Abstract
Several members of the family of guanine nucleotide-binding protein (G protein)-coupled receptors have recently been shown to induce agonist-dependent foci development in NIH 3T3 cells and tumors in nude mice. We selected the five subtypes of the muscarinic acetylcholine receptor family to investigate their role in tumor suppression. When transfected and expressed in CHO-K1 Chinese hamster ovary cells, m1, m3, and m5 muscarinic acetylcholine receptor activation resulted in a morphology change. Receptor activation did not slow or inhibit monolayer growth of CHOm5 cells in culture but markedly inhibited density-independent growth in soft agar and suppressed tumor formation in nude mice. Receptor-mediated tumor suppression was found to be agonist-dependent and reversible and was blocked with a muscarinic receptor antagonist. Of the five signaling pathways associated with the m1, m3, and m5 receptors, only receptor-operated, and inositol trisphosphate-independent, calcium influx was found to correlate with inhibition of tumorigenicity. These data suggest a pivotal role for inositol trisphosphate-independent receptor-regulated calcium homeostasis in CHO-K1 tumor suppression.
Full text
PDF


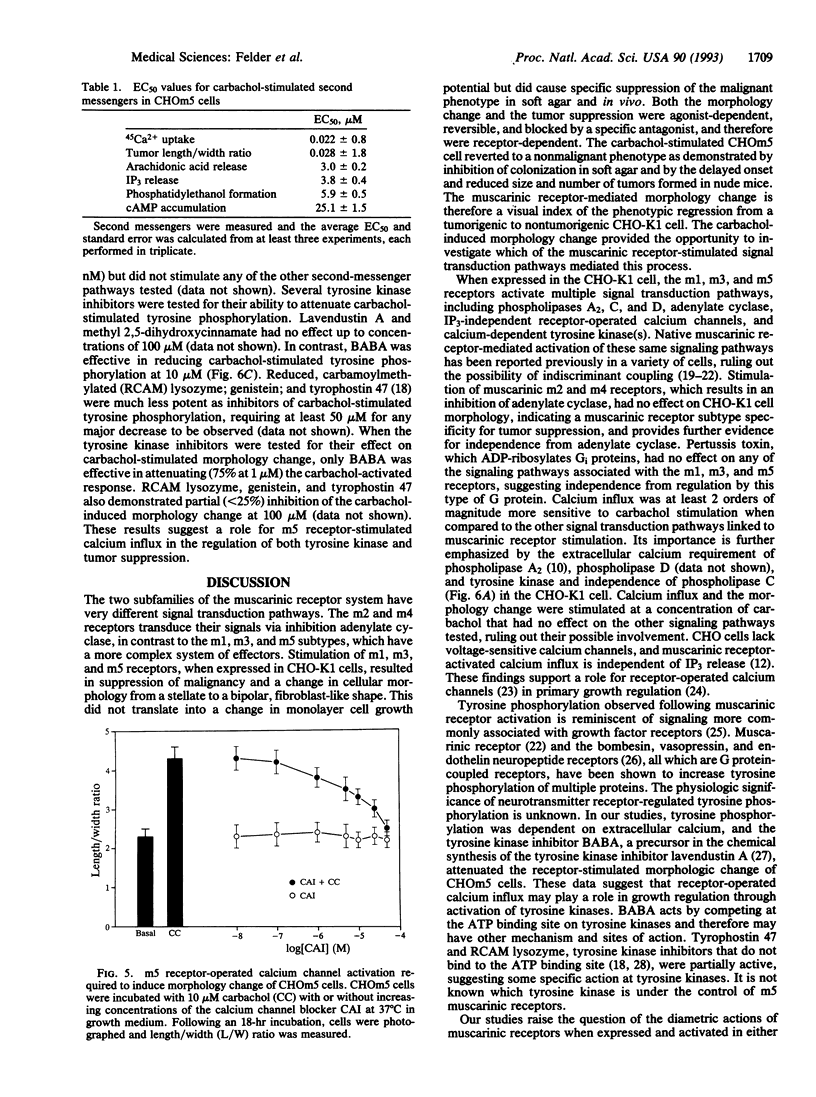
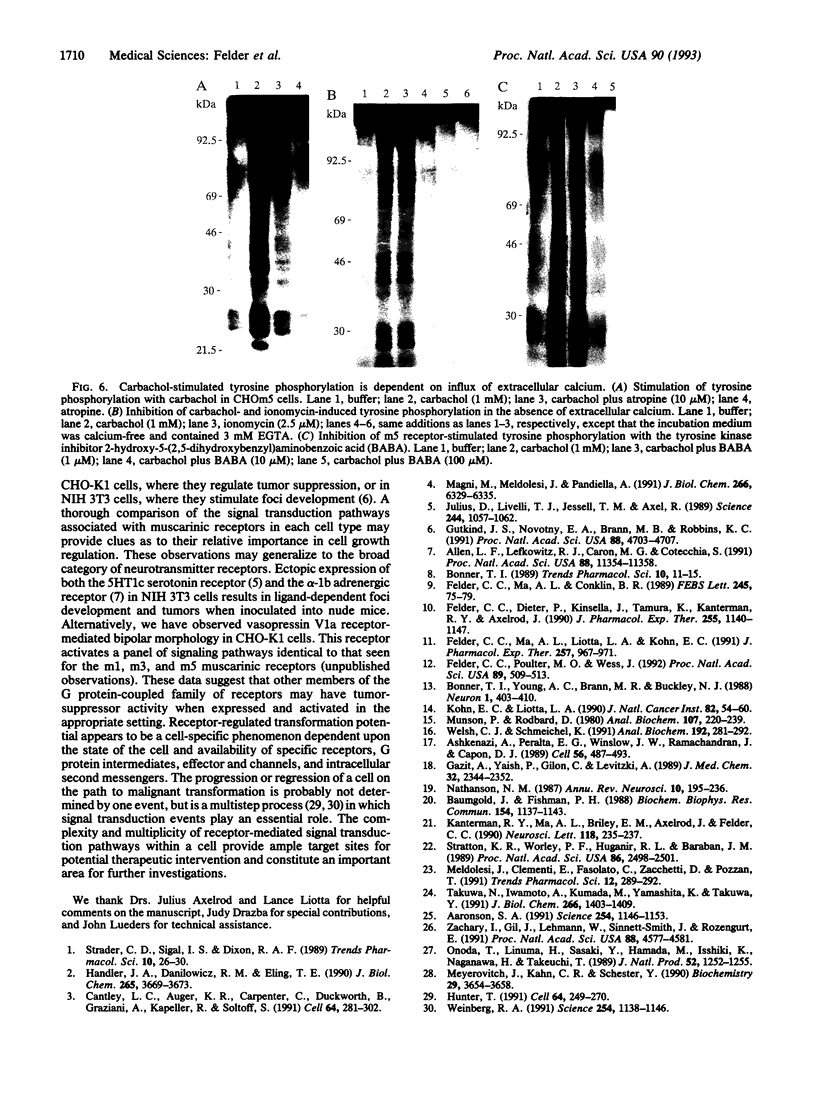
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Allen L. F., Lefkowitz R. J., Caron M. G., Cotecchia S. G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11354–11358. doi: 10.1073/pnas.88.24.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Baumgold J., Fishman P. H. Muscarinic receptor-mediated increase in cAMP levels in SK-N-SH human neuroblastoma cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1137–1143. doi: 10.1016/0006-291x(88)90259-8. [DOI] [PubMed] [Google Scholar]
- Bonner T. I. New subtypes of muscarinic acetylcholine receptors. Trends Pharmacol Sci. 1989 Dec;Suppl:11–15. [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Dieter P., Kinsella J., Tamura K., Kanterman R. Y., Axelrod J. A transfected m5 muscarinic acetylcholine receptor stimulates phospholipase A2 by inducing both calcium influx and activation of protein kinase C. J Pharmacol Exp Ther. 1990 Dec;255(3):1140–1147. [PubMed] [Google Scholar]
- Felder C. C., Ma A. L., Conklin B. R. Carbachol-induced reverse transformation of Chinese hamster ovary cells transfected with and expressing the m5 muscarinic acetylcholine receptor. FEBS Lett. 1989 Mar 13;245(1-2):75–79. doi: 10.1016/0014-5793(89)80195-4. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Ma A. L., Liotta L. A., Kohn E. C. The antiproliferative and antimetastatic compound L651582 inhibits muscarinic acetylcholine receptor-stimulated calcium influx and arachidonic acid release. J Pharmacol Exp Ther. 1991 Jun;257(3):967–971. [PubMed] [Google Scholar]
- Felder C. C., Poulter M. O., Wess J. Muscarinic receptor-operated Ca2+ influx in transfected fibroblast cells is independent of inositol phosphates and release of intracellular Ca2+. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):509–513. doi: 10.1073/pnas.89.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Gutkind J. S., Novotny E. A., Brann M. R., Robbins K. C. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4703–4707. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. A., Danilowicz R. M., Eling T. E. Mitogenic signaling by epidermal growth factor (EGF), but not platelet-derived growth factor, requires arachidonic acid metabolism in BALB/c 3T3 cells. Modulation of EGF-dependent c-myc expression by prostaglandins. J Biol Chem. 1990 Mar 5;265(7):3669–3673. [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Julius D., Livelli T. J., Jessell T. M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989 Jun 2;244(4908):1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Kanterman R. Y., Ma A. L., Briley E. M., Axelrod J., Felder C. C. Muscarinic receptors mediate the release of arachidonic acid from spinal cord and hippocampal neurons in primary culture. Neurosci Lett. 1990 Oct 16;118(2):235–237. doi: 10.1016/0304-3940(90)90635-m. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Liotta L. A. L651582: a novel antiproliferative and antimetastasis agent. J Natl Cancer Inst. 1990 Jan 3;82(1):54–60. doi: 10.1093/jnci/82.1.54. [DOI] [PubMed] [Google Scholar]
- Magni M., Meldolesi J., Pandiella A. Ionic events induced by epidermal growth factor. Evidence that hyperpolarization and stimulated cation influx play a role in the stimulation of cell growth. J Biol Chem. 1991 Apr 5;266(10):6329–6335. [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Kahn C. R., Shechter Y. A family of polypeptide substrates and inhibitors of insulin receptor kinase. Biochemistry. 1990 Apr 17;29(15):3654–3660. doi: 10.1021/bi00467a010. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- Onoda T., Iinuma H., Sasaki Y., Hamada M., Isshiki K., Naganawa H., Takeuchi T., Tatsuta K., Umezawa K. Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus. J Nat Prod. 1989 Nov-Dec;52(6):1252–1257. doi: 10.1021/np50066a009. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Genetic approaches to the determination of structure-function relationships of G protein-coupled receptors. Trends Pharmacol Sci. 1989 Dec;Suppl:26–30. [PubMed] [Google Scholar]
- Stratton K. R., Worley P. F., Huganir R. L., Baraban J. M. Muscarinic agonists and phorbol esters increase tyrosine phosphorylation of a 40-kilodalton protein in hippocampal slices. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2498–2501. doi: 10.1073/pnas.86.7.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Iwamoto A., Kumada M., Yamashita K., Takuwa Y. Role of Ca2+ influx in bombesin-induced mitogenesis in Swiss 3T3 fibroblasts. J Biol Chem. 1991 Jan 25;266(3):1403–1409. [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Welsh C. J., Schmeichel K. Assays for investigations of signal transduction mechanisms involving phospholipase D: mass measurements of phosphatidate, phosphatidylethanol, and diacylglycerol in cultured cells. Anal Biochem. 1991 Feb 1;192(2):281–292. doi: 10.1016/0003-2697(91)90537-4. [DOI] [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]