Abstract
VEGFR1 is a receptor tyrosine kinase that has been implicated in cancer pathogenesis. It is upregulated in angiogenic endothelial cells and expressed on human tumor cells as well. VEGFR1 positive hematopoietic progenitor cells home to sites of distant metastases prior to the arrival of the tumor cells thus establishing a pre-metastatic niche. To discover high affinity human antibodies selective for VEGFR1 molecular imaging or for molecularly targeted therapy, a novel phage display scFv library was assembled and characterized. The library was constructed from the humanized 4D5 framework that was mostly comprised tyrosine and serine residues in four complimentarity determining regions (CDRs). The library produced diverse and functional antibodies against a panel of proteins, some of which are of biomedical interest including, CD44, VEGFA, and VEGFR1. After panning, these antibodies had affinity strong enough for molecular imaging or targeted drug delivery without the need for affinity maturation. One of the anti-VEGFR1 scFvs recognized its cognate receptor and was selective for the VEGFR1.
Keywords: scFv, Phage display, Phage library, Binary code, VEGFR1
Highlights
-
•
VEGFR1 contributes to the pathogenesis cancer.
-
•
To obtain VEGFR1 specific antibodies, a phage displayed scFv library was constructed.
-
•
Four complimentarity determining regions were principally comprised of tyrosine and serine.
-
•
High affinity antibody fragments were isolated and characterized.
-
•
This is the first human antibody fragment specific for VEGFR1 from a phage displayed library.
1. Introduction
The human vascular endothelial growth factor receptor-1 (VEGFR1 or Flt-1) contributes to the pathogenesis of both neoplastic and inflammatory diseases [1]. In human cancer, VEGFR1 mediated signaling is responsible for angiogenesis. In animal models for example, inhibition of VEGFR1 signaling by peptides reduces angiogenesis of xenografted human tumors [2]. VEGFR-1 mediated activation of nonmalignant supporting cells such as tumor associated macrophages, are also likely important for cancer pathogenesis. The expression of VEGFR1 on these macrophages is associated with a more aggressive clinical phenotype of breast cancer [3]. Finally, in response to chemokine activity within the primary tumor, VEGFR1 positive hematopoietic progenitor cells preferentially localize to pre-metastatic sites [4]. Because of the involvement of VEGFR1 in cancer pathogenesis, our goal is to develop high affinity antibodies for molecular imaging or molecularly targeted therapy of cancer. To supply these antibodies, we assembled and characterized a phage displayed scFv library.
While most approaches in creating diversity through degenerate codons draw from all or most of the genetically encoded amino acids, previous work with a phage displayed Fab library has shown that restricting diversity to only two amino acids consisting of tyrosine (Tyr) and serine (Ser) can yield high affinity antibodies in a few rounds of panning without the need for further affinity maturation which is a highly prized characteristic for a phage displayed library [5], [6]. Whereas Fab molecules are generally regarded as more stable than single chain variable fragment antibodies (scFvs), the scFv format offers some distinct advantages. Fab antibodies are heterodimers composed of a VH–CH1 and a VL–CL domain while scFvs, comprised the VH and VL chains linked by a flexible peptide linker, are monomeric. As single molecules, scFvs have a greater efficiency of functional display on filamentous phage and they are amenable to fusion with other monomeric proteins [7], [8], [9]. Additionally, like Fabs, scFvs have demonstrated efficacy in vivo, as tumor imaging agents, when labeled with near infrared dyes or radioisotopes and as mediators of molecularly targeted gene delivery [10]. Further, as monomers, scFvs are readily converted to either minibodies or a diabodies for multivalent effects [11]. These bivalent formats offer superior properties for molecular imaging in experimental animal models, without concern for a loss of affinity upon conversion and are attractive for the development of high-affinity ligands for cell surface receptors [11].
Therefore, we have designed and constructed a scFv phage display library for selection of high affinity functional antibodies for molecular targeting of VEGRF1. The library (BCscFv library) was constructed with binary code mutations comprised Tyr and Ser residues in all of the CDRs of variable heavy chain (VH) and in the CDR3 of variable light chain VL [12]. The library was built upon the humanized and stable 4D5 framework [13]. After the new library was characterized, and the diversity was determined, the functionality of the library was tested by screening against a variety of antigens which included hemoglobin, ubiquitin, VEGFA, CD44, as well as our antigen of interest, VEGFR1.
2. Materials and methods
Phusion high-fidelity DNA polymerase, restriction endonucleases NcoI, DpnI and NotI, and T4 DNA ligase are purchased from New England BioLabs. TG1 phage display competent cells were purchased from Lucigen. Gel Extraction Kit, PCR Purification Kit, and plasmid Spin Miniprep Kit were purchased from Qiagen. Protein Prestained Standards are purchased from BioRad.
2.1. Phagemid construction
The pIT2 vector was used as a parent vector for a phagemid construction. Nucleotide sequences of scFvs of the humanized 4D5 antibody were spanned by restriction enzyme sites NcoI and NotI and synthesized for cloning into pUC57 vector from GenScript. The synthesized sequences were digested and purified using agarose gel electrophoresis, and ligated into NcoI and NotI digested phagemid pIT2 using T4 DNA ligase. After transformation, positives were selected by colony PCR screening and further confirmed by DNA sequencing (Genewiz) using purified phagemids.
2.2. Site-directed mutation by PCR and TG1 electroporation
To introduce mutations, primers containing binary code of Tyr and Ser were designed as previously described and synthesized from Integrated DNA technology with 5′ phosphate modification (Table S1) [12]. Site-directed mutagenic PCRs were prepared using Phusion polymerase (2 U/reaction), with a reaction system containing 80 ng of template plasmid, 1×phusion HF buffer, 7% DMSO (v/v), 55 pmol of forward and backward primers, 10 pmol of dNTPs, and H2O added to final volume as 50 μL. PCR reactions were performed using a BioRad T100 Thermal Cycler programmed as follows: 2 min at 98 °C; 35 cycles of 40 s at 98 °C, 1 min at 68 °C, 3 min at 72 °C; 10 min at 72 °C; hold at 4 °C. PCR products were incubated with 1 unit endonuclease DpnI overnight at 37 °C to digest the template plasmid. The next day, DpnI was inactivated by heating for 10 min at 80 °C. PCR products were resolved on a 1% agarose gel. The target DNA fragment was excised and purified with a Gel Purification Kit. Self-ligation was accomplished with T4 ligase for 72 h at 16 °C. Ligation reactions were heat-inactivated for 15 min at 70 °C.
The ligated plasmids were purified from the ligation reactions with a PCR Purification Kit, and were electroporated into TG1 electrocompetent cells (Lucigen) as described in the manufacture’s protocol. Briefly, 1 μL of ligated plasmid was mixed with 25 μL of ice-thawed TG1 and transferred into an ice-cooled 0.1 cm gap cuvette (BioRad). Electroporation was carried out with a BioRad Gene Pulser using the following conditions: 10 μF, 600 Ω, and 1800 V. Within 10 s of the pulse, 975 μL of warm kit-provided recovery medium was added to resuspend the cells, and they were transferred to a culture tube. After cells were recovered for 1 h at 37 °C, 10 μL of the cells were used for titration with serial dilutions. The rest was cultured for phage library preparation.
2.3. Screening the BCscFv library
BCscFv library was constructed (Fig. S1, Table S2) and characterized (Fig. S2).
The antigens, ubiquitin (R&D Systems), hemoglobin (Sigma), VEGFA (Sino Biological), VEGFR1 (R&D Systems), and CD44 (R&D Systems), were incubated in 96-well Nunc Maxisorp plates (Thermo Scientific) at 4 °C for 16–20 h for immobilization. For stringent phage selections, the amount of antigen for immobilization was gradually decreased: first round: 5 µg/mL, second round: 2.5 µg/mL, and third round: 1.25 μg/mL in PBS (100 µL). After each well was rinsed with PBST (PBS containing 0.05% Tween-20), non-specific binding sites were blocked with alternating proteins (300 μL) to prevent the emergence of phage that bind the blocking protein for 2 h (first round: 0.5% BSA in PBS, second round: 0.5% ovalbumin in PBS, and third round: 0.5% casein in PBS). The plate was rinsed with PBST, and phages in blocking buffer (100 μL, 1013 CFU/mL) were added to the plates for affinity selection and incubated for 2 h at room temperature. The wells were rinsed three times with PBST in the first and second rounds of screening and ten times in the third round of screening. The remaining scFv-expressing phages were eluted by a treatment with trypsin (100 μL/well, 1 mg/mL in PBS) for 10 min. The eluted phage were collected and stored at 4 °C for titration or amplification.
2.4. Phage ELISA
96-well plates were coated with antigens or BSA overnight (0.5 μg in 100 μL of PBS) and washed three times with PBST. The non-specific binding was blocked with a blocking solution (5% non-fat milk in PBS, 300 μL) for 2 h followed by washing (3×, PBST). Blank wells received only the blocking solution (n=96). The phages in 5% non-fat milk in PBS (100 μL, 1013 CFU/mL) were added to the antigen- or BSA-immobilized wells and incubated for 1 h followed by rinsing with PBST (10×, 300 μL). Each well was incubated with an HRP-anti-M13 antibody (1:5000, 100 μL; GE Healthcare Life Sciences, 27-9421-01) for 1 h. The wells were washed (8×, PBST), and HRP enzyme substrate solution (100 μL/well; BioRad, 172-1064) was added into the wells. The enzymatic reaction was stopped using H2SO4 (2 N, 100 μL), and the plate was read at OD 450 nm (SynergyH4 Hybrid Reader, BioTek). Means of the BSA-controls (n=96) plus three times the standard deviation (SD) are set as cut-off for positives [14].
2.5. Confocal microscopy of VEGFR1 expressing cell lines
The cell lines SK-BR3, U87 was seeded for 48 h and fixed with Methanol/Acetone (V:V=1:1) for 10 min at −20 °C, following by modified protocol descripted in supplemental data. Fluorescence microscopic images were taken with a Zeiss Observer.Z1/Apotome 2 Microscope (Carl Zeiss) equipped with an EMCCD camera (Evolve 512 Delta, Photometrics, Tuscon, AZ).
3. Results
3.1. Characterization of the library with phage display against five human antigens
To assess the functionality of the BCscFv phage library, biopanning was performed with five human antigens: hemoglobin, VEGFA, ubiquitin, CD44 and VEGFR1. The antigen amounts were decreased during panning to increase stringency. In some cases the enrichment values decreased for the third round of panning (hemoglobin and ubiquitin) (Table S3). To verify antigen mediated enrichment between rounds of panning, the phage eluents were further amplified and polyclonal phage ELISA was performed (Fig. S1). Higher signals from target antigen-bound phages were observed compared to the BSA or the blank controls, indicating a successful enrichment for all of the antigens (results for VEGR1 and hemoglobin shown, Fig. S3). Individual phage colonies (96) were then randomly picked and amplified for monoclonal phage ELISA, and positive monoclonal phage clones were obtained (14.6–100% positive) from the screened clones (Table 1). From these clones, 24 were selected for sequencing (additional clones were selected at random for hemoglobin and VEGFA) of the CDRs that contained the binary code. The selection process yielded sequences that were highly unique for all the antibodies that were selected (Tables 1 and S4). All 24 soluble scFvs for each antigen were subjected to analysis by SPR at a single concentration. In each group, there was a mixture of low and high affinity antibodies (Table 1).
Table 1.
Screening results of human BCscFv library with five antigens.
| Human protein antigen | Monoclonal phage ELISAa | Sequenceb | KD (M)c |
|---|---|---|---|
| Hemoglobin | 15/96 | 24/24 | 1.2×10−9–9.3×10−7 |
| VEGFR-1 | 76/96 | 22/24 | 1.0×10−9–1.9×10−6 |
| VEGF-A | 14/96 | 24/24 | 1.6×10−9–1.9×10−5 |
| Ubiquitin | 40/96 | 24/24 | 3.7×10−8–1.3×10−6 |
| CD44 | 96/96 | 24/24 | 1.0×10−9–1.0×10−5 |
Numbers of positive clones/total clones.
Numbers of unique clones/total clones.
KD from a single concentration assuming a 1:1 binding model.
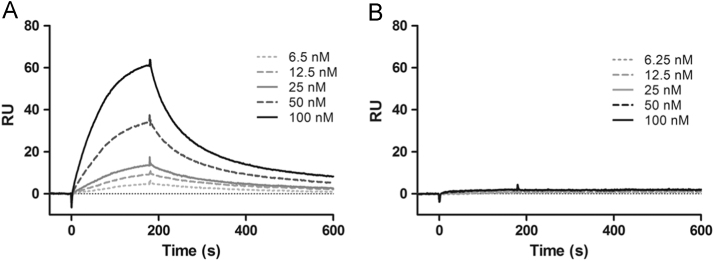
Due to the interest of VEGFR1 as a marker for cancer pathogenesis, clones obtained from panning against VEGFR1 were examined in detail. Unique sequences (22) were among the SPR-tested antibodies (Table 1). Amino acid sequences of 4 mutated CDR regions in 10 unique full-length clones had different lengths of CDR-VH3, with 10–17 mutated binary amino acids and different code patterns in all CDRs (Table S4). Clones (24) with robust ELISA signals were selected for the production of soluble scFvs (~27 kDa) on a small-scale (8 mL of bacterial culture) for affinity determination by SPR. These scFvs (22/24, where one clone appeared three times (B09/C01/C02), bound to the immobilized VEGFR1 with equilibrium dissociation constants (KD) of 7.1×10−9 to 3.8×10−7 M. SPR analysis of clones (A03, A07, A08, B06, B09/C01/C02, B11, C04, C06, E05, E06, F02, F06, and G08) demonstrated specific binding to VEGFR1 (Table S4). The KD of 10 unique scFvs is in the low-nanomolar range, 7.1–29.4 nM. The affinity of scFv A07 was investigated at multiple concentrations to immobilized VEGFR1 and VEGFR2 which shares high homology with VEGFR1 (Fig. 1). There was an approximate 5-fold decrease in affinity as determined by multi-concentration analysis compared to the affinity determined from a single concentration (KD=40.1 nM from KD=7.1 nM) and there was no observed binding to VEGFR2 (Fig. 1). This data indicates that the single concentration analysis is a reasonable estimate of affinity and that scFv A07 is not cross-reactive to a protein that shares high homology with its antigen. In addition to the 10 unique full-length scFv clones against VEGFR1, we also discovered 12 clones that contained truncated sequences (in correct frame with the pIII protein). The molecular weights of the truncated scFv clones (~16 kDa) were confirmed by electrophoresis in SDS-PAGE gels (Fig. S4). The binding properties of the truncated antibodies were measured by SPR: 3/12 clones (B06, C04 and C06) showed relatively high affinity (KD=22.1–375.0 nM), whereas the rest of the clones (9/12) showed relatively weak affinities (KD>400 nM). Sequencing analysis of the three high binders from the truncated clones (B06, C04 and C06) demonstrated that they contained only the heavy chain.
Fig. 1.
Sensorgrams of anti-VEGFR1 scFv clone A07 to immobilized (A) VEGFR1 (KD=40.1 nM) and (B) VEGFR2 (no binding observed). Each experiment was repeated twice.
3.2. Immunofluorescence microscopy of VEGFR1 expression in tissue using anti-VEGFR1 scFv (A07)
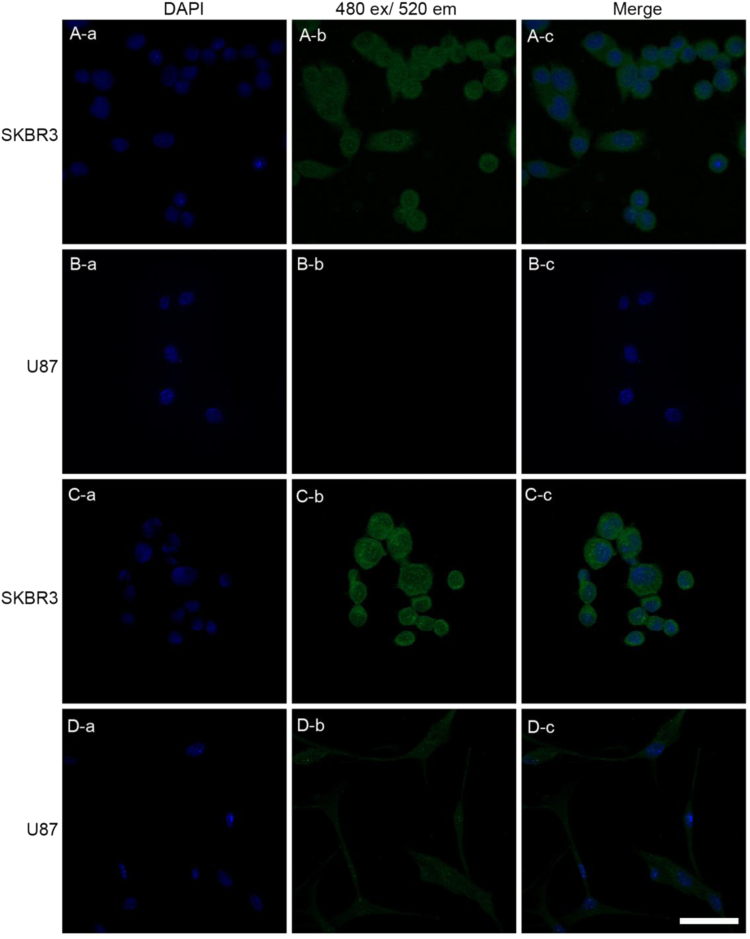
Human breast carcinoma cells (SK-BR3) are known to express VEGFR1 which plays key regulatory roles in tumor cell growth and survival [15]. Utilizing confocal microscopy, Anti-VEGFR1 scFv (A07) was tested with this cell line to determine whether it could recognize cognate receptor. Incubation of the SK-BR3 with anti-VEGFR1 scFv (A07) followed with anti-His tag mAb Alexa Fluor 488 conjugate showed intense pericellular staining (Fig. 2A) in contrast to the staining of cell line U87 (Fig. 2A), a VEGFR1-negative control, with no detectable signal [16]. As a positive control, a commercially available anti-VEGFR1 antibody conjugated with Flour 488 was employed. As predicted, there was higher staining in SK-BR3 cell line relative to the VEGFR1-negative (Fig. 2C) U87 cell line (Fig. 2D). Additionally, tumor xenografts from mice developed from SK-BR3 were used for preparing slides and immunohistochemistry (Fig. S5). The greatly reduced fluorescence from anti-VEGFR1 Mab DyLight 488 by co-treatment with 100-fold molar excess anti-VEGFR1 scFv (A07) indicates that most of VEGFR1 binding sites were blocked by anti-VEGFR1 scFv (A07) (Fig. S5C).
Fig. 2.
Confocal microscopy images of VEGFR1 expressing human cell lines (SKBR3 and U87). The cells were incubated with anti-VEGFR1 mAb DyLight 488 (C and D). Cells were incubated with anti-VEGFR1 scFv (clone A07, 30 µg/mL) followed by anti-His tag mAb Alexa Fluor 488 at room temperature (A and B). DAPI (a) was used for nucleus counter staining in blue. Fluor 488 staining appears in red (b). Column (c) shows the merged picture of (a) and (b). (Scale bar=10 µm).
4. Discussion
The technology of phage display allows the assembly of a large repertoire of antibodies, and through affinity based selection, the isolation of antibodies of high affinity and specificity for the targeted antigens or haptens [17]. A general and successful approach has been to maximize the natural diversity of genetically encoded amino acids in the hypervariable regions. Generally, greater diversity corresponds to the discovery of higher affinity antibodies [18], [19]. In contrast, the formulation of minimalist synthetic antibodies, constructed from a simple binary code consisting of Tyr and Ser in the hypervariable regions has also been successful [20]. When screened against a panel of diverse protein antigens, a Fab library comprised mostly Tyr and Ser in the hypervariable regions yielded antibodies with affinities in the single-digit nanomolar range with unique sequences [20]. Antibodies with such affinities could be used directly for a variety of purposes such as molecular based therapies or affinity matured for even higher affinities. We constructed a scFv library based on the binary code and demonstrated that the library covered the theoretical diversity and produced high affinity antibodies to a variety of proteins that rivaled libraries with a high degree of utility and greater diversity [13], [21].
After assessing the quality of the library through PCR colony screening, immunoblotting, and DNA sequencing, we found that a very high percentage of clones expressed the full scFv sequence (Table S2 and Fig. S3A and S3B). These results compare favorably to other synthetic libraries of high utility [13], [21]. The diversity of the library was reduced over the previously described Fab library; however, we were still able to discover soluble and functional antibodies for a number of antigens [20].
After confirming that the construction of the library was sound, it was tested to determine whether it could deliver a diverse set of functional antibodies for a variety of antigens. Included in these antigens are proteins of relevance in cancer and other diseases. Human VEGFA is over expressed in most human tumors and promotes angiogenesis of tumors. The receptor for VEGFA, VEGFR1, is implicated in tumor progression and dissemination [1], [2], [3], [4], [15]. CD44, a hyaluronic acid receptor, is upregulated in tumor cells and has been identified as a cancer stem cell marker [22]. These proteins, together with other common proteins, were assembled as a panel to test the BCscFv library. After three rounds of panning, high affinity antibodies, based on an apparent KD determined at one concentration, were discovered for all tested antigens, except for ubiquitin. Additionally, all or most of the sequences recovered were unique. The intended use of the library is for the discovery of ligands to cell surface antigens for non-invasive diagnostic imaging or molecular therapies in cancer and other diseases [23]. To be effective for molecular imaging, the minimum affinities (KD) should be less than 100 nM. The best tumor accumulations generally occur with scFvs of KD<10 nM and kd at least on the order of 1×10−3 s−1 [24].
Immunofluorescence microscopy of cell lines and frozen tumor tissue confirmed that the anti-VEFGR1 scFv (A07) discovered from BCscFv library is fully functional (Figs. 2 and S5). To demonstrate the selectivity of anti-VEFGR1 scFv (A07), commercially available anti-VEGFR1-Mab was co-incubated with a 100-fold molar excess of A07 on tumor tissue (SK-BR3 human breast cancer xenograft tissue) and inhibited the binding of the anti-VEGFR1-Mab thus demonstrating that A07 binds an epitope of VEGFR1 in tumor tissue. Additionally, A07 neither binds immobilized VEGFR2 in an SPR experiment nor U87 human glioma cells, which do not express VEGFR1 [16]. This data indicates that the A07 is highly selective for VEGFR1 and is not likely to suffer from significant off-target binding.
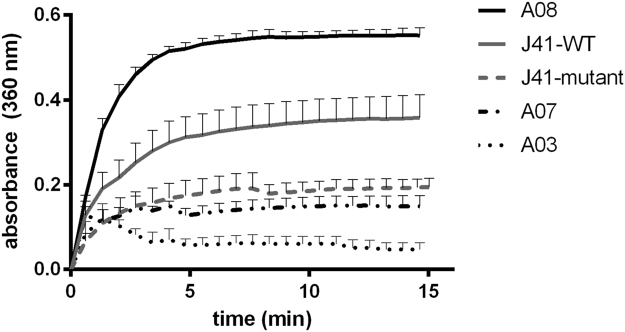
To demonstrate function of the exemplar VEGFR1 scFvs beyond affinity, their propensity towards heat-induced aggregation was examined. Recent findings have shown that antibodies with resistance toward heat-induced aggregation results in higher yields in bacterial expression systems relative to those antibodies which aggregate readily [25]. The turbidity test is a measure of colloidal stability rather than thermodynamic stability, where a colloid is a non-native state consisting of aggregates formed from unfolded or partially folded species [26]. Some proteins can be highly thermodynamically stable but possess low colloidal stability [25]. For scFvs that will be formulated under conditions requiring high colloidal stability, such as lyophilization, filtrations, and high concentrations for small animal injections, the turbidity assay is a rapid and inexpensive method of assessing this important parameter of stability. As shown in Fig. 3, we compared the aggregation propensity of an scFv, J41, that was isolated from the Tomlinson I+J library with those from the BCscFv library [8]. Included in the comparison was a mutated version of J41 that incorporated a DED triad at the C-terminal and N-terminal ends of VH CDR3 and within VH CDR1, and a DD mutation within VL CDR2 (Table S1). This mutant did display greater resistance toward aggregation than the wild type (J41-WT), which is consistent with the observed stability of scFvs with the Asp and Glu mutation at the beginning or end of the CDR regions [25]. By comparison, the exemplar scFvs, A03, A07, and A08, had a range of aggregation propensities. A08 was not aggregation resistant while A03 and A07 were more aggregation resistant than the mutant (J41-mutant). These results suggest that the Ser and Tyr residues that comprise the VH CDRs and VL CDR2 can contribute to relatively stable scFv antibodies that are resistant to aggregation and do not specifically contribute to aggregation which is apparently governed by other sequence determinants.
Fig. 3.
Turbidity measurements of scFvs. Binary code scFvs (A03, A07, and A08) were resistant to aggregation compared to the scFvs (J41-WT and J41-mutant) containing normal complement of amino acids. Any pair differ significantly (P<0.01) from each other. Each measurement was repeated for 3 times. Error bars represent standard deviation of the mean.
In conclusion, to supply high affinity antibodies for molecular targeting of VEGFR1, a new scFv phage library was constructed with the humanized 4D5 framework that incorporated a predominate fraction of tyrosine and serine residues in the CDRs. When the library was panned against a panel of proteins that included proteins of biomedical interest, high affinity antibodies were isolated. Therefore, in three rounds of enrichment, the BCscFv library has delivered a group of three scFvs for CD44, VEGFR1, and VEGFA that are suitable in affinity for further development as molecularly targeted agents without the need for affinity maturation. The screening results suggest that this new library is highly functional. Further analysis of anti-VEGFR1-scFvs, demonstrated that one of them, A07, recognized cognate receptor on tumor tissue and demonstrated high affinity in a multi-concentration analysis of binding kinetics by surface plasmon resonance. This antibody, which is resistant to aggregation, is suitable for molecular imaging or targeted therapies of cancer where VEGFR1 is upregulated as a result of the disease. To our best knowledge, this is the first functional anti-VEGFR1 scFv with CDRs comprised of the binary code that would be suitable for formulation as a bivalent construct such as a minibody or diabody for molecular imaging or for fusion with other proteins for viral targeting [27], [28].
Acknowledgments
The authors thank Jamee Bresee for editing.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.08.004.
Appendix A. Supplementary materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Schwartz J.D., Rowinsky E.K., Youssoufian H., Pytowski B., Wu Y. Vascular endothelial growth factor receptor-1 in human cancer: concise review and rationale for development of IMC-18F1 (Human antibody targeting vascular endothelial growth factor receptor-1) Cancer. 2010;116:1027–1032. doi: 10.1002/cncr.24789. [DOI] [PubMed] [Google Scholar]
- 2.Bae D.G., Kim T.D., Li G., Yoon W.H., Chae C.B. Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis. Clin. Cancer Res. – Off. J. Am. Assoc. Cancer Res. 2005;11:2651–2661. doi: 10.1158/1078-0432.CCR-04-1564. [DOI] [PubMed] [Google Scholar]
- 3.Pollard J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan R.N., Rafii S., Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fellouse F.A., Barthelemy P.A., Kelley R.F., Sidhu S.S. Tyrosine plays a dominant functional role in the paratope of a synthetic antibody derived from a four amino acid code. J. Mol. Biol. 2006;357:100–114. doi: 10.1016/j.jmb.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 6.Fellouse F.A., Esaki K., Birtalan S., Raptis D., Cancasci V.J., Koide A., Jhurani P., Vasser M., Wiesmann C., Kossiakoff A.A., Koide S., Sidhu S.S. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J. Mol. Biol. 2007;373:924–940. doi: 10.1016/j.jmb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 7.de Haard H.J., van Neer N., Reurs A., Hufton S.E., Roovers R.C., Henderikx P., de Bruine A.P., Arends J.W., Hoogenboom H.R. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J. Biol. Chem. 1999;274:18218–18230. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 8.Kim H.Y., Wang X., Wahlberg B., Edwards W.B. Discovery of hapten-specific scFv from a phage display library and applications for HER2-positive tumor imaging. Bioconjug. Chem. 2014;25:1311–1322. doi: 10.1021/bc500173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yazaki P.J., Kassa T., Cheung C.-w, Crow D.M., Sherman M.A., Bading J.R., Anderson A.-L.J., Colcher D., Raubitschek A. Biodistribution and tumor imaging of an anti-CEA single-chain antibody–albumin fusion protein. Nucl. Med. Biol. 2008;35:151–158. doi: 10.1016/j.nucmedbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad Z.A., Yeap S.K., Ali A.M., Ho W.Y., Alitheen N.B., Hamid M. scFv antibody: principles and clinical application. Clin. Develop. Immunol. 2012;2012:980250. doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowles S.M., Wu A.M. Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J. Clin. Oncol. – Off. J. Am. Soc. Clin. Oncol. 2012;30:3884–3892. doi: 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajan S., Sidhu S.S. Simplified synthetic antibody libraries. Methods Enzymol. 2012;502:3–23. doi: 10.1016/B978-0-12-416039-2.00001-X. [DOI] [PubMed] [Google Scholar]
- 13.Lee C.V., Liang W.C., Dennis M.S., Eigenbrot C., Sidhu S.S., Fuh G. High-affinity human antibodies from phage-displayed synthetic Fab libraries with a single framework scaffold. J. Mol. Biol. 2004;340:1073–1093. doi: 10.1016/j.jmb.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Greiner M., Sohr D., Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y., Hooper A.T., Zhong Z., Witte L., Bohlen P., Rafii S., Hicklin D.J. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int. J. Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 16.Yao J., Wu X., Zhuang G., Kasman I.M., Vogt T., Phan V., Shibuya M., Ferrara N., Bais C. Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. Proc. Natl. Acad. Sci. USA. 2011;108:11590–11595. doi: 10.1073/pnas.1109029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidhu S.S., Fellouse F.A. Synthetic therapeutic antibodies. Nat. Chem. Biol. 2006;2:682–688. doi: 10.1038/nchembio843. [DOI] [PubMed] [Google Scholar]
- 18.Ling M.M. Large antibody display libraries for isolation of high-affinity antibodies. Comb. Chem. High Throughput Screen. 2003;6:421–432. doi: 10.2174/138620703106298608. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd C., Lowe D., Edwards B., Welsh F., Dilks T., Hardman C., Vaughan T. Modelling the human immune response: performance of a 1011 human antibody repertoire against a broad panel of therapeutically relevant antigens. Protein Eng. Des. Sel. 2009;22:159–168. doi: 10.1093/protein/gzn058. [DOI] [PubMed] [Google Scholar]
- 20.Fellouse F.A., Esaki K., Birtalan S., Raptis D., Cancasci V.J., Koide A., Jhurani P., Vasser M., Wiesmann C., Kossiakoff A.A., Koide S., Sidhu S.S. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J. Mol. Biol. 2007;373:924–940. doi: 10.1016/j.jmb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Silacci M., Brack S., Schirru G., Marlind J., Ettorre A., Merlo A., Viti F., Neri D. Design, construction, and characterization of a large synthetic human antibody phage display library. Proteomics. 2005;5:2340–2350. doi: 10.1002/pmic.200401273. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Huang K., Li X., Lin X., Zhu Z., Wu Y. Generation of a stable anti-human CD44v6 scFv and analysis of its cancer-targeting ability in vitro. Cancer Immunol. Immunother. 2010;59:933–942. doi: 10.1007/s00262-010-0819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu A.M., Senter P.D. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 24.Adams G.P., Tai M.-S., McCartney J.E., Marks J.D., Stafford W.F., Houston L., Huston J.S., Weiner L.M. Avidity-mediated enhancement of in vivo tumor targeting by single-chain Fv dimers. Clin. Cancer Res. 2006;12:1599–1605. doi: 10.1158/1078-0432.CCR-05-2217. [DOI] [PubMed] [Google Scholar]
- 25.Dudgeon K., Rouet R., Kokmeijer I., Schofield P., Stolp J., Langley D., Stock D., Christ D. General strategy for the generation of human antibody variable domains with increased aggregation resistance. Proc. Natl. Acad. Sci. USA. 2012;109:10879–10884. doi: 10.1073/pnas.1202866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouet R., Lowe D., Christ D. Stability engineering of the human antibody repertoire. FEBS Lett. 2014;588:269–277. doi: 10.1016/j.febslet.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Olafsen T., Wu A.M. Antibody vectors for imaging. Semin. Nucl. Med. 2010;40:167–181. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube R., Zhu Q., Xu C., Diaz-Griffero F., Sui J., Kamau E., Dwyer M., Aird D., Marasco W.A. Lentivirus display: stable expression of human antibodies on the surface of human cells and virus particles. PLoS One. 2008;3:e3181. doi: 10.1371/journal.pone.0003181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material