Abstract
Purpose of review
The purpose of the review is to summarize and comment on recent developments regarding the safety of engineered immunotherapy vaccines.
Recent findings
In the last 2 years, several studies were published in which allergy vaccines were developed on the basis of chemical modification of natural allergen extracts, the engineering of allergen molecules by recombinant DNA technology and synthetic peptide chemistry, allergen genes, new application routes and conjugation with immune modulatory molecules. Several studies exemplified the general applicability of hypoallergenic vaccines on the basis of recombinant fusion proteins consisting of nonallergenic allergen-derived peptides fused to allergen-unrelated carrier molecules. These vaccines are engineered to reduce both, immunoglobulin E (IgE) as well as allergen-specific T cell epitopes in the vaccines, and thus should provoke less IgE and T-cell-mediated side-effects. They are made to induce allergen-specific IgG antibodies against the IgE-binding sites of allergens with the T-cell help of the carrier molecule.
Summary
Several interesting examples of allergy vaccines with potentially increased safety profiles have been published. The concept of fusion proteins consisting of allergen-derived hypoallergenic peptides fused to allergen-unrelated proteins that seems to be broadly applicable for a variety of allergens appears to be of particular interest because it promises not only to reduce side-effects but also to increase efficacy and convenience of allergy vaccines.
Keywords: allergen, allergy, engineered vaccines, immunotherapy, safety
INTRODUCTION
Type I allergy is a hypersensitivity disease based on the recognition of harmless, mainly environmental antigens by specific immunoglobulin E (IgE) [1]. It is the most frequent hypersensitivity disease and affects more than 25% of the population [2]. Over 100 years ago, it was shown that the vaccination of allergic patients with allergen extracts reduced their clinical sensitivity [3]. This finding seems paradoxical at first glance because it is difficult to explain how immunization of patients with an allergen that they have already developed a hyperimmune response with can improve the disease [4]. More than 20 years later, it could be shown that allergen-specific immunotherapy (SIT) induces a counter immune response consisting of allergen-specific IgG antibodies that block the binding of the disease-causing IgE antibodies to the allergen, and thus reduce IgE-mediated pathologies [5,6]. Today, we also know that SIT is the only allergen-specific form of treatment that modulates the course of the disease (i.e. prevents the progression toward severe manifestations), and there is evidence for a long-lasting effect even after discontinuation of SIT [7–9]. However, the insufficient quality of natural allergen extracts, (such as the presence of contaminations, varying amounts of allergens or the poor immunogenicity of certain allergens) and the risk that the administration of allergen to patients can induce severe and life-threatening side-effects are major obstacles for global allergy vaccination [10].
This article reviews studies published mainly in the last 2 years that have provided strategies for increasing the safety of SIT, its efficacy and convenience regarding application
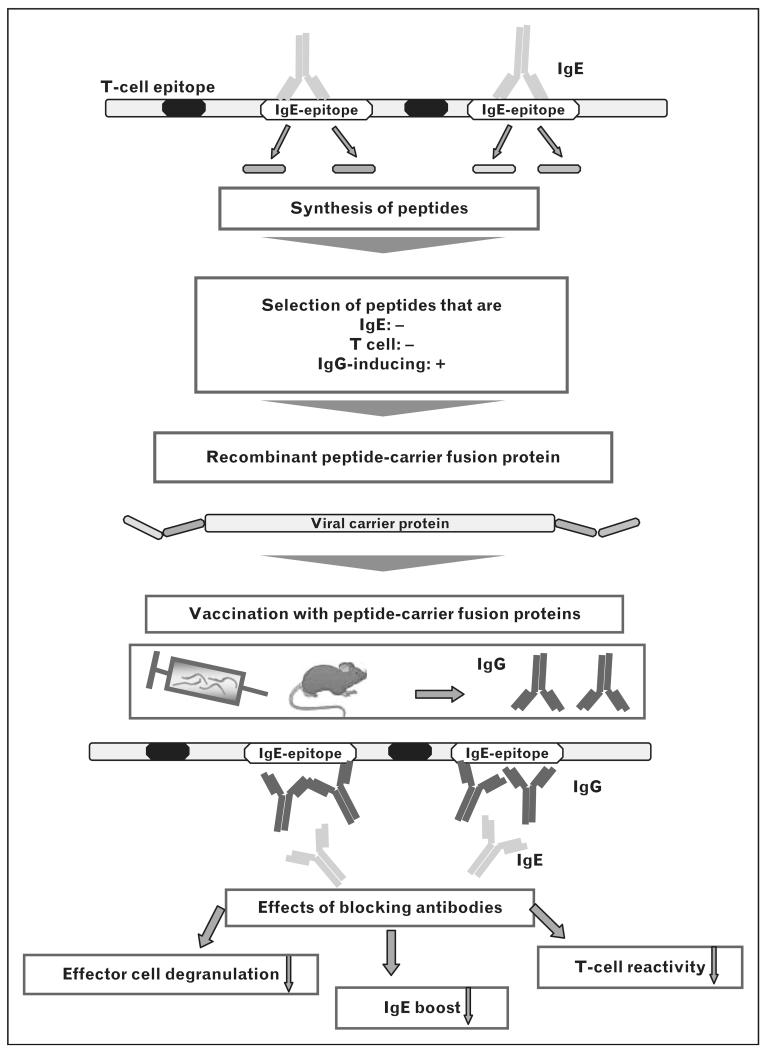
The availability of allergen sequences and structures due to allergen characterization using recombinant DNA technology [6] has greatly increased our knowledge of the epitopes involved in allergic inflammation and allowed us to develop SIT strategies that selectively target different aspects of allergic immune responses. Table 1 summarizes three generally applicable concepts that are based on recombinant or synthetic molecules for SIT. ‘T-cell peptides’ are synthetic peptides that react with allergen-specific T cells but do not bind allergen-specific IgE antibodies [11]. T-cell peptides should induce tolerance in allergen-specific T cells but do not induce allergen-specific IgG antibodies due to their small size and low immunogenicity. Recombinant hypoallergenic allergen derivatives also show reduced IgE reactivity but include the majority of the allergen-specific T cell epitopes. These derivatives are large and immunogenic enough to induce allergen-specific IgG antibodies upon SIT [12▪]. They may be used for SIT aiming at the induction of blocking IgG antibodies as well as for tolerance induction in T cells [13]. The third concept is based on hypoallergenic allergen peptides derived from the IgE-binding sites that are fused to allergen-unrelated carrier molecules. These vaccines are made to reduce IgE-mediated and T-cell-mediated side-effects and aim to induce allergen-specific IgG blocking antibodies without activation of allergen-specific T cells as the T-cell help derives from the allergen-unrelated carrier molecules [14] (Fig. 1).
Table 1. General concepts for improvement of safety of immunotherapy vaccines.
| Strategies | IgE reactivity |
T-cell reactivity |
|---|---|---|
| T-cell peptides | − | + |
| Recombinant technology (mutants, fragments, oligomers and hybrids) |
reduced | + |
| Peptide-carrier fusion proteins |
− | − |
IgE, immunoglobulin E.
FIGURE 1.
Design and immunological effects of immunotherapy performed with recombinant peptide-carrier fusion proteins. Peptides from or close to immunoglobulin E (IgE) binding sites (white) are selected in order to bypass allergen-specific T-cell epitopes (black). These peptides are then fused to allergen-unrelated viral carrier molecules and expressed as recombinant fusion proteins. The peptide vaccine induces peptide-specific and consequently allergen-specific IgG antibodies receiving T-cell help from carrier-derived T-cell epitopes. Fusion proteins are tested in experimental animal models to select those peptide vaccines that induce high titer, high-affinity allergen-specific IgG responses that strongly block allergic patients’ IgE binding to the allergen.
Several other strategies for improving SIT have been described in studies published within the last 2 years. They include vaccines that are constructed to reduce T helper 2 (Th2) responses, for example, by denaturation of allergen preparations (i.e. allergoids) and by the use of DNA or RNA vaccines. Furthermore, several alternative routes of delivery as compared with subcutaneous injection have been described. They include oral SIT, sublingual immunotherapy, edible vaccines, intralymphatic and epicutaneous administration.
T-CELL PEPTIDES
T-cell epitope-containing peptides were originally designed to modulate allergen-specific T-cell responses without IgE-mediated activation of effector cells. The first immunotherapy trials using T-cell epitopes for Fel d 1, the major cat allergen were associated with adverse events, primarily late-onset symptoms of rhinitis, asthma and pruritus, demonstrating the existence of IgE-independent allergic inflammation through the stimulation of allergen-specific T cells [11].
In a new study, immunodominant T-cell epitopes displaying promiscuous major histocompatibility complex (MHC) binding for high population coverage were identified on the basis of proliferative and cytokine responses [interleukin (IL)-10, IL-13 and interferon (IFN)-γ]. A single administration of the peptide vaccine in various doses was found to be safe and well tolerated [15▪]. On the basis of this study, the dose resulting in the greatest reduction in late phase skin response will be subjected to further clinical evaluation.
RECOMBINANT-BASED INNOVATIONS
Due to the fact that with the exception of certain food allergens, most IgE epitopes are conformational (i.e. discontinuous) epitopes, formed only by the correct folding of a protein, IgE binding to allergens is often depending on their tertiary structure. Alterations of the amino acid sequence of allergens can influence protein folding, and thus the IgE-binding capacity [13].
Already by 1970, Marsh et al. [16] showed that chemical modification of allergen extracts by aldehyde treatment resulted in ‘allergoids’ that were characterized by reduced IgE reactivity but retained immunogenicity (i.e. ability to induce IgG responses).
Several recent studies describe further examples of such allergoids. For instance, extracts from house dust mite or mixed tree pollen extracts or grass pollen were shown to be clinically effective and well tolerated when used with a rush immunotherapy build-up schedule [17–24]. Yet the old problems related to the manufacturing process of allergoids leading to relatively ill-defined products remain as long as natural allergen extracts are used for the preparation of the vaccines.
The use of recombinant allergens for the formulation of allergy vaccines can eliminate many of the problems related to poor quality of natural allergen extracts. A reduction of IgE reactivity and allergenic activity of recombinant allergens can be achieved by chemical denaturation of recombinant allergens as has been done by producing a folding variant of the recombinant birch pollen allergen by alkaline treatment of the recombinant Bet v 1 allergen [25]. A vaccine based on this folding variant has been successfully evaluated in clinical trials up to phase III [26].
A similar approach was used for Pru p 3, the major peach allergen. This folding variant was generated using reduction and alkylation and was evaluated in a mouse model. It showed reduced IgE and allergenic reactivity, but retained T-cell reactivity. Unfortunately the immunogenicity of this molecule was basically lost so that no relevant allergen-specific IgG antibodies were achieved upon immunization [27].
A more reproducible way of generating recombinant hypoallergenic allergen derivatives is based on the reduction of allergenic activity by recombinant technologies. A recent review describes this approach and the mechanisms underlying SIT with recombinant hypoallergenic allergen derivatives [12▪]. Among the recent examples of hypoallergens is a structure-guided single point mutation, done for Mus m 1, a mouse urinary protein and major mouse allergen. This mutation induced a spatial rearrangement of aromatic side chains and a lower allergenic activity, although the T-cell reactivity was preserved [28].
Patients sensitized to Art v 1 commonly display IgE antibodies against the cysteine-stabilized defensin fold. Site-directed mutagenesis of eight cysteines was used to disrupt disulfide bonds to generate molecules with altered IgE-binding capacity. The low allergenicity and high immunogenic activity of Art v 1 variant C49S renders the molecule a possible candidate for SIT of mugwort pollen allergy [29].
Another way is the fragmentation of allergens to destroy conformational IgE epitopes. Interestingly it could be shown that trimers made out of the hypoallergenic fragments of the birch pollen allergen Bet v 1 enhanced its immunogenicity so that higher levels of blocking allergen-specific IgG antibodies were achieved upon immunization [30].
For Fel d 1, the major cat allergen, IgE binding was reduced by disruption of the disulphide bonds that link the 2 Fel d 1 chains and additionally duplications of T-cell epitopes were inserted. This molecule was tested in a mouse model of cat allergy in which the mice were sensitized with rFel d 1 and subsequently treated with rFel d 1 or the hypoallergenic rFel d 1 derivative. All treated mice produced rFel d 1-specific IgG with blocking capacity and treatment with high doses of the hypoallergen tended to reduce airway hyperreactivity in the murine asthma model. All mice from groups treated with hypoallergenic Fel d 1 tolerated the treatment, whereas only four of 10 mice survived treatment with the rFel d 1 allergen. SPT reactivity in cat-allergic patients was evaluated in which the hypoallergen indeed induced less skin inflammation than the rFel d 1 allergen [31].
Fusion proteins combining different allergens within one molecule have been suggested for making vaccine production easier and increasing immunogenicity of the vaccines [32]. This approach was used for the major allergens from house dust mite but these molecules appeared to be not stable and it was difficult to keep them in solution [33].
Interesting variants of the major cat allergen, Fel d 1, were generated by the introduction of random mutations and expression in large phage libraries using IgE antibodies of allergic patients for phage enrichment. This approach was thought to obtain variants with preserved structure but reduced allergenic and T-cell activity. Obtained mutants could induce blocking antibodies in mice [34].
PEPTIDE CARRIER FUSION PROTEINS
More than 10 years ago, the idea arose for the concept of peptide carrier fusion proteins [35,36]. The idea behind this approach was to identify allergen-derived peptides that are part of conformational IgE epitopes of allergens but which due to lack of structure do not react with IgE antibodies, and hence lack allergenic activity (Fig. 1). When such peptides are coupled covalently to an allergen-unrelated carrier molecule, it should be possible to induce blocking IgG antibodies toward the IgE-binding sites. According to the hapten–carrier principle [37], the T-cell help for the IgG antibody induction would come from the carrier molecules so that the activation of allergen-specific T cells would be low, which should also reduce T-cell-mediated side-effects in addition to the IgE-mediated side-effects (Fig. 1). Peptide carrier fusion proteins can be obtained by chemical coupling of allergen-derived peptides to carrier molecules as has been shown for the major allergens from timothy grass pollen Phl p 1, birch pollen Bet v 1, olive pollen Ole e 1, the mold Alternaria alternata Alt a 1 and house dust mite Der p 2 [36,38–41].
However, chemical coupling of allergen-derived peptides is not a process that is suitable for the production of allergy vaccines that has to follow strict guidelines for quality and reproducibility (i.e. good manufacture practice GMP guidelines). Furthermore, one has to choose carrier proteins that can be considered well tolerated and may eventually bring additional advantages in addition to the antiallergic effects of the vaccine [42]. As possible carriers, rhinovirus-derived coat protein VP1 and the PreS domain of hepatitis B have been evaluated in some more recent studies [43,44▪]. These vaccines were produced in the form of recombinant fusion proteins that can be obtained by expression in Escherichia coli as stable fusion proteins. PreS is part of hepatitis B vaccines that have been safely administered even to newborns and generated protective immunity against hepatitis B [45]. Immunization with VP1 fusion proteins and with VP1 alone was shown to induce antibodies that could reduce rhinovirus infections of cultured epithelial cells [42,43,46]. It is, therefore, quite possible that vaccines based on fusion proteins consisting of allergen peptides and viral proteins will exhibit not only an antiallergic activity but also be useful for inducing an antivirus immunity. A hypoallergenic vaccine consisting of PreS of hepatitis B fused to nonallergenic peptides from the four major timothy grass pollen allergens Phl p 1, 2, 5 and 6, designated BM32, has been developed and has already been evaluated in clinical studies [Clinical trials.gov identifier NCT01350635, NCT01445002].
OTHER STRATEGIES
In the next part, additional strategies for SIT that are not based on novel proteins or peptides but other molecules, conjugates or routes of application are described.
Genetic vaccines
DNA vaccines differ from traditional vaccines because instead of the antigens, the protein-encoding DNA is used for immunization. These vaccines have been proposed for allergy because they seem to favour specific Th1 responses. Major problems of DNA vaccines are safety concerns because the DNA should not stably integrate into the host genome and uncontrolled expression of allergens in different tissues may cause anaphylaxis. Therefore, current studies focus on delivering DNA coding for hypoallergenic allergen variants.
A DNA vaccine encoding a hypoallergenic Der p 2 variant was shown to prevent allergic airway inflammation and due to the presence of cytosine-phosphate-guanosine (CpG) motifs in the DNA vaccine to upregulate Toll-like receptor (TLR) 9 in lung tissues in a mouse model for allergic asthma. Thus, a Th1-like pattern of cytokine release and reduced IgE production was obtained [47]. A DNA vaccine encoding mature Der p 1 yielded a hypoallergenic Der p 1 due to the lack of the prosequence that has a critical role in the correct folding of Der p 1 and its IgE reactivity. The combination of the DNA with lipoplex (cationic liposomes) induced a faster and stronger Th1 response, holding promise that the amount of DNA can be reduced compared with naked DNA immunization [48].
Vaccination with plasmid DNA encoding the major Bermuda grass pollen allergen Cyn d 1 was used in combination with different adjuvants such as bupivacaine, bestatin, liposome or CpG and induced Th1 responses associated with IgG2a responses in mice. This vaccine prevented the induction of IgE responses and also suppressed ongoing IgE production in a murine model [49].
mRNAs encoding allergens may represent a possible strategy for prophylactic vaccination. For mRNA vaccines, only the genetic information of the allergen can be used, without a plasmid backbone or a viral promoter and mRNA cannot integrate into the host genome and is quickly degraded. The latter features may improve the safety of therapeutic nucleic acid-based vaccines. In a murine model, it was also investigated whether mRNA constructs would prevent the development of allergy. Immunization with mRNAs induced Th1-biased immune responses similar to DNA vaccines but addressed different danger signals (TLR3/4 instead of 9). It was shown that IL-4 and IL-5 levels were reduced, IFN-γ-producing cells were induced and a reduction of airway hyperreactivity and eosinophil counts in the lung was observed. The possible advantages of mRNA vaccines are summarized in a recent review article [50].
Routes other than subcutaneous specific immunotherapy
Subcutaneous application is the most frequently used and effective form of application of immunotherapy vaccines. During the last decades, several other routes of delivery such as oral, nasal, bronchial, epicutaneous, intraepithelial, intralymphatic or sublingual have been investigated. Oral administration of peanut flour seemed to induce desensitization in peanut-allergic patients [51,52].
Transgenic rice-expressing allergens have been proposed as another possibility for mucosal allergy vaccination. The protection of orally administered allergens from proteolytic digestion in the gastrointestinal tract may be important to prevent loss of immunogenicity. Several plant seeds contain protein bodies for protein storage, resistant to proteolytic degradation, and thus should be well suited for bioencapsulation of allergens. Furthermore, storage and transport of these vaccines is very economical due to the fact that in seeds accumulated antigens are stable for years [53]. However, the protection of allergens from degradation may lead to severe anaphylactic side-effects due to the release of intact allergens into the gut and their systemic uptake.
Therefore, transgenic rice that accumulates in the edible part (seed endosperm), a modified hypoallergenic version of the cedar pollen allergen, Cyr j 2, was proposed. Several constructs were made that express wild-type Cry j 2 without signal peptide, Cyr j 2 fragments or a tail to head mosaic protein. These vectors were introduced into the rice genome by Agrobacterium-mediated transformation, but the reduction of allergenic activity of the different transgenic rice variants has not yet been determined [54].
Another study tested the feasibility of oral immunotherapy using transgenic rice seeds containing an immunodominant fragment of Der p 1 in a murine model of asthma. Prophylactic oral vaccination was able to prevent the development of allergen-specific IgE and IgG, the production of Th2 cytokines and of allergic airway inflammation. The effects of the prophylactic vaccine were antigen-specific and there was no induction of bystander immune suppression, but the therapeutic activity has not yet been investigated [55,56].
Likewise, the administration of transgenic rice seeds containing Der f 2 derivatives with reduced IgE reactivity prevented allergy in a mouse model, but the therapeutic approach has not been studied [57].
A recombinant Der p 2, a major house dust mite allergen was produced for sublingual immunotherapy and tested in Der p 2-sensitized mice. Sublingual treatment with rDer p 2 decreased airway hyperresponsiveness and Th2 responses [58].
Another route that is currently under investigation is epicutaneous delivery for immunotherapy [59]. Allergens are either delivered by simple patches [60] or by using special epicutaneous delivery systems [61].
One study describes intralymphatic immunotherapy for cat allergy using a recombinant version of the major cat allergen Fel d 1 that was obtained by fusing the allergen to a translocation sequence and to part of the human invariant chain. This construct was supposed to be targeted to the MHC class II pathway. After three intralymphatic injections increased cat allergen-specific IgG4 levels and a stimulation of regulatory T-cell responses was observed [62▪].
Use of immunomodulatory substances
Bacterial DNA and synthetic oligodeoxynucleotides-containing CpG is thought to promote Th1 responses and the administration of allergens conjugated to CpG has already been evaluated in clinical trials [63]. In a recent study, Cry j 1, a major Japanese cedar pollen allergen was conjugated to CpGs and tested in a mouse model. It was observed that immunization with the conjugate suppressed allergen-specific IgE responses and enhanced IgG2a antibody production [64].
1α 25 dihydroxyvitamin D3 (VD3) has been shown to induce dendritic cells with tolerogenic properties, thus, increasing regulatory T-cell responses [65]. In an attempt to use this effect, VD3 was covalently coupled to Fel d 1, the major cat allergen and tested in a mouse model of cat allergy. This construct was more potent than rFel d 1 in preventing pulmonary eosinophilia, and therefore has been suggested for treatment of allergic asthma [66].
Table 2 [15▪,17–24,27–31,33,34,39–41,43,44▪, 47–50,52,54–58,60,61,62▪,64,66] provides a summary of the different approaches for improvement of SIT that have been described above.
Table 2. Strategies for improving specific immunotherapy.
| Strategies | Examples | References | |
|---|---|---|---|
| Molecule-based | |||
| Natural molecules | Chemical treatment of extracts | Depigmented aldehyde-treated extracts (house dust mite, grass and tree pollen) |
[17–24] |
| Synthetic molecules | Peptides | T-cell peptides of Fel d 1 | [15▪] |
| Recombinant molecules | Folding variants | Pru p 3 folding variant | [27] |
| Mutants | Mus m 1 structural mutant | [28] | |
| Fragments/oligomers | Art v 1 mutation of disulfide bonds | [29] | |
| Hybrids | Trimer of Bet v 1 fragment | [30] | |
| Fel d 1 with duplicated T-cell epitopes and disrupting of disulfide bonds |
[31] | ||
| Feld 1 random mutations | [34] | ||
| Der p 1–2 hybrid | [33] | ||
| Peptide-carrier fusion proteins | Ole e 1 peptides coupled to KLH | [39] | |
| Der p 2 peptides coupled to KLH | [40] | ||
| Alt a 1 peptides coupled to KLH | [41] | ||
| Phl p 1 peptides fused to VP1 | [43] | ||
| Fel d 1 peptides fused to PreS | [44▪] | ||
| DNA vaccines | Der p 2 mutant | [47] | |
| Der p 1 mature | [48] | ||
| Cyn d 1 | [49] | ||
| RNA vaccines | [50] | ||
| Routes | |||
| Oral | Desensitization in food allergy | Peanut flour | [52] |
| Edible seeds | Rice seeds with shuffled Cry j 2,Der p 1 fragment, Der f 2 with disrupted disulfide bonds [57] |
[54–56] | |
| Sublingual | Recombinant allergens | Natural-like recDer p 2 | [58] |
| Epicutaneaous | Pollen extract patches | Grass pollen extract | [60] |
| Food allergens | Peanut extract | [61] | |
| Intralymphatic | MAT | MAT-Fel d 1 | [62▪] |
| Immunomodulation | |||
| Conjugated molecules | CpGs | Cry j 1-CpG | [64] |
| Vitamin D3 | Fel d 1 Vit D3 | [66] |
KLH, keyhole limpet hemocyanin; MAT, modular antigen transporter.
CONCLUSION
In the last 2 years, several studies explored concepts for improving the safety of allergen-specific immunotherapy. These studies either used alternative routes of application and/or engineered allergen molecules that have been made in attempts to increase safety and efficacy of treatment. Among the various technologies of producing hypoallergenic allergen derivatives, the approach of engineering fusion proteins consisting of carrier-bound allergen-derived peptides was of particular interest because it seemed to be generally applicable to a large variety of different major allergens. Furthermore, it appeared that this approach allowed reducing IgE as well as T-cell-mediated side-effects of SIT.
KEY POINTS.
More than 25% of the population suffer from allergy.
Immunotherapy is an allergen-specific, disease-modifying and clinically effective treatment for allergy.
Detailed knowledge of allergen sequences, structures and epitopes allows engineering new vaccines.
New allergy vaccines based on defined molecules hold promise to increase safety, efficacy and convenience of immunotherapy.
Acknowledgements
This study was supported by research grants from the Christian Doppler Research Association and Biomay AG, Vienna, Austria and by grant F4605 of the Austrian Science Fund (FWF).
Footnotes
Conflicts of interest
M.F.T. and R.V. have received research grants from Biomay AG, Vienna, Austria. R.V. serves as a consultant for Biomay AG.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Kay AB. Allergy and Allergic Diseases. 2nd ed Blackwell Science; Oxford: 2008. [Google Scholar]
- 2.Floistrup H, Swartz J, Bergstrom A, et al. Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol. 2006;117:59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Noon L. Prophylactic inoculation against hayfever. Lancet. 1911;1:1572–1573. [Google Scholar]
- 4.Linhart B, Valenta R. Vaccines for allergy. Curr Opin Immunol. 2012;24:354–360. doi: 10.1016/j.coi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy. J Exp Med. 1935;62:733–750. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenta R, Ferreira F, Focke-Tejkl M, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 8.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 9.Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein DI, Wanner M, Borish L, et al. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004;113:1129–1136. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Larché M. T cell epitope-based allergy vaccines. Curr Top Microbiol Immunol. 2011;352:107–119. doi: 10.1007/82_2011_131. [DOI] [PubMed] [Google Scholar]
- 12 ▪.Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2012;30:4328–4335. doi: 10.1016/j.vaccine.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides a summary of the mechanisms underlying SIT with recombinant hypoallergens.
- 13.Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002;2:446–453. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- 14.Focke M, Swoboda I, Marth K, et al. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 15 ▪.Worm M, Lee HH, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]; Study regarding safety of a clinical trial performed with T cell epitope-containing peptides of the major cat allergen, Fel d 1.
- 16.Marsh DG, Lichtenstein LM, Campbell DH. Studies on ‘allergoids’ prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970;18:705–722. [PMC free article] [PubMed] [Google Scholar]
- 17.Riechelmann H, Schmutzhard J, van der Werf JF, et al. Efficacy and safety of a glutaraldehyde-modified house dust mite extract in allergic rhinitis. Am J Rhinol Allergy. 2010;24:e104–e109. doi: 10.2500/ajra.2010.24.3508. [DOI] [PubMed] [Google Scholar]
- 18.Hernández N, Ibero M, Ridao M, et al. Safety of specific immunotherapy using a depigmented and polymerised extract of dermatophagoides pteronyssinus in children under five years of age. Allergol Immunopathol (Madr) 2011;39:267–270. doi: 10.1016/j.aller.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Gallego MT, Iraola V, Himly M, et al. Depigmented and polymerised house dust mite allergoid: allergen content, induction of IgG4 and clinical response. Int Arch Allergy Immunol. 2010;153:61–69. doi: 10.1159/000301580. [DOI] [PubMed] [Google Scholar]
- 20.Pfaar O, Klimek L, Sager A, et al. Safety of a depigmented, polymerized vaccine for the treatment of allergic rhinoconjunctivitis and allergic asthma. Am J Rhinol Allergy. 2010;24:220–225. doi: 10.2500/ajra.2010.24.3437. [DOI] [PubMed] [Google Scholar]
- 21.Pfaar O, Robinson DS, Sager A, et al. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2011;65:1614–1621. doi: 10.1111/j.1398-9995.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- 22.Höiby AS, Strand V, Robinson DS, et al. Efficacy, safety, and immunological effects of a 2-year immunotherapy with Depigoid birch pollen extract: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy. 2010;40:1062–1070. doi: 10.1111/j.1365-2222.2010.03521.x. [DOI] [PubMed] [Google Scholar]
- 23.Brehler R, Klimek L, Pfaar O, et al. Safety of a rush immunotherapy build-up schedule with depigmented polymerized allergen extracts. Allergy Asthma Proc. 2010;31:e31–e38. doi: 10.2500/aap.2010.31.3334. [DOI] [PubMed] [Google Scholar]
- 24.Pfaar O, Urry Z, Robinson DS, et al. A randomized placebo-controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy. Allergy. 2012;67:272–279. doi: 10.1111/j.1398-9995.2011.02736.x. [DOI] [PubMed] [Google Scholar]
- 25.Kahlert H, Suck R, Weber B, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008;145:193–206. doi: 10.1159/000109288. [DOI] [PubMed] [Google Scholar]
- 26.Rak S. Clinical results with a hypoallergenic recombinant birch pollen allergen derivative [abstract]. Presented at XXVIII EAACI congress; Warsaw, Poland. 6–10 June 2009. [Google Scholar]
- 27.Toda M, Reese G, Gadermaier G, et al. Protein unfolding strongly modulates the allergenicity and immunogenicity of Pru p 3, the major peach allergen. J Allergy Clin Immunol. 2011;128:1022–1030. doi: 10.1016/j.jaci.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari E, Breda D, Longhi R, et al. In search of a vaccine for mouse allergy: significant reduction of Mus m 1 allergenicity by structure-guided single-point mutations. Int Arch Allergy Immunol. 2012;157:226–237. doi: 10.1159/000327551. [DOI] [PubMed] [Google Scholar]
- 29.Gadermaier G, Jahn-Schmid B, Vogel L, et al. Targeting the cysteine-stabilized fold of Art v 1 for immunotherapy of Artemisia pollen allergy. Mol Immunol. 2010;47:1292–1298. doi: 10.1016/j.molimm.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Vrtala S, Fohr M, Campana R, et al. Genetic engineering of trimers of hypoallergenic fragments of the major birch pollen allergen, Bet v 1, for allergy vaccination. Vaccine. 2011;29:2140–2148. doi: 10.1016/j.vaccine.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saarne T, Neimert-Andersson T, Grönlund H, et al. Treatment with a Fel d 1 hypoallergen reduces allergic responses in a mouse model for cat allergy. Allergy. 2011;66:255–263. doi: 10.1111/j.1398-9995.2010.02468.x. [DOI] [PubMed] [Google Scholar]
- 32.Linhart B, Valenta R. Vaccine engineering improved by hybrid technology. Int Arch Allergy Immunol. 2004;134:324–331. doi: 10.1159/000079535. [DOI] [PubMed] [Google Scholar]
- 33.Bussières L, Bordas-Le Floch V, Bulder I, et al. Recombinant fusion proteins assembling Der p 1 and Der p 2 allergens from Dermatophagoides pteronyssinus. Int Arch Allergy Immunol. 2010;153:141–151. doi: 10.1159/000312631. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson OB, Adedoyin J, Rhyner C, et al. In vitro evolution of allergy vaccine candidates, with maintained structure, but reduced B cell and T cell activation capacity. PLoS One. 2011;6:e24558. doi: 10.1371/journal.pone.0024558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valenta R, Vrtala S, Focke-Tejkl M, et al. Genetically engineered and synthetic allergen derivatives: candidates for vaccination against type I allergy. Biol Chem. 1999;380:815–824. doi: 10.1515/BC.1999.101. [DOI] [PubMed] [Google Scholar]
- 36.Focke M, Mahler V, Ball T, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–2044. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 37.Paul WE, Katz DH, Goidl EA, et al. Carrier function in antihapten immune responses. II. Specific properties of carrier cells capable of enhancing antihapten antibody responses. J Exp Med. 1970;132:283–299. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Focke M, Linhart B, Hartl A, et al. Nonanaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–1533. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 39.Twaroch TE, Focke M, Civaj V, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–184. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Chen KW, Focke-Tejkl M, Blatt K, et al. Carrier-bound nonallergenic Der p 2 peptides induce IgG antibodies blocking allergen-induced basophil activation in allergic patients: Experimental Allergy and Immunology. Allergy. 2012;67:609–621. doi: 10.1111/j.1398-9995.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Twaroch TE, Focke M, Fleischmann K, et al. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternata allergy. Clin Exp Allergy. doi: 10.1111/j.1365-2222.2012.03996.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edlmayr J, Niespodziana K, Focke-Tejkl M, et al. Allergen-specific immunotherapy: towards combination vaccines for allergic and infectious diseases. Curr Top Microbiol Immunol. 2011;352:121–140. doi: 10.1007/82_2011_130. [DOI] [PubMed] [Google Scholar]
- 43.Edlmayr J, Niespodziana K, Linhart B, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–6306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 44▪.Niespodziana K, Focke-Tejkl M, Linhart B, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–1570. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes an allergy vaccine based on recombinant fusion proteins consisting of allergen-derived peptides fused to a viral carrier protein.
- 45.Rendi-Wagner P, Shouval D, Genton B, et al. Comparative immunogenicity of a PreS/S hepatitis B vaccine in non and low responders to conventional vaccine. Vaccine. 2006;24:2781–2789. doi: 10.1016/j.vaccine.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Edlmayr J, Niespodziana K, Popow-Kraupp T, et al. Antibodies induced with recombinant VP1 from human rhinovirus exhibit cross-neutralisation. Eur Respir J. 2011;37:44–52. doi: 10.1183/09031936.00149109. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Yang Q, Wang P, et al. Derp2-mutant gene vaccine inhibits airway inflammation and up-regulates Toll-like receptor 9 in an allergic asthmatic mouse model. Asian Pac J Allergy Immunol. 2010;28:287–293. [PubMed] [Google Scholar]
- 48.Pulsawat P, Piboonpocanun S, Sirivichayakul S, et al. Production and immunogenicity of hypoallergenic codon-optimized DNA vaccine encoding mature Der p 1 allergen. J Investig Allergol Clin Immunol. 2010;20:582–590. [PubMed] [Google Scholar]
- 49.Huang CF, Chu CH, Wu CC, et al. Induction of specific Th1responses and suppression of IgE antibody formation by vaccination with plasmid DNA encoding Cyn d 1. Int Arch Allergy Immunol. 2012;158:142–150. doi: 10.1159/000331140. [DOI] [PubMed] [Google Scholar]
- 50.Weiss R, Scheiblhofer S, Roesler E, et al. J. Prophylactic mRNA vaccination against allergy. Curr Opin Allergy Clin Immunol. 2010;10:567–574. doi: 10.1097/ACI.0b013e32833fd5b6. [DOI] [PubMed] [Google Scholar]
- 51.Rancitelli P, Hofmann A, Burks AW. Vaccine approaches for food allergy. Curr Top Microbiol Immunol. 2011;352:55–69. doi: 10.1007/82_2011_126. [DOI] [PubMed] [Google Scholar]
- 52.Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takaiwa F. Seed-based oral vaccines as allergen-specific immunotherapies. Hum Vaccin. 2011;7:357–366. doi: 10.4161/hv.7.3.14302. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, Yang L, Takaiwa F. Transgenic rice accumulating modified cedar pollen allergen Cry j 2 derivatives. J Biosci Bioeng. 2012;113:249–251. doi: 10.1016/j.jbiosc.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki K, Kaminuma O, Yang L, et al. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol J. 2011;9:982–990. doi: 10.1111/j.1467-7652.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- 56.Hiroi T, Kaminuma O, Takaiwa F. Vaccination with transgenic rice seed expressing mite allergen: a new option for asthma sufferers? Expert Rev Vaccines. 2011;10:1249–1251. doi: 10.1586/erv.11.102. [DOI] [PubMed] [Google Scholar]
- 57.Yang L, Hirose S, Suzuki K, et al. Expression of hypoallergenic Der f 2 derivatives with altered intramolecular disulphide bonds induces the formation of novel ER-derived protein bodies in transgenic rice seeds. J Exp Bot. 2012;63:2947–2959. doi: 10.1093/jxb/ers006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bordas-Le Floch V, Bussières L, et al. Expression and characterization of natural-like recombinant Der p 2 for sublingual immunotherapy. Int Arch Allergy Immunol. 2012;158:157–167. doi: 10.1159/000331143. [DOI] [PubMed] [Google Scholar]
- 59.Dioszeghy V, Mondoulet L, Dhelft V, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186:5629–5637. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 60.Senti G, von Moos S, Tay F, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Mondoulet L, Dioszeghy V, Vanoirbeek JA, et al. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol. 2011;154:299–309. doi: 10.1159/000321822. [DOI] [PubMed] [Google Scholar]
- 62▪.Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–1296. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]; Clinical study showing efficacy of SIT with a recombinant Fel d 1 hypoallergen.
- 63.Creticos PS, Schroeder JT, Hamilton RG, et al. Immune Tolerance Network Group Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 64.Kaburaki Y, Fujimura T, Kurata K, et al. Induction of Th1 immune responses to Japanese cedar pollen allergen (Cry j 1) in mice immunized with Cry j 1 conjugated with CpG oligodeoxynucleotide. Comp Immunol Microbiol Infect Dis. 2011;34:157–161. doi: 10.1016/j.cimid.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grundström J, Neimert-Andersson T, Kemi C, et al. Covalent coupling of vitamin D3 to the major cat allergen Fel d 1 improves the effects of allergenspecific immunotherapy in a mouse model for cat allergy. Int Arch Allergy Immunol. 2012;157:136–146. doi: 10.1159/000327546. [DOI] [PubMed] [Google Scholar]