Abstract
Objectives
Smoking during pregnancy is causally associated with many adverse health outcomes. Quitting smoking, even late in pregnancy, improves some outcomes. Among adults in general and reproductive-aged women, we sought to understand knowledge and attitudes towards prenatal smoking and its effects on pregnancy outcomes.
Methods
Using data from the 2008 HealthStyles© survey, we assessed knowledge and attitudes about prenatal smoking and smoking cessation. We classified respondents as having high knowledge if they gave ≥5 correct responses to 6 knowledge questions regarding the health effects of prenatal smoking. We calculated frequencies of correct responses to assess knowledge about prenatal smoking and estimated relative risk (RR) to examine knowledge by demographic and lifestyle factors.
Results
Only 15% of all respondents and 23% of reproductive-aged women had high knowledge of the adverse effects of prenatal smoking on pregnancy outcomes. Preterm birth and low birth weight were most often recognized as adverse outcomes associated with prenatal smoking. Nearly 70% of reproductive-aged women smokers reported they would quit smoking if they became pregnant without any specific reasons from their doctor. Few respondents recognized the benefits of quitting smoking after the first trimester of pregnancy.
Conclusions
Our results suggest that many women lack knowledge regarding the increased risks for adverse outcomes associated with prenatal smoking. Healthcare providers should follow the recommendations provided by the American Congress of Obstetricians and Gynecologists, which include educating women about the health risks of prenatal smoking and the benefits of quitting. Healthcare providers should emphasize quitting smoking even after the first trimester of pregnancy.
Keywords: pregnancy, knowledge, attitudes, smoking, adverse outcomes
Introduction
Smoking during pregnancy is associated with increased risk for numerous maternal, fetal, and infant complications, including infertility (1–3), placenta previa (4–7), placental abruption (5–9), spontaneous abortion (10–12), preterm birth (7, 11, 13–18), low birth weight (11, 13–15, 18), intrauterine growth retardation (14, 18), stillbirth (7, 19), sudden infant death syndrome (SIDS) (11, 15, 20–22), and orofacial clefts (birth defects of the upper lip and mouth) (23–26). While quitting smoking before conception is optimal because some effects of smoking, such as increased risk for orofacial clefts, occur in early pregnancy, smoking cessation later in pregnancy has been shown to improve a number of pregnancy outcomes, including birth weight (27–30).
Although the harmful effects of smoking during pregnancy on maternal, fetal, and infant health are well-recognized, about 12–21% of U.S. women smoke during pregnancy (31–33). Previous studies have identified many factors associated with smoking during pregnancy, including younger maternal age, lower education level, single marital status, low socioeconomic status, high parity, second hand smoke exposure in the home, smoking behavior of the woman’s social network, younger age when smoking was initiated, increased level of addiction, and increased stress (32, 34–40). Research by Ershoff and colleagues suggested that women who had low intentions to quit smoking were less convinced that smoking was harmful to their pregnancy compared with women who had high intentions to quit (41). Pregnant women who had low intentions to quit smoking were also more likely to have relatives and friends who smoked compared with pregnant women smokers who had higher intentions to quit smoking (41). Understanding common knowledge and attitudes among adults, in general, toward smoking during pregnancy is important because partners, family members, and colleagues of pregnant women may influence their smoking behavior (38, 42–44). Although much has been published on trends and patterns of smoking during pregnancy (45–47), data are limited regarding knowledge and attitudes of adults in the United States towards smoking during pregnancy.
Our objectives were to examine the knowledge and attitudes of adults, in general, and women of reproductive age, specifically, toward smoking during pregnancy, smoking cessation, and the effects of smoking and quitting smoking on pregnancy outcomes. Results from this study can be used to guide the development of future messages aimed at decreasing the prevalence of smoking during pregnancy.
Methods
Study Design
Four questions focused on prenatal smoking and smoking cessation were added to the Porter Novelli 2008 HealthStyles© survey, an annual postal mail survey, which is designed to collect data on health-related knowledge and behavior and is distributed to a sample of adults 18 years or older in the United States. The survey is conducted for Porter Novelli by Synovate, Inc., a global marketing and research firm. The HealthStyles© survey is sent as a follow-up to the ConsumerStyles© survey, which collects data on media habits, product use, services, and personal interests. For the 2008 ConsumerStyles© survey, a stratified random sample (balanced on region, household income, population density, age, and household size) of 20,000 consumers in the United States was drawn from an existing consumer mail panel of 340,000 potential respondents previously recruited to participate in consumer marketing surveys (48). Low-income and minority households were oversampled to ensure adequate representation from these groups. The response rate for the 2008 ConsumerStyles© survey was 50.5% (10,108 households) (48). From the pool of participants that completed the 2008 ConsumerStyles© survey, 7,000 households were selected to receive the 2008 HealthStyles© survey (49–51). More details on the survey are described elsewhere (49–52). The CDC licensed the results of the 2008 HealthStyles survey from Porter Novelli after data collection was complete, and analysis of these data was exempt from institutional review board approval because personal identifiers were not included in the data file.
To create a sample with a demographic breakdown similar to that of the U.S. population and account for non-response among HealthStyles© survey participants, data were post-stratified and weighted to the U.S. Census Current Population Survey on five variables: gender, age, income, race/ethnicity, and household size.
Study variables
Four questions focused on prenatal smoking and smoking cessation were added to the 2008 HealthStyles© survey. Two questions were added to the section for female respondents only: the first assessed current smoking status; if respondents marked yes, the second question asked smokers what messages from a doctor might influence them to quit smoking if, hypothetically, they were thinking about becoming pregnant (Questions #1 & #2, Table 1). Two additional questions were included in the section for all respondents: the first assessed knowledge of the possible consequences of smoking during pregnancy and the second asked respondents if there are health benefits of smoking cessation at different points before and during pregnancy (Questions #3 & #4, Table 1).
Table 1.
Questions related to smoking during pregnancy in the 2008 Healthstyles© Survey
| FOR WOMEN ONLY | ||
| 1. | Do you currently smoke cigarettes? | |
| Yes □ → (Go to the Question below) No □ →(Skip the Question below) | ||
| 2. | If you were thinking about becoming pregnant, what would your doctor or health professional need to say to you before you would consider quitting smoking? (“X” ALL THAT APPLY) | |
| My doctor would not have to give any specific reason—I would quit smoking | □ | |
| Smoking before pregnancy makes it harder to become pregnant | □ | |
| Smoking during pregnancy increases the chance of having a miscarriage | □ | |
| Smoking during pregnancy increases the chance of having a baby born too small or born too early | □ | |
| Smoking during pregnancy increases the chance of a baby being born with cleft lip or cleft palate (birth defects of the upper lip and mouth) | □ | |
| Smoking increases the chance of a baby dying of Sudden Infant Death Syndrome (SIDS) | □ | |
| I would not consider quitting smoking for any of these reasons | □ | |
| FOR EVERYONE | ||
| 3. | Which of the following have you heard could result from smoking during pregnancy? (“X” ALL THAT APPLY) | |
| Miscarriage | □ | |
| Problems with the placenta (mother’s organ that supplies the baby’s oxygen and nutrition) | □ | |
| Baby born too small (low birth weight) or too early | □ | |
| Baby born with cleft lip or cleft palate (birth defects of the upper lip and mouth) | □ | |
| Baby born with hearing loss | □* | |
| Baby is more likely to die from Sudden Infant Death Syndrome (SIDS) | □ | |
| 4. | A woman who smokes can improve the health of her unborn baby if she quits smoking during which, if any, of the following times? (“X” ALL THAT APPLY) | |
| Before becoming pregnant | □ | |
| Early in the pregnancy, before the 3rd month | □ | |
| Later in pregnancy, after the 3rd month | □ | |
| None of these | □ | |
‘Baby born with hearing loss’ was added as a distracter among the responses to differentiate the respondents who checked all the responses indiscriminately.
To assess knowledge of the health effects of smoking during pregnancy, respondents were asked to check all adverse effects which they had previously heard could result from smoking during pregnancy (question #3, Table 1). The response options included five “correct” responses (miscarriage, baby born too small or too early, problems with placenta, baby born with cleft lip or cleft palate, and baby dies from SIDS) and one “incorrect” response (baby born with hearing loss) that was included as a distracter to differentiate persons who were checking all answers indiscriminately. To analyze these data, we created a knowledge scale that ranged from 0 to 6 to assess the number of answers checked appropriately. For each appropriate response, meaning the correct response checked and absence of selecting the incorrect response, the respondent received one point. The resulting scale ranged from 0 (no appropriate responses) to 6 (all appropriate responses). If respondents checked all 6 boxes, including the incorrect response, indicating they might have been checking all answers indiscriminately, they were considered in a separate category, “checked all”. After examining the knowledge score distribution (Table 2), we created three categories: low knowledge (scored 0–2), moderate knowledge (scored 3–4), and high knowledge (scored 5–6). The respondents who checked all responses technically scored 5 on the knowledge scale, but because they might be answering arbitrarily, the respondents in the “checked all” category were included in the moderate knowledge category.
Table 2.
Knowledge of Adverse Pregnancy Outcomes (number, percent correct responses, and 95% CI) associated with Smoking during Pregnancy, Among All Respondents, Women of Reproductive Age (18–44 years), and Women of Reproductive Age Planning a Pregnancy in the Next Year, United States, HealthStyles©, 2008 1,2
| Knowledge about adverse pregnancy outcomes associated with smoking during pregnancy | Correct responses from all respondents (n = 5,399) | Correct responses from women of reproductive age (n = 1,437) | Correct responses from women of reproductive age who are planning to get pregnant in the next year (n = 180) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | |
|
| ||||||
| Smoking during pregnancy can cause miscarriage (correct response = yes) | 3,425 | 63.4 (62.2–64.7) | 1,047 | 72.9 (70.6–75.2) | 143 | 79.4 (73.5–85.3) |
|
| ||||||
| Smoking during pregnancy can cause problems with placenta (correct response = yes) | 3,091 | 57.3 (55.9–58.6) | 961 | 66.9 (64.4–69.3) | 134 | 74.4 (68.0–80.8) |
|
| ||||||
| Smoking during pregnancy can cause the baby to be born too small or too early (correct response = yes) | 4,440 | 82.2 (81.2–83.3) | 1,333 | 92.7 (91.4–94.0) | 159 | 88.5 (83.9–93.2) |
|
| ||||||
| Smoking during pregnancy can cause the baby to be born with cleft lip or cleft palate (correct response = yes) | 1,039 | 19.2 (18.2–20.3) | 350 | 24.4 (22.2–26.6) | 69 | 38.5 (31.4–45.6) |
|
| ||||||
| Smoking during pregnancy can cause the baby to be born with hearing loss (correct response = no) | 915 | 16.9 (15.9–17.9) | 273 | 19.0 (17.0–21.0) | 47 | 25.9 (19.5–32.2) |
|
| ||||||
| Smoking during pregnancy can cause the baby to be more likely to die from SIDS (correct response = yes) | 1,993 | 36.9 (35.6–38.2) | 729 | 50.7 (48.1–53.3) | 103 | 57.2 (50.0–64.4) |
|
| ||||||
| None checked3 | 545 | 10.1 (9.3–10.9) | 36 | 2.5 (1.7–3.3) | 2 | 1.3 (0.0–3.0) |
|
| ||||||
| Number of correct responses | ||||||
| High Knowledge | 784 | 14.5 (13.6–15.5) | 333 | 23.2 (21.0–25.3) | 38 | 20.8 (14.9–26.8) |
| 6 correct responses | 99 | 1.8 (1.5–2.2) | 44 | 3.1 (2.2–4.0) | 12 | 6.5 (2.9–10.1) |
| 5 correct responses | 685 | 12.7 (11.8–13.6) | 289 | 20.1 (18.0–22.2) | 26 | 14.4 (9.2–19.5) |
| Moderate Knowledge | 3,133 | 58.0 (56.7–59.4) | 882 | 61.4 (58.9–63.9) | 122 | 68.4 (61.6–75.2) |
| Checked all | 676 | 12.5 (11.6–13.4) | 221 | 15.4 (13.5–17.2) | 39 | 21.9 (15.8–27.9) |
| 4 correct responses | 1,294 | 24.0 (22.8–25.1) | 381 | 26.5 (24.2–28.8) | 58 | 32.5 (25.6–39.3) |
| 3 correct responses | 1,163 | 21.5 (20.4–22.6) | 280 | 19.5 (17.4–21.5) | 25 | 14.1 (9.0–19.1) |
| Low Knowledge | 1,481 | 27.4 (26.3–28.6) | 223 | 15.5 (13.6–17.3) | 19 | 10.8 (6.3–15.3) |
| 2 correct responses | 927 | 17.2 (16.2–18.2) | 185 | 12.9 (11.1–14.6) | 17 | 9.5 (5.2–13.7) |
| 1 correct responses | 552 | 10.2 (9.4–11.0) | 37 | 2.6 (1.8–3.4) | 2 | 1.3 (0.0–3.0) |
| 0 correct responses | 2 | 0.0 (0.0–0.1) | 1 | 0.0 (0.0–0.1) | 0 | |
The total of these percentages will not add up to 100% because multiple responses were allowed.
All data were weighted, and the resulting numbers were rounded to the nearest whole number. Thus, frequencies may not add to the total.
The participant did not check any responses.
Statistical analyses
To examine respondent knowledge regarding the adverse health effects associated with smoking during pregnancy, we calculated frequencies of responses to Question #3 (Table 1) among all respondents, among women of reproductive age (18–44 years), and among women of reproductive age who reported planning a pregnancy in the next year. We compared the knowledge score (described previously) among each group. We used SAS PROC FREQ to estimate relative risk (RR) and 95% confidence intervals (CIs) for demographic and lifestyle factors potentially associated with high level and moderate level knowledge, including age of respondent, household income and size, race/ethnicity, education level, marital status, and whether the respondent was a current smoker.
To examine what messages from a doctor or health professional might motivate a woman to quit smoking if she, hypothetically, was thinking about becoming pregnant, we calculated the frequency of responses to Question #2 (Table 1) among women of reproductive age who reported currently smoking cigarettes. To examine beliefs about the benefits of quitting smoking at different points during pregnancy, we calculated the frequency of responses to Question #4 (Table 1) among all respondents, women of reproductive age (18–44 years), and women of reproductive age who reported planning a pregnancy in the next year. We used descriptive statistics to assess respondents’ attitudes regarding messages that might influence a woman to quit smoking if she, hypothetically, was thinking about becoming pregnant and her knowledge regarding the benefits of quitting smoking before, early, and late in pregnancy.
After reviewing preliminary results, we wanted to examine if the “checked all” category of respondents affected the findings related to the knowledge scale (described previously). To investigate this sub-group’s impact, we repeated the analyses categorizing the “checked all” respondents in two different ways—1) including the “checked all” respondents in the low knowledge category and 2) excluding them from the analyses.
We performed all statistical analyses after the data was post-stratified and weighted, as described previously. We analyzed the data using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
A total of 5,399 out of 7,000 distributed HealthStyles© surveys were completed and returned (participation rate = 77 %). Before post-stratification and weighting, 2,408 (45%) respondents were men and 2,991 (55%) were women, including 1,053 (19.5%) women of reproductive age (18–44 years). Regarding race/ethnicity, 3,664 (68%) respondents were white, 670 (12%) were black, 656 (12%) were Hispanic, and 409 (8%) were of other race/ethnicity. Respondents were most often married (68%), had some college education (37%), had a household size of 2 (31%), and had a household income greater than $75,000 (34%). Among reproductive-aged women, 19% reported currently smoking, 64% were married, 44% had attended some college, 62% were between 35–44 years of age, and 9% were planning a pregnancy in the next year. After post-stratification and weighting, the sample consisted of 2,613 (48%) men and 2,786 (52%) women, including 1,437 (27%) women of reproductive age. In this weighted sample, 180 (12.5%) of the reproductive aged women were planning a pregnancy in the next year. The weighted sample included 68% of respondents being white, 12% black, 13% Hispanic, and 7% of other race/ethnicity. All further results are for the post-stratified, weighted sample.
Knowledge about smoking during pregnancy (Question #3)
We examined knowledge of the adverse effects of prenatal smoking on pregnancy outcomes among three groups (all respondents combined, women of reproductive age, and women of reproductive age who reported planning a pregnancy in the next year); each group is a subset of the previous group (Table 2). Of all the adverse pregnancy outcomes, “smoking during pregnancy can cause the baby to be born too small or too early” was the outcome most often recognized as associated with smoking during pregnancy among each group (>80%). Fewer respondents in each of the groups were aware of the associations between smoking during pregnancy and having a baby born with a cleft lip or cleft palate (19% of all respondents, 24% of women of reproductive age, and 39% of women of reproductive age planning a pregnancy in the next year).
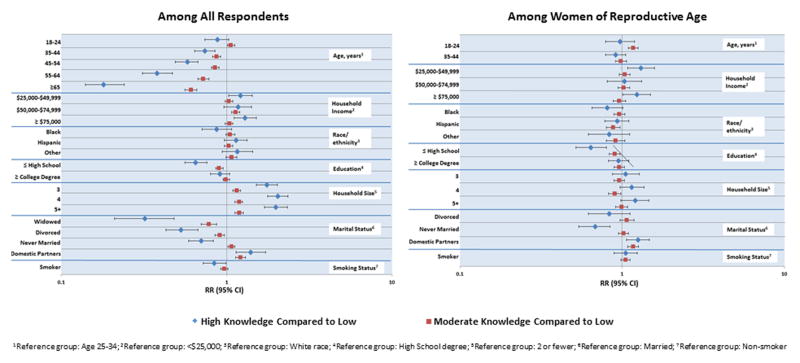
We also compared the knowledge levels about smoking during pregnancy and potential adverse pregnancy outcomes. Among all respondents, 784 respondents (15%) had high knowledge, 3,133 respondents (58%) had moderate knowledge, and 1,483 respondents (27%) had low knowledge (Table 2). Factors such as higher education level (> high school), married or domestic partnership status, larger household size (>2 members), and non-smoking status were positively associated with high knowledge (Figure 1). Among all respondents, women were more likely than men to have high knowledge of adverse pregnancy outcomes related to smoking during pregnancy (RR 1.68, 95% CI 1.49, 1.90). Among women of reproductive age, 333 (23%) had high knowledge, 882 (61%) had moderate knowledge, and 223 (16%) had low knowledge (Table 2). More than a high school education was associated with high knowledge among women of reproductive age (Figure 1).
Figure 1.
Knowledge Level about Adverse Pregnancy Outcomes Associated with Smoking During Pregnancy Among All Respondents and Among Women of Reproductive Age (18–44 years), by Demographic Characteristics, United States, HealthStyles©, 2008
In the aforementioned results, the respondents who checked all options were placed in the moderate knowledge category. To assess this sub-group’s impact on our results, we repeated the analyses post-hoc and categorized this sub-group in two different ways—1) placing this group in the low knowledge level and 2) excluding them all together. When we included these respondents in the low knowledge level, 14% had high knowledge, 46% had moderate knowledge, and 40% had low knowledge of the health effects of smoking during pregnancy. When we excluded these respondents, 17% of all respondents had high level knowledge, 52% had moderate knowledge, and 31% had low knowledge. Although the percentages per category changed, the direction and magnitude of most of the associations with demographic and lifestyle characteristics were similar, with the exception of few racial/ethnic comparisons (data not shown). For example, in the main analysis when this group of respondents who checked all was included in the moderate knowledge category, high and moderate knowledge were increased among respondents of other race/ethnicities compared to white race/ethnicity. However, when this group moved to the low knowledge category, high and moderate knowledge were decreased among respondents of other race/ethnicities compared to white race/ethnicity. In this example, the differences are not significant, as the confidence intervals in both instances contain 1.0.
Advice from a health professional to quit smoking (Question #2)
We examined what messages describing the effects of smoking during pregnancy provided by a doctor or health professional might motivate a current female smoker to quit smoking if she, hypothetically, was thinking about becoming pregnant; multiple responses were allowed (Table 3). The majority of female smokers of reproductive age (70%) checked that they would quit without any specific reasons from their doctor. About 30% of these women also checked one or more specific reasons provided by a doctor that might motivate them to quit smoking (data not shown). In total, 41% of the female smokers of reproductive age checked at least one reason that might motivate them to consider quitting smoking (data not shown). Of those respondents who checked at least one reason, about 46% of them were less than 25 years of age and 85% had less than a college education. The most often reported reasons that might motivate current women smokers of reproductive age to quit smoking were increased chances of having a baby born too early or too small (34%), increased risk of a baby dying of SIDS (27%), and increased risk of miscarriage (25%). Fewer women reported that increased risk of having a baby born with cleft lip or cleft palate (20%) or difficulty getting pregnant (11%) would influence quitting smoking. About 5% of current women smokers of reproductive age checked that they would not consider quitting smoking for any reason if they were thinking about becoming pregnant (Table 3). Of the 5% who would not consider quitting, the majority had a household income less than $50,000, were of white race/ethnicity, had less than a college education, and were married (data not shown).
Table 3.
Potential Influence of Health Professional Advice on Smoking Cessation in Women Smokers of Reproductive Age (18–44 years), United States, Healthstyles©, 2008 1,2
| Advice from a doctor or health professional to consider quitting smoking | Women Smokers of Reproductive Age (n = 290) | |
|---|---|---|
| N | % (95% CI) | |
| No specific reason checked | ||
| My doctor would not have to give any specific reason- I would quit | 203 | 69.7 (64.6–75.1) |
| I would not consider quitting smoking for any of these reasons | 16 | 5.3 (2.8–7.9) |
| Specific reasons checked | ||
| Smoking before pregnancy makes it harder to become pregnant | 31 | 10.8 (7.2–14.4) |
| Smoking during pregnancy increases the chance of having a miscarriage | 73 | 25.1 (20.1–30.1) |
| Smoking during pregnancy increases the chance of having a baby born too small or too early | 98 | 33.9 (28.4–39.3) |
| Smoking during pregnancy increases the chance of having a baby being born with cleft lip or cleft palate | 59 | 20.4 (15.7–25.0) |
| Smoking increases the chance of baby dying of Sudden Infant Death Syndrome (SIDS) | 80 | 27.4 (22.3–32.6) |
| None checked3 | 11 | 3.9 (1.7–6.2) |
The total of these percentages will not add up to 100% because multiple responses were allowed.
All the data were weighted, and the resulting numbers were rounded to the nearest whole number. Thus, frequencies may not add exactly to the total.
The participant did not check any option.
Knowledge about timing of smoking cessation (Question #4)
More than 80% of the respondents in each group (all respondents, women of reproductive age, and women of reproductive age planning a pregnancy in the next year) agreed that quitting smoking before becoming pregnant improves the health of the unborn baby (Table 4). Almost half of the women of reproductive age agreed that quitting smoking during early pregnancy can improve the health of the unborn baby (Table 4). However, very few respondents recognized the benefits of quitting smoking after the first trimester (17% of all respondents and 21% of reproductive-aged women).
Table 4.
Knowledge of Timing of Smoking Cessation to Improve the Health of Unborn Baby, United States, HealthStyles©, 2008 1,2
| A female smoker can improve the health of her unborn baby if she quits smoking when? | All respondents (n = 5,399) | Women of reproductive Age (n = 1,437) | Women of reproductive age who are planning to get pregnant in the next year (n = 180) | |||
|---|---|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | |
| Before becoming pregnant | 4,385 | 81.2 (80.2–82.3) | 1276 | 88.8 (87.1–90.4) | 157 | 87.4 (82.6–92.3) |
| Early in the pregnancy, before the 3rd month | 2,242 | 41.5 (40.2–42.8) | 729 | 50.7 (48.2–53.3) | 94 | 52.0 (44.7–59.3) |
| Later in the pregnancy, after the 3rd month | 923 | 17.1 (16.1–18.1) | 305 | 21.2 (19.1–23.3) | 38 | 21.1 (15.1–27.1) |
| None of these | 256 | 4.7 (4.2–5.3) | 39 | 2.7 (1.9–3.5) | 6 | 3.2 (0.7–5.8) |
| None checked3 | 285 | 5.3 (4.7–5.9) | 19 | 1.4 (0.8–2.0) | 2 | 1.0 (0.0–2.5) |
The total of these percentages will not add up to 100% because multiple responses were allowed.
All the data were weighted, and the resulting numbers were rounded to the nearest whole number. Thus, frequencies may not add exactly to the total.
The participant did not check any option.
Discussion
In general, high knowledge of the adverse effects of smoking during pregnancy was low among respondents overall, but was somewhat higher among women of reproductive age. For all respondents combined, factors associated with high knowledge included being married or in a domestic partnership, higher education level, larger household size, and non-smoking status. Among women of reproductive age, higher education level was associated with high knowledge. The majority of reproductive-aged women smokers indicated that they would not need a specific reason from their doctor to influence them to quit smoking if, hypothetically, they were thinking about becoming pregnant. Among women who indicated that at least one reason would influence them to quit, the reasons that were most often reported were increased risks of preterm birth, SIDS, and miscarriage. Less than 20% of all respondents were aware that quitting smoking after the first trimester of pregnancy has benefits.
Although individuals in the public health community assume that nearly all women are aware that smoking during pregnancy may have negative effects on the developing baby (53, 54), our results suggest that some women still lack knowledge about the specific adverse reproductive outcomes associated with prenatal smoking. Although we observed that more than 90% of women of reproductive age were aware of the association between smoking and preterm birth and low birth weight, fewer women of reproductive age were aware of other potential adverse outcomes, such as miscarriage or cleft lip/palate. Our finding that lower level of education is associated with less knowledge of adverse effects of smoking during pregnancy is consistent with results from numerous studies in which smokers with lower levels of education or limited literacy skills were less knowledgeable about the effects of prenatal smoking on their own health or the health of their offspring (55–57). Previous research has shown that behavioral intervention programs focused on increasing education, including multi-media campaigns and telephone quit lines, have achieved modest success in increasing smoking cessation in pregnant women (58, 59). Other methods that have the potential to target smokers with lower levels of education or SES include interventions conducted via Women, Infants, and Children (WIC) clinics or community health centers and interventions that include incentives (60–62). Additionally, as of 2012, 43 state Medicaid programs cover tobacco-cessation counseling, which could improve cessation efforts targeting women of lower education or SES who might have previously had less access to these services (63).
Pregnancy presents a unique opportunity for smoking cessation interventions because pregnant women may be more likely to make changes in their lifestyle and health behaviors than non-pregnant women. Current best practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) support clinic-based interventions in which healthcare providers use the 5As: (1) Ask all pregnant women about their smoking status, (2) Advise all pregnant smokers about the risks of smoking in pregnancy and emphasize the benefits of quitting, (3) Assess the pregnant women’s readiness to change and set a quit date, (4) Assist pregnant smokers to quit using a range of interventions, and (5) Arrange follow-up. Pregnant smokers should be asked about their smoking status and, if still smoking, assisted with quitting at each subsequent visit (64). However, research has suggested that providers’ discussions of smoking behavior during prenatal visits is limited to inquiring about and documenting smoking status and advising pregnant patients to quit. Few physicians provide smoking cessation counseling and arrange for follow-up visits (65–69). Our data suggest that when smoking is discussed with female smokers of reproductive age, a more detailed explanation of the risks, including possible adverse pregnancy outcomes associated with smoking during pregnancy, might be effective in motivating some to quit smoking, if they were thinking about becoming pregnant. While improved knowledge doesn’t directly translate into behavior change, it can impact efforts to quit, helping to increase smokers’ readiness to quit (70, 71).
Smoking during pregnancy affects fetal health in a variety of ways, depending on the amount and timing of exposure. For example, the rate of fetal growth varies over the course of a pregnancy, and the development of organ systems occur during different periods of pregnancy (72–76). Because some effects of smoking, such as increased risk of orofacial clefts, occur early in pregnancy, continued emphasis on the benefits of smoking cessation before conception is important. However, previous research has shown that quitting smoking during the first or second trimester of pregnancy may reduce a number of adverse pregnancy outcomes, including low birth weight and preterm delivery (28, 30, 77). In our study, respondents appeared to be unaware of the benefits of smoking cessation after the first trimester of pregnancy. Increasing knowledge about the benefits of smoking cessation after the first trimester of pregnancy might encourage pregnant smokers to continue pursuing quitting even after the first trimester, to reduce potential adverse outcomes, such as low birth weight.
Our study has several important limitations. First, women responding to the current survey might have been more likely to provide socially favorable responses, especially regarding smoking cessation during pregnancy, rather than being completely truthful. Another important limitation is that we know that predicted behavior does not reflect actual behavior. Respondents might say they would quit smoking if their doctor gave specific reasons to quit, but they might not recognize the difficulty in actually quitting. Further, respondents might not have understood the hypothetical nature of the question asking about advice from a healthcare provider. The survey was written and conducted in English. Thefore, women who lack fluency in English, women who have difficulty reading, or women who are illiterate likely did not participate. The survey is also only distributed to adults 18 years or older, so our results do not include knowledge and attitudes held by younger individuals. Some of the segments, including women of reproductive age planning a pregnancy in the next year, contain small numbers, which limits our ability to characterize them by demographic and lifestyle factors. Further, women who participated in the HealthStyles© panel might have been more interested in health topics (and thus might be more knowledgeable) or have had more time to complete surveys than people who declined to participate. Therefore, our results might not represent the knowledge and attitudes of the general population. However, previous analyses have showed that responses to the HealthStyles© survey on health conditions, attitudes, and behaviors were comparable with responses to similar survey questions asked as part of the Behavioral Risk Factor Surveillance System (BRFSS), a large probability sample survey conducted in the United States (78). Finally, respondents might have indiscriminately checked all responses to the question used to develop the knowledge scale, which might affect the findings related to knowledge of the health effects of smoking during pregnancy. When we re-evaluated the results categorizing these respondents as low knowledge or excluding them, we found similar magnitude and direction of associations with demographic and lifestyle factors.
Conclusions
These results suggest that many women lack knowledge regarding the increased risks for some adverse pregnancy outcomes associated with smoking during pregnancy. Overall, the outcome most often recognized as associated with prenatal smoking was “smoking during pregnancy can cause the baby to be born too small or too early.” Fewer respondents reported knowledge of the associations between smoking during pregnancy and having a baby born with a cleft lip or cleft palate. While our data do not support that this knowledge would greatly increase the percentage of women interested in quitting during pregnancy, educating women about this risk factor could have modest benefits. Although many societal and personal influences impact smoking behavior, healthcare providers play an important role in improving smoking cessation among pregnant women. When providing smoking cessation counseling, providers should follow the entire 5As carefully, as recommended by ACOG. Further, healthcare providers should emphasize the importance of quitting smoking even after the first trimester of pregnancy. Additional research is needed to better understand if increased knowledge among women smokers of reproductive age would result in smoking cessation behavior.
Acknowledgments
We thank Porter Novelli for data collection efforts and their assistance with appropriate interpretation of the data. We also thank all respondents to this survey for their participation. The authors also would like to thank Lucinda England, M.D., M.S.P.H, for her valuable insights and contributions towards the project.
Footnotes
Author Disclosure Statement
Paramjit K. Sandhu and Kara ND Polen were supported by an appointment to the Research Participation Program for the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an agreement between the Department of Energy and CDC.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Hull MG, North K, Taylor H, et al. Delayed conception and active and passive smoking. The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000;74(4):725–33. doi: 10.1016/s0015-0282(00)01501-6. [DOI] [PubMed] [Google Scholar]
- 2.Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–9. doi: 10.1093/humrep/13.6.1532. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri RL. The initial fertility consultation: recommendations concerning cigarette smoking, body mass index, and alcohol and caffeine consumption. Am J Obstet Gynecol. 2001;185(5):1168–73. doi: 10.1067/mob.2001.117667. [DOI] [PubMed] [Google Scholar]
- 4.Chelmow D, Andrew DE, Baker ER. Maternal cigarette smoking and placenta previa. Obstet Gynecol. 1996;87(5 Pt 1):703–6. doi: 10.1016/0029-7844(95)00471-8. [DOI] [PubMed] [Google Scholar]
- 5.Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol. 1996;144(9):881–9. doi: 10.1093/oxfordjournals.aje.a009022. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen JT, Thulstrup AM, Larsen H, et al. Smoking, sex of the offspring, and risk of placental abruption, placenta previa, and preeclampsia: a population-based cohort study. Acta Obstet Gynecol Scand. 2001;80(10):894–8. doi: 10.1034/j.1600-0412.2001.801005.x. [DOI] [PubMed] [Google Scholar]
- 7.Aliyu MH, Lynch O, Wilson RE, et al. Association between tobacco use in pregnancy and placenta-associated syndromes: a population-based study. Arch Gynecol Obstet. 2011;283(4):729–34. doi: 10.1007/s00404-010-1447-8. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K, Matsuda Y, Kawamichi Y, et al. Smoking during pregnancy increases risks of various obstetric complications: a case-cohort study of the Japan Perinatal Registry Network database. Journal of Epidemiology / Japan Epidemiological Association. 2011;21(1):61–6. doi: 10.2188/jea.JE20100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011;90(2):140–9. doi: 10.1111/j.1600-0412.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 10.Chatenoud L, Parazzini F, di Cintio E, et al. Paternal and maternal smoking habits before conception and during the first trimester: relation to spontaneous abortion. Ann Epidemiol. 1998;8(8):520–6. doi: 10.1016/s1047-2797(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 11.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40(4):385–94. [PubMed] [Google Scholar]
- 12.George L, Granath F, Johansson AL, et al. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiol. 2006;17(5):500–5. doi: 10.1097/01.ede.0000229984.53726.33. [DOI] [PubMed] [Google Scholar]
- 13.Delpisheh A, Attia E, Drammond S, et al. Adolescent smoking in pregnancy and birth outcomes. Eur J Public Health. 2006;16(2):168–72. doi: 10.1093/eurpub/cki219. [DOI] [PubMed] [Google Scholar]
- 14.Horta BL, Victora CG, Menezes AM, et al. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol. 1997;11(2):140–51. doi: 10.1046/j.1365-3016.1997.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 15.Dietz PM, England LJ, Shapiro-Mendoza CK, et al. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 39(1):45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Aliyu MH, Lynch O, Saidu R, et al. Intrauterine exposure to tobacco and risk of medically indicated and spontaneous preterm birth. Am J Perinatol. 2010;27(5):405–10. doi: 10.1055/s-0029-1243316. [DOI] [PubMed] [Google Scholar]
- 17.Baba S, Wikstrom AK, Stephansson O, et al. Influence of smoking and snuff cessation on risk of preterm birth. Eur J Epidemiol. 2012;27(4):297–304. doi: 10.1007/s10654-012-9676-8. [DOI] [PubMed] [Google Scholar]
- 18.Vardavas CI, Chatzi L, Patelarou E, et al. Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. Eur J Pediatr. 2010;169(6):741–8. doi: 10.1007/s00431-009-1107-9. [DOI] [PubMed] [Google Scholar]
- 19.Fretts R. Stillbirth epidemiology, risk factors, and opportunities for stillbirth prevention. Clin Obstet Gynecol. 2010;53(3):588–96. doi: 10.1097/GRF.0b013e3181eb63fc. [DOI] [PubMed] [Google Scholar]
- 20.Schoendorf KC, Kiely JL. Relationship of sudden infant death syndrome to maternal smoking during and after pregnancy. Pediatr. 1992;90(6):905–8. [PubMed] [Google Scholar]
- 21.Mitchell EA, Milerad J. Smoking and the sudden infant death syndrome. Rev Environ Health. 2006;21(2):81–103. doi: 10.1515/reveh.2006.21.2.81. [DOI] [PubMed] [Google Scholar]
- 22.Pinho AP, Aerts D, Nunes ML. Risk factors for sudden infant death syndrome in a developing country. Rev Saude Publica. 2008;42(3):396–401. doi: 10.1590/s0034-89102008000300002. [DOI] [PubMed] [Google Scholar]
- 23.Little J, Cardy A, Munger RG. Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ. 2004;82(3):213–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Honein MA, Rasmussen SA, Reefhuis J, et al. Maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts. Epidemiol. 2007;18(2):226–33. doi: 10.1097/01.ede.0000254430.61294.c0. [DOI] [PubMed] [Google Scholar]
- 25.Shaw GM, Carmichael SL, Vollset SE, et al. Mid-Pregnancy Cotinine and Risks of Orofacial Clefts and Neural Tube Defects. J Pediatr. 2009;154:17–9. doi: 10.1016/j.jpeds.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking—50 Years of Progress. Printed with corrections, January 2014. [Google Scholar]
- 27.Shaw GM, Wasserman CR, Lammer EJ, et al. Orofacial clefts, parental cigarette smoking, and transforming growth factor-alpha gene variants. Am J Hum Genet. 1996;58(3):551–61. [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman E, Gremy I, Lang JM, et al. Low birthweight at term and the timing of fetal exposure to maternal smoking. Am J Public Health. 1994;84(7):1127–31. doi: 10.2105/ajph.84.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiger JS, Beaty TH, Liang KY. Oral clefts, maternal smoking, and TGFA: a meta-analysis of gene-environment interaction. Cleft Palate Craniofac J. 2005;42(1):58–63. doi: 10.1597/02-128.1. [DOI] [PubMed] [Google Scholar]
- 30.Bickerstaff M, Beckmann M, Gibbons K, et al. Recent cessation of smoking and its effect on pregnancy outcomes. Aust NZ J Obstet Gyn. 2012;52(1):54–8. doi: 10.1111/j.1479-828X.2011.01387.x. [DOI] [PubMed] [Google Scholar]
- 31.Allen AM, Dietz PM, Tong VT, et al. Prenatal smoking prevalence ascertained from two population-based data sources: birth certificates and PRAMS questionnaires, 2004. Public Health Rep. 2008;123(5):586–92. doi: 10.1177/003335490812300508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderka M, Romitti PA, Sun L, et al. Patterns of tobacco exposure before and during pregnancy. Acta Obstet Gynecol Scand. 89(4):505–14. doi: 10.3109/00016341003692261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC. Trends in Smoking Before, During, and After Pregnancy-- Pregnancy Risk Assessment Monitoring System, United States, 40 Sites, 2000–2010. MMWR. 2013;62(6):1–19. [PubMed] [Google Scholar]
- 34.Cnattingius S, Lindmark G, Meirik O. Who continues to smoke while pregnant? J Epidemiol Community Health. 1992;46(3):218–21. doi: 10.1136/jech.46.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobel M, Cannella DL, Graham JE, et al. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27(5):604–15. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Tong S, Oldenburg B. Determinants of smoking and cessation during and after pregnancy. Health Promot Int. 2001;16(4):355–65. doi: 10.1093/heapro/16.4.355. [DOI] [PubMed] [Google Scholar]
- 37.Mohsin M, Bauman AE. Socio-demographic factors associated with smoking and smoking cessation among 426,344 pregnant women in New South Wales, Australia. BMC Public Health. 2005;5:138. doi: 10.1186/1471-2458-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller LL, Munk C, Thomsen BL, et al. The influence of parity and smoking in the social environment on tobacco consumption among daily smoking women in Denmark. Eur Addict Res. 2007;13(3):177–84. doi: 10.1159/000101554. [DOI] [PubMed] [Google Scholar]
- 39.Ockene J, Ma Y, Zapka J, et al. Spontaneous cessation of smoking and alcohol use among low-income pregnant women. Am J Prev Med. 2002;23(3):150–9. doi: 10.1016/s0749-3797(02)00492-0. [DOI] [PubMed] [Google Scholar]
- 40.Schneider S, Huy C, Schutz J, et al. Smoking cessation during pregnancy: a systematic literature review. Drug and Alcohol review. 29(1):81–90. doi: 10.1111/j.1465-3362.2009.00098.x. [DOI] [PubMed] [Google Scholar]
- 41.Ershoff DH, Solomon LJ, Dolan-Mullen P. Predictors of intentions to stop smoking early in prenatal care. Tob Control. 2000;9(Suppl 3):III41–5. doi: 10.1136/tc.9.suppl_3.iii41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haslam C. A targeted approach to reducing maternal smoking. Br J Gen Pract. 2000;50(457):661–3. [PMC free article] [PubMed] [Google Scholar]
- 43.Lawrence WT, Haslam C. Smoking during pregnancy: where next for stage-based interventions? J Health Psychol. 2007;12(1):159–69. doi: 10.1177/1359105307071750. [DOI] [PubMed] [Google Scholar]
- 44.Koshy P, Mackenzie M, Tappin D, et al. Smoking cessation during pregnancy: the influence of partners, family and friends on quitters and non-quitters. Health & Social Care in the Community. 18(5):500–10. doi: 10.1111/j.1365-2524.2010.00926.x. [DOI] [PubMed] [Google Scholar]
- 45.Ebrahim SH, Floyd RL, Merritt RK, 2nd, et al. Trends in pregnancy-related smoking rates in the United States, 1987–1996. JAMA. 2000;283(3):361–6. doi: 10.1001/jama.283.3.361. [DOI] [PubMed] [Google Scholar]
- 46.Mathews TJ. Smoking during pregnancy in the 1990s. Natl Vital Stat Rep. 2001;49(7):1–14. [PubMed] [Google Scholar]
- 47.Owen L, McNeill A, Callum C. Trends in smoking during pregnancy in England, 1992–7: quota sampling surveys. BMJ. 1998;317(7160):728. doi: 10.1136/bmj.317.7160.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClave-Regan AK, Berkowitz J. Smokers who are also using smokeless tobacco products in the US: a national assessment of characteristics, behaviours and beliefs of ‘dual users’. Tob Control. 2011;20(3):239–42. doi: 10.1136/tc.2010.039115. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy AM, Brown CJ, Gust DA. Vaccine beliefs of parents who oppose compulsory vaccination. Public Health Rep. 2005;120(3):252–8. doi: 10.1177/003335490512000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobau R, Gilliam F, Thurman DJ. Prevalence of self-reported epilepsy or seizure disorder and its associations with self-reported depression and anxiety: results from the 2004 HealthStyles Survey. Epilepsia. 2006;47(11):1915–21. doi: 10.1111/j.1528-1167.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 51.Ayala C, Tong X, Keenan NL. Regular use of a home blood pressure monitor by hypertensive adults--HealthStyles, 2005 and 2008. J Clin Hypertens. 2012;14(3):172–7. doi: 10.1111/j.1751-7176.2011.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maibach EW, Maxfield A, Ladin K, et al. Translating Health Psychology into Effective Health Communication: The American Healthstyles Audience Segmentation Project. J Health Psychol. 1996;1(3):261–77. doi: 10.1177/135910539600100302. [DOI] [PubMed] [Google Scholar]
- 53.Einarson A, Riordan S. Smoking in pregnancy and lactation: a review of risks and cessation strategies. Eur J Clin Pharmacol. 2009;65(4):325–30. doi: 10.1007/s00228-008-0609-0. [DOI] [PubMed] [Google Scholar]
- 54.Roth LK, Taylor HS. Risks of smoking to reproductive health: assessment of women’s knowledge. Am J Obstet Gynecol. 2001;184(5):934–9. doi: 10.1067/mob.2001.112103. [DOI] [PubMed] [Google Scholar]
- 55.Arnold CL, Davis TC, Berkel HJ, et al. Smoking status, reading level, and knowledge of tobacco effects among low-income pregnant women. Prev Med. 2001;32(4):313–20. doi: 10.1006/pmed.2000.0815. [DOI] [PubMed] [Google Scholar]
- 56.Brownson RC, Jackson-Thompson J, Wilkerson JC, et al. Demographic and socioeconomic differences in beliefs about the health effects of smoking. Am J Public Health. 1992;82(1):99–103. doi: 10.2105/ajph.82.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siahpush M, McNeill A, Hammond D, et al. Socioeconomic and country variations in knowledge of health risks of tobacco smoking and toxic constituents of smoke: results from the 2002 International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15(Suppl 3):iii65–70. doi: 10.1136/tc.2005.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rigotti NA, Park ER, Regan S, et al. Efficacy of telephone counseling for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2006;108(1):83–92. doi: 10.1097/01.AOG.0000218100.05601.f8. [DOI] [PubMed] [Google Scholar]
- 59.Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Womens Health. 2007;16(8):1211–8. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- 60.Cluss PA, Levine MD, Landsittel D. The Pittsburgh STOP program: disseminating an evidence-informed intervention for low-income pregnant smokers. Am J Health Promot. 2011;25(5 Suppl):S75–81. doi: 10.4278/ajhp.100616-QUAN-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gadomski A, Adams L, Tallman N, et al. Effectiveness of a combined prenatal and postpartum smoking cessation program. Matern Child Health J. 2011;15(2):188–97. doi: 10.1007/s10995-010-0568-9. [DOI] [PubMed] [Google Scholar]
- 62.Higgins ST, Washio Y, Heil SH, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55 (Suppl):S33–40. doi: 10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McMenamin SB, Halpin HA, Ganiats TG. Medicaid coverage of tobacco-dependence treatment for pregnant women: impact of the Affordable Care Act. Am J Prev Med. 2012;43(4):e27–9. doi: 10.1016/j.amepre.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 64.ACOG committee opinion. Number 316, October 2005. Smoking cessation during pregnancy. Obstet Gynecol. 2005;106(4):883–8. doi: 10.1097/00006250-200510000-00052. [DOI] [PubMed] [Google Scholar]
- 65.Bailey BA, Cole LK. Are Obstetricians Following Best-Practice Guidelines for Addressing Pregnancy Smoking? Results from Northeast Tennessee. South Med J. 2009;102(9):894–9. doi: 10.1097/smj.0b013e3181aa579c. [DOI] [PubMed] [Google Scholar]
- 66.Grimley DM, Bellis JM, Raczynski JM, et al. Smoking cessation counseling practices: a survey of Alabama obstetrician-gynecologists. South Med J. 2001;94(3):297–303. [PubMed] [Google Scholar]
- 67.Jordan TR, Dake JR, Price JH. Best practices for smoking cessation in pregnancy: do obstetrician/gynecologists use them in practice? J Womens Health. 2006;15(4):400–41. doi: 10.1089/jwh.2006.15.400. [DOI] [PubMed] [Google Scholar]
- 68.Mullen PD, Pollak KI, Titus JP, et al. Prenatal smoking cessation counseling by Texas obstetricians. Birth. 1998;25(1):25–31. doi: 10.1046/j.1523-536x.1998.00025.x. [DOI] [PubMed] [Google Scholar]
- 69.Okoli CT, Greaves L, Bottorff JL, et al. Healthcare providers’ engagement in smoking cessation with pregnant smokers. J Obstet Gynecol Neonatal Nurs. 39(1):64–77. doi: 10.1111/j.1552-6909.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 70.Albrecht SA, Higgins LW, Lebow H. Knowledge about the deleterious effects of smoking and its relationship to smoking cessation among pregnant adolescents. Adolescence. 2000;35(140):709–16. [PubMed] [Google Scholar]
- 71.Durkin S, Brennan E, Wakefield M. Mass media campaigns to promote smoking cessation among adults: an integrative review. Tob Control. 2012;21(2):127–38. doi: 10.1136/tobaccocontrol-2011-050345. [DOI] [PubMed] [Google Scholar]
- 72.Jaddoe VW, Verburg BO, de Ridder MA, et al. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epidemiol. 2007;165(10):1207–15. doi: 10.1093/aje/kwm014. [DOI] [PubMed] [Google Scholar]
- 73.Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10(1–2):1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- 74.Toft PB, Leth H, Ring PB, et al. Volumetric analysis of the normal infant brain and in intrauterine growth retardation. Early Hum Dev. 1995;43(1):15–29. doi: 10.1016/0378-3782(95)01657-o. [DOI] [PubMed] [Google Scholar]
- 75.Falkner F. Ultrasonography and fetal growth: key perinatal factors. J Perinatol. 1995;15(2):114–8. [PubMed] [Google Scholar]
- 76.Bernstein IM, Goran MI, Amini SB, et al. Differential growth of fetal tissues during the second half of pregnancy. Am J Obstet Gynecol. 1997;176(1 Pt 1):28–32. doi: 10.1016/s0002-9378(97)80006-3. [DOI] [PubMed] [Google Scholar]
- 77.McCowan LM, Dekker GA, Chan E, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338:b1081. doi: 10.1136/bmj.b1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pollard W, editor. Use of consumer panel survey data for public health communication planning: An evaluation of survey results; Proceedings of the Annual Meeting of the American Statistical Association, Joint Statistical Meetings; New York, NY: 2002. [Google Scholar]