Abstract
Phosphatases are recognized to have important functions in the initiation of skeletal mineralization. Tissue-nonspecific alkaline phosphatase (TNAP) and PHOSPHO1 are indispensable for bone and cartilage mineralization but their functional relationship in the mineralization process remains unclear. In this study, we have used osteoblast and ex-vivo metatarsal cultures to obtain biochemical evidence for co-operativity and cross-talk between PHOSPHO1 and TNAP in the initiation of mineralization. Clones 14 and 24 of the MC3T3-E1 cell line were used in the initial studies. Clone 14 cells expressed high levels of PHOSPHO1 and low levels of TNAP and in the presence of β-glycerol phosphate (βGP) or phosphocholine (P-Cho) as substrates and they mineralized their matrix strongly. In contrast clone 24 cells expressed high levels of TNAP and low levels of PHOSPHO1 and mineralized their matrix poorly. Lentiviral Phospho1 overexpression in clone 24 cells resulted in higher PHOSPHO1 and TNAP protein expression and increased levels of matrix mineralization. To uncouple the roles of PHOSPHO1 and TNAP in promoting matrix mineralization we used PHOSPHO1 (MLS-0263839) and TNAP (MLS-0038949) specific inhibitors, which individually reduced mineralization levels of Phospho1 overexpressing C24 cells, whereas the simultaneous addition of both inhibitors essentially abolished matrix mineralization (85%; P<0.001). Using metatarsals from E15 mice as a physiological ex vivo model of mineralization, the response to both TNAP and PHOSPHO1 inhibitors appeared to be substrate dependent. Nevertheless, in the presence of βGP, mineralization was reduced by the TNAP inhibitor alone and almost completely eliminated by the co-incubation of both inhibitors. These data suggest critical non-redundant roles for PHOSPHO1 and TNAP during the initiation of osteoblast and chondrocyte mineralization.
Keywords: Mineralization, Osteoblasts, Chondrocytes, Metatarsals, PHOSPHO1, Alkaline phosphatase
Highlights
-
•
PHOSPHO1 activity is required for osteoblast and chondrocyte mineralization.
-
•
PHOSPHO1 and TNAP have non-redundant roles in the initiation of mineralization.
-
•
Both PHOSPHO1 and TNAP activity are required for complete matrix mineralization.
-
•
Phosphocholine is a suitable substrate for mineralization studies.
-
•
Embryonic metatarsals are a valuable model to study matrix mineralization.
1. Introduction
Tissue-nonspecific alkaline phosphatase (TNAP) is the isozyme of the alkaline phosphatase family whose activity is linked to the promotion of matrix mineralization in bone and cartilage [1]. Indeed, both a Pi-generating function, as well as the ability to hydrolyze a mineralization inhibitor were proposed for TNAP since the discovery of this enzyme in bone [2]. A primary inhibitor of ECM mineralization is extracellular inorganic pyrophosphate (PPi) [3], produced ectoplasmically by the enzymatic action of nucleotide pyrophosphatase/phosphodiesterase-1 (NPP1) on extracellular ATP [4]. Intracellularly produced PPi is also transported to the extracellular milieu by the channeling function of the ankylosis protein (ANK) [5]. TNAP activity is crucial for restricting the concentration of extracellular PPi to maintain a Pi/PPi ratio permissive for normal bone mineralization [6], [7]. Indeed, lack of TNAP function (Alpl−/−) leads to the soft bones condition known as hypophosphatasia (HPP), caused by accumulation of extracellular PPi that blocks the propagation of HA onto the ECM [8], [9]. However, chondrocyte- and osteoblast-derived matrix vesicles (MV) derived from both HPP patients and Alpl−/− mice retain the ability to initiate intravesicular mineral formation and contain HA crystals [10], [11] demonstrating that TNAP is not essential for the initiation of MV mediated ECM mineralization.
Instead, PHOSPHO1, a member of the haloacid dehalogenase superfamily [12], [13] highly expressed in mineralizing cartilage, bone and dentin [14], [15], [16], appears to be involved in the initiation of MV-mediated mineralization. PHOSPHO1 shows high phosphohydrolase activity towards phosphoethanolamine (P-Etn) and phosphocholine (P-Cho) and is active inside chondrocyte- and osteoblast derived MVs where it may have a role scavenging Pi from MV membrane phospholipids to favor intra-vesicular HA deposition [17], [18]. Small molecule compounds that inhibit PHOSPHO1 activity in Alpl−/− MVs cause a significant decrease in MV-mediated calcification in vitro [15] and the absence of PHOSPHO1 results in a lower accumulation of mineral, which leads to a more deformable bone [19]. Therefore, Phospho1−/− mice show skeletal abnormalities that include decreased bone mineral density, spontaneous fractures, osteomalacia and scoliosis. However, lack of PHOSPHO1 does not prevent the intravesicular deposition of mineral, although the double ablation of Phospho1 and Alpl lead to complete lack of skeletal mineralization [20]. Here, we have used osteoblast and ex-vivo metatarsal cultures to obtain biochemical evidence for co-operativity and cross-talk between PHOSPHO1 and TNAP in the initiation of mineralization.
2. Methods
2.1. Animals
Phospho1‐R74X null mutant (Phospho1−/−) mice were generated by N‐ethyl‐N‐nitrosourea mutagenesis in a C3HeB/FeJ (Stock No. 000658, Jackson Laboratories, Bar Harbor, ME, USA) background, then bred to C57BL/6 mice to segregate other possible undesired mutations [20]. All animal experiments were approved by Roslin Institute's Animal Users Committee, and the animals were maintained in accordance with Home Office (UK) guidelines for the care and use of laboratory animals.
2.2. Cell culture
Previously characterized clones 14 and 24 of the MC3T3-E1 cell line ([21]; a generous gift of Prof Renny Franceschi, Michigan, USA) were cultured in maintenance medium (α-MEM (Invitrogen, Paisley, UK) containing 10% (v/v) fetal bovine serum (FBS, (Invitrogen) and 0.05 mg/ml gentamycin (Invitrogen)) at 37° C in 5% CO2.
2.3. Lentiviral vectors and cell infection
Mouse Phospho1 sequence was amplified from mouse primary osteoblast cDNA adding a FLAG tag sequence to the 5′ end and cloned into a commercially available pLVX vector (Clonetech Mountain View, CA, USA). An empty vector was used as control. For Lentivirus packaging, a T25 tissue culture flask was seeded with 1.6×106 HEK293T cells in 6mls medium (DMEM, 10% FBS, 1% NEAA; Invitrogen), incubated for 24 h and transfected when 70–90% confluent. The transfection mix was set up in 145 μl Opti-Mem (Invitrogen) containing 2 μg psPAX2, 1 μg of VSV-G and 1.5 μg of the desired pLVX plasmid and 17 μl of Fugene HD (Roche, East Sussex, UK). The transfection mix was incubated for 15 min at room temperature prior to adding to the cells. The transfected cells were incubated at 37 °C in 5% CO2 overnight and the medium was collected 24 and 48 h post transfection to concentrate and titrate the virus. MC3T3-E1 clones were plated at 2×105 cells per T25 flask and transduced the next day with the desired lentivirus at 2 virus particles per cell plated. Selection was done by antibiotic selection with puromycin (Invitrogen) at a final concentration 2 µg/ml.
2.4. Expression and preparation of test enzymes
Recombinant human PHOSPHO1 and TNAP protein was produced and purified as previously described [17]. Enzyme reactions were initiated by the addition of P-Cho and allowed to proceed for 60 min at room temperature and pH 7.3 as previously described [22].
2.5. Mineralization cultures
Cells were plated at 2.5×104 cells per well in 12 well plates and cultured in maintenance medium for two days before changing to mineralization medium (maintenance medium+50 µg/ml ascorbic acid and 5 mM β-glycerol phosphate (βGP; Sigma) or 3 mM P-Cho (Sigma) as phosphate donors. PHOSPHO1 and TNAP inhibitors, MLS-0263839 and MLS-0038949 (both 30 µM) were added where indicated. This concentration was derived from our previous dose response experiments and showed maximum enzyme inhibition and no cellular toxicity [22]. Medium was changed every 3–4 days for up to 21 days. Cells were fixed for alizarin red staining or lysed for protein or mRNA extraction.
2.6. Alizarin red staining for matrix mineralization
Cells were fixed with 4% paraformaldehyde for 30 min at RT, prior to staining with 2% alizarin red, pH 4.2. After image capture, the cells were destained with 10% cetylpyridium chloride and the optical density determined at 570 nm [23].
2.7. Western Blotting
Cells were lysed in RIPA buffer (20 mM Tris–HCl, pH. 8.0, 135 mM NaCL, 10% glycerol, 1% IGEPAL, 0.1% SDS, 0.5% Na deoxycholate, 2 mM EDTA; Invitrogen) containing “complete” protease inhibitor cocktail according to manufacturer's instructions (Roche) and protein concentration determined using the standard DC assay (Bio-Rad, Hemel Hempsted, UK). Proteins (8 µg) were run in a 10% Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane. The membrane was blocked with Odyssey blocking buffer (LI-COR Biosciences, Nebraska, USA) for 1 h and probed overnight at 4 °C with anti-PHOSPHO1 (recombinant Fab, AbD Serotec, Martinsried/Planegg, Germany) anti-TNAP (R&D, Abingdon, UK) and anti ß-actin (Cell signaling technology, Hitchin, UK) antibodies diluted 1:1000,1:500 and 1:1000 respectively in Odyssey blocking buffer. After washing in PBS the membranes were incubated with goat anti-Human (800CW), goat anti-Rat (800CW) and goat anti-Rabbit (680 RD) for 50 min (LI-COR Biosciences, 1:1250 dilution in Odyssey blocking buffer). The resulting blots were subsequently washed in PBS and visualized using the LI-COR Odyssey infrared scanner and software (LI-COR biosciences) with a scan resolution of 169 µm.
2.8. Analysis of gene expression using quantitative RT-PCR
RNA was extracted from cells using RNeasy total RNA (Qiagen Ltd., Crawley, UK), according to the manufacturer's instructions. For each sample, total RNA content was assessed by absorbance at 260 nm and purity by A260/A280 ratios by NanoDrop (Fischer Scientific, Loughborough, UK). RNA was reverse transcribed and the PCR reaction undertaken as described previously [24]. All genes were analyzed with the SYBR green detection method using the Stratagene Mx3000P real-time QPCR system (Stratagene, CA, USA). Each sample was assayed in triplicate. All gene expression data were normalized against the house keeping gene, Atp5b (Primer Design, Southampton, UK). The control values are expressed as 1 to indicate a precise fold change value for each gene of interest. Primers for Phospho1 (Forward; TTCTCATTTCGGATGCCAACA, Reverse; TGAGGATGCGGCGGAATAA) and Alpl (Forward 5′GGG ACG AAT CTC AGG GTA CA3′, Reverse 5′AGT AAC TGG GGT CTC TCT CTT T3′). Data were analysed by the delta delta Ct method.
2.9. Metatarsal culture
Embryonic metatarsal organ cultures provide a well-established ex vivo model of endochondral bone growth [25], [26]. In addition, 15-day-old fetal metatarsal bones (E15) are comprised of early proliferating chondrocytes with no evidence of a mineralized core and are therefore an informative physiological model to investigate the effects of TNAP and PHOSPHO1 on the initiation of bone mineralization [23], [27]. Metatarsals from E15 WT and Phospho1−/− mice were cultured as previously described [23] and PHOSPHO1 (30 µM) and TNAP (30 µM) inhibitors were added where indicated. Metatarsals were maintained in medium for at least 7 days (no medium change) and the length of the mineralization zone and of the whole bone was measured as previously described [23]. Bone mineralization status of each culture was assessed by calculating the ratio of the length of the mineralized zone over the total length of the bone.
2.10. Statistics
SigmaStat v 11.0 was used for statistical analysis. Data (mean±SEM) comparison was conducted by regular t-tests or ANOVA unless the data was not normally distributed, in which case a suitable non-parametric test was selected.
3. Results
3.1. Effect of substrate choice on in vitro osteoblast ECM mineralization
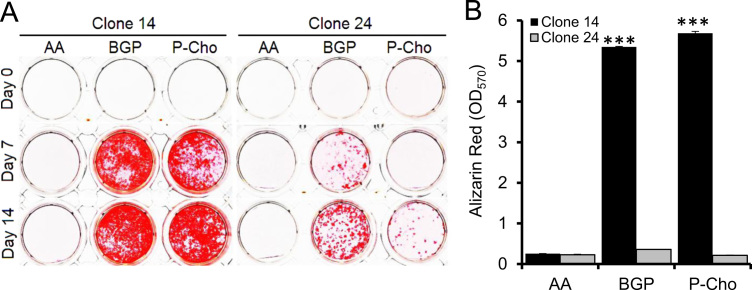
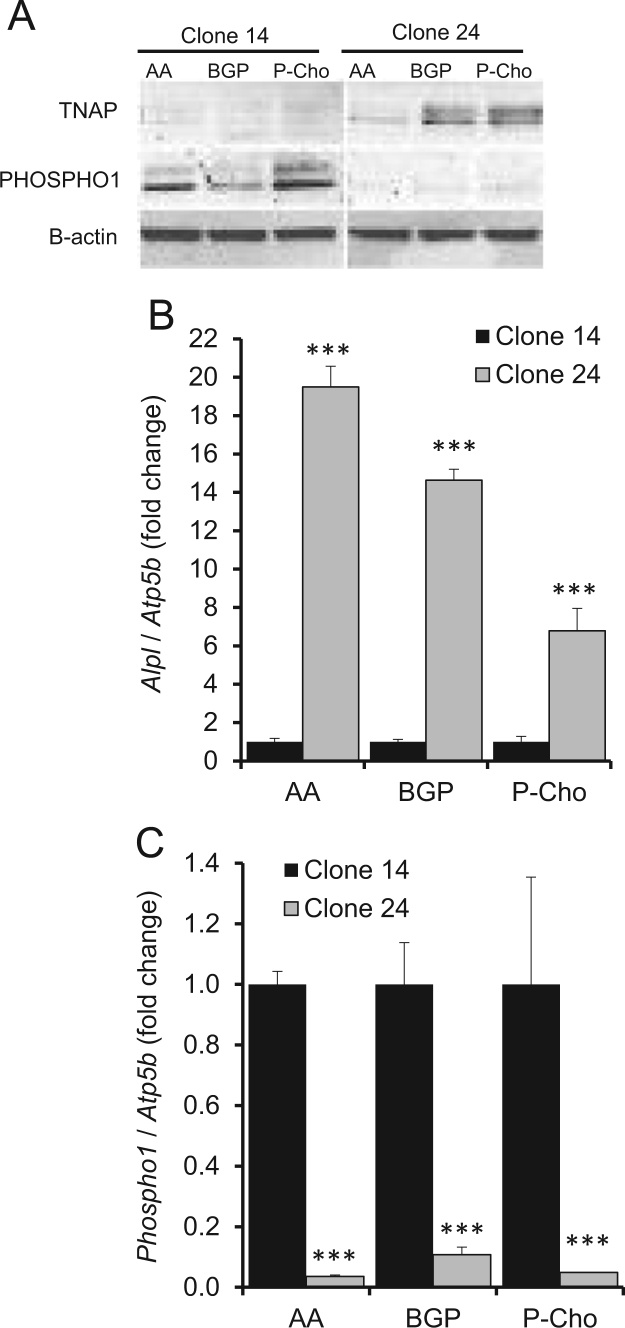
Although βGP can be hydrolyzed by PHOSPHO1 to release Pi it has been previously reported to be a poor substrate for PHOSPHO1 in comparison to TNAP [17], [22]. We therefore first determined the potential of P-Cho and βGP as phosphate donors for ECM mineralization of MC3T3-E1 clones 14 and 24. These clones were chosen because of their reported differences in TNAP expression and mineralization ability [21]. The supplementation of clone 14 cultures by βGP or P-Cho promoted mineralization from day 7 onwards (Fig. 1A and B). It is possible that during the process of mineralization and the destruction of MVs, PHOSPHO1 is released into the extracellular milieu and is able to generate Pi from the added substrates. NPP1 can also act as an efficient phosphatase in the absence of TNAP and this may be another potential source of Pi for mineral formation [28]. Matrix mineralization of clone 24 cells in the presence of βGP also was noted from day 7 onwards but, as expected, was less than their clone 14 counterparts. Mineralization in the presence of P-Cho was also less and its initiation was delayed till day 14 of culture (Fig. 1A and B). Whilst clone 24 mineralizes its matrix poorly it had greater TNAP expression than the more highly mineralizing clone 14 (Fig. 2A and B). In contrast, PHOSPHO1 expression by clone 14 was higher than that of clone 24 at both the gene and protein level (Fig. 2A and C). These data revealed that P-Cho, which can be cleaved by both TNAP and PHOSPHO1 (Supplemental Fig. 1), was a suitable phosphate substrate for the promotion of ECM mineralization and that poor ECM mineralization of MC3T3-E1 clone 24 cells was associated with very low PHOSPHO1 expression.
Fig. 1.
In vitro substrate mineralization assay on MC3T3 clones 14 and 24. MC3T3s were cultured in the presence of 50 µg/ml Ascorbic Acid (AA) with 5 mM βGP or 3 mM P-Cho as substrates and (A) stained with alizarin red and (B) quantified after leaching. n=3. ***P<0.001.
Fig. 2.
TNAP and PHOSPHO1 expression in MC3T3 clones 14 and 24. MC3T3s were cultured in the presence of 50 µg/ml Ascorbic Acid (AA) with 5 mM βGP or 3 mM P-Cho as substrates and analysed for (A) TNAP and PHOSHO1 protein expression (B) Alpl gene expression and (C) and Phospho1 gene expression. n=3. ***P<0.001.
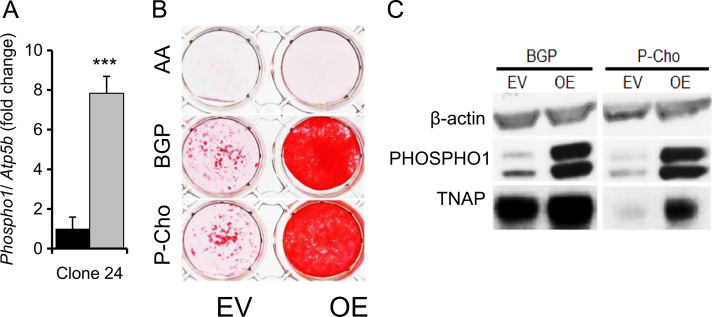
3.2. Phospho1 overexpression induces ECM mineralization
To directly assess the co-operativity and cross-talk between PHOSPHO1 and TNAP expression in ECM mineralization we overexpressed Phospho1 in clone 24 cells which had low Phospho1 expression and mineralized their ECM poorly. This resulted in an 8-fold increase in Phospho1 expression in cells maintained under non-mineralizing conditions (Fig. 3A). When cultured in the presence of βGP or P-Cho, Phospho1 overexpression resulted in higher PHOSPHO1 and TNAP protein expression and increased levels of matrix mineralization (Fig. 3B and C). The increased TNAP expression by βGP (Fig. 3C) has been noted previously [29]. Despite increased TNAP expression it was, in the absence of higher PHOSPHO1 expression, unable to promote matrix mineralization possibly implicating the necessity for PHOSPHO1 in mineral initiation.
Fig. 3.
Overexpression of Phospho1 in MC3T3 clone 24 cells. (A) Phospho1 expression levels in cells transfected with empty vector (black bars) or Phospho1 overexpression vector (gray bars). (B) Mineralization after 14 days in cells transfected with empty vector (EV) or Phospho1 overexpression vector (OE) and cultured with 50 µg/ml of AA and 5 mM βGP or 3 mM P-Cho. (C) Western blotting of TNAP and PHOSPHO1 protein expression in EV or OE cells cultured with 50 µg/ml of AA and 5 mM βGP or 3 mM P-Cho. n=3. ***P<0.001.
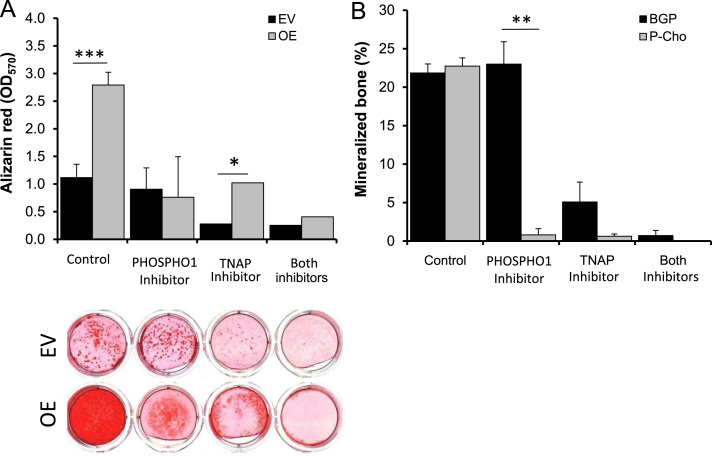
3.3. Joint PHOSPHO1 and TNAP activity is required for MC3T3 matrix mineralization
TNAP (MLS-0038949) and PHOSPHO1 (MLS-0263839) inhibitors were tested on Phospho1 overexpressing clone 24 cells in the presence of 3 mM P-Cho to avoid interference from βGP induced TNAP expression (Fig. 3C). At 30 µM no cell toxicity was observed and this is in agreement with our previous reports [22]. Phospho1 overexpression induced a 2.8 fold increase (P<0.01) in alizarin red staining, which was reduced (72%; P<0.01) to control (empty vector transduced) levels after treatment with MLS-0263839. The PHOSPHO1 inhibitor did not however alter ECM mineralization of control cells possibly reflecting their low level of endogenous PHOSPHO1 expression (Fig. 4A). The TNAP inhibitor also reduced matrix mineralization of both control (75%; P<0.05) and Phospho1 overexpressing (63%; P<0.01) cells, possibly reflecting the inhibition of both basal (control) and Phospho1-induced TNAP activity. The simultaneous addition of both inhibitors essentially abolished ECM mineralization in Phospho1 overexpressing cells (85%; P<0.001) (Fig. 4A).
Fig. 4.
Inhibition of osteoblast and metatarsal matrix mineralization. (A) Clone 14 cells and (B) metatarsals show reduced mineralization in the presence of PHOSPHO1 and TNAP inhibitors alone and in combination. n=4. * P<0.05, ***P<0.001.
3.4. Joint PHOSPHO1 and TNAP activity is required for metatarsal mineralization
Next we used metatarsals from WT E15 mice, in the presence or absence of MLS-0038949 and MLS-0263839 (Fig. 4B), to probe the co-operativity of PHOSPHO1 and TNAP in a physiological ex vivo model of mineralization. The inhibitors did not affect metatarsal linear growth indicating a lack of toxicity at the concentrations used (data not shown). Mineralization of metatarsals cultured in the presence of βGP was unaffected by the addition of the PHOSPHO1 inhibitors whereas TNAP inhibitors reduced mineralization by 73% (P<0.001). Conversely, mineralization was almost completely inhibited by either inhibitor when P-Cho was the substrate. Also, in the presence of both inhibitors when using βGP as substrate, mineralization is almost completely eliminated (Fig. 4B). These experiments were repeated 4 times and similar results were obtained in all occasions.
4. Discussion
PHOSPHO1 is now recognized to be essential for the initiation of skeletal mineralization. Its genetic ablation in mice results in osteoidosis and decreased BMD which are likely to explain the observed spontaneous fractures, bowed long bones, osteomalacia and scoliosis in early life [19], [20]. PHOSPHO1 is present and active inside chondrocyte- and osteoblast-derived MVs through its ability to scavenge Pi from MV membrane phospholipids [17]. Furthermore, whilst small molecule compounds that inhibit PHOSPHO1 activity decreased the ability of chick chondrocyte micromass cultures and isolated MVs to mineralize this was to a lesser extent than similarly treated MVs extracted from cultured Alpl−/− osteoblasts [15], [30]. This is a revealing observation and suggests that TNAP's Pi generating ability can at least, in part, compensate for the lack of PHOSPHO1 function in driving the mineralization process. Moreover, the complete ablation of PHOSPHO1 function only leads to a decrease in the calcification ability of MVs and not to a complete lack of calcification [20]. Conversely, chondrocyte- and osteoblast-derived MVs in hypophosphatasia patients and Alpl−/− mice retain the ability to initiate intra-vesicular mineral formation and contain HA crystals and this, we speculate, is through the actions of PHOSPHO1 [11], [31]. Both scenarios are consistent with our recent observations in mice with a double ablation of PHOSPHO1 and TNAP function where there is complete lack of MV and skeletal mineralization due to a complete lack of Pi generation from intra- and extra-vesicular sources [20]. This current biochemical study was designed to extend these observations and examine the functional interplay of both phosphatases in the regulation of ECM mineralization.
We first exploited MC3T3-E1 clones 14 and 24 because of their reported differences in TNAP expression and mineralization ability [21]. In this original report mineralizing clone 14 did not express Alpl but was able to induce in vivo osteogenesis whereas the poorly-mineralizing clone 24 cells expressed Alpl but failed to promote in vivo osteogenesis [21]. A possible explanation for the differing mineralization abilities of the clones are the differences in matrix gene expression between the two clones. Previous reports have shown that clone 14 cells express high levels of bone sialoprotein (BSP), osteocalcin and Runx2 compared with clone 24 cells [21]. BSP shows a temporo-spatial pattern of expression that closely parallels early mineral formation and is a potent nucleator of HA crystal formation under conditions where osteopontin is inactive or inhibitory [32]. We confirmed that clone 14 cells mineralize their ECM strongly despite having very low expression levels of TNAP but we now extend these observations by showing that these cells express high levels of PHOSPHO1 which may provide sufficient intra-vesicular Pi for mineralization to be initiated. The importance of PHOSPHO1 to the mineralization process was further underscored by poor PHOSPHO1 expression in clone 24 cells which mineralized their ECM less well. Deletion of PHOSPHO1 would suppress intra-vesicular generation of Pi but would leave extra-vesicular Pi generation via TNAP activity and influx via Pi transporters unaffected [20], [31]. Therefore mineralization in the absence of PHOSPHO1 is a slower process. This observation is consistent with the reduced mineralization noted in the skeletons of Phospho1−/− mice [20].
Although it is recognized that the inactivation of PHOSPHO1 in the developing chick limb or its genetic ablation in mice results in a osteoidosis and osteomalacia [20], [30] we further exploited clone 24 cells to show that Phospho1 overexpression was sufficient to promote robust matrix mineralization in this poorly mineralizing cell line. These data provide persuasive evidence for the importance of this phosphatase to the mineralization process. Interestingly, when cultured in the presence of βGP or P-Cho, Phospho1 overexpression resulted in higher TNAP protein expression; an observation consistent with our previous reports which noted that Phospho1-/- mice exhibit reduced TNAP expression and activity [20]. Although the mechanism(s) by which the ablation of PHOSPHO1 expression results in low TNAP expression is unclear, the transgenic overexpression of TNAP does not correct the bone osteomalacia of Phospho1−/− mice despite normalization of their plasma PPi levels [20]. This indicates that the elevation of serum PPi in the Phospho1−/− mouse (as a result of reduced TNAP) is not the cause of the hypomineralized phenotype. Indeed, our more recent studies have implicated a role for PPi induced osteopontin expression in the hypomineralization phenotype of the Phospho1−/− mouse [33].
More definitive data on the function of PHOSPHO1 and TNAP in promoting matrix mineralization was provided by the use of previously validated PHOSPHO1 (MLS-0263839) and TNAP (MLS-0038949) inhibitors [22], [33]. Both inhibitors when used individually reduced mineralization levels of the Phospho1 overexpressing C24 cells to control (empty vector transduced) levels. Only when both inhibitors were used in combination did we note an almost complete inhibition of all mineralization. These observations suggest non-redundant actions of PHOSPHO1 and TNAP on the mineralization process and this supports our in vivo observations where the ablation of either PHOSPHO1 or TNAP results in a hypomineralized skeleton whereas the double ablation of both phosphatases leads to the complete absence of skeletal mineralization [21]. These data suggest that both PHOSPHO1 and TNAP are required for the formation of a fully mineralized matrix. Using the more physiological metatarsal mineralization model, the response to both TNAP and PHOSPHO1 inhibitors appeared to be substrate dependent. We know that βGP is a poor substrate for PHOSPHO1 and therefore normal metatarsal mineralization in the presence of BGP and MLS-0263839 was as expected [17]. Unexpectedly, in the presence of P-Cho, a substrate for both TNAP and PHOSPHO1, mineralization was almost completely inhibited when either TNAP or PHOSPHO1 activity was inhibited alone and it is unclear why residual TNAP (in the presence of PHOSPHO1 inhibitor) or PHOSPHO1 (in the presence of TNAP inhibitor) was not able to maintain limited mineralization. Nevertheless, in the presence of βGP, the reduction in mineralization by the TNAP inhibitor and the almost complete elimination of mineralization by both inhibitors is again supportive of our previous observations that both phosphatases are required for full matrix mineralization. These data highlight the important non-redundant role of PHOSPHO1 and TNAP in the control of ECM mineralization. It is possible that the initiation of skeletal mineralization involves the action of PHOSPHO1 inside MVs together with transporter mediated influx of extra-vesicular Pi, produced by TNAP activity [31]. This consequence of events, whilst attractive, needs to be examined in vivo using the judicious use of genetic mouse models.
Acknowledgments
This work was supported by Institute Strategic Programme Grant funding from the Biotechnology and Biological Sciences Research Council (BBSRC), UK and Grants DE12889 from the National Institute of Dental and Craniofacial Research (NICDR) and AR53102 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH), USA. The authors wish to thank Elaine Seawright for technical support.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.09.013.
Contributor Information
Carmen Huesa, Email: Carmen.Huesa@uws.ac.uk.
Dean Houston, Email: Dean.Houston@roslin.ed.ac.uk.
Tina Kiffer-Moreira, Email: tkbay0226@gmail.com.
Manisha C. Yadav, Email: myadav@sanfordburnham.org.
Jose Luis Millan, Email: millan@sanfordburnham.org.
Colin Farquharson, Email: Colin.Farquharson@roslin.ed.ac.uk.
Appendix A. Supplementary material
Supplementary material Supplementary Fig. 1. Measurement of recombinant human (A) TNAP and (B) PHOSPHO1 phosphatase activities over a 60 min period in the presence of phosphocholine as substrate.
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Anderson H.C. Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. 1995;314:266–280. [PubMed] [Google Scholar]
- 2.Robison R. The possible significance of hexosephosphoric esters in ossification. Biochem. J. 1923;17:286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer J.L. Can biological calcification occur in the presence of pyrophosphate? Arch. Biochem. Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 4.Terkeltaub R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 5.Ho A.M., Johnson M.D., Kingsley D.M. Role of mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–269. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 6.Johnson K.A., Hessle L., Vaingankar S., Wennberg C., Mauro S., Narisawa S., Goding J.W., Sano K., Millán J.L., Terkeltaub R. Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1365–R1377. doi: 10.1152/ajpregu.2000.279.4.R1365. [DOI] [PubMed] [Google Scholar]
- 7.Hessle L., Johnsson K.A., Anderson H.C., Narisawa S., Sali A., Goding J.W., Terkeltaub R., Millán J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narisawa S., Fröhlander N., Millán J.L. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev. Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Fedde K.N., Blair L., Silverstein J., Coburn S.P., Ryan L.M., Weinstein R.S., Waymire K., Narisawa S., Millán J.L., MacGregor G.R., Whyte M.P. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner. Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson H.C., Hsu H.H.T., Morris D.C., Fedde K.N., Whyte M.P. Matrix vesicles in osteomalacic hypophosphatasia bone contain apatite-like mineral crystals. Am. J. Pathol. 1997;151:1555–1561. [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson H.C., Sipe J.E., Hessle L., Dhamayamraju R., Atti E., Camacho N.P., Millán J.L. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am. J. Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houston B., Seawright E., Jefferies D., Hoogland E., Lester D., Whitehead C.C., Farquharson C. Identification and cloning of a novel phosphatase expressed at high levels in differentiating growth plate chondrocytes. Biochim. Biophys. Acta. 1999;1448:500–506. doi: 10.1016/s0167-4889(98)00153-0. [DOI] [PubMed] [Google Scholar]
- 13.Stewart A.J., Schmid R., Blindauer C.A., Paisey S.J., Farquharson C. Comparative modelling of human PHOSPHO1 reveals a new group of phosphatases within the haloacid dehalogenase superfamily. Protein Eng. 2003;16:889–895. doi: 10.1093/protein/gzg126. [DOI] [PubMed] [Google Scholar]
- 14.Houston B., Stewart A.J., Farquharson C. PHOSPHO1-A novel phosphatase specifically expressed at sites of mineralization in bone and cartilage. Bone. 2004;34:629–637. doi: 10.1016/j.bone.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Roberts S.J., Narisawa S., Harmey D., Millán J.L., Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J. Bone Miner. Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 16.McKee M.D., Yadav M.C., Foster B.L., Somerman M.J., Farquharson C., Millán J.L. Compounded PHOSPHO1/ALPL deficiencies reduce dentin mineralization. J. Dent. Res. 2013;92:721–727. doi: 10.1177/0022034513490958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts S.J., Stewart A.J., Sadler P.J., Farquharson C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem. J. 2004;382:59–65. doi: 10.1042/BJ20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart A.J., Roberts S.J., Seawright E., Davey M.G., Fleming R.H., Farquharson C. The presence of PHOSPHO1 in matrix vesicles and its developmental expression prior to skeletal mineralization. Bone. 2006;39:1000–1007. doi: 10.1016/j.bone.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Huesa C., Yadav M.C., Finnilä M.A.J., Goodyear S.R., Robins S.P., Tanner K.E., Aspden R.M., Millán J.L., Farquharson C. PHOSPHO1 is essential for mechanically competent mineralization and the avoidance of spontaneous fractures. Bone. 2011;48:1066–1074. doi: 10.1016/j.bone.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav M.C., Simão A.M., Narisawa S., Huesa C., McKee M.D., Farquharson C., Millán J.L. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function-a unified model of the mechanisms of initiation of skeletal calcification. J. Bone Miner. Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Christensen K., Chawla K., Xiao G., Krebsbach P.H., Franceschi R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 22.Kiffer-Moreira T., Yadav M.C., Zhu D., Narisawa S., Sheen C., Stec B B., Cosford N.D., Dahl, R R., Farquharson C., Hoylaerts M.F., Macrae V.E., Millán J.L. Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification. J. Bone Miner. Res. 2013;28:81–91. doi: 10.1002/jbmr.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staines K.A., Mackenzie N.C., Clarkin C.E., Zelenchuk L., Rowe P.S., Macrae V.E., Farquharson C. MEPE is a novel regulator of growth plate cartilage mineralization. Bone. 2012;51:418–430. doi: 10.1016/j.bone.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacRae V.E., Horvat S., Pells S., Dale H., Collinson R.S., Pitsillides A.A., Ahmed S.F., Farquharson C C. Increased bone mass, altered trabecular architecture and modified growth plate organization in the growing skeleton of SOCS2 deficient mice. J. Cell Physiol. 2009;218:276–284. doi: 10.1002/jcp.21593. [DOI] [PubMed] [Google Scholar]
- 25.Mushtaq T., Bijman P., Ahmed S.F., Farquharson C. Insulin like growth factor-I augments chondrocyte hypertrophy and reverses glucocorticoid mediated growth retardation in fetal mice metatarsal cultures. Endocrinology. 2004;145:2478–2486. doi: 10.1210/en.2003-1435. [DOI] [PubMed] [Google Scholar]
- 26.Owen H., Miner J.N., Ahmed S.F., Farquharson C. The growth plate sparing effects of the selective glucocorticoid receptor modulator, AL-438. Mol. Cell. Endocrinol. 2007;264:164–170. doi: 10.1016/j.mce.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Van Loon J.J., Bervoets D.J., Burger E.H., Dieudonné S.C., Hagen J.W., Semeins C.M., Doulabi B.Z., Veldhuijzen J.P. Decreased mineralization and increased calcium release in isolated fetal mouse long bones under near weightlessness. J. Bone Miner. Res. 1995;10:550–557. doi: 10.1002/jbmr.5650100407. [DOI] [PubMed] [Google Scholar]
- 28.Ciancaglini P., Yadav M.C., Simão A.M.S., Narisawa S., Pizauro J.M., Farquharson C., Hoylaerts M.F., Millán J.L. Kinetic analysis of substrate utilization by native and TNAP-, NPP1- or PHOSPHO1-deficient matrix vesicles. J. Bone Miner. Res. 2010;25:716–723. doi: 10.1359/jbmr.091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orimo H., Shimada T T. The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol. Cell. Biochem. 2008;315:51–60. doi: 10.1007/s11010-008-9788-3. [DOI] [PubMed] [Google Scholar]
- 30.Macrae V.E., Davey M.G., McTeir L., Narisawa S., Yadav M.C., Millan J.L., Farquharson C. Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick. Bone. 2010;46:1146–1155. doi: 10.1016/j.bone.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millán J.L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 2013;93:299–306. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Shapiro H.S., Sodek J. Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner. Res. 1992;7:987–997. doi: 10.1002/jbmr.5650070816. [DOI] [PubMed] [Google Scholar]
- 33.Yadav M.C., Huesa C., Narisawa S., Hoylaerts M.F., Moreau A., Farquharson C., Millán J.L. Ablation of osteopontin prevents the skeletal deformities in Phospho1−/− mice. J. Bone Miner. Res. 2014;29:2369–2381. doi: 10.1002/jbmr.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bravo Y., Teriete P., Dhanya R.-P., Dahl R., Lee P.S., Kiffer-Moreira T., Ganji S.R., Sergienko E., Smith L.H., Farquharson C., Millán J.L., Cosford N.D.P. Design, Synthesis and Evaluation of Benzoisothiazolones as Selective Inhibitors of PHOSPHO1. Bioorg. Med. Chem. Lett. 2014;24:4308–4311. doi: 10.1016/j.bmcl.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Supplementary Fig. 1. Measurement of recombinant human (A) TNAP and (B) PHOSPHO1 phosphatase activities over a 60 min period in the presence of phosphocholine as substrate.
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material