Abstract
Glutaraldehyde cross-linked bioprosthetic heart valves fail within 12–15 years of implantation due to limited durability. Glutaraldehyde does not adequately stabilize extracellular matrix components such as glycosaminoglycans and elastin, and loss of these components could be a major cause of degeneration of valve after implantation. We have shown earlier that neomycin-based cross-linking stabilizes glycosaminoglycans in the tissue but fails to stabilize elastin component. Here, we report a new treatment where neomycin and pentagalloyl glucose (PGG) were incorporated into glutaraldehyde cross-linking neomycin-PGG-Glutaraldehyde (NPG) to stabilize both glycosaminoglycans and elastin in porcine aortic valves. In vitro studies demonstrated a marked increase in extracellular matrix stability against enzymatic degradation after cross-linking and 10 month storage in NPG group when compared to glutaraldehyde controls. Tensile properties showed increased lower elastic modulus in both radial and circumferential directions in NPG group as compared to glutaraldehyde, probably due to increased elastin stabilization with no changes in upper elastic modulus and extensibility. The enhanced extracellular matrix stability was further maintained in NPG-treated tissues after rat subdermal implantation for three weeks. NPG group also showed reduced calcification when compared to glutaraldehyde controls. We conclude that NPG cross-linking would be an excellent alternative to glutaraldehyde cross-linking of bioprosthetic heart valves to improve its durability.
Keywords: Heart valve implant, extracellular matrix, tissue preservation, glycosaminoglycans, tissue biomechanics
Introduction
Bioprosthetic heart valves (BHVs) have been used for valve replacement since the 1960s.1 BHVs are xenograft heart valves comprised of glutaraldehyde (GLUT) cross-linked porcine aortic valves or bovine pericardium, intended to supplement the limited supply of cadaver valves for tissue valve replacement.1–4 Benefits over mechanical replacement valves include improved hemodynamic properties, and the absence of lifelong anticoagulant therapy required to counteract the thrombogenicity of mechanical heart valve materials. These properties make BHVs a preferred choice for patients who cannot tolerate anticoagulant therapy. However, the limited durability of current BHVs restricted their use to either elderly patients or those with contraindications for mechanical heart valves.1,3 However, over the last five years use of BHVs in younger patients (<50 years) has increased significantly and therefore long-term durability of BHVs is of prime importance. Durability improvements could lead to increased patient quality of life, longer implant time, and a broadened patient demographic.
Clinically used BHVs are treated with GLUT to reduce tissue antigenicity and cross-link the tissue. These valves suffer from inadequate durability; the majority fail within 12–15 years of implantation due to tissue degeneration and calcification.1,5 A new generation of BHVs has added anticalcification treatments to prevent calcification; however, there are no treatments to prevent tissue degeneration in the absence of calcification that is seen in up to 50% of failed valves. While GLUT provides adequate stabilization of collagen, it fails to stabilize other extracellular matrix (ECM) components such as elastin and glycosaminoglycans (GAGs).1,6–10 Both of these compounds may play a vital role in valve durability.11 Additions to GLUT cross-linking, which provide additional ECM stabilization and reduced calcification, could prove critical to improve valve durability.
The present study utilizes the addition of neomycin trisulfate and pentagalloyl glucose (PGG) to GLUT-fixed valves. Neomycin trisulfate is a hyaluronidase inhibitor which is cross-linked to the cusps, and has previously been shown to inhibit GAG loss in BHVs, but it was unable to stabilize elastin component.12–16 PGG is derived from a plant polyphenol. It forms bonds with elastin via GLUT cross-linking to block enzyme-binding sites and improves elastin stability.17,18 Here, we tested if combination of neomycin and PGG would enhance ECM stability in BHVs and improve their durability. We tested the resistance of valve tissue to enzymatic degradation in vitro after cross-linking and after long-term storage. We also tested if this new cross-linking significantly alters tissue mechanical properties. We further tested if ECM stability was maintained in vivo.
Methods
Tissue fixation
Fresh porcine aortic valves were harvested onsite at a local abattoir, Snow Creek Meat Processing (Seneca, SC). Hearts were excised immediately following death, and valves were removed via incisions just above the aortic sinuses and below the aortic cusps. Valves were transported on ice to the laboratory and rinsed thoroughly in 0.9% saline. Excess muscle tissue, fat, and aortic wall were removed, leaving approximately 2 cm of aortic wall connected to the leaflet. Whole valves were fixed and stuffed with cotton balls for mechanical testing, or cusps were separated and fixed immediately. All fixation steps used 33 mL solution per leaflet, and were carried out at room temperature within 4 h of harvest.
GLUT
Leaflets were treated with 0.6% GLUT in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer at pH 7.4 and room temperature for 24 h. This was followed by fixation with 0.2% GLUT in 50 mM HEPES buffer at pH 7.4 and room temperature for 6 days.
NPG
Leaflets were treated with 1 mM neomycin trisulfate in 50 mM 4-morpholinoethanesulfonic acid (MES) buffer at pH 5.5 for 1 h under constant shaking. This was followed by incubation in PGG in 0.6% GLUT and 50 mM MES buffer at pH 5.5 for 24 h. Leaflets were transferred to 0.2% GLUT in 50 mM HEPES at pH 7.4 for 6 days. PGG was dissolved in 1% isopropanol in MES prior to addition of GLUT. PGG concentration studies were performed utilizing 0.05%, 0.10%, and 0.15% PGG in the NPG treatment. Further studies utilized 0.15% PGG as an optimum concentration.
NPG tissues were also stored for 10 months in 0.2% GLUT in 50 mM HEPES at pH 7.4, and assessed for ECM stability.
Number of samples used were n = 6 per test group unless otherwise noted in the text.
Collagenase and elastase assays
Enzyme resistance was used as a measure of ECM stability. Freshly treated or implanted cusps were cut in half symmetrically, rinsed in water, and lyophilized. Initial weight was recorded. Half cusps were incubated in 1.2 mL of 5.0 U/mL elastase (100 mM Tris, 1 mM CaCl2, 0.02% NaN3, pH 7.8) for 24 h or in 1.2 mL of 75.0 U/mL collagenase (VII) (50 mM Tris, 10 mM CaCl2, 0.02% NaN3, pH 8.0) for 48 h. Samples were rinsed in DI water, lyophilized and final weight recorded. Degree of enzyme degradation was determined by quantification of percent weight loss, calculated by dividing the difference of weights by the initial weight.
Enzymatic degradation of GAGs
Treatment with GAG degrading enzymes (GAGase) was used to measure GAG stability. Cusps were excised from the aortic wall, rinsed in 100 mM ammonium acetate buffer (AAB) at pH 7.0 3 × for 5 min, and cut in half symmetrically. One half was incubated in 1.2 mL AAB, the other in 1.2 GAGase (5 U/mL hyaluronidase, 0.1 U/mL chondroitinase ABC in 100 mM AAB, pH 7.0). Samples were shaken vigorously at 37°C for 24 h, washed thoroughly in DI water, lyophilized, and weight recorded. Tissue was used for the hexosamine assay, and enzyme liquid for the dimethyl methylene blue (DMMB) assay. Pairwise testing was not conducted on half-leaflets since experience has shown there is intrinsic tissue variability in GAG levels (different levels in leaflet areas). Therefore, all half-leaflets that were treated with control buffer gave us an average value (n = 6) that was compared to the average value of all other half-leaflets (n = 6) that were treated with the enzyme.
GAG quantification by hexosamine analysis
Total GAG content was measured using the Elson and Morgan’s modified hexosamine assay as previously reported.7 Lyophilized GAG-digested samples were weighed, digested in 2 mL 6 N HCl (24 h, 96°C) and dried under nitrogen gas. Samples were resuspended in 2 mL 1 M sodium chloride and reacted with 2 mL of 3% acetyl acetone in 1.25 M sodium carbonate (1 h, 96 C). Ethanol of 4 mL and 2 mL of Ehrlich’s reagent (0.18 M p-dimethylaminobenzaldehyde in 50% ethanol containing 3 N HCl) were added, and solutions left for 45 min to allow the color to develop. A pinkish-red color is indicative of tissue hexosamine quantities, and the absorbance was read at 540 nm. A set of D(+)-glucosamine standards were used.
Sulfated GAG quantification by DMMB assay
Tissue lysates from GAG digestion were analyzed using the DMMB assay for sulfated GAGs, which are released into the enzyme or buffer solution. The following solutions were pipetted into each well of a 96-well plate: 20 µL sample, 30 µL PBE buffer (100 mM Na2HPO4, 5 mM EDTA, pH 7.5) and 200 µL DMMB reagent (40 mM NaCl, 40 mM glycine, 46 mM DMMB, pH 3.0). These were compared to chondroitin sulfate (0–1.25 µg) standards, and absorbance measured at 525 nm. Absorbance was read immediately to prevent degradation of the GAG-DMMB dye complex, using the mQuant spectrophotometer (BIO-TEK instruments, Winooski, VT).
Subdermal implantation
All tissues were treated with 80% ethanol in HEPES, pH 7.4 for 24 h and rinsed thoroughly in sterile saline prior to implantation. Male juvenile Sprague-Dawley rats (35–40 g; Harlan Laboratories; Indianapolis, IN) were anesthetized by inhalation of 3% isoflurane gas. A dorsal surgical incision was made, and two subdermal pockets created on either side of the sagittal plane. One cusp was placed per pocket. Incisions were closed with surgical staples. A total of 10 animals were used (5 for NPG group and 5 for GLUT group). Animals were sacrificed at 3 weeks using carbon dioxide asphyxiation. Implants and tissue capsule were saved for further analysis. All animals received humane care in compliance with protocols that have been approved by the Clemson University Animal Research Committee and NIH.
Calcium and phosphorous analysis of explants
Half cusps were immediately frozen on dry ice following subdermal implantation. Samples were frozen, lyophilized, weighed and hydrolyzed in 1 mL 6 N Ultrex II HCl for 20 h at 95°C. Samples were then dried under nitrogen gas, and dissolved in 1 mL 0.1 N Ultrex II HCl. Samples were diluted in water, and calcium and phosphorus analyses were performed using Spectro Acros ICP Spectrophotometer (SPECTRO Analytical Instruments; Kleve, Germany). Resulting calcium and phosphorous concentrations were normalized by dry weight of the tissue.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was performed on cross-linked tissue samples to assess the collagen denaturation temperature (Td), which is represented by an endothermic peak in the heating curve. Tissue samples (3–8 mg) were excised, blotted dry with tissue paper, and placed in hermetically sealed aluminum pans. Samples were equilibrated at 20°C and heated at 10°C/min. The resulting heating curves were analyzed using Thermal Analysis software. The collagen denaturation temperature was recorded at the maximum endothermic peak.
Uniaxial tensile testing
The MTS Synergie 100 (MTS Systems, Eden Prairie, MN) was used for uniaxial tensile testing with a 10 N load cell, and Testworks 4 software for analysis. Tissue strips of 4 mm wide were cut from the center of the leaflet using a die in the radial and circumferential directions. Tissue hydration was maintained by water, and samples were loaded into custom grips lined with fine grit sandpaper to prevent slippage. Samples were preloaded (less than 0.1 N), measured, and stretched at an extension rate of 12.5 mm/min. Stress–strain curves were analyzed for elastic moduli, ultimate tensile stress, and extensibility. Any samples that failed at the grip were removed from the analysis.
Histological assessment
Radial cross-sections were taken from the center of cusps, fixed in 10% neutral buffered formalin, embedded in paraffin wax, and sectioned at 5 µm for light microscopy analysis. Dahl’s Alizarin red stain with light green counterstain was used to visualize calcium deposition in implanted samples; calcium deposits appear red. Verhoeff van Giesen’s (VVG) stain was used to verify elastin integrity; elastin appears black while collagen appears red. Alcian blue staining with nuclear fast red counterstain was used to visualize GAGs; the blue color is indicative of the presence of GAG. Representative photomicrographs were taken using Zeiss Axioscope 2 Plus (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Statistical analysis
Results are expressed as means of 6 samples ± standard error of the mean with each sample run in triplicate. Statistical analyses of the data were performed using the Student’s t-test and probability values (p) for significance were calculated. P-values smaller than 0.05 were considered statistically significant.
Results
Stability of cusps against enzyme degradation
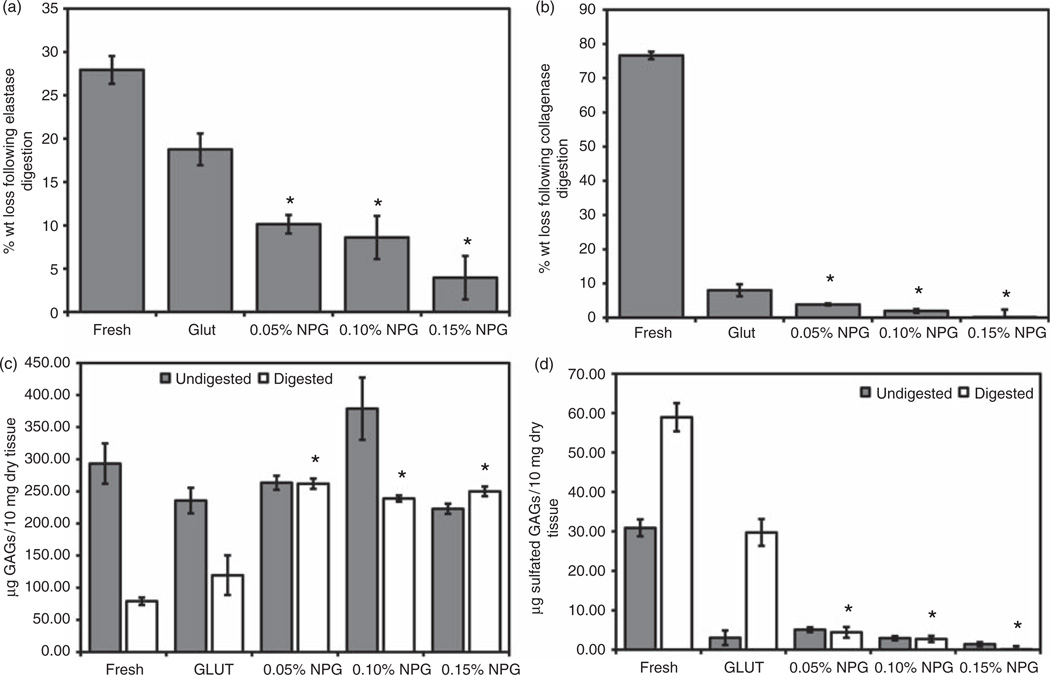
Elastin stability was assessed by weight loss after enzyme degradation (Figure 1(a)). Resistance to elastase in NPG groups was concentration dependent but all NPG groups exhibited higher stability than GLUT or fresh tissue (p < 0.05). The use of 0.15% NPG showed the highest elastin stability. These results suggest elastin stabilization against elastase can be achieved by a concentration of at least 0.05% PGG. The resistance to elastase was also visualized via the VVG elastic stain. Following enzyme treatment, this stain demonstrated higher content of elastic fibers in NPG (Figure 2(a)) than GLUT (Figure 2(b)) or fresh (Figure 2(c)).
Figure 1.
Extracellular matrix stability of treated tissue samples, using (a) elastase, (b) collagenase, (c) hexosamine assay in the tissue and (d) DMMB assay on the enzyme liquid. Results demonstrate improved collagen, elastin and GAG stability following NPG treatment.
*Statistical difference between GLUT and experimental group (p < 0.05, n = 6).
Figure 2.
Histological staining of tissue following enzyme treatments: (a–c) VVG elastic stain following elastase digestion on (a) NPG, (b) GLUT and (c) fresh tissue treatments. Note that black elastic fibers are maintained in NPG tissue, but not GLUT or fresh. (d–f) Alcian blue stain for GAGs following GAGase digestion on (d) NPG, (e) GLUT and (f) fresh tissue treatments. Staining demonstrates higher GAG levels in NPG tissues. Leaflet surfaces are labeled ventricularis (V) and fibrosa (F).
A similar trend was observed when collagen stability was evaluated on NPG, GLUT, and fresh tissue (Figure 1(b)). GLUT is known to stabilize collagen, so this test was intended to show that NPG does not negatively affect the GLUT cross-linking. All cross-linked tissue exhibited higher resistance to collagenase degradation than fresh tissue (p < 0.001). NPG, at all concentrations, improved enzyme resistance when compared to GLUT, as observed from a reduction in weight loss. However, only the 0.15% and 0.10% treatments produced statistically significant results (p < 0.05).
GAG stability was evaluated by treating samples with GAGase. The hexosamine assay, which detects GAG-related hexosamines, was used to quantitatively measure GAGs in tissue, and to evaluate the effect of PGG concentration on preserving GAGs (Figure 1(c)). All NPG groups improved GAG stabilization over GLUT or fresh tissue, as evidenced by higher levels of GAGs remaining following enzyme digestion (p < 0.003). Statistical difference was found between 0.15% and 0.10% NPG groups (p = 0.034). The 0.15% NPG group exhibited much higher resistance to GAGase than any other fixation group.
The GAG stabilization trends were confirmed by measuring quantities of GAGs lost to enzyme digestion by DMMB assays (Figure 1(d)).19 NPG group lost fewer GAGs than GLUT or fresh tissue after GAGase digestion (p < 0.05). GAG stability was further confirmed histologically via the Alcian blue stain with nuclear fast red counterstain. NPG tissue retained higher GAG levels, both when untreated and treated with GAGase (Figure 2(d) to (f)), than GLUT tissues. This indicates higher resistance to GAGase and improved GAG stability within the tissue in NPG groups. These results corroborated hexosamine assay results for tissues, and showed that GAGs in NPG-treated tissue exposed to enzymes are stable and resist enzyme-mediated degradation. Due to the high stabilization against elastase, collagenase, and GAGase, the 0.15% NPG group was chosen for further studies.
The comparison in the ECM stability after cross-linking and after 10 month storage for GLUT and NPG group (0.15% PGG) is shown in Table 1. GLUT tissue showed poor stability for GAGs and elastin (~35% GAG content loss after GAGase degradation and ~18% weight loss after elastase degradation) while GAGs and elastin remain stable in NPG group. GLUT group showed continuous degradation of GAGs and elastin after storage (~19% loss in weight after elastase degradation and ~35% loss in GAG content) while NPG group showed minimal loss (~7% and ~3%, respectively).
Table 1.
ECM stability after cross-linking and 10 month storage in 0.2% GLUT solution. PGG of 0.15% was used in NPG cross-linking. After cross-linking or storage, leaflets were treated with elastase, collagenase or GAGase. Percent GAG loss is referenced to the unstored buffer group of the same treatment.
| After cross-linking | After 10 months storage | |||||
|---|---|---|---|---|---|---|
| NPG | GLUT | p-Value | NPG | GLUT | p-Value | |
| Elastin stability (% wt loss) | 3.97 ± 2.50 | 18.77 ± 1.83 | 0.009 | 2.68 ± 1.35 | 19.00 ± 4.31 | 0.014 |
| Collagen stability (% wt loss) | Undetected | 8.03 ± 1.77 | 0.014 | 2.80 ± 3.06 | Undetected | 0.041 |
| GAG stability (% GAG loss) | Undetected | 35.68 ± 9.49 | 0.001 | 7.31 ± 4.02 | 32.92 ± 2.43 | <0.001 |
ECM: extracellular matrix; GLUT: glutaraldehyde; GAG: glycosaminoglycans; GAGase: glycosaminoglycans degrading enzymes; PGG: pentagalloyl glucose; NPG: Neomycin-PGG-Glutaraldehyde.
Thermal denaturation temperature
Collagen thermal denaturation temperature (Td) as measured by DSC is an indicator for the degree of tissue cross-linking.8 Td-values of 85°C and above are considered to be adequate for tissue cross-linking. NPG-treated tissue showed higher Td-values than GLUT (NPG: 94.89 ± 0.61°C, GLUT: 88.90 ± 0.43°C, p < 0.05), indicating more than adequate collagen stabilization and tissue cross-linking.
Tissue mechanical properties
Uniaxial tensile testing was used to evaluate the upper and lower elastic moduli, ultimate tensile stress, and extensibility in both radial and circumferential directions.
The elastic modulus (E) was used as a measure of tissue stiffness (Figure 3).
Figure 3.
Mechanical uniaxial tensile testing summary: (a) lower elastic modulus (elastin response region), (b) upper elastic modulus (collagen response region), (c) peak stress and (d) tissue extensibility.
*Statistical difference between GLUT and NPG (p < 0.05, n = 6 radial, n = 8 circumferential).
NPG tissues exhibited a greater lower modulus than GLUT tissue in both radial (p = 0.001) and circumferential (p = 0.014) directions (Figure 3(a)). The elevated lower modulus in NPG tissue is likely due to increased elastin preservation and cross-linking. In the upper modulus region, NPG tissue was less stiff than GLUT tissue in the radial (p = 0.017) and similar to GLUT in the circumferential (p = 0.147) directions (Figure 3(b)). It is important to note that NPG presented a marked improvement in elasticity over GLUT.
NPG exhibited higher peak tensile stresses than GLUT in the circumferential direction (p = 0.011), but similar tensile strength to GLUT in the radial direction (Figure 3(c)).
Tissue extensibility showed that NPG and GLUT tissue were equivalent in both directions (Figure 3(d)).
In vivo calcification and ECM stability
Cross-linked tissues were implanted for 3 weeks to evaluate mineralization levels, GAG stabilization, and elastin stabilization in vivo. Both groups utilized 24 h ethanol treatments prior to implantation intended to reduce calcification levels.20
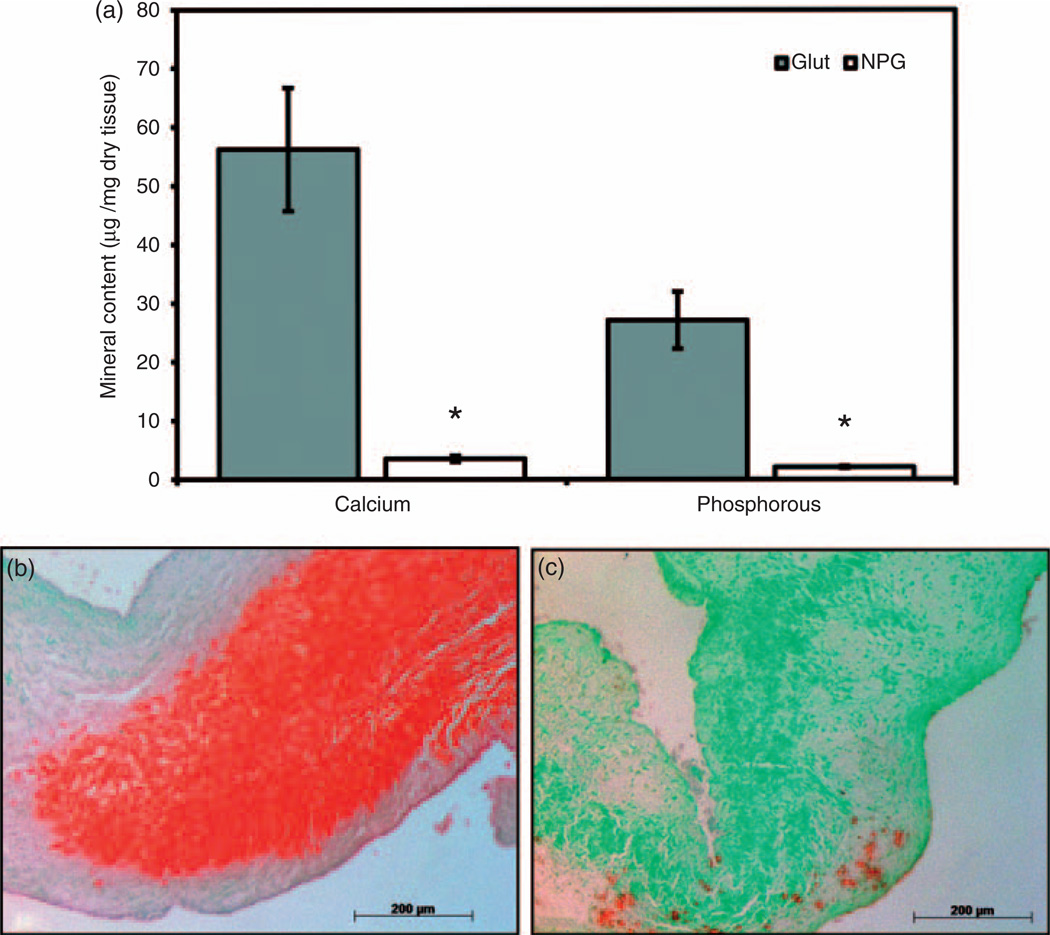
NPG accumulated lower calcium levels than GLUT tissue (p = 0.001) (Figure 4(a)). NPG tissue also contained less phosphorous than GLUT-treated tissue (p = 0.001) (Figure 4(a)). The Ca:P ratios in NPG group (1.67) were similar to poorly crystalline hydroxyapatite while the minerals formed in the GLUT group were more calcium-rich, poorly crystalline hydroxyapatite (Ca:P = 2.06). Dahl’s Alizarin red stain for calcium corroborated quantitative calcium results showing more staining in GLUT group as compared to NPG (Figure 4(b) and (c)).
Figure 4.
Calcification of tissue implanted for 3 weeks in a rat subdermal model: (a) mineral content. Decreased mineral content observed in NPG than GLUT tissue. (b, c) Alizarin red stain for calcium shows increased calcium levels in (b) GLUT over (c) NPG tissue.
*Statistical difference between GLUT and NPG (p < 0.05, n = 6).
GAG and elastin stability was evaluated histologically using the Alcian blue and VVG stain, respectively. NPG implants showed higher GAG staining over GLUT tissues following 21 days subdermal implantation. Explanted NPG tissues clearly showed the presence of elastic fibers in the tissue in VVG stain while no elastin was present in the GLUT tissue (Figure 5(a)–(c)).
Figure 5.
Histological staining of tissue following three weeks subdermal implantation: (a, d) GLUT and (b, c, e) NPG. (a–c) VVG elastic stain and (d, e) Alcian blue stain for GAGs. Stains demonstrate improved elastin and GAG stability following implantation. Leaflet layers are labeled ventricularis (V), spongiosa (S) and fibrosa (F). Original magnification is × 100, except (c) which is × 400, arrows in (c) show black elastic fibers.
Discussion
GLUT has been used for valve fixation for many decades. Calcification is considered to be one of the major issues for valve failure and many treatments, such as amino-oleic acid and ethanol treatment, are currently available to prevent calcification in vivo. However, many valves also fail due to tissue degeneration in the absence of calcification and degradation of GAGs, and elastin may play a role in this failure. Stabilization of elastin in heart valve leaflets with chemical cross-linking has never been demonstrated. The main objective in this study was to investigate elastin stabilization in addition to GAG and collagen preservation in BHV leaflets. Our laboratory has developed multiple treatments to improve GAG stability in porcine heart valve leaflets, including the addition of neomycin,4,10,12–16 carbodiimide cross-linking to replace GLUT10,12,15,16,22 and periodate oxidation.22,23 None of these treatments demonstrated improved stabilization against elasteolytic degradation. Elastin plays a critical role in heart valve mechanics, providing elasticity to the tissue, and contributing to a low level of hysteresis via links with collagen.24 Elastin degradation may promote calcification in vivo;25 consequently, stabilization of elastin may improve resistance to calcium deposition.26
The primary mechanism of GLUT cross-linking utilizes amine functionalities in proteins. Native elastin contains a negligible concentration of amine-based residues, and therefore may not allow sufficient cross-linking.24 This inability of GLUT to cross-link elastin was previously shown in vitro by using tritium-labeled GLUT in attempts to cross-link pure elastin.28 GLUT was previously shown to be ineffective at stabilizing both pure elastin and elastin in porcine aortic wall by Isenburg et al.27,28 However, the introduction of tannic acid or PGG demonstrated that using polyphenols in conjunction with GLUT cross-linking shows a reduction in elasteolytic degradation in the aortic walls.27,28
In the present study, PGG treatment was tested in conjunction with neomycin pre-treatment and GLUT cross-linking to stabilize both GAGs and elastin in porcine aortic valve leaflet tissue. This stabilization was evaluated by measuring resistance to enzyme digestion following treatment over a range of PGG concentrations. We tested that such additional PGG cross-linking does not alter collagen and GAG stability in the tissue endowed with GLUT and neomycin (as shown previously).15 We re-evaluated ECM stability in tissues following 10 months of storage. We further evaluated if PGG cross-linking negatively affect materials properties, specifically thermal denaturation temperature and mechanical properties. Finally, an in vivo model was used to assess the calcification and ECM stability.
The incorporation of additional molecules (such as PGG) and cross-linking modalities can negatively affect cross-linking benefits via steric hindrance and competitive binding. Elastase digestion demonstrated PGG’s properties to effectively protect leaflet elastin from enzymatic degradation. The addition of PGG and neomycin showed a marked resistance to elastase or collagenase digestion compared to GLUT cross-linking, gravimetrically (Figure 1), histologically (Figure 2), and using zymography (data not shown). Polyphenols such as PGG have a high affinity for hydrophobic regions;17 therefore, they likely bind to the hydrophobic elastase cleavage sites in elastin. The high reduction in enzymatic degradation of elastin suggests this binding occurs.
GAG loss in BHVs may occur in two phases. The tissue preparation technique is critical to maintaining near-physiological GAG levels in porcine aortic valve leaflets.29 Despite minimized GAG loss during treatment, GAG loss may still occur due to storage, cyclic fatigue, or enzymatic degradation.7,8,12 Chondroitin sulfate and hyaluronic acid are the two primary GAG types present in heart valves.30 Therefore, an in vitro combination of chondroitinase and hyaluronidase was used to evaluate the resistance to enzymatic GAG loss. We have shown earlier that the addition of neomycin enhances GAG stabilization in BHVs,12 but how PGG addition will affect neomycin-based GAG stabilization was not previously investigated. Incorporation of PGG into neomycin and GLUT cross-linking demonstrated that PGG binding improved GAG stabilization achieved by neomycin alone, published elsewhere.12 This demonstrates that the combination of neomycin and PGG not only maintains GAG stabilization potential of neomycin in porcine aortic leaflets but also improves it probably due to synergistic effects of reactions with additional functional groups. Neomycin is a hyaluronidase inhibitor with multiple amine functionalities,15,31 while PGG has multiple phenolic hydroxyl groups.17 The presence of multiple functionalities for linkages to proteins presents that a synergistic effect may exist; however, we do not know the exact mechanisms of this synergistic effect. Collagen stability against enzymatic degradation was measured gravimetrically via collagenase digestion.
Collagen cross-linking of tissue was verified using DSC to measure the collagen thermal denaturation temperature (Td) of the tissue.32 The NPG group exhibited an increased collagen thermal denaturation temperature over GLUT, indicating additional cross-linking. PGG hydroxyl groups can also take part in GLUT cross-linking, thus increasing collagen stability. This is evidenced by the improvement in collagenase resistance following NPG treatment.
Cross-linking of tissue alters mechanical properties by strengthening interactions between ECM components. Elastic moduli in cross-linked heart valves can be defined in two regions, each affected primarily by certain proteins. The collagen interactions dominate the upper elastic modulus, while elastin interactions affect the lower elastic modulus. For the purpose of this study, the strain at which the transition between these responses occurs is defined as tissue extensibility.16 Excessive stiffness increase has been correlated with decreased durability, while decreased extensibility is also detrimental.33
Adding neomycin and PGG to GLUT treated tissue increased the lower elastic moduli, both in circumferential and in radial directions. This is due to elastin cross-linking; cross-links between elastic fibers increases tissue stiffness. The effect of this increase in lower modulus in ultimate durability of leaflets remains to be seen. Upper elastic moduli remained unchanged in the circumferential direction, but decreased in the radial direction when treated with NPG. This stiffness decrease may improve valve performance during opening and closing phases. Peak tensile stresses and extensibility remained unchanged. The higher elastin region moduli and unchanged extensibility suggests an increase in elastin region toughness. Future fatigue testing may elucidate this effect on durability. The data show that adding neomycin and PGG to GLUT treatment does not adversely affect uniaxial mechanical properties.
While the uniaxial tensile model can show one-dimensional biomechanics of valve tissue, the aortic valve is much more complex.34,35 Biaxial testing and further examination of molecular mechanics, while not performed in this study, would prove beneficial to understanding the cross-linking mechanisms and suitability for aortic valve material. Additionally, comparison to fresh (untreated) tissue would be important to further understand the effects of NPG cross-linking on valve tissue.
Rat subdermal implantation was carried out to test calcification and leaflet degeneration. Both tissues were treated with ethanol, which reduces calcification as shown earlier.20 NPG exhibited significantly lower calcium and phosphorous levels than GLUT-treated tissue. Ethanol treatment should have completely prevented calcification of both groups. GLUT cross-linked tissues after ethanol treatment in this study calcified. This is in contrast to what is reported in the literature. One reason could be the source of leaflets and ethanol in the present study. The calcification was almost completely prevented in NPG group; this can be an added advantage of NPG cross-linking.
The in vivo model was also used to characterize the preservation of ECM through histological staining. Histological examination of the tissue following implantation showed that both higher levels of GAGs and elastin were retained in the NPG tissue than GLUT. This demonstrates that the addition of neomycin and PGG preserves both GAGs and elastin during implantation.
Conclusion
An elastin-targeted chemical cross-linking treatment for use in porcine aortic valves which further stabilizes the ECM against enzymatic degradation over GLUT alone has been demonstrated. In vitro testing indicated extensive cross-linking which protected tissue against degradation by individual enzymes in a single-factor approach. Subdermal implantation demonstrated the novel treatment maintained resistance to enzyme degradation as well as the inhibition of mineralization. The combined results show a method which can stabilize all components of the ECM. Further optimization and investigation into this treatment may prove beneficial to long-term valve durability.
Acknowledgments
The authors wish to thank Snow Creek Meat Processing, Seneca, SC for supply of porcine aortic valves. They thank Mrs Linda Jenkins for histology facility support and Nitin Balakrishnan for assisting with mechanical testing.
Funding
This work was funded by NIHHL070969, HL108330 and P20GM103444.
Footnotes
Conflict of interest
None declared.
References
- 1.Schoen FJ, Levy RJ. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999;47(4):439–465. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Johansen P, Andersen TS, Hasenkam JM, et al. In-vivo prediction of cavitation near a Medtronic Hall valve. J Heart Valve Dis. 2004;13(4):651–658. [PubMed] [Google Scholar]
- 3.Vesely I. The evolution of bioprosthetic heart valve design and its impact on durability. Cardiovasc Pathol. 2003;12(5):277–286. doi: 10.1016/s1054-8807(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 4.Munnelly AE, Cochrane L, Leong J, et al. Porcine vena cava as an alternative to bovine pericardium in bioprosthetic percutaneous heart valves. Biomaterials. 2012;33(1):1–8. doi: 10.1016/j.biomaterials.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55(2):135–144. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee TC, Midura RJ, Hascall VC, et al. The effect of elastin damage on the mechanics of the aortic valve. J Biomech. 2001;34(2):203–210. doi: 10.1016/s0021-9290(00)00187-1. [DOI] [PubMed] [Google Scholar]
- 7.Lovekamp JJ, Simionescu DT, Mercuri JJ, et al. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006;27(8):1507–1518. doi: 10.1016/j.biomaterials.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyavahare N, Ogle M, Schoen FJ, et al. Mechanisms of bioprosthetic heart valve failure: fatigue causes collagen denaturation and glycosaminoglycan loss. J Biomed Mater Res. 1999;46(1):44–50. doi: 10.1002/(sici)1097-4636(199907)46:1<44::aid-jbm5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Mirnajafi A, Zubiate B, Sacks MS. Effects of cyclic flexural fatigue on porcine bioprosthetic heart valve heterograft biomaterials. J Biomed Mater Res A. 2010;94(1):205–213. doi: 10.1002/jbm.a.32659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah SR, Vyavahare NR. The effect of glycosaminoglycan stabilization on tissue buckling in bioprosthetic heart valves. Biomaterials. 2008;29(11):1645–1653. doi: 10.1016/j.biomaterials.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoen FJ. Cardiac valves and valvular pathology: update on function, disease, repair, and replacement. Cardiovasc Pathol. 2005;14(4):189–194. doi: 10.1016/j.carpath.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Raghavan D, Starcher BC, Vyavahare NR. Neomycin binding preserves extracellular matrix in bioprosthetic heart valves during in vitro cyclic fatigue and storage. Acta Biomater. 2009;5(4):983–992. doi: 10.1016/j.actbio.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghavan D, Shah SR, Vyavahare NR. Neomycin fixation followed by ethanol pretreatment leads to reduced buckling and inhibition of calcification in bioprosthetic valves. J Biomed Mater Res B Appl Biomater. 2010;92(1):168–177. doi: 10.1002/jbm.b.31503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friebe VM, Mikulis B, Kole S, et al. Neomycin enhances extracellular matrix stability of glutaraldehyde cross-linked bioprosthetic heart valves. J Biomed Mater Res B Appl Biomater. 2011;99(2):217–229. doi: 10.1002/jbm.b.31889. [DOI] [PubMed] [Google Scholar]
- 15.Raghavan D, Simionescu DT, Vyavahare NR. Neomycin prevents enzyme-mediated glycosaminoglycan degradation in bioprosthetic heart valves. Biomaterials. 2007;28(18):2861–2868. doi: 10.1016/j.biomaterials.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong J, Munnelly A, Liberio B, et al. Neomycin and carbodiimide crosslinking as an alternative to glutaraldehyde for enhanced durability of bioprosthetic heart valves. J Biomater Appl. 2011 doi: 10.1177/0885328211430542. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Isenburg JC, Karamchandani NV, Simionescu DT, et al. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials. 2006;27(19):3645–3651. doi: 10.1016/j.biomaterials.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Basalyga DM, Simionescu A, et al. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am J Pathol. 2006;168(2):490–498. doi: 10.2353/ajpath.2006.050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 20.Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation. 1997;95(2):479–488. doi: 10.1161/01.cir.95.2.479. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Schoen FJ, Levy RJ. Mechanism of efficacy of 2-amino oleic acid for inhibition of calcification of glutaraldehyde-pretreated porcine bioprosthetic heart valves. Circulation. 1994;90(1):323–329. doi: 10.1161/01.cir.90.1.323. [DOI] [PubMed] [Google Scholar]
- 22.Mercuri JJ, Lovekamp JJ, Simionescu DT, et al. Glycosaminoglycan-targeted fixation for improved bioprosthetic heart valve stabilization. Biomaterials. 2007;28(3):496–503. doi: 10.1016/j.biomaterials.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Lovekamp J, Vyavahare N. Periodate-mediated glycosaminoglycan stabilization in bioprosthetic heart valves. J Biomed Mater Res. 2001;56(4):478–486. doi: 10.1002/1097-4636(20010915)56:4<478::aid-jbm1119>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 24.Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31(2):115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 25.Bailey MT, Pillarisetti S, Xiao H, et al. Role of elastin in pathologic calcification of xenograft heart valves. J Biomed Mater Res A. 2003;66(1):93–102. doi: 10.1002/jbm.a.10543. [DOI] [PubMed] [Google Scholar]
- 26.Vyavahare N, Ogle M, Schoen FJ, et al. Elastin calcification and its prevention with aluminum chloride pretreatment. Am J Pathol. 1999;155(3):973–982. doi: 10.1016/S0002-9440(10)65197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isenburg JC, Simionescu DT, Vyavahare NR. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials. 2004;25(16):3293–3302. doi: 10.1016/j.biomaterials.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Isenburg JC, Simionescu DT, Vyavahare NR. Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials. 2005;26(11):1237–1245. doi: 10.1016/j.biomaterials.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Simionescu DT, Lovekamp JJ, Vyavahare NR. Degeneration of bioprosthetic heart valve cusp and wall tissues is initiated during tissue preparation: an ultrastructural study. J Heart Valve Dis. 2003;12(2):226–234. [PubMed] [Google Scholar]
- 30.Lowther DA, Preston BN, Meyer FA. Isolation and properties of chondroitin sulphates from bovine heart valves. Biochem J. 1970;118(4):595–601. doi: 10.1042/bj1180595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmen S, Hoechstetter J, Käsbauer C, et al. Sulphated oligosaccharides as inhibitors of hyaluronidases from bovine testis, bee venom and Streptococcus agalactiae. Planta Med. 2005;71(8):727–732. doi: 10.1055/s-2005-871255. [DOI] [PubMed] [Google Scholar]
- 32.Vyavahare NR, Hirsch D, Lerner E, et al. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998;40(4):577–585. doi: 10.1002/(sici)1097-4636(19980615)40:4<577::aid-jbm9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 33.Christie GW, Barratt-Boyes BG. Age-dependent changes in the radial stretch of human aortic valve leaflets determined by biaxial testing. Ann Thorac Surg. 1995;60(2 Suppl.):S156–S158. doi: 10.1016/0003-4975(95)00219-b. discussion S159. [DOI] [PubMed] [Google Scholar]
- 34.Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng. 2007;129(5):757–766. doi: 10.1115/1.2768111. [DOI] [PubMed] [Google Scholar]
- 35.Sacks MS, David Merryman W, Schmidt DE. On the biomechanics of heart valve function. J Biomech. 2009;42(12):1804–1824. doi: 10.1016/j.jbiomech.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]