Abstract
Background
Diagnosis and immunotherapy of house-dust mite (HDM) allergy is still based on natural allergen extracts. The aim of this study was to analyze commercially available Dermatophagoides pteronyssinus extracts from different manufacturers regarding allergen composition and content and whether variations may affect their allergenic activity.
Methods
Antibodies specific for several D. pteronyssinus allergens (Der p 1, 2, 5, 7, 10 and 21) were used to analyze extracts from 10 different manufacturers by immunoblotting. Sandwich ELISAs were used to quantify Der p 1 and Der p 2 in the extracts. Mite-allergic patients (n = 45) were skin-tested with the extracts and tested for immunoglobulin E (IgE) reactivity to a panel of 10 mite allergens (Der p 1, 2, 4, 5, 7, 8, 10, 14, 20 and 21) by dot blot.
Results
Only Der p 1 and Der p 2 were detected in all extracts but their concentrations and ratios showed high variability (Der p 1: 6.0–40.8 μg ml−1; Der p 2: 1.7–45.0 μg ml−1). At least 1 out of 4 allergens (i.e. Der p 5, 7, 10 and 21) was not detected in 8 of the studied extracts. Mite-allergic subjects showed different IgE reactivity profiles to the individual mite allergens, the extracts showed different allergenic activity in skin-prick tests and false-negative results.
Conclusions
Commercially available D. pteronyssinus extracts lack important allergens, show great variability regarding allergen composition and content and some gave false-negative diagnostic test results in certain patients.
Keywords: House-dust mites, Allergen extracts, Dermatophagoides pteronyssinus, Skin prick tests, Recombinant allergens
Introduction
House dust has been known as a triggering factor of respiratory diseases, like rhinitis and asthma, for decades [1] and mites from the genus Dermatophagoides sp. have been found to be the most important allergen source in house dust [2]. House-dust mites (HDMs) represent one of the most common causes of allergy worldwide, against which more than 50% of allergic patients are sensitized [3] and in which, so far, more than 20 allergens have been identified [4, 5].
Skin-prick testing (SPT) with allergen extracts represents one of the most common methods of diagnosing allergy and has been used since the 19th century [6-8]. Early attempts for a quality control of allergen extracts were based on the measurement of total protein content defining protein nitrogen units (PNU) [9]. Further attempts to characterize diagnostic and therapeutic extracts included in-house standards and units [e.g. allergy unit (AU), biological unit (BU) and index of reactivity (IR)] defined by skin testing [7] or in vitro testing using serum samples of available allergic patients in methods such as direct RAST, RAST inhibition or basophil activation assays [9-12]. Today, allergen standardization mainly concentrates on the safety aspect by determining the overall immunoglobulin E (IgE)-binding potency of the allergen extracts [13]. However, each manufacturer uses company-specific units which are not suitable for the comparison of different products. It has been shown that the concentration of major allergens correlated with the biological potency and IgE reactivity of allergen extracts [14-15]; therefore, the quantification of major allergens in extracts using recombinant reference allergens has been initiated [16].
However, major problems in the preparation of HDM allergen extracts are due to the fact that these extracts contain several different important allergens and several proteases which may lead to degradation, and the allergen composition varies depending on culture conditions, source material (mite bodies and mite cultures), extraction procedures and storage conditions [17-20]. A recent study indicated that there is considerable variation of the major HDM allergens, Der p 1 and Der p 2 in commercial D. pteronyssinus extracts, but there are no data available regarding other important HDM allergens [21].
The aim of our study was to perform an in-depth analysis of commercially available D. pteronyssinus extracts from different European manufacturers regarding a panel of 6 important HDM allergens (Der p 1, 2, 5, 7, 10 and 21) [5, 22], and to study if variations in allergen composition and content may affect diagnostic skin-test results.
Material and Methods
Allergens and Antibodies
Natural Der p 1 was affinity-purified from a D. pteronyssinus extract using the monoclonal antibody 4C1 and Der p 4 was purified by cyclodextrin affinity chromatography [23-24]. The recombinant allergens rDer p 5, 7, 10 and 21 were expressed in the vector pET 17b as nonfusion proteins [5, 25, 26 and Casset and Vrtala, unpubl.]. rDer p 2 was expressed in the vector pET 17b with a C-terminal hexahistidine tag [27] and an rDer p 14 fragment (aa 1–260) was expressed in the vector pET 19b with an N-terminal hexahistidine tag [28]. rDer p 8 was expressed in the vector pGEX as a GST fusion protein and rDer p 20 in the vector pET 19b as a nonfusion protein [Thomas et al., unpubl.]. Rabbits were immunized with natural (nDer p 1) and recombinant allergens (rDer p 2, 5, 7, 10 and 21) using Freund’s adjuvant (Charles River Laboratories, Kißlegg, Germany). The immunizations consisted of 3 injections; the 1st contained 200 μg allergen in complete Freund’s adjuvant and the 2nd and 3rd contained 200 μg protein in incomplete Freund’s adjuvant. A monoclonal anti-HSA (human serum albumin) antibody (clone HSA-11, A6684) was purchased from Sigma.
Subject Characteristics
Adult subjects with clear-cut clinical symptoms appearing on exposure to house dust (e.g. sneezing and running nose at home, wheezing at night and worsening of symptoms upon greater exposure) were recruited. Twenty-six adult subjects from Italy and 19 from France were tested and had blood taken at the time the SPT was performed. Patients had a history of mite allergy with symptoms indicative of this (asthma and/or rhinitis and/or conjunctivitis), a positive SPT to D. pteronyssinus extract and/or D. pteronyssinus-specific IgE antibodies (>0.35 kUA l−1) measured by ImmunoCAP (Phadia, Uppsala, Sweden). Subjects who were using oral corticosteroids and/or antihistamines and pregnant or breast-feeding women were excluded. As a control group, 5 subjects with no history of mite allergy and negative in vitro and/or in vivo tests for D. pteronyssinus were included.
Allergen Extracts and Skin Prick Testing
As a part of the diagnostic testing procedure, SPTs were performed at the Centre for Molecular Allergology in Rome, Italy and in the Division of Asthma and Allergology in the Strasbourg University Hospital, France, with permission from the local ethics committees and informed consent from the patients (IDI-EC 2006-202). Italian mite-allergic patients were tested with D. pteronyssinus SPT solutions from 9 international suppliers that are available in Italy (extract 1: Leti batch 753795-06/E, extract 2: ALK-Abello batch A1667, extract 3: Lofarma batch 06101, extract 4: Anallergo batch 062511, extract 5: Bial batch 61129, extract 6: HAL Allergy batch B1605034, extract 7: Merck batch 30012523-IT, extract 8: Stallergenes batch 64891 and extract 9: SARM Allergeni batch N156). French mite-allergic and non-mite-allergic subjects (controls) were tested in duplicate with D. pteronyssinus SPT solutions available from 4 international suppliers in France (extract A: Leti batch 821111-06/S, extract B: Stallergenes batch 73933, extract C: HAL batch B1725029 and extract D: Allerbio batch 0701678). Histamine dihydrochloride (10 mg ml−1) served as positive control and a glycerol-saline solution as negative control. SPT procedures were performed according to the published practice guidelines [6, 7] on the volar aspect of the patient’s forearms, 3 cm apart. Wheal sizes were marked with a pen after 15 min and transferred to a record sheet with an adhesive tape. SPT responses were evaluated as the mean wheal area calculated by computer scanning (Autocad 2008, Autodesk, Pratteln, Switzerland) as previously described [29]. Only wheals of an area of at least 7 mm2 were considered as positive [6].
The protein and allergen contents of aliquots of the extracts used for the SPTs were analyzed in parallel.
All tests were performed with coded anonymous preparations. Investigators in the clinical and laboratory settings were not aware at the time of the study of the kind of preparation they were using in vivo or in vitro.
The total protein content of the extracts was determined with the Micro BCA Protein Assay Reagent Kit (Pierce, Rockford, Ill., USA) using the bicinchoninic acid method.
SDS-PAGE and Silver Staining
Equal volumes of each D. pteronyssinus extract (10 μl) were applied to 14% SDS-PAGE and proteins were visualised by silver-staining [30].
Detection of D. pteronyssinus Allergens Der p 1, 2, 5, 7, 10 and 21 in D. pteronyssinus Extracts by Immunoblotting
Equal volumes of each D. pteronyssinus extract (15 μl cm−1) were separated by 14% SDS-PAGE and blotted onto nitrocellulose (Protan®, Whatman, Dassel, Germany). Membranes were blocked in buffer A [50 mm sodium phosphate pH 7.5, 0.5% (w/v) BSA, 0.5% (v/v) Tween-20 and 0.05% (w/v) sodium azide] and incubated overnight at 4°C with rabbit antisera specific for Der p 1 (diluted 1/10,000 in buffer A), Der p 2 (1/100,000), Der p 5 (1/10,000), Der p 7 (1/10,000), Der p 10 (1/100,000) and Der p 21 (1/10,000) and the corresponding preimmune sera at the same dilutions. Bound antibodies were detected as described [31].
A semiquantitative analysis of the blot signals was achieved with the NIH ImageJ software (version 1.42; http://rsb.info.nih.gov/ij/). The scanned film was analyzed after conversion into an 8-bit greyscale image, background correction and inversion of the image. Thus, pixel values ranged from 0 (black) to 255 (white). After outlining the individual bands using the square tool, the integrated density was calculated as a product of ‘area’ value (area of selection in square pixels) and ‘mean grey value’ (sum of the grey values of all the pixels in the selection divided by the number of pixels). Three independent measurements were done and averaged for each dot.
ELISA Assay
Natural and recombinant mite allergens (nDer p 1 and rDer p 2, 5, 7, 10 and 21) as well as BSA as control protein (each 5 μg/ml in PBS) were coated onto an ELISA plate (Nunc, Roskilde, Denmark) at 4°C overnight. The plate was washed 5 times with PBS/0.05% (v/v) Tween-20 (PBST) and blocked for 2.5 h at room temperature with PBST containing 1% (w/v) BSA. The ELISA plate-bound proteins were incubated with rabbit antisera specific for Der p 1, 2, 5, 7, 10 and 21 and serum from a nonimmunized rabbit [dilution 1/20,000 in PBST/0.5% (w/v) BSA] at 4°C overnight. The color reaction was performed as described [5]. The optical density values were corrected by subtracting the values obtained with the serum from a nonimmunized rabbit from the values obtained with the immune sera.
Quantification of Der p 1 and Der p 2 in D. pteronyssinus Extracts by Sandwich ELISA
One-milliliter aliquots from extracts 1–9 and 500 μl from extracts A–D were dialyzed in cassettes with 3,500 Da MW cutoff (Slide-A-Lyzer®, Pierce, Rockford, Ill., USA) for 24 h against 2 liters of 0.9% NaCl and for 24 h against 2 liters of PBS at 4°C to remove the glycerol. The volume obtained after dialysis was measured in order to calculate the dilution factor. Quantification of Der p 1 and Der p 2 in the extracts was performed by quantitative sandwich ELISA according to the manufacturer’s instructions (Indoor Biotechnologies, Charlottesville, Va., USA). Results were expressed in μg ml−1 after taking into account the dilution factor of dialysis. All measurements were performed in duplicate. The intra-assay coefficient of variation (CV) of the Der p 1 ELISA was 11.3% and the inter-assay CV 3.1%. The intra-assay CV of the Der p 2 ELISA was 9.5% and the inter-assay CV 7.6%. Both assay detection limits were 0.5 ng ml−1.
IgE Immunoblots and Dot Blots
Equal volumes (15 μl cm−1) of each commercial D. pteronyssinus extract were separated by 14% SDS-PAGE and blotted onto nitrocellulose (Protan). Two microliters of the purified D. pteronyssinus allergens, nDer p 1, rDer p 2, nDer p 4 and rDer p 5, 7, 8, 10, 14, 20 and 21 and BSA as a negative control were dotted onto nitrocellulose strips (0.25 μg μl−1). Nitrocellulose strips containing extracts or purified allergens were incubated with serum from the subjects diluted 1/10 in buffer A overnight at 4°C. Bound IgE antibodies were detected as described [31].
Statistical Analysis
Correlations between Der p 1 and Der p 2 concentrations in the extracts and the mean wheal area obtained in SPTs with the different extracts were analyzed by nonparametric Kendall correlation. Analyses were performed using the SPSS 11.01 statistical software (Chicago, Ill., USA).
Results
Amounts of Protein and Protein Compositions Differ in Commercial D. pteronyssinus Extracts
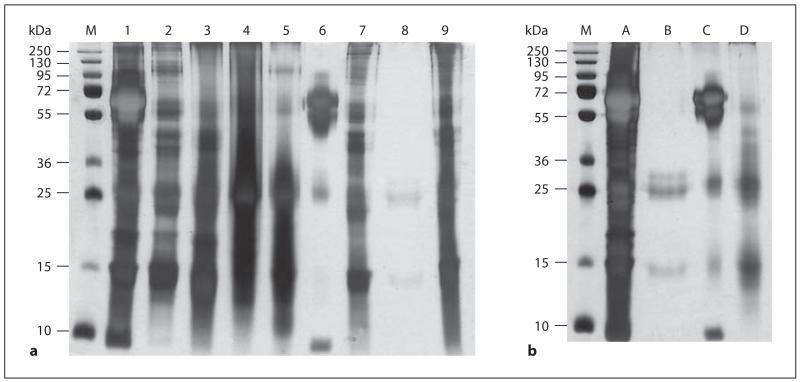
The commercial D. pteronyssinus extracts from different companies were separated by SDS-PAGE and silver-stained (fig. 1a: extracts 1–9, fig. 1b: extracts A–D). The protein content and composition differed considerably between the commercial extracts. Extracts 4 and A contained large amounts of proteins between 10 and 250 kDa, whereas extract 8 showed only 2 weak bands at around 25 and 14 kDa, a molecular weight corresponding to the D. pteronyssinus allergens Der p 2, 5, 21 and possibly to group 12 and 13 allergens. The 14-kDa band was detected in all commercial extracts except in extract 6, which showed 1 prominent band at approximately 66 kDa, which was also visible in extracts 1, A and C (fig. 1). Western blots of the commercial extracts with a monoclonal anti-HSA antibody, which specifically recognized HSA but not other albumins, showed that the band at 66 kDa found in extracts 1, 6, A and C represented HSA, which may have been added to increase the stability of the extracts (data not shown). Differences of the protein content between the commercial extracts were also reflected by the protein quantifications summarized in table 1. The total protein concentrations ranged in extracts 1–9 from 0.7 (extract 9) to 6.0 mg ml−1 (extract 2). In extracts A, B, C and D, the total protein concentrations ranged from 5.2 to 7.7 mg ml−1 (table 1). However, the total protein concentrations did not correlate with the amounts seen in the silver-stained SDS-PAGE (fig. 1). This might be caused by degradation products in some extracts which are not visible on the SDS gel. We therefore dialysed the extracts in cassettes with 3,500 Da MW cutoff before protein quantification (data not shown). The protein concentrations measured after dialysis corresponded well with the protein amounts seen in silver-stained SDS-PAGE, indicating that degradation took place in some of the commercial extracts (data not shown).
Fig. 1.
Silver-stained SDS-PAGE containing D. pteronyssinus extracts from different companies, i.e. lanes 1–9 (a) and A–D (b). Lane M = Molecular mass marker.
Table 1.
Concentrations of total proteins, Der p 1 and Der p 2 and intensity of Der p 5, 7, 10 and 21 in 9 different D. pteronyssinus extracts (Italy: 1–9; France: A–D)
| Extracts |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 2 | 3 | 4 | 5 | 6b | 7 | 8c | 9 | Aa | Bc | Cb | D | |
| Total protein concentration, mg ml−1 | 4.8 | 6.0 | 4.4 | 1.7 | 3.1 | 5.6 | 2.6 | 4.7 | 0.7 | 7.7 | 5.2 | 6.5 | 5.5 |
| Der p 1 concentration, μg ml−1 | 12.7 | 40.8 | 23.3 | 14.4 | 32.7 | 11.0 | 7.7 | 8.8 | 7.1 | 22.5 | 14.0 | 13.8 | 6.0 |
| Der p 2 concentration, μg ml−1 | 26.6 | 43.4 | 28.5 | 2.7 | 9.3 | 2.0 | 14.4 | 3.7 | 20.6 | 45.0 | 4.3 | 1.7 | 2.1 |
| Ratio Der p 1/Der p 2 | 0.5 | 0.9 | 0.8 | 5.3 | 3.5 | 5.5 | 0.5 | 2.4 | 0.3 | 0.5 | 3.2 | 8.1 | 2.9 |
|
| |||||||||||||
| Der p 5 | – | – | 8.17 | – | – | – | 16.1 | – | 77.5 | 7.76 | – | – | 6.55 |
| Der p 7 | 7.73 | 2.96 | 72.2 | – | 12 | – | 60.8 | – | 15.4 | 35.2 | – | – | 28.1 |
| Der p 10 | 217 | 109 | 201 | – | – | – | 164 | – | 144 | 177 | – | – | – |
| Der p 21 | – | – | – | – | – | – | 101 | – | 52.7 | – | – | – | – |
Maximum and minimum values are in italics. An absence of allergen in the extract is shown by –. The integrated density ×104 is indicated for Der p 5, Der p 7, Der p 10 and Der p 21.
Extracts 1 and A represent different batches from Leti.
Extracts 6 and C represent different batches from HAL.
Extracts 8 and B represent different batches from Stallergenes.
Concentrations of the Major Allergens Der p 1 and Der p 2 Differ Considerably among the Commercial D. pteronyssinus Extracts
Because Der p 1 and Der p 2 represent the major allergens of D. pteronyssinus, we determined their concentrations in the different commercially available extracts by quantitative ELISA (table 1). They were detected in all extracts tested, but the amounts varied considerably. In extracts 1–9, Der p 1 levels ranged from 7.1 μg ml−1 (extract 9) to 40.8 μg ml−1 (extract 2) and in extracts A to D, from 6.0 μg ml−1 (extract D) to 22.5 μg ml−1 (extract A) (table 1). An even greater variation was seen for the Der p 2 concentrations in the different extracts, with levels varying up to 22-fold in extracts 1–9, from 2.0 μg ml−1 (extract 6) to 43.4 μg ml−1 (extract 2), and up to 26-fold in extracts A–D, from 1.7 μg ml−1 (extract C) to 45.0 μg ml−1 (extract A) (table 1). Strong differences regarding the ratios of Der p 1 and Der p 2 were observed, ranging (on the weight basis) from 0.3 in extract 9 to 8.1 in extract C (table 1).
Important Allergens Der p 5, 7, 10 and 21 Are Absent in Certain Commercial D. pteronyssinus Extracts
Highly specific rabbit antisera raised by immunization with purified allergens (nDer p 1 and rDer p 2, 5, 7, 10 and 21) were used to search for these allergens in the extracts. In ELISA assays, each of the rabbit antisera showed strong and specific reactivity to the respective allergen against which they were raised. No cross-reactivity with the other allergens was observed, except for the anti-Der p 5 antiserum which showed low cross-reactivity to Der p 21, presumably because of the high sequence homology between Der p 5 and Der p 21 [5] (table 2). The rabbit antisera were used to detect Der p 1, 2, 5, 7, 10 and 21 in nitrocellulose-blotted commercial D. pteronyssinus extracts (table 1, lower part). Der p 2 was the only allergen which was detected in all of the tested extracts, but in varying amounts which corresponded well to the amounts measured by quantitative ELISA (table 1). Although quantitative ELISA showed that Der p 1 was also present in all extracts, this allergen cannot be detected in immunoblots due to its heat lability [32]. The presence of the other allergens varied strongly between the extracts. Only in 2 of the extracts (extracts 7 and 9) were all tested allergens detected (table 1, lower part). Der p 7 was detected in 8 extracts (extracts 1, 2, 3, 5, 7, 9 and A–D), Der p 10 in 6 (extracts 1, 2, 3, 7, 9 and A), Der p 5 in 5 (extracts 3, 7, 9, A and D) and Der p 21 in 2 (extracts 7 and 9). The reactivity of the allergens varied considerably. Whereas Der p 10 was present in high amounts in all 6 extracts where it was detected (integrated density × 104: 109–217), the reactivity to Der p 7 varied 24-fold between extract 2 (2.96) and extract 3 (72.2) (table 1, lower part). In 5 extracts (4, 6, 8, B and C), Der p 2 was the only allergen which was detected with the rabbit antisera (table 1, lower part). No background reactivity was found with the preimmune sera (data not shown).
Table 2.
Specificity of allergen-specific rabbit antisera as tested with purified natural and recombinant D. pteronyssinus allergens by ELISA
| Rabbit immune sera |
||||||
|---|---|---|---|---|---|---|
| anti-Der p 1 | anti-Der p 2 | anti-Der p 5 | anti-Der p 7 | anti-Der p 10 | anti-Der p 21 | |
| nDer p 1 | 2.312 | 0.000 | 0.036 | 0.001 | 0.000 | 0.017 |
| rDer p 2 | 0.000 | 0.802 | 0.134 | 0.000 | 0.000 | 0.008 |
| rDer p 5 | 0.000 | 0.000 | 2.767 | 0.054 | 0.045 | 0.001 |
| rDer p 7 | 0.000 | 0.000 | 0.130 | 0.528 | 0.049 | 0.034 |
| rDer p 10 | 0.000 | 0.000 | 0.045 | 0.010 | 1.165 | 0.000 |
| rDer p 21 | 0.000 | 0.000 | 0.855 | 0.000 | 0.126 | 0.564 |
| BSA | 0.002 | 0.005 | 0.006 | 0.004 | 0.001 | 0.000 |
The mean OD values of two determinations are shown.
We also found that the allergen contents and compositions between different batches from the same manufacturer (e.g. extracts 1 and A, 6 and C and 8 and B) varied considerably (table 1).
HDM-Allergic Subjects Show Different IgE Reactivity Profiles
The IgE reactivity profiles of the HDM-allergic subjects and the frequencies of sensitization to the individual allergens in the 2 countries are displayed in table 3.
Table 3a.
Clinical and serological characteristics of HDM-allergic subjects from Italy
| Subject No. | Symptoms | Der p-specific IgE, kUA l−1 |
IgE reactivity to Der p allergens |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 5 | 7 | 8 | 10 | 14 | 20 | 21 | |||
| 1 | R, C | 2.4 | + | + | − | + | − | − | − | − | − | − |
| 2 | R, C | 46.7 | + | + | − | − | − | + | + | − | − | − |
| 3 | R | 18.8 | + | + | − | + | + | − | − | − | − | + |
| 4 | R, C | >100 | + | + | + | − | − | + | + | − | − | + |
| 5 | R | <0.3 | − | − | − | − | − | − | − | − | − | − |
| 6 | R, C | 28.8 | + | + | + | − | + | − | − | − | − | − |
| 7 | R | 1.5 | + | − | − | − | − | − | − | − | − | − |
| 8 | C | 3.0 | + | + | − | + | − | − | − | − | − | − |
| 9 | R, C | n.d. | + | + | − | − | + | + | + | − | − | − |
| 10 | R | 12.1 | + | − | + | − | − | − | − | − | − | − |
| 11 | R, A | 3.9 | + | + | − | + | − | − | − | − | − | + |
| 12 | R, C | 0.5 | + | + | − | − | − | − | − | − | − | − |
| 13 | R, C | 19.0 | + | + | − | − | + | + | − | − | − | − |
| 14 | R | 3.1 | + | − | + | − | − | − | + | − | − | − |
| 15 | R, C | 9.7 | + | + | − | − | − | − | − | − | − | − |
| 16 | R, C | 10.5 | + | − | + | − | − | + | − | − | − | + |
| 17 | R, C | 2.0 | + | − | − | − | − | − | − | − | − | − |
| 18 | R, C | <0.3 | + | + | − | − | − | − | − | − | − | − |
| 19 | R | 6.9 | + | + | − | − | − | − | − | − | − | − |
| 20 | R | 6.7 | + | + | − | − | + | − | − | − | − | − |
| 21 | R, C | 91.2 | + | + | + | + | + | + | + | − | − | − |
| 22 | R, C | 56.2 | + | + | + | + | + | + | − | − | − | + |
| 23 | R | 19.6 | + | + | + | − | + | − | − | − | − | + |
| 24 | R | 2.8 | + | + | − | − | − | + | − | − | − | − |
| 25 | R | 7.5 | + | + | − | + | − | − | − | − | − | + |
| 26 | R, C | 8.1 | + | + | − | + | − | − | − | − | − | + |
|
| ||||||||||||
| Frequency of IgE reactivity (%) | 96 | 77 | 31 | 31 | 31 | 31 | 19 | 0 | 0 | 31 | ||
When the sera of 26 Italian mite-allergic patients were tested in dot blot assays, 25 (96%) reacted specifically with nDer p 1 and 20 (77%) specifically with rDer p 2. Twenty (77%) showed IgE reactivity both with nDer p 1 and rDer p 2. Five (19%) reacted with nDer p 1 but not with rDer p 2 (table 3a). The mite-allergic subject No. 5 did not react with any of the tested allergens. When the 19 mite-allergic French subjects were tested in dot blots, again, Der p 1 was the most frequently recognized allergen. Sera from 18 mite-allergic subjects (95%) reacted specifically with nDer p 1 and 17 (89%) with rDer p 2. Sixteen (84%) showed IgE reactivity both with nDer p 1 and rDer p 2. Two (11%) reacted with nDer p 1 but not with rDer p 2, and 1 (5%) reacted with rDer p 2 but not with nDer p 1 (table 3b).
Table 3b.
Clinical and serological characteristics of HDM-allergic subjects from France
| Subject No. | Symptoms | Der p-specific IgE, kUA l−1 |
IgE reactivity to Der p allergens |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 5 | 7 | 8 | 10 | 14 | 20 | 21 | |||
| 27 | R, C, A | 12.2 | + | + | − | + | + | − | + | − | − | + |
| 28 | R, C, A | 12.6 | + | − | − | − | − | − | − | − | − | − |
| 29 | R, C, A | 16.5 | + | + | − | − | − | − | − | − | − | − |
| 30 | R, C | 5.3 | + | + | − | + | − | − | − | − | − | − |
| 31 | R, C | 21.2 | + | + | − | + | − | − | − | − | − | + |
| 32 | R, C | 4.7 | + | + | − | + | − | − | − | − | − | + |
| 33 | R, C | 1.3 | + | + | − | − | − | − | − | − | − | − |
| 34 | R, C, A | 5.0 | + | − | − | − | − | − | − | − | − | − |
| 35 | R, C | 67.8 | + | + | − | + | − | − | − | − | − | − |
| 36 | R, C | 91.3 | + | + | + | − | + | − | − | − | − | − |
| 37 | R, C | 64.4 | + | + | − | + | + | − | − | − | − | + |
| 38 | R, C | 3.1 | + | + | − | − | − | − | − | − | − | − |
| 39 | R, C | 3.9 | + | + | − | − | − | − | − | − | − | − |
| 40 | R, C | 22.2 | + | + | − | + | − | − | − | − | − | + |
| 41 | R, C | 4.7 | + | + | − | − | − | − | − | − | − | − |
| 42 | R, C, A | 44.8 | + | + | − | − | + | − | + | − | − | + |
| 43 | R, C | 14.5 | − | + | − | − | − | − | − | − | − | − |
| 44 | R, C, A | 9.1 | + | + | − | + | − | − | − | − | − | − |
| 45 | R, C, A | 38.4 | + | + | − | + | − | − | − | − | − | + |
|
| ||||||||||||
| Frequency of IgE reactivity (%) | 95 | 89 | 5 | 47 | 21 | 0 | 11 | 0 | 0 | 37 | ||
A = Asthma; C = conjunctivitis; n.d. = not determined; R = rhinitis.
Der p 4, 7 and 8 were less frequently recognized by the French sera compared to the Italian sera (5, 21 and 0% vs. 31% for the 3 allergens) whereas IgE reactivity frequencies to Der p 1, 2, 10 and 21 were comparable in the 2 populations (table 3). None of the subjects showed IgE reactivity to Der p 14 and Der p 20. The 5 non-mite-allergic subjects from the control group did not show IgE reactivity with any of the allergens tested (data not shown).
Commercial D. pteronyssinus Extracts Show Different Allergenic Activity in Skin Prick Tests
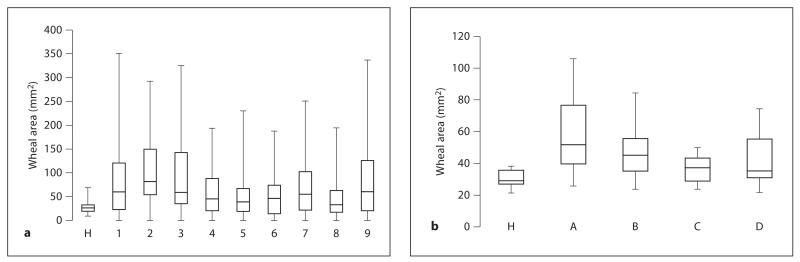
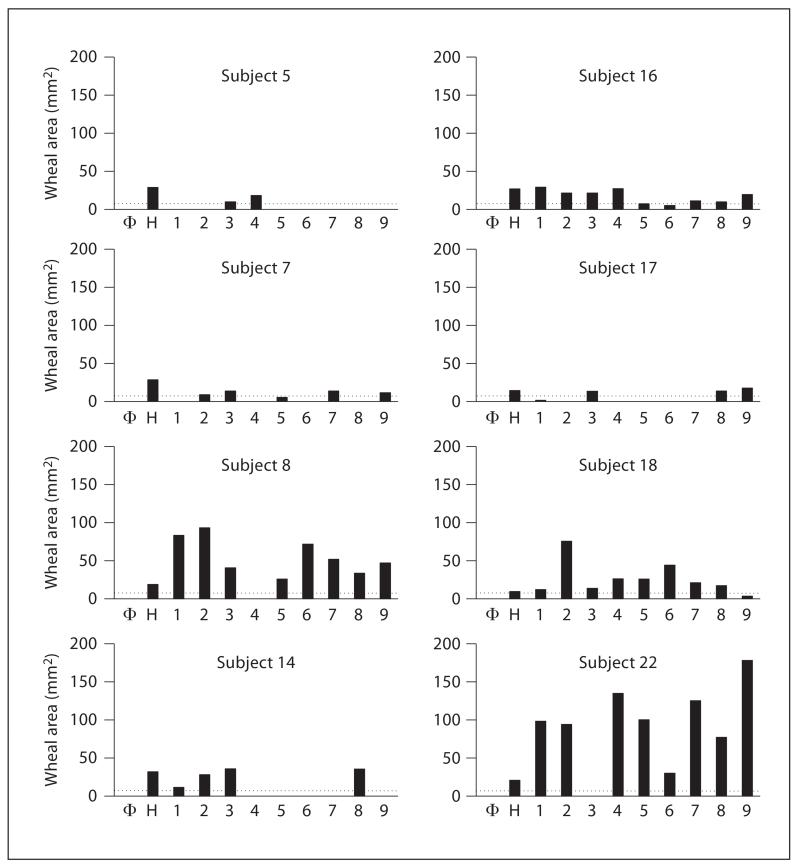
SPTs were performed in 26 Italian mite-allergic subjects using extracts 1–9 and in 19 French mite-allergic subjects applying extracts A–D. The wheal reactions induced with the different D. pteronyssinus extracts varied considerably (fig. 2). For extracts 1–9, the mean wheal area (± SD) ranged from 46.4 ± 44.4 mm2 (extract 8) to 107.0 ± 83.5 mm2 (extract 2), and for extracts A to D, from 36.9 ± 8.0 mm2 (extract C) to 58.4 ± 24.9 mm2 (extract A). For the Italian patients, negative cutaneous reactions (wheal area ≤ 7 mm2) were obtained for 8 of the 26 mite-allergic subjects (Nos. 5, 7, 8, 14, 16, 17, 18 and 22) with at least 1 of the 9 extracts (fig. 3). None of the 9 extracts diagnosed all mite-allergic subjects, and extracts 4, 5 and 6 showed negative SPT results in 4 subjects or more. For the French patients, diagnosis could be done with extracts A–D, but the wheal reactions differed considerably (fig. 2). In all the subjects tested, histamine induced a positive wheal reaction, whereas the glycerol-saline control was negative. None of the 5 nonallergic subjects showed a cutaneous response with any of the extracts used (data not shown). No correlations were observed between Der p 1 and Der p 2 concentrations in the extracts and the mean wheal areas (data not shown).
Fig. 2.
Variability of skin responses to 9 D. pteronyssinus extracts (1–9) in the Italian population and to 4 extracts (A–D) in the French population. Wheal areas of the extracts and of histamine (H), expressed in mm2 (y-axes), are presented in box plots (maximum, 3rd quartile, median value, 1st quartile and minimum).
Fig. 3.
Wheal areas to 9 D. pteronyssinus extracts (1–9) in mite-allergic subjects (No. 5, 7, 8, 14, 16, 17, 18 and 22) which could not be diagnosed with certain extracts. Wheal areas are expressed in mm2 with the cutoff at 7 mm2. Φ = Glycerol-saline solution; H = histamine dihydrochloride 10 mg ml−1.
Discussion
The results of our study showed that certain natural D. pteronyssinus extracts lack important allergens and that the extracts show a considerable variability in allergen composition and content as well as in allergenic activity in SPT studies.
The levels of the major allergens, Der p 1 and Der p 2, ranged from 6.0–40.8 μg ml−1 and from 1.7–45 μg ml−1, respectively. This is similar to the differences described in a recent study by Brunetto et al. [21] (Der p 1: 9.6–36.2 μg ml−1, Der p 2: 0.7–31.7 μg ml−1). SPT studies with natural and recombinant Der p 1 and Der p 2 indicated that the best results were obtained with allergen concentrations of 100 μg ml−1, and that several allergic patients were not diagnosed with the major mite allergens when concentrations of less than 10 μg ml−1 were used for skin testing [27, 33]. Der p 1 and Der p 2 levels <10 μg ml−1 were found in 4 and in 7 of our extracts, respectively. In addition, the Der p 1/Der p 2 ratios differed between 0.3 and 8.1. As Der p 1 is predominantly a component of mite feces and Der p 2 of mite bodies, the different ratios might be caused by the source material used for extraction by the different manufacturers (e.g. mite bodies or mite culture) [19, 32]. The contents of Der p 1 and 2 have been well investigated in allergen extracts [21, 32], but no studies have been performed to investigate mite extracts regarding other important allergens such as Der p 5, 7, 10 and 21 [5, 25, 26, 34, 35]. We found that certain of these allergens were absent in 8 of the 10 extracts studied. As a consequence, mite-allergic subjects who are highly sensitized to the other allergens may not be diagnosed with these extracts. We observed no correlation between Der p 1 or Der p 2 concentrations in the extracts and the intensity of the skin response indicating the importance also of other allergens (e.g. Der p 5, 7 and 21) for the biological response. In asthmatics, it has previously been shown that the late-phase bronchial response is more severe with a natural HDM extract than with the purified allergens Der p 1 or Der p 2, which also suggests the clinical relevance of allergens other than Der p 1 or Der p 2 [23, 36]. Our results showed that in 30% of the Italian mite-allergic subjects (8 subjects), negative SPTs were obtained with at least 1 of the extracts tested. These results are in agreement with previous studies which also showed marked differences in results between extracts from different manufacturers [37-40]. Interestingly, several patients who had negative SPTs with certain of the extracts were sensitized to Der p 1 but not to Der p 2 (Nos. 7, 14, 16 and 17) (fig. 3). The negative results with certain extracts may be due to their low levels of Der p 1. However, positive SPTs were obtained with some extracts which contained only tiny amounts of Der p 1. This may be caused by different sensitivity of patients and sensitization to other allergens which were present in sufficient amounts in these extracts.
The lack of certain important allergens from extracts might also have an influence on the success of immunotherapy. Certain allergens, such as Der p 5 or Der p 21 elicit strong allergic reactions, but are not present in sufficient amounts in the extracts to induce protective immune responses during immunotherapy [5, 25, 34]. To overcome this problem, it would be possible to spike natural extracts with allergens not present in sufficient amounts in these extracts – as has been shown for latex extracts [41] – to induce protective immune responses in immunotherapy.
However, the content and ratio of allergens in extracts cannot be influenced; therefore, it would be advantageous to use recombinant allergens for diagnosis and immunotherapy. These can be produced in large quantities and high purity with reproducible batch-to-batch consistency. For certain allergen sources (e.g. grass pollen), panels of recombinant allergens have been defined which are sufficient for the diagnosis and immunotherapy of allergic patients [42, 43]. Relevant allergens can also be fused in the form of recombinant hybrid molecules which contain the allergens in 1 molecule [44]. In fact, SPTs have shown that grass-pollen-allergic patients were diagnosed with a hybrid molecule consisting of 4 Timothy grass pollen allergens [45].
In the case of mite allergy, more than 20 allergens have been identified so far and most of them were produced as recombinant allergens [4, 5]. Recently, it was shown that a cocktail of only 4 mite allergens (Der p 1, 2, 5 and 7) was sufficient to diagnose all mite-allergic subjects in 4 different European populations (Austria, France, Italy and Sweden) [22]. To identify the panel of HDM allergens necessary for immunotherapy, the allergenic activity of individual mite allergens has to be determined in order to identify those components with high clinical importance, as has been done recently for grass-pollen allergens [42].
In summary, our study showed that commercially available D. pteronyssinus extracts lack certain important allergens and may therefore fail in the diagnosis and treatment of HDM-allergic patients. These problems might be overcome by the use of defined recombinant HDM allergens for diagnosis and in the near future for the immunotherapy of allergic respiratory diseases.
Acknowledgments
The authors thank P. Meyer, MD, PhD, for the statistical analysis. The study was supported by grants F01803, F01815, F4602 and F4605 of the Austrian Science Fund, a research grant from Phadia, Uppsala, Sweden, the Christian Doppler Research Association, Vienna, Austria, and the Italian Ministry of Health Research Program 2006–2007.
References
- 1.Kern RA. Dust sensitization in bronchial asthma. Med Clin North Am. 1921;5:751–758. [Google Scholar]
- 2.Voorhorst R, Spieksma-Boezman MIA, Spieksma FTHM. Is a mite (Dermatophagoides spp.) the producer of the house dust allergen? Allerg Asthma. 1964;10:329–334. [PubMed] [Google Scholar]
- 3.Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- 4.Thomas WR, Heinrich TK, Smith WA, Hales BJ. Pyroglyphid house dust mite allergens. Protein Pept Lett. 2007;14:943–953. doi: 10.2174/092986607782541169. [DOI] [PubMed] [Google Scholar]
- 5.Weghofer M, Dall’Antonia Y, Grote M, Stöcklinger A, Kneidinger M, Balic N, Krauth MT, Fernandez-Caldas E, Thomas WR, van Hage M, Vieths S, Spitzauer S, Horak F, Svergun DI, Konarev PV, Valent P, Thalhamer J, Keller W, Valenta R, Vrtala S. Characterization of Der p 21, a new important allergen derived from the gut of house dust mites. Allergy. 2008;63:758–767. doi: 10.1111/j.1398-9995.2008.01647.x. [DOI] [PubMed] [Google Scholar]
- 6.Sub-Committee on Skin Tests of the European Academy of Allergology and Clinical Immunology Position paper: skin tests used in type I allergy testing. Allergy. 1989;44:1–59. [PubMed] [Google Scholar]
- 7.The European Academy of Allergology and Clinical Immunology Position paper: allergen standardization and skin tests. Allergy. 1993;48:48–82. [PubMed] [Google Scholar]
- 8.Board of Directors of the American Academy of Allergy, Asthma and Immunology Position statement: allergen skin testing. J Allergy Clin Immunol. 1993;92:636–637. [PubMed] [Google Scholar]
- 9.Reed CE, Yunginger JW, Evans R. Quality assurance and standardization of allergy extracts in allergy practice. J Allergy Clin Immunol. 1989;84:4–8. doi: 10.1016/0091-6749(89)90171-1. [DOI] [PubMed] [Google Scholar]
- 10.Larenas-Linnemann D, Cox LS, Immunotherapy and Allergy Diagnostics Committee of the American Academy of Allergy, Asthma and Immunology European allergen extract units and potency: review of available information. Ann Allergy Asthma Immunol. 2008;100:137–145. doi: 10.1016/S1081-1206(10)60422-X. [DOI] [PubMed] [Google Scholar]
- 11.Vogel L, Lüttkopf D, Hatahet D, Vieths S. Development of a functional in vitro assay as a novel tool for the standardization of allergen extracts in the human system. Allergy. 2005;60:1021–1028. doi: 10.1111/j.1398-9995.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A, Jamin A, Foetisch K, May S, Aulepp H, Haustein D, Vieths S. Determination of the allergenic activity of birch pollen and apple prick test solutions by measurement of beta-hexosaminidase release from RBL-2H3 cells. Comparison with classical methods in allergen standardization. Allergy. 1999;54:446–454. doi: 10.1034/j.1398-9995.1999.00917.x. [DOI] [PubMed] [Google Scholar]
- 13.Larsen JN, Dreborg S. Standardization of allergen extracts. Methods Mol Med. 2008;138:133–145. doi: 10.1007/978-1-59745-366-0_12. [DOI] [PubMed] [Google Scholar]
- 14.Dreborg S, Einarsson R. The major allergen content of allergenic preparations reflect their biological activity. Allergy. 1992;47:418–423. doi: 10.1111/j.1398-9995.1992.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 15.van Ree R. Indoor allergens: relevance of major allergen measurements and standardization. J Allergy Clin Immunol. 2007;119:270–277. doi: 10.1016/j.jaci.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–326. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 17.Eraso E, Martinez J, Garcia-Ortega P, Martinez A, Palacios R, Cisterna R, Guisantes JA. Influence of mite growth culture phases on the biological standardisation of allergenic extracts. J Investig Allergol Clin Immunol. 1998;8:201–206. [PubMed] [Google Scholar]
- 18.Maasch HJ, Wahl R, Fuchs T. Application of the first international standard of Dermatophagoides pteronyssinus (house dust mite) in the evaluation of allergen extracts produced from two different source materials. Int Arch Allergy Appl Immunol. 1987;84:363–372. doi: 10.1159/000234451. [DOI] [PubMed] [Google Scholar]
- 19.Tovey ER, Baldo BA. Comparison by electroblotting of IgE-binding components in extracts of house dust mite bodies and spent mite culture. J Allergy Clin Immunol. 1987;79:93–102. doi: 10.1016/s0091-6749(87)80022-2. [DOI] [PubMed] [Google Scholar]
- 20.Esch RE. Allergen source materials and quality control of allergenic extracts. Methods. 1997;13:2–13. doi: 10.1006/meth.1997.0491. [DOI] [PubMed] [Google Scholar]
- 21.Brunetto B, Tinghino R, Braschi MC, Antonicelli L, Pini C, Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65:184–190. doi: 10.1111/j.1398-9995.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 22.Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, Horak F, Grönlund H, van Hage M, Valenta R, Vrtala S. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008;12:959–965. doi: 10.1111/j.1365-2362.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 23.Hales BJ, Shen HD, Thomas WR. Cytokine responses to Der p 1 and Der p 7: house dust mite allergens with different IgE-binding activities. Clin Exp Allergy. 2000;30:934–943. doi: 10.1046/j.1365-2222.2000.00901.x. [DOI] [PubMed] [Google Scholar]
- 24.Mills KL, Hart BJ, Lynch NR, Thomas WR, Smith W. Molecular characterization of the Group 4 house dust mite allergen from Dermatophagoides pteronyssinus and its amylase homologue from Euroglyphus maynei. Int Arch Allergy Immunol. 1999;120:100–107. doi: 10.1159/000024227. [DOI] [PubMed] [Google Scholar]
- 25.Weghofer M, Grote M, Dall’Antonia Y, Fernández-Caldas E, Krauth M-T, van Hage M, Horak F, Thomas WR, Valent P, Keller W, Valenta R, Vrtala S. Characterization of folded recombinant Der p 5, a potential diagnostic marker allergen for house dust mite allergy. Int Arch Allergy Immunol. 2008;147:101–109. doi: 10.1159/000135696. [DOI] [PubMed] [Google Scholar]
- 26.Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, et al. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy. 2011;41:1468–1477. doi: 10.1111/j.1365-2222.2011.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen KW, Fuchs G, Sonneck K, Gieras A, Swoboda I, Douladiris N, Linhart B, Jankovic M, Pavkov T, Keller W, Papadopoulos NG, Valent P, Valenta R, Vrtala S. Reduction of the in vivo allergenicity of Der p 2, the major house-dust mite allergen, by genetic engineering. Mol Immunol. 2008;45:2486–2498. doi: 10.1016/j.molimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Epton MJ, Dilworth RJ, Smith W, Thomas WR. Sensitisation to the lipid-binding apolipophorin Der p 14 and the peptide Mag-1. Int Arch Allergy Immunol. 2001;124:57–60. doi: 10.1159/000053668. [DOI] [PubMed] [Google Scholar]
- 29.Mari A, Di Felice G, Afferni C, Barletta B, Tinghino R, Sallusto F, Pini C. Assessment of skin prick test and serum specific IgE detection in the diagnosis of Cupressaceae pollinosis. J Allergy Clin Immunol. 1996;98:21–31. doi: 10.1016/s0091-6749(96)70222-1. [DOI] [PubMed] [Google Scholar]
- 30.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 31.Vrtala S, Focke M, Kopec J, Verdino P, Hartl A, Sperr WR, Fedorov AA, Ball T, Almo S, Valent P, Thalhamer J, Keller W, Valenta R. Genetic engineering of the major Timothy grass pollen allergen, Phl p 6, to reduce allergenic activity and preserve immunogenicity. J Immunol. 2007;179:1730–1739. doi: 10.4049/jimmunol.179.3.1730. [DOI] [PubMed] [Google Scholar]
- 32.Meyer CH, Bond JF, Chen MS, Kasaian MT. Comparison of the levels of the major allergens Der p I and Der p II in standardized extracts of the house dust mite, Dermatophagoides pteronyssinus. Clin Exp Allergy. 1994;24:1041–1048. doi: 10.1111/j.1365-2222.1994.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 33.Asturias JA, Ibarrola I, Arilla MC, Vidal C, Ferrer A, Gamboa PM, Vinuela JE, Sanz ML, Andrei C, Martinez A. Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin Exp Allergy. 2009;39:1088–1098. doi: 10.1111/j.1365-2222.2009.03264.x. [DOI] [PubMed] [Google Scholar]
- 34.Lynch NR, Thomas WR, Garcia NM, Di Prisco MC, Puccio FA, Lopez RI, Hazell LA, Shen HD, Lin KL, Chua KY. Biological activity of recombinant Der p 2, Der p 5 and Der p 7 allergens of the house dust mite Dermatophagoides pteronyssinus. Int Arch Allergy Immunol. 1997;114:59–67. doi: 10.1159/000237644. [DOI] [PubMed] [Google Scholar]
- 35.Asturias JA, Arilla MC, Gomez-Bayon N, Martinez A, Martinez J, Palacios R. Sequencing and high level expression in Escherichia coli of the tropomyosin allergen (Der p 10) from Dermatophagoides pteronyssinus. Biochim Biophys Acta. 1998;1397:27–30. doi: 10.1016/s0167-4781(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 36.Van der Veen MJ, Jansen HM, Aalberse RC, van der Zee JS. Der p 1 and Der p 2 induce less severe late asthmatic responses than native Dermatophagoides pteronyssinus extract after a similar early asthmatic response. Clin Exp Allergy. 2001;31:705–714. doi: 10.1046/j.1365-2222.2001.01120.x. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen NH, Dirksen A, Mosbech H, Launbjerg J, Biering I, Søborg M. Skin prick testing with standardized extracts from 3 different manufacturers. A comparative randomized study. Allergol Immunopathol. 1992;20:246–248. [PubMed] [Google Scholar]
- 38.Eichler I, Götz M, Jarisch R, Eichler HG, Moss R. Reproducibility of skin prick testing with allergen extracts from different manufacturers. Allergy. 1988;43:458–463. doi: 10.1111/j.1398-9995.1988.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 39.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial Timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–1408. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 40.Focke M, Marth K, Valenta R. Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Invest. 2009;39:429–436. doi: 10.1111/j.1365-2362.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 41.Lundberg M, Chen Z, Rihs HP, Wrangsjö K. Recombinant spiked allergen extract. Allergy. 2001;56:794–795. doi: 10.1034/j.1398-9995.2001.056008794.x. [DOI] [PubMed] [Google Scholar]
- 42.Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, Fiebig H, Suck R, Cromwell O, Valenta R. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–267. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 43.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Linhart B, Hartl A, Jahn-Schmid B, Verdino P, Keller W, Krauth MT, Valent P, Horak F, Wiedermann U, Thalhamer J, Ebner C, Kraft D, Valenta R. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115:1010–1016. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 45.Metz-Favre C, Linhart B, Focke-Tejkl M, Purohit A, de Blay F, Valenta R, Pauli G. Skin test diagnosis of grass pollen allergy with a recombinant hybrid molecule. J Allergy Clin Immunol. 2007;120:315–321. doi: 10.1016/j.jaci.2007.03.046. [DOI] [PubMed] [Google Scholar]