neither model makes predictions about the absolute duration of percepts, they both predict that a reduction in inhibitory connection strength reduces exclusivity of the two percepts, or raises the proportion of mixed percepts, due to incomplete mutual suppression between pools of neurons coding for the opposing percepts. It should be noted that in one model, the same increase in mixed percepts occurs when excitatory connection strength amongst pools of neurons coding for the same percept is reduced (Said et al., 2012), indicating that atypical rivalry dynamics may be agnostic to the direction of an E/I imbalance. Future work linking the duration of mixed percepts to E/I balance in the brain is required to resolve these computational predictions.
A previous experiment did not confirm atypical dynamics of binocular rivalry in ASC using low-level stimuli. However, the reported results were consistent with the direction of our findings: the authors reported a higher proportion of mixed percepts in ASC (t(22) = 1.76, p = .09, Said et al., 2012). We therefore suggest that the current literature, as a whole, supports the hypothesis of atypical dynamics of binocular rivalry in autism across multiple levels of stimulus complexity. However, we highlight one aspect of our stimulus parameters that may have contributed to the strength of the observed effects in the current study, which future work should explore. The proportion of mixed percepts reported during rivalry is known to increase with stimulus size (Blake, O’Shea, & Mueller, 1992), and our stimuli were larger than those used by Said and colleagues in order to match our object stimuli (3.5°, as opposed to 1°). This difference may have increased the dynamic range of rivalry dynamics measured in our experiments, and allowed for a group difference to become evident. It should also be noted that larger stimuli could also lead to larger eye movements, which are known to trigger perceptual switches during bistable perception (Bonneh et al., 2010; van Dam & van Ee, 2006). However, our results are not consistent with the concern that a clinical population might show a higher frequency of eye movements, as we report fewer perceptual switches in ASC.
Our primary motivation in comparing the grating and object rivalry in ASC was to explore whether atypical rivalry dynamics in ASC would generalize across various types of visual stimuli. Binocular rivalry between complex stimuli is thought to employ competitive interactions between pools of neurons at both early (eye-selective) and late (percept-selective) stages of visual processing (Freeman, 2005; Said & Heeger, 2013; Wilson, 2003). Consistent with these models, rivalry oscillations are mirrored in fluctuations in activity across levels of the visual hierarchy (Tong & Engel, 2001; Tong, Nakayama, Vaughan, & Kanwisher, 1998). Our findings of reduced perceptual exclusivity in ASC with both grating and object rivalry suggest that an E/I imbalance may affect multiple types of competitive interactions in the autistic visual system.
Although our results demonstrate that atypical rivalry dynamics in ASC are robust with respect to stimulus choice, they also indicate an interaction between stimulus type and diagnosis. Consistent with previous studies of binocular rivalry (Klink et al., 2010), we observed a main effect of Stimulus Type on percept durations: in both groups, coloured object stimuli elicited more perceptual exclusivity than grayscale grating stimuli, although this may also be influenced by luminance contrast (Brascamp et al., 2015). Interestingly, this effect interacted with Diagnosis: although mixed percepts were longer for ASC participants in both stimulus conditions, this difference between groups was exaggerated with the grating stimuli. Additionally, although we find an overall slower switch rate in ASC, this effect was particularly driven by grating trials in this study, as the numerically lower switch rate in ASC on object trials did not statistically differ between ASC and controls. Our two stimulus types were chosen to match the stimuli of prior studies (Robertson et al., 2013; Said et al., 2012), and therefore differed on many dimensions: colour (chromatic/achromatic), spatial frequency variation (varied/uniform), orientation variation (varied/uniform), shape (objects/lines), and contrast. As a result, it is impossible to establish whether differences in autistic visual processing on a particular one of these dimensions could explain the observed interaction between Stimulus Type and Diagnosis, or whether these findings reflect an increase in sensitivity to the diminished number of levels of cortical competition between object and grating stimuli. There is some evidence to suggest that stimulus complexity may be processed differently in ASC (Bertone, Mottron, Jelenic, & Faubert, 2003, 2005), but future work is needed to explore the influence of stimulus strength as modulated by, for example, colour contrast, luminance contrast or spatial frequency on mixed percepts in ASC.
In summary, these findings demonstrate a reliable perturbation in the dynamics of binocular rivalry in individuals with ASC. This replicable difference between individuals with and without ASC in such a fundamental aspect of vision, and across a diverse range of stimuli, suggests that an E/I imbalance may be pervasive in the autistic visual system, and might be predicted to occur in other sensory modalities. Rivalry may therefore have the potential to serve as a behavioural marker of atypical function in a canonical neural computation in the autistic brain.
Acknowledgments
We thank Dwight Kravitz and Chris Baker for their help with the conception of this work, as well as the analysis and stimulus design. We also thank John Mollon for the use of his mirror stereoscope. This work was supported by a Medical Research Council (MRC) UK grant to JF, a Harvard Society of Fellows grant to CER, and grants from the MRC, the Wellcome Trust, and the Autism Research Trust to SBC. This study was conducted in association with the NIHR CLAHRC EoE and Cambridgeshire and Peterborough NHS Foundation Trust.
Footnotes
Financial Disclosures
The authors report no financial interests or conflicts of interest.
References
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bayerl P, Neumann H. Disambiguating visual motion through contextual feedback modulation. Neural Computation. 2004;16(10):2041–2066. doi: 10.1162/0899766041732404. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience. 2002;3(9):728–739. doi: 10.1038/nrn920. http://doi.org/10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion Perception in Autism: A “Complex” Issue. Journal of Cognitive Neuroscience. 2003;15(2):218–225. doi: 10.1162/089892903321208150. http://doi.org/10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain : A Journal of Neurology. 2005;128(Pt 10):2430–41. doi: 10.1093/brain/awh561. http://doi.org/10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Blake R. A neural theory of binocular rivalry. Psychological Review. 1989;96(1):145–167. doi: 10.1037/0033-295x.96.1.145. http://doi.org/10.1037/0033-295X.96.1.145. [DOI] [PubMed] [Google Scholar]
- Blake R, O’Shea RP, Mueller TJ. Spatial zones of binocular rivalry in central and peripheral vision. Visual Neuroscience. 1992;8(05):469–478. doi: 10.1017/s0952523800004971. http://doi.org/10.1017/S0952523800004971. [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Donner TH, Sagi D, Fried M, Cooperman A, Heeger DJ, Arieli A. Motion-induced blindness and microsaccades: cause and effect. Journal of Vision. 2010;10(14):22. doi: 10.1167/10.14.22. http://doi.org/10.1167/10.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brascamp JW, Klink PC, Levelt WJM. The “laws” of binocular rivalry: 50 years of Levelt’s propositions. Vision Research. 2015;109(Part A):20–37. doi: 10.1016/j.visres.2015.02.019. http://doi.org/10.1016/j.visres.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Bundey S, Hardy C, Vickers S, Kilpatrick MW, Corbett JA. Duplication of the 15q11-13 region in a patient with autism, epilepsy and ataxia. Developmental Medicine and Child Neurology. 1994;36(8):736–742. doi: 10.1111/j.1469-8749.1994.tb11916.x. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Vitale R. Association between a GABRB3 polymorphism and autism. Molecular Psychiatry. 2002;7(3):311–316. doi: 10.1038/sj.mp.4001011. http://doi.org/10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Canitano R. Epilepsy in autism spectrum disorders. European Child & Adolescent Psychiatry. 2007;16(1):61–6. doi: 10.1007/s00787-006-0563-2. http://doi.org/10.1007/s00787-006-0563-2. [DOI] [PubMed] [Google Scholar]
- Chao H-T, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468(7321):263–9. doi: 10.1038/nature09582. http://doi.org/10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABAB Receptors Is Altered in Brains of Subjects with Autism. The Cerebellum. 2009;8(1):64–69. doi: 10.1007/s12311-008-0075-3. http://doi.org/10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABAa receptor downregulation in brains of subjects with autism. Journal of Autism and Developmental Disorders. 2009;39(2):223–230. doi: 10.1007/s10803-008-0646-7. http://doi.org/10.1007/s10803-008-0646-7.GABA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2013;33(19):8243–9. doi: 10.1523/JNEUROSCI.1608-12.2013. http://doi.org/10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AW. Multistage model for binocular rivalry. Journal of Neurophysiology. 2005;94(6):4412–20. doi: 10.1152/jn.00557.2005. http://doi.org/10.1152/jn.00557.2005. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. Journal of Neurodevelopmental Disorders. 2009;1(2):172–81. doi: 10.1007/s11689-009-9023-x. http://doi.org/10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J-D, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nature Neuroscience. 2005;8(5):686–691. doi: 10.1038/nn1445. http://doi.org/10.1038/nn1445. [DOI] [PubMed] [Google Scholar]
- Hohwy J, Roepstorff A, Friston K. Predictive coding explains binocular rivalry: an epistemological review. Cognition. 2008;108(3):687–701. doi: 10.1016/j.cognition.2008.05.010. http://doi.org/10.1016/j.cognition.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Hollins M, Hudnell K. Adaptation of the binocular rivalry mechanism. Investigative Ophthalmology & Visual Science. 1980;19(9):1117–1120. [PubMed] [Google Scholar]
- Kim H-G, Kishikawa S, Higgins AW, Seong I-S, Donovan DJ, Shen Y, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. The American Journal of Human Genetics. 2008;82(1):199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink PC, Brascamp JW, Blake R, Van Wezel RJA. Experience-driven plasticity in binocular vision. Current Biology. 2010;20(16):1464–1469. doi: 10.1016/j.cub.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Sheinberg DL. Visual Object Recognition. Annual Review of Neuroscience. 1996;19(1):577–621. doi: 10.1146/annurev.ne.19.030196.003045. http://doi.org/10.1146/annurev.ne.19.030196.003045. [DOI] [PubMed] [Google Scholar]
- Lunghi C, Emir UE, Morrone MC, Bridge H. Short-Term Monocular Deprivation Alters GABA in the Adult Human Visual Cortex. Current Biology. 2015 doi: 10.1016/j.cub.2015.04.021. http://doi.org/10.1016/j.cub.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menold MM, Shao Y, Wolpert CM, Donnelly SL, Raiford KL, Martin ER, Gilbert JR. Association analysis of chromosome 15 GABAa receptor subunit genes in autistic disorder. Journal of Neurogenetics. 2001;15(3-4):245–59. doi: 10.3109/01677060109167380. http://doi.org/10.3109/01677060109167380. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Strupp JP, Uğurbil K. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. Journal of Neurophysiology. 1997;77(5):2780–2787. doi: 10.1152/jn.1997.77.5.2780. [DOI] [PubMed] [Google Scholar]
- Miller SM, Gynther BD, Heslop KR, Liu GB, Mitchell PB, Ngo TT, Geffen LB. Slow binocular rivalry in bipolar disorder. Psychological Medicine. 2003;(04):683–692. doi: 10.1017/s0033291703007475. null. http://doi.org/10.1017/S0033291703007475. [DOI] [PubMed] [Google Scholar]
- Moreno-Bote R, Rinzel J, Rubin N. Noise-Induced Alternations in an Attractor Network Model of Perceptual Bistability. Journal of Neurophysiology. 2007;98(3):1125–1139. doi: 10.1152/jn.00116.2007. http://doi.org/10.1152/jn.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine M, Yoshino A, Miyazaki M, Takahashi Y, Nomura S. Difference in binocular rivalry rate between patients with bipolar I and bipolar II disorders. Bipolar Disorders. 2009;11(5):539–546. doi: 10.1111/j.1399-5618.2009.00719.x. http://doi.org/10.1111/j.1399-5618.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Research. 2011;1380:218–228. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew JD, Miller SM. A ’Sticky’ Interhemispheric Switch in Bipolar Disorder? Proceedings: Biological Sciences. 1998:2141–2148. doi: 10.1098/rspb.1998.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AE, Simmons DR. The Relationship between Sensory Sensitivity and Autistic Traits in the General Population. Journal of Autism and Developmental Disorders. 2012;43(4):775–84. doi: 10.1007/s10803-012-1608-7. http://doi.org/10.1007/s10803-012-1608-7. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Slower Rate of Binocular Rivalry in Autism. The Journal of Neuroscience. 2013;33(43):16983–16991. doi: 10.1523/JNEUROSCI.0448-13.2013. http://doi.org/10.1523/JNEUROSCI.0448-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Thomas C, Kravitz DJ, Wallace GL, Baron-Cohen S, Martin A, Baker CI. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain. 2014 doi: 10.1093/brain/awu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism : increased ratio of excitation / inhibition in key neural systems. Brain. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. http://doi.org/10.1046/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Egan RD, Minshew NJ, Behrmann M, Heeger DJ. Normal binocular rivalry in autism : Implications for the excitation / inhibition imbalance hypothesis. Vision Research. 2012 Dec;77:59–66. doi: 10.1016/j.visres.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Heeger DJ. A Model of Binocular Rivalry and Cross-orientation Suppression. PLoS Computational Biology. 2013;9(3) doi: 10.1371/journal.pcbi.1002991. http://doi.org/10.1371/journal.pcbi.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl BJ, Nakayama K. Causal capture: Contextual effects on the perception of collision events. Psychological Science. 2002;13(6):493–498. doi: 10.1111/1467-9280.00487. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf DS, Anderson EJ, de Haas B, White SJ, Rees G. Larger extrastriate population receptive fields in autism spectrum disorders. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2014;34(7):2713–24. doi: 10.1523/JNEUROSCI.4416-13.2014. http://doi.org/10.1523/JNEUROSCI.4416-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Hoekstra RA, Baron-Cohen S. The Sensory Perception Quotient (SPQ): development and validation of a new sensory questionnaire for adults with and without autism. Molecular Autism. 2014;5(1):29. doi: 10.1186/2040-2392-5-29. http://doi.org/10.1186/2040-2392-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Engel SA. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. 2001;411(6834):195–199. doi: 10.1038/35075583. http://doi.org/10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends in Cognitive Sciences. 2006;10(11):502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21(4):753–9. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488(7413):647–651. doi: 10.1038/nature11310. http://doi.org/10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam LCJ, van Ee R. The role of saccades in exerting voluntary control in perceptual and binocular rivalry. Vision Research. 2006;46(6):787–799. doi: 10.1016/j.visres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Van Loon AM, Knapen T, Scholte HS, St. John-Saaltink E, Donner TH, Lamme VAF. GABA Shapes the Dynamics of Bistable Perception. Current Biology. 2013 doi: 10.1016/j.cub.2013.03.067. http://doi.org/10.1016/j.cub.2013.03.067. [DOI] [PubMed] [Google Scholar]
- Vattikuti S, Chow CC. A Computational Model for Cerebral Cortical Dysfunction in Autism Spectrum Disorders. Biological Psychiatry. 2010;67(7):672–678. doi: 10.1016/j.biopsych.2009.09.008. http://doi.org/10.1016/j.biopsych.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier V, Baron-Cohen S, Chakrabarti B. Genetic variation in GABRB3 is associated with Asperger syndrome and multiple endophenotypes relevant to autism. Molecular Autism. 2013;4(1):48. doi: 10.1186/2040-2392-4-48. http://doi.org/10.1186/2040-2392-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR. Computational evidence for a rivalry hierarchy in vision. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14499–503. doi: 10.1073/pnas.2333622100. http://doi.org/10.1073/pnas.2333622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian J-J, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathologica. 2007;113(5):559–68. doi: 10.1007/s00401-006-0176-3. http://doi.org/10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–8. doi: 10.1038/nature10360. http://doi.org/10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]