Abstract
Background
More than 90% of house dust mite-allergic patients are sensitized to the major Dermatophagoides pteronyssinus allergen, Der p 2. The aim of this study was to develop and characterize an allergy vaccine based on carrier-bound Der p 2 peptides, which should allow reducing IgE- and T-cell-mediated side-effects during specific immunotherapy (SIT).
Methods
Five Der p 2 peptides (P1–P5) were synthesized and analyzed regarding IgE reactivity and allergenic activity. Lymphoproliferative and cytokine responses induced with Der p 2 and Der p 2 peptides were determined in peripheral blood mononuclear cells from mite-allergic patients. Der p 2-specific IgG antibodies induced with carrier-bound Der p 2 peptides in mice and rabbits were tested for their capacity to inhibit IgE binding and basophil activation in allergic patients.
Results
Of five overlapping peptides (P1–P5) covering the Der p 2 sequence, two peptides (P2 and P4) were identified, which showed no relevant IgE reactivity, allergenic activity, and induced lower Der p 2-specific T-cell activation than Der p 2. However, when coupled to a carrier, P2 and P4 induced Der p 2-specific IgG anti-bodies in animals, which inhibited allergic patients’ IgE binding to the allergen and allergen-induced basophil activation similar as antibodies induced with Der p 2.
Conclusions
Carrier-bound Der p 2 peptides should allow avoiding IgE-mediated side-effects, and because of their low potential to activate allergen-specific T cells, they may reduce late-phase side-effects during SIT. Further, these peptides may be also useful for prophylactic vaccination.
Keywords: Der p 2, house dust mite allergy, immunotherapy, peptides
The Dermatophagoides pteronyssinus allergen, Der p 2, is one of the most potent and frequent house dust mite (HDM) allergens, which is recognized by more than 90% of HDM-allergic patients (1). It represents a 15-kDa β-sheet protein that exhibits extensive sequence and structural similarity with group 2 allergens from other mites species (2). Furthermore, it cross-reacts with group 2 allergens from other dust mite species at the IgE antibody and at the T-cell level (1).
Several approaches have been taken to engineer recombinant hypoallergenic derivatives of group two mite allergens for improving the safety of HDM specific immunotherapy (SIT). With the aim to disrupt the conformational IgE epitopes of group 2 allergens, recombinant mutants and deletion variants have been produced (3-6). Furthermore, hypoallergenic fragments and hybrids of Der p 2 have been engineered (7). These hypoallergenic derivatives exhibit reduced IgE reactivity and allergenic activity, but the allergen-specific T-cell epitopes have been preserved in these constructs. This may represent a possible disadvantage because it has been shown in clinical studies performed with recombinant hypoallergens and T-cell-reactive peptides that IgE-mediated side-effects can be reduced but T-cell-mediated side-effects still occur (8-11).
Here, we present a strategy for generating a Der p 2-based vaccine that should eliminate IgE- and T-cell-mediated side-effects. Using synthetic peptide chemistry, we prepared five Der p 2-derived peptides that showed no relevant IgE reactivity and IgE-mediated allergenic activity. Using cultured peripheral blood mononuclear cells (PBMCs) from HDM-allergic patients, peptides were identified, which induced lower T-cell proliferation and pro-inflammatory cytokine release than Der p 2. Among these peptides, two were identified which, when coupled to a carrier molecule, induced allergen-specific IgG antibodies upon immunization, which were able to block allergic patients’ IgE recognition and allergen-induced basophil degranulation equally well as antibodies raised against complete Der p 2.
Material and methods
Sera from allergic patients, rDer p 2, and recombinant hypoallergenic Der p 2 derivatives
HDM-allergic patients (n = 41) were selected according to case history, skin prick testing, and serological analysis as described (12). HLA typing of the patients was performed by nucleotide sequencing as described (13).
Sera from nonallergic individuals were included for control purposes. rDer p 2 and recombinant hypoallergenic Der p 2 derivatives (rDerp 2 fragments: aa 1-53; aa 54-129 and hybrid aa 54–129 + 1–53) were expressed in E. coli strain BL21 (DE3) (Novagen Inc., Darmstadt, Germany) and purified as described (7). Purified rDer p 2 was subjected to affinity chromatography step using immobilized polymyxin (Affi-Prep Polymyxin Matrix; Bio-Rad, Hercules, CA, USA) to reduce endotoxin contents. The endotoxin contents in the rDer p 2 preparations were determined with the Limulus-Amebocyte-Lysate assay (BioWhittaker, Walkersville, MD, USA) and were typically in the range of 25–110 EU/ml (endotoxin unit).
Chemical synthesis and characterization of Der p 2 peptides
Five overlapping peptides spanning the Der p 2 sequence (Fig. 1A) with a length between 31 and 41 amino acids were synthesized using a Fmoc (9-fluorenylmethoxycarbonyl) strategy with 2-(1H-Benzotriazol-1-yl) 1,1,3,3, tetramethyluronium hexafluorophosphat (HBTU)-activation (14). The peptides were purified by preparative HPLC, and their identities were confirmed by mass spectrometry.
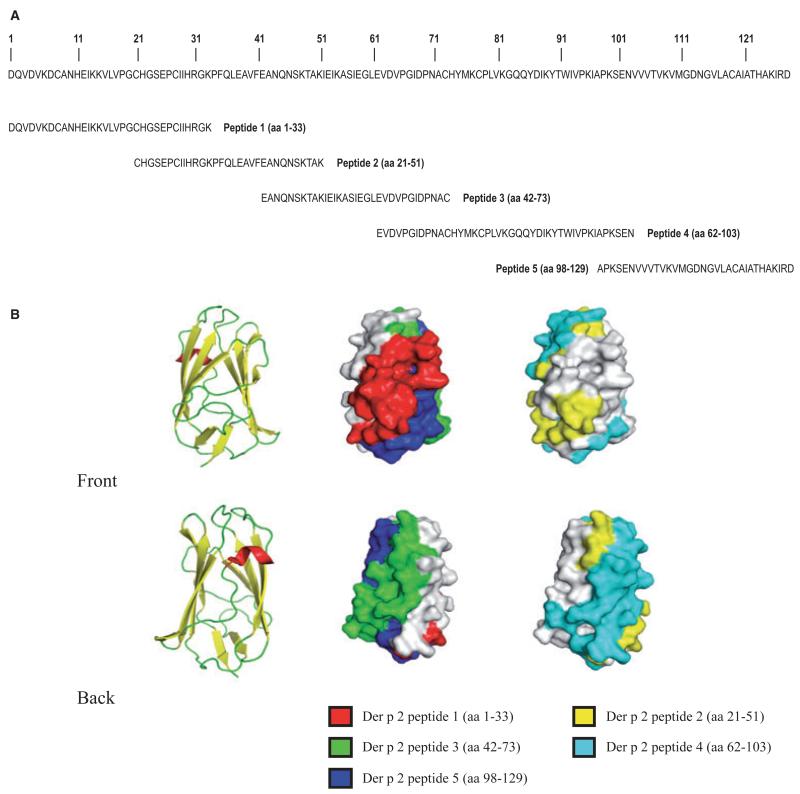
Figure 1.
(A) Amino acid sequence of Der p 2. Synthetic Der p 2-derived peptides 1–5 are indicated. (B) Left images: Ribbon representations of the Der p 2 structure from the front and back. Alpha-helices and beta-sheets are indicated in red and yellow, respectively. Center and right images: corresponding surface representations of the Der p 2 structure with peptides 1–5 in different colors.
Each of the Der p 2 peptides was coupled to keyhole limpet hemocyanin (KLH) (MW 4.5 × 105–1.3 × 107 Daltons; Pierce, ThermoFisher Scientific, Waltham, MA, USA) and purified using a conjugation kit (Pierce, ThermoFisher Scientific).
The solvent-accessible surface (SAS) of each amino acid residue was calculated with the program MSMS (15), which is determined by a spheric solvent probe (r = 1.4 Å) rolling over the van der Waals surface of the protein and is displayed in Å2. The surface exposure of the peptides was calculated as follows: Surface exposure [%] = SAS of all aminoacids of the peptide/SAS of all aminoacids of the protein*100.
IgE reactivity of dot-blotted rDer p 2 and Der p 2 peptides
The concentration of Der p 2 and the KLH-conjugated Der p 2 peptides was measured using the Micro BCA Assay Kit (Pierce, Rockford, IL, USA). Aliquots containing 0.2 μg of purified rDer p 2, KLH-conjugated Der p 2 peptides 1–5, and for control purposes, KLH, were dotted onto nitrocellulose (Schleicher & Schuell, Dassel, Germany), and IgE reactivity was determined using sera from 26 HDM-allergic patients as described (7).
T-cell proliferation assays and cytokine measurements
Heparinized venous blood was collected from HDM-allergic individuals (n = 18) after informed consent was given. Peripheral blood mononuclear cells were isolated by Ficoll (Amersham, GE Healthcare, Buckinghamshire, UK) density gradient centrifugation, and T-cell proliferation assays were performed as described (16). Peripheral blood mononuclear cells were stimulated in triplicates with equimolar concentrations (0.03 nmol/well) of Der p 2, the recombinant Der p 2 derivatives, the unconjugated Der p 2 peptides, the KLH-conjugated Der p 2 peptides or KLH. Proliferation was measured by [3H]-thymidine uptake and is given in counts per minute (cpm). The results were displayed as stimulation index (SI) calculated as quotient of the mean stimulated values and mean background values. A SI of two or more was considered to be indicative of a specific response.
Supernatants from PBMCs cultured in parallel to T-cell proliferation assays were harvested at day 6. The levels of cytokines (IFN-gamma, GM-CSF, IL-5, IL-10, and IL-13) released into the supernatants were measured using a Human Fluorokine MAP Base Kit (R&D Systems, Minneapolis, MN, USA).
Immunization of rabbits and mice
Peptide-specific IgG antibodies were obtained by immunizing rabbits three times with each of the KLH-conjugated peptides (200 μg/injection) and, for control purposes, with recombinant Der p 2 protein (200 μg/injection) using once Freund’s complete and twice Freund’s incomplete adjuvant (Charles River, Kisslegg, Germany).
Eight-week-old female BALB/c mice were purchased from Charles River (Sulzfeld, Germany) and maintained according to the local animal care guidelines in the Animal Care Unit of the Department of Pathophysiology and Allergy Research of the Medical University of Vienna. Six groups consisting of five mice each were monthly immunized subcutaneously in the neck with 5 μg rDer p 2 or 5 μg of each of the five KLH-conjugated Der p 2 peptides, absorbed to 200 μl AluGel-S (SERVA Electrophoresis, Heidelberg, Germany). Blood samples were collected at the day before each immunization and stored at −20°C.
IgG antibody responses in mice and rabbits
Der p 2-specific IgG antibody responses in mice were determined by ELISA as described (7). Specific IgG antibody responses in rabbits were measured in dot-blot assays. Aliquots of 2 μl containing 0.2 μg of rDer p 2, each of the five Der p 2 peptides and, for control purposes, BSA, were dotted onto nitrocellulose membrane strips (Schleicher & Schuell). The strips were washed twice for 5 min and once for 30 min with buffer A. The nitrocellulose strips were then incubated with rabbit anti-sera raised against each of the five Der p 2 peptides (dilution 1 : 10 000 in buffer A) or with the corresponding pre-immune sera (dilution 1: 10 000 in buffer A) overnight at 4°C. The nitrocellulose strips were washed with buffer A (2 × 10 min, 1 × 60 min), and bound rabbit IgG antibodies were detected with 125I-labeled donkey anti-rabbit IgG (GE Healthcare) (dilution 1: 1000 in buffer A) and visualized by autoradiography.
Inhibition of allergic patients’ IgE binding to rDer p 2 with peptide-specific antibodies as determined by ELISA
ELISA plates (Nunc, Roskilde, Denmark) were coated with rDer p 2 (0.5 μg/well in PBS) overnight at 4°C. The plates were washed twice with PBST (PBS; 0.05% [v/v] Tween 20) and blocked in blocking buffer (PBST, 1% [w/v] BSA) for 3 h at room temperature. Then, rabbit anti-rDer p 2 antiserum, each of the rabbit anti-Der p 2 peptide antisera (anti-peptide 1-anti-peptide 5), mixes of the rabbit antisera (anti-peptide 1–anti-peptide 5 and anti-peptide 2+anti-peptide 4 antiserum), or the corresponding pre-immune sera [1: 100 dilution in PBST, 0.5% (w/v) BSA] were added to the plates and incubated overnight at 4°C. After washing, plates were incubated with HDM-allergic patients’ sera [1: 10 dilution in PBST, 0.5% (w/v) BSA] overnight at 4°C. Bound human IgE antibodies were detected with HRP-coupled goat anti-human IgE antibodies (KPL, Gaithersburg, MD, USA) diluted 1: 2500 in PBST, 0.5% (w/v) BSA as described (17). The percentage inhibition of IgE binding was calculated as follows: 100–(ODs/ODp) × 100. ODs and ODp represent the extinctions after pre-incubation with the rabbit immune serum and pre-immune serum, respectively.
CD203c expression on allergic patients’ basophils and inhibition of CD203c expression with specific antibodies
Aliquots of 100 μl heparinized blood were incubated with increasing concentrations of rDer p 2 (0.066–660 nM), an equimolar mix of the five peptides, an equimolar mix of the five KLH-coupled peptides and, for control purposes, with a monoclonal anti-IgE antibody (1 μg/ml) (Immunotech, Vaudreuil-Dorion, QC, Canada) or PBS for 15 min (37°C). After incubation, cells were washed in PBS-EDTA and then incubated with phycoerythrin-labeled CD203c mAb 97A6. Thereafter, samples were subjected to erythrocyte lysis with FACS lysing solution (Becton Dickinson, San Diego, CA, USA). Cells were then washed, resuspended in PBS, and analyzed by means of 2-color flow cytometry on a FACScan (Becton Dickinson). Basophils were detected on the basis of forward side-scatter characteristics and expression of CD203c and analyzed with Painta-gate (Becton Dickinson). Peripheral blood mononuclear cells of allergic individuals were sorted as CD203c+ and CD203c− cells by using a FACS-Vantage (Becton Dickinson) to confirm specificity of CD203c for basophils. Allergen-induced CD203c up-regulation was calculated from mean fluorescence intensities (MFIs) obtained with stimulated (MFIstim) and unstimulated (MFIcontrol) basophils and is expressed as SI (SI = MFIstim:MFIcontrol) (18).
To test whether peptide-specific antibodies can inhibit allergen-induced up-regulation of CD203c expression, increasing concentrations of rDer p 2 (0.0005–0.5 μg/ml) were pre-incubated with a 1: 20 dilution of the rabbit anti-Der p 2 antiserum, the corresponding pre-immune serum, or a mix of the rabbit anti-peptide 2 and the anti-peptide 4 antisera at 37°C for 60 min. Thereafter, blood samples from HDM-allergic patients (n = 8) were incubated with these mixes, and CD203c expression and up-regulation was assessed as described (18).
In vivo allergenicity of peptides, rat basophil leukemia cell degranulation assay
Serum samples were obtained from mice that had been immunized with aluminum-hydroxide-adsorbed rDer p 2 (n = 5) or with the KLH-coupled Der p 2 peptides (five mice per peptide) before or 16 weeks after immunization. Aliquots of 2 μl serum from each mouse were then exposed to rat basophil leukemia cells [subline rat basophil leukemia cells (RBL)-2H3] cultivated in 96-well tissue-culture plates (Nunc) (4 × 104 cells in 100 μl) for 2 h at 37°C. After the addition of Der p 2, the releases of β-hexosaminidase in the cultures were measured and are shown as mean percentages ± SD of total β-hexosaminidase releases (7). Unpaired Mann–Whitney tests were used to assess statistically significant differences between the mouse groups, and spss statistical software system (SPSS Inc., Chicago, IL, USA) was used for calculations. The reported P-values are the results of a two-sided test, corrected by Shaffer coefficient. A P-value ≤0.05 has been considered statistically significant.
Results
Characterization of overlapping synthetic peptides covering different areas of the Der p 2 surface
We have synthesized five overlapping peptides of a length between 31 and 42 amino acids, which span the complete sequence of Der p 2 (Fig. 1A). The identity of each of the synthetic peptides was confirmed by MALDI-TOF analysis (data not shown). When the peptides were superimposed to the 3D structure of Der p 2, the N-terminal peptide 1 and the C-terminal peptide 5 get close and define a surface patch on one side of the Der p 2 molecule (19) (Fig. 1B, front), whereas peptides 2–4 appear on the other side of Der p 2 (Fig. 1B, back). The coverage of surface by the individual Der p 2 peptides was as follows: peptide 1: 27%, peptide 2: 22%, peptide 3: 25%, peptide 4: 40%, and peptide 5: 19%.
KLH-conjugated Der p 2 peptides showed no relevant IgE reactivity and allergenic activity
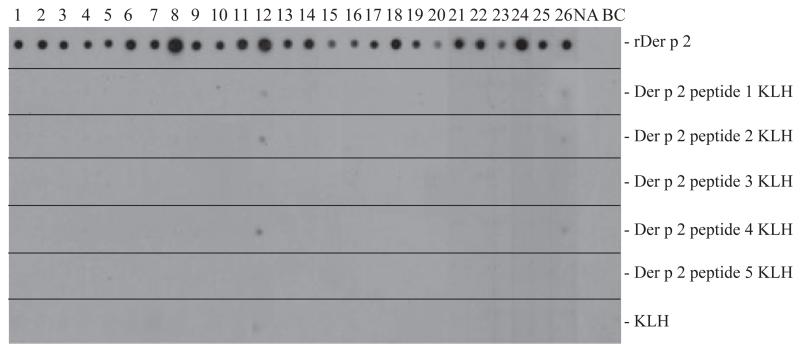
The IgE-binding capacity of the five Der p 2 peptides was compared with that of the complete rDer p 2 allergen in non-denaturing dot-blot RAST-based IgE-binding dot-blot assays using sera from 26 HDM-allergic individuals (Fig. 2). Each of the patients showed IgE reactivity of varying intensity to rDer p 2, whereas only two patients (12 and 26) showed very weak IgE reactivity to the KLH-conjugated peptides and to KLH (Fig. 2). When serum from a nonallergic person (NA) or buffer without serum (BC) were used, no reactivities to rDer p 2 or to Der p 2 peptides were found (Fig. 2, lanes NA, BC).
Figure 2.
IgE reactivity of rDer p 2 and Der p 2 peptides. Dot-blotted rDer p 2, keyhole limpet hemocyanin (KLH)-conjugated Der p 2 peptides, and KLH were tested for IgE reactivity with sera from 26 house dust mite-allergic patients (1-26), serum from a non-allergic individual (NA) and buffer without serum (BC). Bound IgE were detected with 125I labeled anti-human IgE antibodies and visualized by autoradiography.
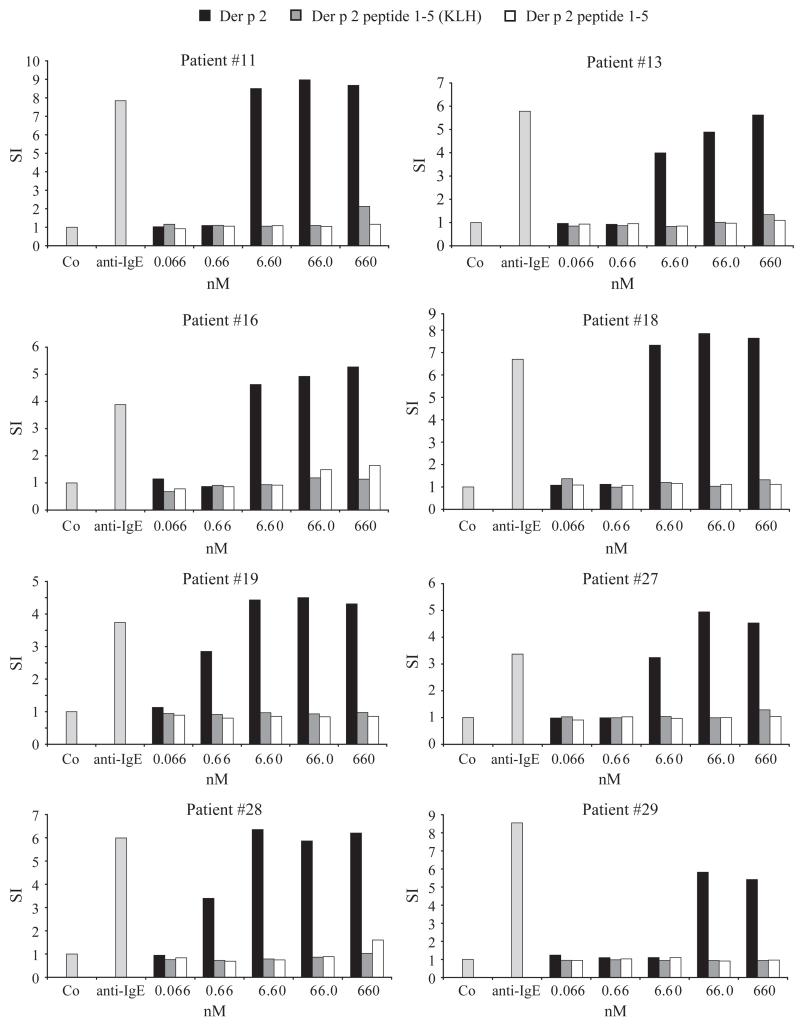
The allergenic activity of Der p 2-derived peptides was compared with rDer p 2 wild type by measuring the up-regulation of CD203c expression on basophils from HDM-allergic individuals, which had been exposed to Der p 2 or the peptides (Fig. 3). rDer p 2 induced a strong up-regulation of CD203c expression in each of the tested mite-allergic individuals at concentrations between 0.66 and 66 nM, whereas no up-regulation was obtained with an equimolar mixture of the five peptides up to a concentration of 660 nM (Fig. 3). Additionally, basophils were incubated with an equimolar mixture of the KLH-coupled peptides to test whether multiple coupling of the peptides to a carrier may influence allergenic activity. Also with KLH-coupled peptides, no up-regulation of CD203c expression was obtained up to 660 nM (Fig. 3). Anti-human IgE antibodies induced up-regulation of CD203c expression on basophils from each of the patients, whereas no up-regulation was obtained with buffer alone (Co) (Fig. 3). Similar results were obtained when basophils were incubated with the individual unconjugated and KLH-conjugated peptides (data not shown).
Figure 3.
Allergenic activity of rDer p 2 and Der p 2 peptides determined by CD203c expression. Basophils from eight house dust mite-allergic patients were incubated with various concentrations of rDer p 2, an equimolar mixture of the five peptides, an equimolar mixture of the five keyhole limpet hemocyanin-coupled peptides, anti-IgE and buffer (Co) (x-axes). Expression of CD203c was determined by FACS analysis and is displayed as stimulation index (y-axes).
Identification of surface-exposed Der p 2 peptides, which induce low lymphoproliferative responses in PBMCs from HDM-allergic patients
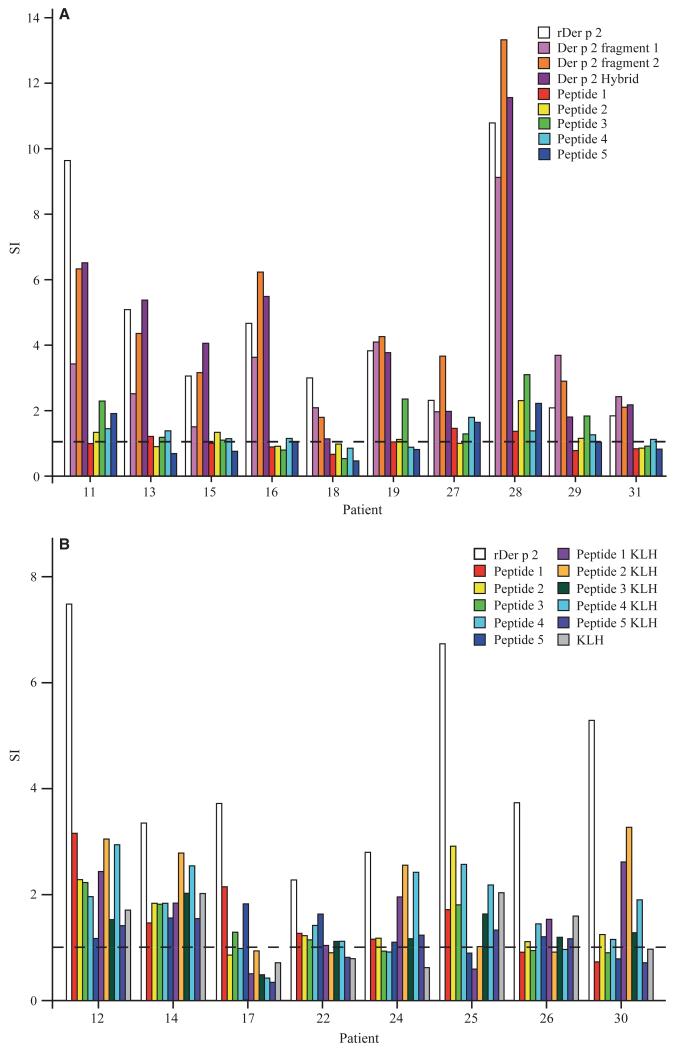
Next, we investigated the lymphoproliferative responses in PBMCs from 18 HDM-allergic patients to Der p 2, to three earlier described recombinant hypoallergenic Der p 2 derivatives (rDer p 2 fragments 1, 2 and hybrid) (7), the individual Der p 2 peptides and the KLH-conjugated peptides (Fig. 4). In a first set of experiments, PBMCs were stimulated with equimolar concentrations of Der p 2, the recombinant Der p 2 derivatives, or the unconjugated Der p 2 peptides (Fig. 4A). Der p 2 and Der p 2-derivatives induced T-cell proliferation in PBMCs from almost all tested patients. Peptide 3 seemed to contain an important T-cell epitope of Der p 2 because it induced proliferative responses in four of the ten patients (patient 11, 19, 28, and 29) and peptide 5 showed activity in three of the ten patients (patient 11, 27, and 28). The other peptides (peptide 1, 2, and 4) induced only low or no T-cell proliferation (Fig. 4A). To investigate whether the KLH-conjugated peptides induce T-cell proliferation, PBMCs from eight additional HDM-allergic patients with diverse HLA backgrounds (patient 12: DRB1*04, DRB1*08, DRB4, DQB1*03, DQB1*04; patient 14: DRB1*11, DRB1*10, DRB3, DQB1*03, DQB1*05; patient 17: DRB1*04, DRB4, DQB1*04; patient 22: DRB1*01, DRB1*15, DRB5, DQB1*05, DQB1*06; patient 24: DRB1*11, DRB1*10, DRB3, DQB1*03, DQB1*05; patient 25: DRB1*01, DQB1*05; patient 26: DRB1*04, DRB1*07, DRB4, DQB1*02, DQB1*03; patient 30: DRB1*01, DRB1*07, DRB4, DQB1*02, DQB1*05) were stimulated with equimolar concentrations of Der p 2, the unconjugated Der p 2 peptides, or the KLH-conjugated Der p 2 peptides in a second set of experiments (Fig. 4B). In each of the tested patients, the KLH-conjugated peptides as well as the unconjugated peptides induced lower T-cell proliferation than the Der p 2 allergen (Fig. 4B). The background values of proliferation obtained with medium alone were between 346 cpm (patient 28) and 1624 cpm (patient 13).
Figure 4.
T-cell proliferative responses of allergic patients to rDer p 2, rDer p 2 derivatives, and Der p 2 peptides. (A) Peripheral blood mononuclear cells (PBMCs) from ten house dust mite (HDM)-allergic patients (x-axis) were stimulated with equimolar amounts of rDer p 2, rDer p 2 derivatives (Der p 2 fragment 1, 2 and hybrid), or Der p 2 peptides. (B) PBMCs from eight additional HDM-allergic patients with different HLA backgrounds (x-axis) were stimulated with equimolar amounts of rDer p 2, unconjugated Der p 2 peptides, keyhole limpet hemocyanin (KLH)-conjugated Der p 2 peptides or KLH. T-cell proliferation was measured by [3H]-thymidine uptake and is displayed as stimulation index (y-axis).
Der p 2 peptides induce less IFN-gamma secretion than Der p 2 or Der p 2 derivatives in allergic patients’ PBMCs
Table 1 shows the mean cytokine production in PBMCs from the ten patients of Fig. 4A, after stimulation with Der p 2, recombinant Der p 2 derivatives, or Der p 2 peptides. We found that Der p 2 and Der p 2 derivatives induced much higher levels of IFN-gamma in allergic patients’ PBMC cultures than any of the Der p 2 peptides. The high IFN-gamma production was accompanied by higher IL-10 production in the Der p 2 and Der p 2-derivative-stimulated cultures compared with peptide-stimulated cultures. The median levels of IL-5 and IL-13 in the Der p 2- and the Der p 2-derivative-exposed cultures were higher than in the peptide-exposed culture, but the differences were not statistically significant (Table 1). The production of GM-CSF was higher in cultures stimulated with Der p 2- and Der p 2 derivatives compared with peptide-containing cultures (Table 1).
Table 1.
Expression of cytokines in PBMC cultures from ten HDM-allergic patients after stimulation with medium, rDer p 2, Der p 2 derivatives, or Der p 2 peptides
| Cytokine |
|||||
|---|---|---|---|---|---|
| IFN-gamma | GM-CSF | IL-5 | IL-10 | IL-13 | |
| Medium | 2.59 | 61.68 | 27.68 | 3.59 | 40.22 |
| Der p 2 | 195.13 | 865.45 | 34.12 | 33.93 | 34.29 |
| Der p 2 fragment 1 | 59.66 | 348.91 | 89.56 | 7.03 | 83.75 |
| Der p 2 fragment 2 | 142.14 | 889.77 | 62.45 | 36.74 | 37.56 |
| Der p 2 hybrid | 164.97 | 953.64 | 38.05 | 25.89 | 36.36 |
| Peptide 1 | 2.59 | 56.12 | 11.52 | 3.59 | 38.94 |
| Peptide 2 | 2.59 | 49.64 | 12.96 | 3.59 | 34.29 |
| Peptide 3 | 2.59 | 78.80 | 18.43 | 3.59 | 39.37 |
| Peptide 4 | 2.59 | 79.68 | 35.38 | 3.59 | 35.82 |
| Peptide 5 | 2.59 | 62.97 | 21.00 | 3.59 | 40.65 |
HDM, house dust mite; PBMC, peripheral blood mononuclear cell.
The results are displayed as median value in pg/ml
Detection limits for IFN-gamma: 2.59 pg/ml and for IL-10: 3.59 pg/ml
Immunization with KLH-coupled Der p 2 peptides induces Der p 2-specific IgG antibodies, which inhibit mite-allergic patients’ IgE binding to the Der p 2 allergen
In a first set of experiments, we studied the IgG reactivity of rabbits, immunized with KLH-coupled peptides, with dot-blotted Der p 2 and Der p 2 peptides. Each of the five peptides induced Der p 2-specific IgG antibodies. The IgG response to Der p 2 was weaker in the peptide 5-immunized rabbit than in the rabbits immunized with peptides 1–4. Certain peptides (e.g. peptides 1, 3, and 4) containing overlapping sequences with other peptides induced IgG antibodies, which recognized also the adjacent peptides (data not shown).
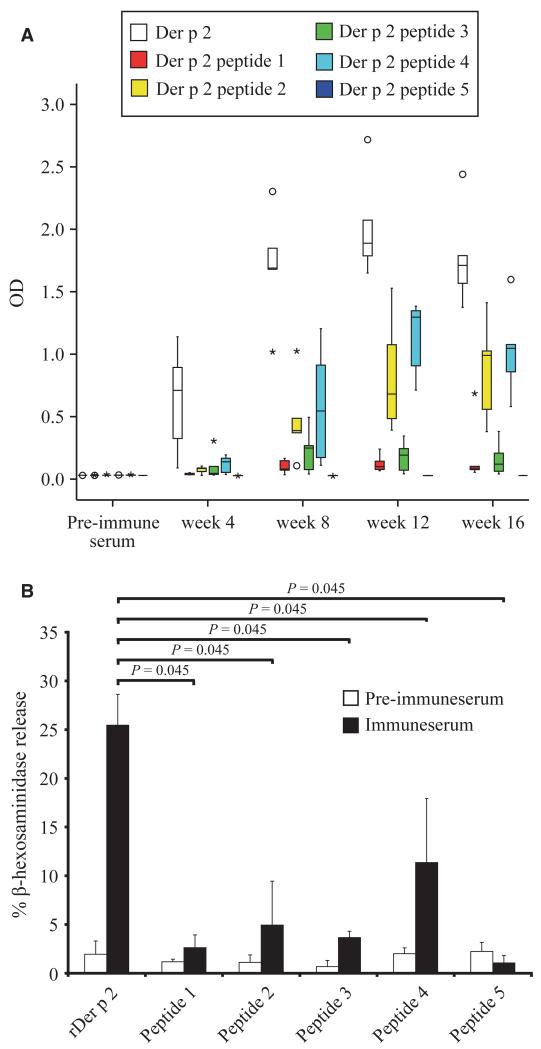
We also immunized mice with Der p 2 or the KLH-coupled peptides using aluminum hydroxide. Der p 2-specific IgG1 responses induced with rDer p 2 were higher and were detected earlier than IgG1 responses induced with the individual KLH-coupled peptides (Fig. 5A). However, after week 8, Der p 2-specific IgG1 responses were induced by immunization with peptides 2, 3, and 4 (Fig. 5A). The other peptides induced low (peptide 1) or no (peptide 5) Der p 2-specific IgG1 responses at the tested serum dilution (i.e. 1: 1000).
Figure 5.
(A) Der p 2-specific IgG1 responses of mice immunized with rDer p 2 or Der p 2 peptides. Groups of five mice each were immunized with rDer p 2 or Der p 2 peptides in 4-week intervals. The optical densities (OD 405 nm) corresponding to the IgG1 antibody levels (y-axis) are displayed for groups of five mice as box plot diagram where 50% of the values are within the boxes and nonoutliers between the bars. Lines within the boxes indicate the median values. Open circles and stars indicate outliers and extreme values of each mouse group. (B) Der p 2-specific basophil degranulation of rat basophil leukemia (RBL) cells loaded with sera from mice immunized with rDer p 2 or Der p 2 peptides. Rat basophil leukemia cells were loaded with serum IgE from mice obtained before (pre-immune serum) or after immunization (immune serum) with Der p 2 or Der p 2 peptides 1–5. Der p 2-specific release of β-hexosaminidase is displayed as percentage of total release on the y-axis. Results are displayed as mean values ± SD from each mouse group. Statistically significant differences are indicated.
Next, we investigated whether antibodies induced by immunization of rabbits with Der p 2 peptides can inhibit mite-allergic patients’ IgE binding to Der p 2 in ELISA competition experiments. The percentage of inhibition of serum IgE binding to rDer p 2 wild type by rabbit antibodies is shown for 20 HDM-allergic patients in Table 2. Rabbit antirDer p 2 antibodies inhibited patients’ IgE binding to rDer p 2 between 56.7 and 94.4% (mean 79.5%). Interestingly, the inhibitions obtained with rabbit anti-peptide 2 (mean 69.9%), anti-peptide 3 (mean 77.7%), and anti-peptide 4 (mean 72.7%) antibodies were comparable with the inhibitions obtained with rabbit antibodies raised by immunization with the complete Der p 2 allergen. The inhibitions of patients’ IgE binding to Der p 2 with anti-peptide 1 antibodies were lower (i.e., 0–64.7%; mean 40.7%). Rabbit anti-peptide 5 antibodies showed no relevant inhibition of patients’ IgE binding to Der p 2 (i.e. 0–16.2%; mean 3.2%). A mixture of the anti-peptide 2 and anti-peptide 4 antibodies inhibited IgE binding between 56.2 and 92.9% (mean 79.3%) and thus equally well as a mixture of the five anti-peptide antibodies (i.e. 43.4–96.0%; mean 77.8%) or anti-rDer p 2 antibodies (Table 2).
Table 2.
Inhibition of allergic patients’ IgE binding to Der p 2 with rabbit anti-Der p 2 or anti-peptide antisera
| Patient no. | Peptide 1 | Peptide 2 | Peptide 3 | Peptide 4 | Peptide 5 | Peptide 1-5 | Peptide 2,4 | Der p 2 |
|---|---|---|---|---|---|---|---|---|
| 2 | 20.89 | 60.85 | 70.76 | 63.16 | 2.69 | 73.43 | 73.07 | 74.98 |
| 13 | 42.93 | 71.02 | 79.55 | 75.44 | 0.83 | 81.27 | 83.27 | 78.35 |
| 15 | 0.00 | 43.56 | 50.52 | 47.28 | 0.00 | 56.19 | 58.13 | 56.70 |
| 16 | 48.15 | 74.60 | 83.13 | 78.97 | 5.59 | 81.82 | 83.65 | 83.56 |
| 18 | 30.33 | 58.36 | 50.94 | 56.49 | 7.76 | 53.60 | 62.71 | 57.03 |
| 19 | 54.12 | 80.63 | 82.64 | 80.94 | 1.10 | 80.02 | 83.73 | 83.21 |
| 26 | 44.71 | 73.62 | 87.29 | 77.19 | 4.97 | 89.15 | 88.15 | 84.34 |
| 27 | 51.43 | 79.64 | 92.08 | 83.25 | 16.16 | 94.69 | 92.88 | 93.51 |
| 28 | 38.46 | 66.79 | 71.20 | 71.25 | 0.00 | 57.89 | 62.07 | 69.06 |
| 31 | 46.06 | 68.54 | 74.05 | 71.32 | 10.05 | 75.99 | 75.73 | 76.46 |
| 32 | 39.20 | 63.55 | 63.94 | 65.30 | 0.00 | 43.35 | 56.15 | 66.20 |
| 33 | 43.62 | 71.82 | 89.94 | 74.54 | 0.51 | 96.03 | 92.83 | 94.39 |
| 34 | 38.09 | 69.94 | 84.08 | 72.45 | 1.29 | 89.09 | 87.53 | 86.83 |
| 35 | 43.63 | 74.16 | 87.12 | 78.50 | 2.98 | 88.45 | 89.00 | 89.10 |
| 36 | 29.09 | 73.75 | 89.97 | 77.59 | 1.38 | 93.29 | 92.53 | 90.66 |
| 37 | 40.44 | 56.77 | 62.09 | 62.30 | 0.00 | 68.62 | 66.00 | 66.16 |
| 38 | 50.63 | 74.41 | 78.36 | 75.50 | 1.07 | 77.58 | 79.15 | 78.26 |
| 39 | 49.61 | 77.15 | 82.95 | 77.85 | 4.16 | 80.56 | 82.92 | 82.74 |
| 40 | 64.73 | 87.41 | 92.13 | 89.25 | 0.00 | 93.08 | 92.70 | 93.34 |
| 41 | 37.98 | 72.24 | 81.08 | 75.60 | 2.48 | 81.76 | 83.67 | 84.25 |
|
| ||||||||
| Mean | 40.71 | 69.94 | 77.69 | 72.71 | 3.15 | 77.79 | 79.29 | 79.46 |
Results are shown in % inhibition of IgE binding.
Reduced in vivo allergenicity of Der p 2 peptides compared with Der p 2
Der p 2 peptides were compared with rDer p 2 regarding their in vivo allergenicity. For this purpose, mice were immunized with the KLH-coupled peptides or Der p 2, and the induction of Der p 2-specific reaginic IgE antibodies was then studied by loading RBL cells with the sera followed by allergen provocation (Fig. 5B). Very low or no relevant release of β-hexosaminidase was obtained when RBLs loaded with mouse anti-peptide IgE were exposed to Der p 2 (peptide 1: mean 2.6%; peptide 2: mean 4.9%; peptide 3: mean 2.9%, peptide 4: mean 11.5%; peptide 5: mean 1.1%). By contrast, immunization with Der p 2 induced high levels of Der p 2-specific reaginic IgE yielding a mean Der p 2-specific degranulation of 25.5% (Fig. 5B).
Anti-Der p 2 peptide antibodies inhibit allergen-induced basophil activation
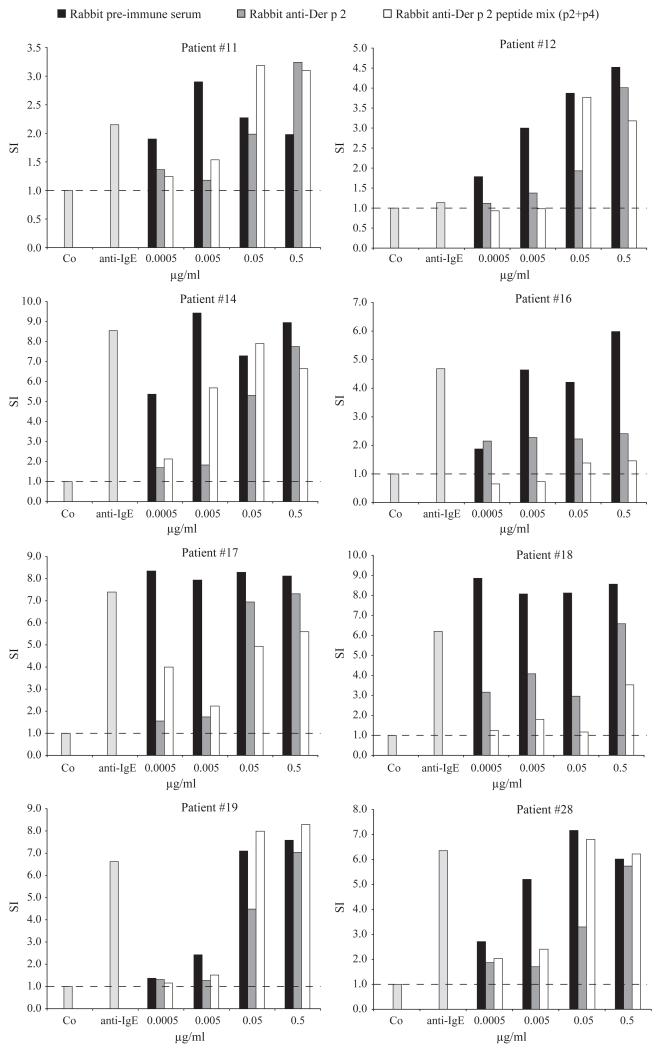
Antibodies induced by immunization with Der p 2 peptides were tested for their ability to inhibit allergen-induced basophil activation. Increasing concentrations of Der p 2 were pre-incubated with a mixture of rabbit anti-peptide 2 and anti-peptide 4 antibodies, anti-Der p 2 antibodies or pre-immune serum. Basophils from eight HDM-allergic patients were then incubated with the Der p 2-immune complexes, and the up-regulation of CD203c expression was measured (Fig. 6). Pre-incubation of rDer p 2 with the mixture of the two anti-peptide antibodies strongly inhibited allergen-induced CD203c up-regulation on basophils. The inhibitory effect achieved with this mixture was in certain patients (e.g. patients 32 and 35) even stronger than the inhibition with antibodies obtained by immunization with the complete Der p 2 allergen. Overall, a more than tenfold reduction and in certain cases (e.g. patient 35) an up to 1000-fold reduction of sensitivity to Der p 2 could be achieved.
Figure 6.
Inhibition of Der p 2-induced basophil activation by allergen-specific antibodies. Various concentrations of rDer p 2 (0.0005–0.5 μg/ml) were pre-incubated with rabbit anti-Der p 2 antibodies, the corresponding pre-immune serum or a mixture of rabbit anti-peptide 2 and anti-peptide 4 antibodies. Basophils from eight house dust mite-allergic patients were then incubated with the pre-incubated rDer p 2, anti-IgE, or buffer (Co) (x-axes). Up-regulation of CD203c expression on basophils determined by FACS analysis is displayed as stimulation index (y-axes).
Discussion
Der p 2 is recognized by more than 90% of HDM-allergic patients and exhibits high in vivo allergenic activity (12, 20). Here, we developed an approach for a Der p 2-based allergy vaccine, which should eliminate IgE-mediated side-effects and induce reduced activation of allergen-specific T cells and thus reduce late-phase side-effects. Such as most other potent respiratory allergens, Der p 2 contains primarily conformational IgE epitopes, and no relevant sequential IgE epitopes have been found so far (21). We therefore synthesized five overlapping Der p 2 peptides spanning the Der p 2 sequence to study whether the disruption of the Der p 2 molecule into small fragments may abolish IgE reactivity and allergenic activity. A length of approximately 30 amino acids was chosen for the peptides because it has been shown for other respiratory allergens that such peptides, when coupled to a carrier molecule, can induce robust IgG antibody responses against the intact wild-type allergen (14, 22, 23). When we tested the five peptides for IgE reactivity with sera from 26 HDM-allergic patients and basophil preparations from eight patients, we found that none of the peptides showed any detectable IgE reactivity or allergenic activity. This result demonstrates that the majority of Der p 2 IgE epitopes belong to the conformational type and provide a set of nonallergenic Der p 2 peptides for vaccine design.
Several recombinant hypoallergenic Der p 2 derivatives with reduced IgE reactivity have been engineered earlier for SIT (3-7, 24). These derivatives were made to reduce IgE-mediated side-effects but preserved the majority of allergen-specific T-cell epitopes. However, several clinical studies have shown that allergen-specific T-cell epitopes can induce IgE-independent, mainly late-phase side-effects (9-11, 25). We therefore compared the five Der p 2 peptides with Der p 2 and three hypoallergenic Der p 2 derivatives regarding their ability to induce specific T-cell proliferation and cytokine responses in PBMC cultures from HDM-allergic patients. Der p 2 and the Der p 2 derivatives induced strong lymphoproliferative responses and the production of pro-inflammatory cytokines, among them IFN-gamma that has been shown to damage respiratory epithelial cells (26). Peptide 3, comprising aa 42–73 and peptide 5, comprising aa 98–129, also induced T-cell proliferation, whereas peptides 1, 2, and 4 induced low proliferation and low production of IFN-gamma. Based on these results, peptides 1, 2, and 4 showed not only no relevant IgE reactivity but should also induce only low T-cell activation and thus T-cell-mediated side-effects when used for SIT. Several immunotherapy studies performed with purified allergen molecules and hypoallergenic allergen derivatives have demonstrated that it is important to induce allergen-specific IgG antibodies that antagonize allergen recognition by IgE antibodies (8, 27, 28). We therefore followed the principle of the hapten carrier concept described by Benaceraff et al. (29) and coupled the peptides to KLH for immunization experiments. Immunization of mice and rabbits showed that KLH-coupled peptides 2, 3, and 4 induced robust Der p 2-specific IgG responses. These rabbit anti-peptide 2, 3, and 4 antibodies inhibited allergic patients’ IgE binding to the allergen Der p 2 almost similar as those induced with the complete allergen. Furthermore, anti-peptide antibodies inhibited allergen-induced basophil activation and thus should suppress acute allergic inflammation.
When we superimposed the areas defined by peptides 2–4 onto the three-dimensional structure of Der p 2 (19), we found that they occupied one side of the Der p 2 molecule, whereas peptide 5 that failed to induce IgE-blocking responses and peptide 1 that induced low IgE-blocking responses defined an area on the other side of the Der p 2 structure. We thus assume that the majority of the Der p 2-specific IgE epitopes are located in the area defined by peptides 2–4. This assumption is supported by an earlier performed in vitro mutagenesis study demonstrating that a Der p 2 mutant in which prolines 34, 95, and 99 have been exchanged retained structural fold but exhibited reduced IgE reactivity (24). Each of the three amino acids mutated in this derivative is located in the area defined by peptides 2 and 4.
Because peptide 3 induced T-cell proliferation, we selected peptides 2 and 4 as minimal structural elements for the Der p 2 vaccine. Comparing a combination of anti-peptide 2 and 4 antibodies with antibodies raised against the complete Der p 2 allergen, we found that the combination of peptide 2- and 4-specific antibodies inhibited Der p 2-induced basophil degranulation in allergic patients equally well as Der p 2-specific antibodies. Moreover, we used a murine model based on injection of aluminum-hydroxide-adsorbed vaccines and found that immunization with peptide 2 and 4 induced only low IgE responses to Der p 2, whereas immunization with Der p 2 induced allergenic IgE responses in mice.
We thus have identified two Der p 2 peptides (peptide 2 and 4) as key elements for a Der p 2 allergy vaccine, which should allow avoiding IgE- and T-cell-mediated side-effects and should not induce allergic sensitization. The peptides may be conjugated to KLH or can be produced as fusion protein with other carrier molecules similar as described for a grass pollen peptide fused to the rhinovirus coat protein VP1 (23). The safety of the vaccine can then be evaluated in a first step by skin prick and atopy patch testing and later in safety and dose-finding vaccination trials in representative numbers of HDM-allergic patients. To cover the spectrum of all clinically important HDM allergens, it will be necessary to include peptides derived from other major HDM allergens such as Der p 1 which should be no problem because the concept of peptide vaccines seems to be broadly applicable to other allergens (30, 31).
The advantage of the carrier-bound peptides over the earlier described approach of using allergen derived T-cell epitope-containing peptides (25) is that they induce robust allergen-specific IgG antibodies and abolish or reduce T-cell-mediated side-effects originating from allergen-specific T cells. According to previous experience regarding the kinetics and efficacy of blocking antibody responses induced by hypo-allergenic vaccines (27), it is envisaged that the peptide vaccines need to be administered approximately four times per year to maintain the levels of allergen-specific IgG antibodies.
The allergen-specific IgG antibodies should inhibit allergen-induced mast cell and basophil degranulation and thus immediate allergic symptoms (8, 27). Furthermore, allergen-specific IgG should reduce allergen-induced boosts of IgE production and thus down-regulate allergen-specific IgE responses (8, 32). Finally, allergen-specific IgG should inhibit IgE-facilitated allergen presentation and thus reduce T-cell activation and the subsequent release of pro-inflammatory cytokines (33-35). This strategy should allow reducing T-cell activation by antibody-mediated effects. However due to the reduction of allergen-specific T-cell epitopes, the vaccine will not induce regulatory T-cell responses.
The peptide vaccine described by us thus should combine all the advantages of allergy vaccines based on wild-type allergens and recombinant hypoallergens (8, 27, 28, 32). Moreover, the carrier-bound peptides should even have advantages over the above-mentioned approaches, because they should neither induce IgE- nor T-cell-mediated side-effects. Furthermore, the Der p 2-based peptide vaccine shows reduced allergenicity compared with the wild-type allergen and therefore may be also used for prophylactic vaccination.
Acknowledgments
This work was supported by grants F1803, F1805, F1809, F1815, F1820, F4602, F4604, F4605, and F4611 of the Austrian Science Fund and by the Christian Doppler Research Association, Vienna, Austria
Abbreviations
- SIT
specific immunotherapy
- HDM
house dust mite
- KLH
keyhole limpet hemocyanin
- PBMC
peripheral blood mononuclear cell
- SI
stimulation index
- RBL
rat basophil leukemia cells
Footnotes
Conflict of interest
None.
References
- 1.Thomas WR, Smith WA, Hales BJ, Mills KL, O’Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002;129:1–18. doi: 10.1159/000065179. [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa S, Takai T, Inoue T, Yuuki T, Okumura Y, Ogura K, et al. NMR study on the major mite allergen Der f 2: its refined tertiary structure, epitopes for monoclonal antibodies and characteristics shared by ML protein group members. J Biochem (Tokyo) 2005;137:255–263. doi: 10.1093/jb/mvi039. [DOI] [PubMed] [Google Scholar]
- 3.Smith AM, Chapman MD. Reduction in IgE binding to allergen variants generated by site-directed mutagenesis: contribution of disulfide bonds to the antigenic structure of the major house dust mite allergen Der p 2. Mol Immunol. 1996;33:399–405. doi: 10.1016/0161-5890(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 4.Takai T, Yokota T, Yasue M, Nishiyama C, Yuuki T, Mori A, et al. Engineering of the major house dust mite allergen Der f 2 for allergen-specific immunotherapy. Nat Biotechnol. 1997;15:754–758. doi: 10.1038/nbt0897-754. [DOI] [PubMed] [Google Scholar]
- 5.Korematsu S, Tanaka Y, Hosoi S, Koyanagi S, Yokota T, Mikami B, et al. C8/119S mutation of major mite allergen Derf-2 leads to degenerate secondary structure and molecular polymerization and induces potent and exclusive Th1 cell differentiation. J Immunol. 2000;165:2895–2902. doi: 10.4049/jimmunol.165.5.2895. [DOI] [PubMed] [Google Scholar]
- 6.Takai T, Mori A, Yuuki T, Okudaira H, Okumura Y. Non-anaphylactic combination of partially deleted fragments of the major house dust mite allergen Der f 2 for allergen-specific immunotherapy. Mol Immunol. 1999;36:1055–1065. doi: 10.1016/s0161-5890(99)00098-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Fuchs G, Sonneck K, Gieras A, Swoboda I, Douladiris N, et al. Reduction of the in vivo allergenicity of Der p 2, the major house-dust mite allergen, by genetic engineering. Mol Immunol. 2008;45:2486–2498. doi: 10.1016/j.molimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purohit A, Niederberger V, Kronqvist M, Horak F, Gronneberg R, Suck R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–1525. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 10.Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campana R, Mothes N, Rauter I, Vrtala S, Reininger R, Focke-Tejkl M, et al. Non-IgE-mediated chronic allergic skin inflammation revealed with rBet v 1 fragments. J Allergy Clin Immunol. 2008;121:528–530. e1. doi: 10.1016/j.jaci.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, et al. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004;34:597–603. doi: 10.1111/j.1365-2222.2004.1930.x. [DOI] [PubMed] [Google Scholar]
- 13.Jahn-Schmid B, Fischer GF, Bohle B, Fae I, Gadermaier G, Dedic A, et al. Antigen presentation of the immunodominant T-cell epitope of the major mugwort pollen allergen, Art v 1, is associated with the expression of HLA-DRB1 *01. J Allergy Clin Immunol. 2005;115:399–404. doi: 10.1016/j.jaci.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–2044. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 15.Sanner MF, Olson AJ, Spehner JC. Reduced surface: an efficient way to compute molecular surfaces. Biopolymers. 1996;38:305–320. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C305::AID-BIP4%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Vrtala S, Fohr M, Campana R, Baumgartner C, Valent P, Valenta R. Genetic engineering of trimers of hypoallergenic fragments of the major birch pollen allergen, Bet v 1, for allergy vaccination. Vaccine. 2011;29:2140–2148. doi: 10.1016/j.vaccine.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, et al. Recombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergy. J Immunol. 2002;168:4576–4584. doi: 10.4049/jimmunol.168.9.4576. [DOI] [PubMed] [Google Scholar]
- 18.Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, et al. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol. 2002;110:102–109. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 19.Derewenda U, Li J, Derewenda Z, Dauter Z, Mueller GA, Rule GS, et al. The crystal structure of a major dust mite allergen Der p 2, and its biological implications. J Mol Biol. 2002;318:189–197. doi: 10.1016/S0022-2836(02)00027-X. [DOI] [PubMed] [Google Scholar]
- 20.Lynch NR, Thomas WR, Garcia NM, Di Prisco MC, Puccio FA, L’Opez RI, et al. Biological activity of recombinant Der p 2, Der p 5 and Der p 7 allergens of the house-dust mite Dermatophagoides pteronyssinus. Int Arch Allergy Immunol. 1997;114:59–67. doi: 10.1159/000237644. [DOI] [PubMed] [Google Scholar]
- 21.Takai T, Yuuki T, Okumura Y, Mori A, Okudaira H. Determination of the N- and C-terminal sequences required to bind human IgE of the major house dust mite allergen Der f 2 and epitope mapping for monoclonal antibodies. Mol Immunol. 1997;34:255–261. doi: 10.1016/s0161-5890(97)00020-5. [DOI] [PubMed] [Google Scholar]
- 22.Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 23.Edlmayr J, Niespodziana K, Linhart B, Focke-Tejkl M, Westritschnig K, Scheiblhofer S, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–6306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 24.Nakazawa T, Takai T, Hatanaka H, Mizuuchi E, Nagamune T, Okumura K, et al. Multiple-mutation at a potential ligand-binding region decreased allergenicity of a mite allergen Der f 2 without disrupting global structure. FEBS Lett. 2005;579:1988–1994. doi: 10.1016/j.febslet.2005.01.088. [DOI] [PubMed] [Google Scholar]
- 25.Oldfield WL, Kay AB, Larche M. Allergen-derived T cell peptide-induced late asthmatic reactions precede the induction of antigen-specific hyporesponsiveness in atopic allergic asthmatic subjects. J Immunol. 2001;167:1734–1739. doi: 10.4049/jimmunol.167.3.1734. [DOI] [PubMed] [Google Scholar]
- 26.Reisinger J, Triendl A, Kuchler E, Bohle B, Krauth MT, Rauter I, et al. IFN-gamma-enhanced allergen penetration across respiratory epithelium augments allergic inflammation. J Allergy Clin Immunol. 2005;115:973–981. doi: 10.1016/j.jaci.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, Konig F, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116:347–354. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Paul WE, Katz DH, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. II. Specific properties of carrier cells capable of enhancing anti-hapten antibody responses. J Exp Med. 1970;132:283–299. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–1570. e6. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–184. e7. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lind-blad R, et al. Immunotherapy with a rag-weed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 33.van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4 + T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–2952. [PubMed] [Google Scholar]
- 34.van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–129. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- 35.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–922. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]