Abstract
Oral prescription drugs are an increasingly important treatment option for cancer. Yet contemporaneous US trends in spending on anticancer drugs known as oral oncologics have not been described. Using nationally representative data, we describe trends in national spending on and use of forty-seven oral oncologics between the first quarter of 2006 and the third quarter of 2011. Average quarterly national spending on oral oncologics increased 37 percent, from $940.3 million to $1.4 billion in 2012 dollars, a significant change. Average quarterly use of oral oncologics in the same time period measured in extended units increased at a significant pace but more slowly than spending (10 percent). Within this broader trend, differences in spending among categories of oral oncologics were observed. High levels of and increases in both spending and use were concentrated among new brand-name and patent-protected oral oncologics, including second-generation tyrosine kinase inhibitors used to treat chronic myelogenous leukemia. Decreased spending but increased use was observed among oral oncologics that lost patent protection during the study period and were available in generic form, including hormonal therapies used to treat breast and prostate cancers. Spending on new and patent-protected oral oncologics and associated price increases are significant drivers of increased spending.
Rapid progress in the fields of tumor biology, genetics, and immunology has spurred the development of many novel cancer treatments in the past two decades.1,2 Anticancer drugs, known as “oncologics,” are often the only life-prolonging treatment available for patients with blood cancers and solid tumor cancers, where tumors have spread beyond their original location to a nonadjacent site in the body.
In 2010, $125 billion was spent in the United States on cancer treatment, which accounted for approximately 5 percent of that year’s total medical care spending.2 National spending on oncologics alone is also significant. Approximately $23 billion was spent on them in 2011.3 Oncologics rank first in terms of national spending on prescription drugs by therapeutic class.4
Total manufacturers’ sales of specialty drugs, including oncologics, have also grown significantly in recent years, with a 9.5 percent increase in 2006 and an 8.9 percent increase in 2007.3,4 This growth has outpaced that of all pharmaceutical manufacturers’ sales (a 1.6 percent increase in 2007 and a 1 percent decline in 2012) and that of recent medical spending (approximately 3 percent in the period 2009–11).4,5
In 2011, 77 percent of national spending on oncologics was on those administered to patients in specialty medical offices and hospital outpatient departments. For these drugs, Medicare was the largest payer under beneficiaries’ outpatient medical benefits, known as Part B (all references to Medicare in this article are to the fee-for-service program).6–8 Most commercial insurers follow Medicare’s coverage and reimbursement policies for these drugs.
Several factors have likely contributed to the high and increasing spending on oncologics overall.3,4,8–10 These factors include the increasing incidence of cancer in the US population, the introduction of new brand-name oncologics and the virtually guaranteed coverage of them by insurers after approval by the Food and Drug Administration (FDA), insurance reimbursement policies that encourage the use of brand-name oncologics, and postlaunch price increases among some brand-name and generic oncologics.
There are two reasons why quantifying national spending on and use of oral oncologics, a subset of all oncologics, may be of interest to policy makers.
First, the Medicare Modernization Act enacted in 2003 and implemented in 2006 created a new pharmacy benefit (Part D) that included coverage within Medicare for most oral oncologics. This new coverage significantly expanded reimbursement by third-party payers for oral oncologics. Before the implementation of Part D, Medicare covered—under the Part B outpatient benefit—only oral oncologics that were reformulations of older parenteral (that is, infused or injected) drugs. According to a recent report by the leading pharmacy benefit manager, Express Scripts, oncologics currently rank first in spending among drugs covered under third-party payer pharmacy benefits.11
Second, several notable oral oncologics have recently been launched to treat highly prevalent cancers.3,4,6,10,11 For example, a number of new oral oncologics target tumors with specific genetic profiles. These drugs include the second-generation tyrosine kinase inhibitors nilotinib (Tasigna) and dasatinib (Sprycel), which are used for the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors.
Despite their clinical and increasing economic importance, national trends in spending on and use of oral oncologics have not been described. Using nationally representative data, we examined levels of spending on, and trends in the use of, oral oncologics between the first quarter of 2006 and the third quarter of 2011. We decomposed these levels and trends using the following categories: the oncologics’ therapeutic class, patent protection status, and coverage under Medicare’s Part D or Part B benefit.
Study Data And Methods
DATA
We used data from IMS Health’s National Sales Perspectives (NSP) that covered the period between the first quarter of 2006 and the third quarter of 2011. IMS Health is a firm that provides detailed data on domestic and global pharmaceutical sales and use. These data have been used in numerous studies of pharmaceutical spending and use.3,4
The data were derived from an audit that described 100 percent of the national unit volume and dollar sales for every major class of trade and distribution channel for US prescription pharmaceuticals. The data provide information on the molecule-specific chemical names and brand names, route of administration (oral versus injected, infused, inhaled, topical, or other), and World Health Organization Anatomic Therapeutic Classification designation.12 Drugs were considered oncologics if they had an L designation, which is given to “preparations used in the treatment of malignant neoplastic diseases and immunomodulating agents.”13 Our reliance on this classification precluded us from examining spending levels and trends related to three commonly used oral oncologics: raloxifene, lenalidomide, and thalidomide.
In the NSP, spending measures the amount of money that retail pharmacies, mail-order pharmacies, nonfederal hospitals, long-term care facilities, and miscellaneous facilities spend to acquire a drug from manufacturers and drug wholesalers. The prices reflected in this sales measure are the actual invoice prices that these outlets pay for drugs, whether they are purchased directly from a manufacturer or indirectly via a wholesaler. Invoice line-item discounts are included, but prompt-payment discounts and invoice bottom-line discounts are not included. Rebates, which are typically paid by the manufacturer directly to a customer, insurer, or pharmacy benefit manager, are not included. To compare spending levels across study time periods, we converted spending in all quarters to 2012 US dollars using the Consumer Price Index inflation calculator.
Use was measured using the NSP variable known as extended units. According to IMS Health, extended units measure the number of single items (such as vials, syringes, bottles, or packets of tablets or capsules) that are contained in a shipping package purchased by providers and pharmacies. For example, a package containing a month’s supply of an oral drug is considered to consist of one extended unit. In this clinical context, a package may also include several different doses and strengths of a given drug.
ANALYSES
We used ordinary least squares regression to examine the relationship between national utilization and inflation-adjusted spending and the passage of time. This approach helped account for potential correlation in these outcomes over time, which is the main advantage over the use of Student t-tests to detect level changes.
We report estimated p values to examine the significance of trend changes. Changes were considered significant if the estimated p values were equal to or less than 0.05.
To examine factors related to these levels and trends, each oncologic in our sample was further categorized. The first category was the oncologic’s therapeutic class. To classify oncologics using this category, we relied on information in the drug’s FDA-approved label, which was reviewed by one of the authors (Sumita Bhatta), who has clinical expertise.14,15
The therapeutic class measures we used were alkylating agents, topoisomerase inhibitors, antimetabolites, hormonal agents, targeted agents, and other. Briefly, alkylating agents in cancer treatment are chemical compounds that inhibit cell division and growth. Topoisomerase inhibitors interfere with enzymes that control changes in DNA structure. Antimetabolites are chemicals that interfere with DNA production, affecting cell division and tumor growth. Hormonal agents manipulate the endocrine system, and targeted agents target specific genetic pathways to interrupt normal cell functioning. More detailed definitions of these measures are provided in Exhibit 1.
Exhibit 1.
Characteristics Of Forty-Seven Oral Oncologics Available In The US Market, First Quarter 2006 Through Third Quarter 2011
| Category | Oncologics
|
|
|---|---|---|
| Number | Percent | |
| THERAPEUTIC CLASS | ||
|
| ||
| Alkylating agentsa | 9 | 19 |
| Topoisomerase inhibitorsb | 2 | 4 |
| Antimetabolitesc | 5 | 11 |
| Hormonal agentsd | 12 | 26 |
| Targeted agentse | 14 | 32 |
| Other | 6 | 131 |
|
| ||
| PATENT PROTECTION STATUS DURING STUDY PERIOD | ||
|
| ||
| Patent protected throughout | 22 | 47 |
| Lost patent protection | 5 | 11 |
| Generic throughout | 20 | 43 |
|
| ||
| FEE-FOR-SERVICE MEDICARE OUTPATIENT COVERAGE | ||
|
| ||
| Medical benefit (Part B) | 9 | 19 |
| Pharmacy benefit (Part D) | 38 | 81 |
SOURCE Authors’ analysis of data from IMS Health’s National Sales Perspectives and the Food and Drug Administration’s approved drug labels.
Agents that cause the replacement of hydrogen by an alkyl group, especially in a biologically important molecule. These agents have mutagenic activity to inhibit cell division and growth.
Agents that block the ligation step of the cell cycle, generating single- and double-stranded breaks that harm the integrity of the genome. Introduction of these breaks leads to apoptosis and cell death.
Agents used to interfere with DNA production and therefore cell division and the growth of tumors. Because cancer cells spend more time dividing than other cells, inhibiting cell division harms cancer cells more than other cells.
Agents that manipulate the endocrine system by adding exogenous-specific hormones, particularly steroid hormones, or by inhibiting the production or activity of such hormones (hormone antagonists). Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing or undergo cell death.
Includes agents that target specific genetic pathways, interrupting normal cell functioning such as cell division.
The second category used to classify oral oncologics was patent protection status. We classified oncologics by whether the brand-name drug had lost patent protection before 2006 and became available in generic form, experienced loss of patent protection and generic entry between the first quarter of 2006 and the second quarter of 2011, or had patent protection throughout the study period. For oral oncologics that lost patent protection before or during the study period, we report quarterly spending on and use of all versions of the same chemical entity (both generic and brand-name versions and all generic versions of the drug, regardless of manufacturer).
The third category we used to classify oral oncologics was whether they were covered by Medicare Part B (that is, whether they were oral equivalents of parenteral formulations) or by Medicare Part D. We used Medicare data from the 2005–12 average sales price drug pricing files to perform this classification.16
For each category, we report summary descriptive statistics. We report national quarterly levels and trends in percentage terms (as a function of total spending and use) to examine compositional levels and trends. We also report p values derived from a chi-square test of average quarterly percentages of total spending and use observed in each category for 2006 compared to the period between the fourth quarter of 2010 and the third quarter of 2011.17
All analyses were performed using the statistical software Stata, version 11.2.
Study Results
DESCRIPTIVE STATISTICS
Forty-seven oral oncologics were available during the study period (Exhibit 1). For each oncologic in the sample, the online Appendix18 gives chemical name, brand name, therapeutic class, FDA approval date (month and year), and FDA-approved cancer indication(s).
The majority of oncologics in the sample were targeted (30 percent), hormonal (26 percent), and alkylating agents (19 percent) (Exhibit 1).
Forty-three percent of the oncologics studied were available throughout the period as generics. The majority of oncologics in this category were alkylating or hormonal agents. Another forty-five percent of the oncologics were available only in brand-name form throughout the study period. More than half of these were introduced into the US market in 2007 or later.
Eleven percent of the oncologics in the sample lost patent protection during the study period and became available in generic form. All of the generic drugs except for one (Vesanoid, an “other” agent) were hormonal agents used to treat breast and prostate cancers.
Eighty-one percent of the oncologics in the sample—including all of the oncologics that lost patent protection during the study period—were covered under Part D. The remaining 19 percent were covered under Part B. The majority of Part B drugs were available in generic form throughout the study period.
NATIONAL SPENDING AND USE
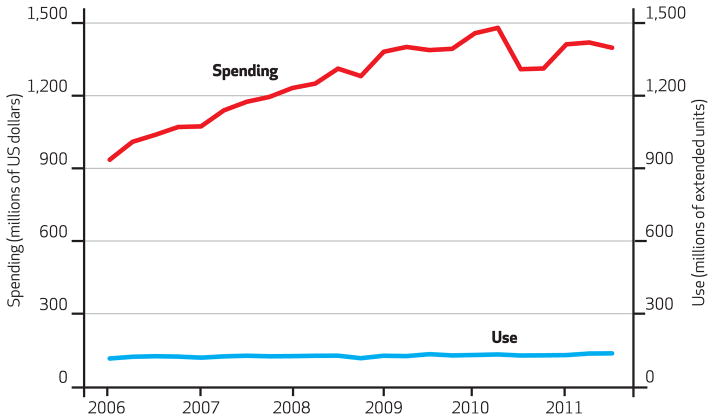
Quarterly national spending on oral oncologics increased 37 percent during the study period from $940.3 million to $1.4 billion, a significant change. The estimated average quarterly increase in national spending on oral oncologics was $20 million during the study period (Exhibit 2).
Exhibit 2.
Spending On And Use Of Oral Oncologics Available In The US Market, First Quarter 2006 Through Third Quarter 2011
SOURCE Authors’ analysis of data from IMS Health’s National Sales Perspectives. NOTES The red line denotes spending and relates to the left-hand y axis. The blue line denotes use and relates to the right-hand y axis. Extended units measure the number of single items (such as vials, syringes, bottles, or packets of tablets or capsules) that are contained in a shipping package purchased by providers and pharmacies. For example, a package containing a month’s supply of an oral drug is considered to be one extended unit.
National use of oral oncologics also increased significantly during the study period (Exhibit 2), albeit at a much lower average quarterly rate (10 percent), compared to spending.
CORRELATES OF NATIONAL SPENDING AND USE
Exhibit 3 shows national spending levels and trends for the oral oncologics classified by category. In 2006 average quarterly national spending was concentrated on hormonal agents (which accounted for approximately 42 percent of total spending), targeted agents (35 percent), antimetabolites (11 percent), and alkylating agents (10 percent). In 2011 average quarterly national spending was concentrated on targeted agents (59 percent), hormonal agents (19 percent), antimetabolites (12 percent), and alkylating agents (8 percent).
Exhibit 3.
Average Quarterly Spending On And Use Of Oral Oncologics Available In The US Market, First Quarter 2006 Through Third Quarter 2011
| Category | Estimated percent
|
|||||
|---|---|---|---|---|---|---|
| Spending ($US 2012)
|
Use (extended units)
|
|||||
| Q1–4 2006 average | Q4 2010–Q3 2011 average | Change | Q1–4 2006 average | Q4 2010–Q3 2011 average | Change | |
| THERAPEUTIC CLASS | ||||||
|
| ||||||
| Alkylating agents | 10.00 | 7.63 | −23.67 | 3.31 | 2.10 | −36.63 |
| Topoisomerase inhibitors | 0.46 | 0.50 | 7.88 | 0.09 | 0.07 | −22.80 |
| Antimetabolites | 11.12 | 12.18 | 9.54 | 18.28 | 17.96 | −1.76 |
| Hormonal agents | 41.82 | 19.13 | −54.26 | 73.04 | 72.79 | −0.34 |
| Targeted agents | 35.26 | 59.32 | 68.26 | 4.41 | 6.32 | 43.11 |
| Other | 1.35 | 1.25 | −7.80 | 0.86 | 0.76 | −11.31 |
|
| ||||||
| PATENT PROTECTION STATUS DURING STUDY PERIOD | ||||||
|
| ||||||
| Patent protected throughout | 45.30 | 72.87 | 60.86 | 11.65 | 15.11 | 29.61 |
| Lost patent protection | 37.38 | 13.19 | −64.72 | 26.39 | 30.64 | 16.12 |
| Generic throughout | 7.41 | 3.26 | −56.01 | 61.96 | 54.36 | −12.43 |
|
| ||||||
| FEE-FOR-SERVICE MEDICARE OUTPATIENT COVERAGE | ||||||
|
| ||||||
| Medical benefit (Part B) | 18.95 | 18.51 | −2.33 | 7.62 | 6.84 | −10.34 |
| Pharmacy benefit (Part D) | 81.05 | 81.49 | 0.54 | 92.38 | 93.16 | 0.85 |
Exhibit 3 also shows average quarterly national use levels and trends. Similar to 2006 spending levels, average quarterly national use levels of hormonal agents (73 percent of the total) and antimetabolites (18 percent) in 2006 were significant. The percentages of total quarterly use and rank of the drug classes in terms of those percentages remained relatively stable between 2006 and 2011. There was a significant increase in the use of targeted agents: a change of 43 percent between 2006 and the period between September 2010 and September 2011.
Perhaps not surprisingly, spending throughout the study period appears to have been concentrated on oral oncologics that either were always patent protected or lost patent protection during the study period (Exhibit 3). Interestingly, use throughout the study period was concentrated on generic oncologics and oncologics that lost patent protection during that period.
Among patent-protected oncologics, we estimated that average quarterly spending increased 61 percent and average quarterly use increased 30 percent between 2006 and the period between September 2010 and September 2011. Among oral oncologics that lost patent protection, we estimated that average quarterly spending decreased 65 percent and average quarterly use increased 16 percent during the same time. For generic oral oncologics, we observed a very large decline (56 percent) in estimated quarterly spending and a smaller decline in estimated quarterly use (12 percent).
Spending on and use of oral oncologics covered under Medicare Part D significantly outweighed spending on and use of oral oncologics covered under Part B (Exhibit 3). We estimated that average quarterly national spending on oral oncologics covered under Part B declined 2.3 percent during the study period and that their use declined 10 percent. We estimated small but significant increases in spending on and use of oral oncologics covered under Part D.
Discussion
This study is the first to report contemporaneous national levels and trends in spending on oral oncologics. We found that in 2011, $1.4 billion (in 2012 dollars) was spent per quarter on oral oncologics. Average quarterly spending increases on the oral oncologics in the sample were also significant (37 percent). National spending on oral oncologics observed during the study period significantly outpaced spending on all prescription drugs and medical care generally.3–5,19 Notably, between 2007 and 2012 the rate of spending growth on all prescription drugs was much lower than earlier in the decade.4
Our results suggest that spending levels and trends are driven by the use of new brand-name oral oncologics. Many of these drugs represent significant therapeutic advances over the standard of care for the treatment of specific cancers.20 For example, spending throughout the study period was observed to be concentrated on targeted agents—notably, the tyrosine kinase inhibitors, which provide dramatic survival benefit in patients with chronic myelogenous leukemia21–25 or gastrointestinal stromal tumors26–29 and decreased toxicity in patients with renal cell carcinoma,30–34 compared to conventional chemotherapy or cytokine-mediated therapies. However, data limitations prevented us from correlating spending and utilization levels and trends according to guideline-consistent indications, including those that may not be approved by the FDA.35
We also found that national spending on and use of oral oncologics was concentrated among those covered under Medicare’s (and likely most commercial insurers’) pharmacy benefits. Interestingly, our results suggest that price increases among brand-name oncologics covered under Medicare Part D may be the most important driver of spending trends, if use is held constant. This finding is consistent with the results of a recent government report that focused solely on parenteral oncologics covered under Medicare Part B.36
These price findings are also noteworthy because of current coverage and reimbursement policies that govern Part D benefits. Commercial insurers, including those that provide Part D coverage for prescription drugs, may have some negotiating power over pricing levels and consequently over spending and use trends.8 Yet this bargaining power may be most efficacious for drugs with brand-name or generic substitutes.37 Bargaining on prices by these commercial insurers may also be limited since they are required by statute to cover all drugs in six protected classes, including oral oncologics.38
We believe that this study is also the first to report empirical evidence of spending decreases, utilization increases, and therefore price declines (spending declines that outstrip utilization increases) among generic oral oncologics that lost patent protection and experienced generic entry during the study period. Specifically, among oral oncologics that lost patent protection during the study period, we estimated that average quarterly spending decreased 65 percent, while average quarterly use increased 16 percent between 2006 and the period between September 2010 and September 2011. This spending result is consistent in magnitude with the findings of previous empirical studies of spending trends on nononcologic oral drugs after the entry of a generic drug into the market.39
Our use results suggest that there may be increasing use of oral oncology drugs, particularly those covered by Part D, after their patents expire. This observation is consistent with the results of a recent economic analysis of use trends after generic entry into the market among specialty drugs, including but not limited to oral oncologics.40 We also found significant use of generic oncologics throughout the study period. This result is consistent with national studies of drug usage generally.3,4,37
Conclusion
The potential clinical benefits of using the oral oncologics discussed in this article must be weighed against their financial costs for patients. Many commercial insurers do not require substantial patient cost sharing for physician-administered drugs.41 However, insurers may impose high cost sharing for oral oncologics.42 Since 2007 twenty-six states have passed laws requiring insurers to offer patients cost-sharing parity between oral and physician-administered oncologics. Whether and how these policies affect clinical outcomes and spending are important topics for future research.
Acknowledgments
The work of Rena M. Conti was funded by the National Cancer Institute (Grant No. K07 CA138906). The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript for publication. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from IMS Health. Conti had full access to all of the data in the study and takes responsibility for the integrity of the data analysis.
Contributor Information
Rena M. Conti, Email: rconti@uchicago.edu, Assistant professor of health policy and economics in the Departments of Pediatrics and Health Studies at the University of Chicago, in Illinois
Adam J. Fein, President of Pembroke Consulting, Inc., in Philadelphia, Pennsylvania
Sumita S. Bhatta, Instructor in the Department of Medicine, Section of Hematology and Oncology, the University of Chicago
NOTES
- 1.Lakdawalla DN, Sun EC, Jena AB, Reyes CM, Goldman GP, Phillipson TJ. An economic evaluation of the war on cancer. J Health Econ. 2010;29(3):333–46. doi: 10.1016/j.jhealeco.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IMS Institute for Healthcare Informatics. Declining medicine use and costs: for better or worse? A review of the use of medicines in the United States in 2012. Falls Church (VA): The Institute; 2013. May, [Google Scholar]
- 4.Aitken M, Berndt ER, Cutler DM. Prescription drug spending trends in the United States: looking beyond the turning point. Health Aff (Millwood) 2009;28(1):w151–60. doi: 10.1377/hlthaff.28.1.w151. [DOI] [PubMed] [Google Scholar]
- 5.Ryu AJ, Gibson TB, McKellar MR, Chernew ME. The slowdown in health care spending in 2009–11 reflected factors other than the weak economy and thus may persist. Health Aff (Millwood) 2013;32(5):835–40. doi: 10.1377/hlthaff.2012.1297. [DOI] [PubMed] [Google Scholar]
- 6.Government Accountability Office. Medicare: high-expenditure Part B drugs [Internet] Washington (DC): GAO; 2012. Oct 12, [cited 2014 Aug 7]. Available from: http://www.gao.gov/assets/650/649459.pdf. [Google Scholar]
- 7.Genentech. The 2012 Genentech oncology trend report: perspectives from managed care, specialty pharmacy providers, oncologists, practice managers, and employers [Internet] South San Francisco (CA): Genentech; [cited 2014 Aug 7]. Available from: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=16168. [Google Scholar]
- 8.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–33. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson M, Earle CC, Price M, Newhouse JP. How Medicare’s payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Aff (Millwood) 2010;29(7):1391–9. doi: 10.1377/hlthaff.2009.0563. [DOI] [PubMed] [Google Scholar]
- 10.Conti RM, Rosenthal MB, Polite BN, Bach PB, Shi YC. Infused chemotherapy use in the elderly after patent expiration. Am J Manag Care. 2012;18(5):e173–8. [PubMed] [Google Scholar]
- 11.Specialty trend forecast: cancer [Internet] St. Louis (MO): Express Scripts; Express Scripts. [cited 2014 Aug 7]. Available from: http://lab.express-scripts.com/drug-trend-report/trend-forecast/forecast-specialty-therapy-classes/forecast-3-cancer. [Google Scholar]
- 12.World Health Organization. The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD) Geneva: WHO; [cited 2014 Aug 7]. Available from: http://www.who.int/classifications/atcddd/en/ [Google Scholar]
- 13.World Health Organization. WHO Collaborating Centre for Drug Statistics Methodology [Internet] Geneva: WHO; [last updated 2013 Dec 19; cited 2014 Aug 13]. Available from: http://www.whocc.no/atc_ddd_index/?code=L&showdescription=yes. [Google Scholar]
- 14.Food and Drug Administration. Approved drug products with therapeutic equivalence evaluations. 34. Silver Spring (MD): FDA; 2014. [Internet] [cited 2014 Aug 25]. Available from: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm071436.pdf. [Google Scholar]
- 15.Food and Drug Administration. Drugs@FDA [Internet] Silver Spring (MD): FDA; [cited 2014 Aug 25]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. [Google Scholar]
- 16.CMS.gov. ASP drug pricing files [Internet] Baltimore (MD): Centers for Medicare and Medicaid Services; 2013. [last modified 2014 Jun 11; cited 2014 Aug 7]. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2013ASPFiles.html. [Google Scholar]
- 17.Dougherty C. Introduction to econometrics. 3. New York (NY): Oxford University Press; 2007. p. 194. [Google Scholar]
- 18.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 19.Martin AB, Lassman D, Washington B, Catlin A National Health Expenditure Accounts Team. Growth in US health spending remained slow in 2010; health share of gross domestic product was unchanged from 2009. Health Aff (Millwood) 2012;31(1):208–19. doi: 10.1377/hlthaff.2011.1135. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Cancer trends progress report—2011/2012 update [Internet] Bethesda (MD): NCI; 2012. Aug, [cited 2014 Aug 7]. Available for download from: http://progressreport.cancer.gov/ [Google Scholar]
- 21.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–42. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 22.Apperley JF, Cortes JE, Kim DW, Roy L, Roboz GJ, Rosti G, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START A trial. J Clin Oncol. 2009;27(21):3472–9. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 24.Hess G, Bunjes D, Siegert W, Schwerdtfeger R, Ledderose G, Wassmann B, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol. 2005;23(30):7583–93. doi: 10.1200/JCO.2005.01.3110. [DOI] [PubMed] [Google Scholar]
- 25.Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome–positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–6. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 26.Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with un-resectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26(4):620–5. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 27.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 28.Kindler HL, Campbell NP, Wroblewski K, Maki RG, D’Adamo DR, Chow WA, et al. Sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): final results of a University of Chicago phase II consortium trial. J Clin Oncol. 2011;29(15 Suppl):10009. [Google Scholar]
- 29.Sawaki A, Nishida T, Doi T, Yamada Y, Komatsu Y, Kanda T, et al. Phase 2 study of Nilotinib as third-line therapy for patients with gastrointestinal stromal tumor. Cancer. 2011;117(20):4633–41. doi: 10.1002/cncr.26120. [DOI] [PubMed] [Google Scholar]
- 30.Castellano D, del Muro XG, Pérez-Gracia JL, González-Larriba JL, Abrio MV, Ruiz MA, et al. Patient-reported outcomes in a phase III, randomized study of sunitinib versus interferon-{alpha} as first-line systemic therapy for patients with metastatic renal cell carcinoma in a European population. Ann Oncol. 2009;20(11):1803–12. doi: 10.1093/annonc/mdp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cella D, Michaelson MD, Bushmakin AG, Cappelleri JC, Charbonneau C, Kim ST, et al. Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon-alpha in a phase III trial: final results and geographical analysis. Br J Cancer. 2010;102(4):658–64. doi: 10.1038/sj.bjc.6605552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28(3):475–80. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 33.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 34.Amato RJ, Jac J, Giessinger S, Saxena S, Willis JP. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer. 2009;115(11):2438–46. doi: 10.1002/cncr.24280. [DOI] [PubMed] [Google Scholar]
- 35.Conti RM, Bernstein AC, Villaflor VM, Schilsky RL, Rosenthal MB, Bach PB. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol. 2013;31(9):1134–9. doi: 10.1200/JCO.2012.42.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Government Accountability Office. Brand-name prescription drug pricing: lack of therapeutically equivalent drugs and limited competition may contribute to extraordinary price increases [Internet] Washington (DC): GAO; 2009. Dec, [cited 2014 Aug 8]. Available from: http://www.gao.gov/new.items/d10201.pdf. [Google Scholar]
- 37.Aitken ML, Berndt ER. Medicare Part D at age five: what has happened to seniors’ prescription drug prices? [Internet] Parsippany (NJ): IMS Institute for Healthcare Informatics; 2011. Jul, [cited 2014 Aug 21]. Available from: http://www.imshealth.com/ims/Global/Content/Home%20Page%20Content/IMS%20News/IHII_Medicare_Part_D2.pdf. [Google Scholar]
- 38.EMD Serono. EMD Serono specialty digest. 10. Darmstadt (Germany): EMD Serono; [serial on the Internet] [cited 2014 Aug 18; login required]. Available from: http://specialtydigest.emdserono.com. [Google Scholar]
- 39.Aitken ML, Berndt ER, Bosworth B, Cockburn IM, Frank R, Kleinrock M, et al. The regulation of prescription drug competition and market responses: patterns in prices and sales following loss of exclusivity [Internet] Cambridge (MA): National Bureau of Economic Research; 2013. Oct, [cited 2014 Aug 18]. (NBER Working Paper No. 19487). Available from: http://www.nber.org/papers/w19487.pdf. [Google Scholar]
- 40.Conti RM, Berndt ER. Specialty drug prices and utilization after loss of US patent exclusivity, 2001–2007 [Internet] Cambridge (MA): National Bureau of Economic Research; 2014. Mar, [cited 2014 Aug 18]. (NBER Working Paper No. 20016). Available from: http://www.nber.org/papers/w20016.pdf. [Google Scholar]
- 41.Banegas MP, Yabroff KR. Out of pocket, out of sight? An unmeasured component of the burden of cancer. J Natl Cancer Inst. 2013;105(4):252–3. doi: 10.1093/jnci/djs641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–11. doi: 10.1200/JCO.2013.52.9123. [DOI] [PubMed] [Google Scholar]