Abstract
Objectives
Eosinophilic esophagitis (EoE) is an increasingly prevalent chronic disease arising from an allergy/immune-mediated process. Generally, the risk of atopic disease differs in rural and urban environments. The relationship between population density and EoE is unknown. Our aim was to assess the relationship between EoE and population density.
Methods
: We conducted a cross-sectional, case-control study of patients with esophageal biopsies in a U.S. national pathology database between January 2009 and June 2012 to assess the relationship between population density and EoE. Using Geographic Information Systems (GIS), the population density (individuals/mile2) was determined for each patient zip code. The odds of esophageal eosinophilia and EoE were estimated for each quintile of population density and adjusted for potential confounders. Sensitivity analyses were conducted with varying case definitions and to evaluate the potential for bias from endoscopy volume and patient factors.
Results
Of 292,621 unique patients in the source population, 89,754 had normal esophageal biopsies and 14,381 had esophageal eosinophilia with ≥15 eosinophils per high-power field (eos/hpf). The odds of esophageal eosinophilia increased with decreasing population density (p for trend < 0.001). Compared to those in the highest quintile of population density, odds of esophageal eosinophilia were significantly higher amongst those in the lowest quintile of population density (aOR 1.27, 95% CI: 1.18, 1.36). A similar dose-response trend was observed across case definitions with odds of EoE increased in the lowest population density quintile (aOR 1.59, 95% CI: 1.45-1.76). Estimates were robust to sensitivity analyses.
Conclusions
Population density is strongly and inversely associated with esophageal eosinophilia and EoE. This association is robust to varying case definitions and adjustment factors. Environmental exposures more prominent in rural areas may be relevant to the pathogenesis of EoE.
Keywords: Eosinophilic esophagitis, Esophageal eosinophilia, Population density, Environmental exposures, Epidemiology
Introduction
Eosinophilic esophagitis (EoE) is a chronic esophageal disease characterized by dense esophageal eosinophilia with clinical symptoms of esophageal dysfunction in the absence of other etiologies (1). EoE is one cause of esophageal eosinophilia, which is a broader term for the histopathologic finding of eosinophils infiltrating the esophageal mucosa, and for which an etiology should be determined (2). While the pathogenesis of EoE is incompletely understood, the underlying mechanism of disease is thought to be immune- or allergen-mediated (3, 4). Individuals with EoE often have concomitant atopy (5-10), there can be seasonal variation in EoE diagnosis (11), and experimental EoE can be induced in murine models with antigen challenge (12, 13). In humans, however, the initial allergen exposure that elicits disease development can rarely be identified (14), and risk factors for the development of EoE have yet to be fully elucidated.
Because EoE is a newly recognized disease with a markedly increasing incidence (15, 16), changes in environmental factors, rather than changes in genetics, likely explain the evolving epidemiology. In other allergic and autoimmune conditions such as eczema, multiple sclerosis, and inflammatory bowel disease, geographical factors may impact the observed incidence and prevalence of disease (17-20). In EoE, environmental factors such as Helicobacter pylori (21) or climate (22) have been shown to impact the prevalence of EoE. A prior survey of physicians suggested that EoE might be more prevalent in rural areas (6). Geographic differences in development of EoE may also provide etiologic clues for EoE pathogenesis. However geographic variation in EoE has not been explored in detail (10, 23, 24), and data from single centers with variable referral patterns cannot reliably detect such geographic variations.
The aim of this study was to use a large national pathology database to estimate the association between population density, as a proxy for rural versus urban residence, and EoE. We hypothesized that population density would be associated with esophageal eosinophilia and EoE.
Methods
Data sources and case definitions
We conducted a cross-sectional, case-control study of patients with esophageal biopsies examined between January 2009 and June 2012 by pathologists at Miraca Life Sciences, a specialized pathology laboratory serving outpatient endoscopy and surgery centers throughout the United States. Details of pathology protocols have been previously reported (21, 25). In brief, samples from 43 states, DC, and Puerto Rico were processed centrally in one of three laboratories (Irving, Texas; Phoenix, Arizona; Boston, Massachusetts) with identical sectioning and staining procedures. Sub-specialty trained gastrointestinal pathologists applied standardized criteria for diagnoses (21, 25, 26). A central database contained biopsy reports, demographic information (patient age, sex, and zip code of residence), indication for esophagogastroduodenoscopy, and zip code location of the gastroenterology practice where the procedure was performed. The University of North Carolina Institutional Review Board approved the study.
For this study, we constructed a de-identified database of pre-existing pathology records from 292,621 unique patients representing all esophageal biopsies examined between January 2009 and June 2012. Next, community–level data, including the number of persons living in each zip code and information on the racial make-up of zip codes, were obtained for 2010 from the United States Census Bureau (27). Combining these data with geographic data, we calculated population density in each zip code unit by dividing the number of individuals living in each zip code unit by the total area (miles2) within that zip code unit in geographic information systems (GIS ArcMap; version 9.3; ESRI Inc., Redlands, CA). Population density information was then linked to patient-level data in the pathology database using the residential zip code. We used population density rather than the U.S. Census Bureau urban/rural definition (a cut-point of 1,000 individuals/mile2) (2) to characterize population density with greater granularity than this simple dichotomization would provide.
To characterize population density into interpretable units of change for analyses, we divided the distribution of population into quintiles. We assigned latitude and longitude coordinates to each patient endoscopy center, using the center of the reported zip code as a proxy for geographic location. We then calculated the distance traveled by each patient to the endoscopy center. We restricted our study population to patients residing in the United States, excluding patients residing in Puerto Rico (n=775), where zip code-based distances from residence to endoscopy center could not be extrapolated in GIS. We further excluded from analyses any patients with missing zip codes (n=11) or patients living in zip codes for which we were missing any census information (n=8,649). Missing data resulted in fewer than 3% of the study population to be excluded from analysis.
Case and control characterization
Patients with esophageal eosinophilia were defined as those with ≥15 eosinophils per high power field (eos/hpf; 400x magnification with 22mm oculars; hpf area = 0.237mm2) on any esophageal biopsy. Because of the standardized pathology coding, these subjects could be readily identified in the database, and the level of esophageal eosinophilia recorded. We excluded subjects with esophageal eosinophilia who had accompanying histologic findings of candidal or viral esophagitis. Next, we applied increasingly stringent criteria to approximate patient disease status. Patients with esophageal eosinophilia were categorized by density of eosinophils, specifically ≥15 eos/hpf, ≥50 eos/hpf, and ≥100 eos/hpf.
For the eosinophilic esophagitis case definition, we selected three increasingly stringent and specific definitions: 1) presence of ≥15 eos/hpf and documentation of dysphagia; 2) presence of ≥15 eos/hpf, documentation of dysphagia, and exclusion of patients with clinical or histologic data suggesting differential diagnoses (reflux/heartburn symptoms, reflux esophagitis, Barrett's esophageal on biopsy, inflammatory bowel disease, and eosinophilic gastroenteritis); 3) presence of ≥15 eos/hpf, documentation of dysphagia, exclusion of the above differential diagnoses, and presence of eosinophilic microabscesses in the esophageal epithelium (defined as clusters of ≥4 contiguous eosinophils) (28). The EoE case definitions were created in this way because of limited clinical information in our database. While guidelines define EoE as symptoms of esophageal dysfunction, ≥ 15 eos/hpf, and exclusion of other competing conditions,(1) we could not fully evaluate the last criterion. Therefore, the above definitions allowed us to assess whether the strength of the observed associations would increase with increasing case definition specificity.
For the control group, we selected patients with histologically normal esophageal biopsies with no indication of a history of esophageal eosinophilia or EoE. Patients were identified as histologically normal if there was no evidence of inflammation of any type, mucosal disruption, infection, dysplasia, or neoplasia in the squamous epithelium.
Statistical analysis
Primary analyses
We first described the distribution of demographic characteristics for patients with normal biopsies and those with esophageal eosinophilia (≥15 eos/hpf). We then compared the distribution of demographic and disease characteristics for patients at the lowest (“rural”) and highest (“urban”) quintiles of population density. Specifically, we assessed for differences in sex, clinical symptoms (dysphagia, heartburn, chest pain, abdominal pain/dyspepsia, nausea/vomiting, or weight loss), histological features (eosinophil counts and microabscesses), distance traveled for endoscopy, and proportion of White subjects in a zip code. We used a binomial test for difference in proportions and the Student t-test to assess for differences in means. P values of < 0.05 were considered as statistically significant.
We used logistic regression to estimate the relationship between population density and levels of esophageal eosinophilia or eosinophilic esophagitis cases definitions. Specifically, we estimated the odds of disease at each quintile of population density (individuals/miles2) as compared to the lowest quintile of population density and assessed for any trend in response at increasing levels of population density. We assessed for possible collinearity between distance traveled to endoscopy center and population density to inform selection of covariates for inclusion in multiple logistic regression models. Models were adjusted for the following potential confounders: age (continuous), sex (male, female), race distribution (continuous measure of proportion White in zip code of residence), and estimated distance traveled for endoscopy (quintiles). Because we had previously identified an association between climate and EoE (22), we also conducted a sensitivity analysis where we adjusted on climate, as defined and described previously (22).
To account for potential residual within-group correlation that can occur when exposure (e.g. population density) is assigned at the group level, we also conducted analyses using generalized estimating equations (GEEs). Because there was little residual within-group correlation in the matrix and GEEs produced nearly identical results to logistic regression (data not shown), we present results of the logistic regression analyses.
Mapping
To assess adequacy of representation of patients throughout the Unites States, we first examined the geographical distribution of patients from which biopsies were obtained. Next, we mapped the predicted the odds of esophageal eosinophilia (≥15 eso/hpf) across the United States using the results obtained from continuous predictor models for population density. Based on visual inspection of smoothed plots we determined that a linear approximation was appropriate across the data range. By mapping relative disease odds we were able to evaluate the presence of geographic disease clustering. The data were mapped in GIS using a continuous color scale with red indicating higher disease odds and blue indicating lower disease odds.
Sensitivity analyses
Given the potential that diagnosis of EoE in rural areas could reflect, at least in part, an artifact of volume of EGD performance, we conducted additional sensitivity analyses. First, we assessed the correlation between EGD frequency and population density. Next, we adjusted for the number of endoscopies in the database conducted at a given endoscopy center. Then, to evaluate the potential that patients from rural areas were more symptomatic (for example, due to limited access to care), we assessed whether the proportion of normal biopsies was different when stratifying by population density quintile. Finally, we repeated the analysis with another disease condition as the comparator group. Specifically, we evaluated whether there were differences in EoE diagnosis by population density as compared to cases of reflux esophagitis, as defined on esophageal biopsy by a mixed active/chronic inflammatory pattern with basal hyperplasia. Reflux esophagitis was chosen because there was no a priori reason that this should be impacted by population density. Therefore, if the estimates from the primary analysis were biased by patient or endoscopy center factors, we would expect the comparison of EoE to reflux esophagitis would generate a null result. All sensitivity analyses were conducted using the first EoE case definition listed above of esophageal eosinophilia of ≥15 eos/hpf, with symptoms of dysphagia.
Results
Primary analyses
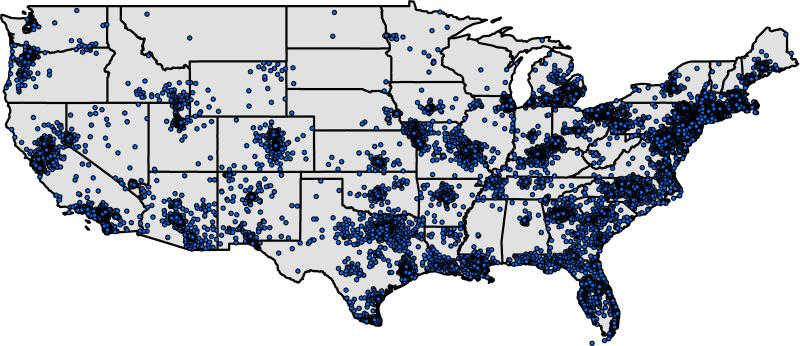
The final study sample included 89,754 patients with normal biopsies and 14,381 patients with esophageal eosinophilia. Examination of demographic and clinical features for the study population indicated that patients with esophageal eosinophilia were typically younger, more commonly male, and had a higher proportion of dysphagia compared with subjects with normal esophageal biopsies (Table 1). A higher proportion of patients with normal esophageal biopsies had documentation of heartburn, weight loss, and abdominal pain/dyspepsia. The proportion of the population that was White was similar between the groups, as was the mean distance travelled to an endoscopy center. The geographical representation of the distribution of patient zip codes revealed that patients with esophageal biopsies were distributed throughout the Unites States, with both rural and urban geographic areas represented (Figure 1).
Table 1.
Demographic characteristics, clinical symptoms, and histological features of patients with esophageal biopsies
| Normal esophageal biopsies (n = 89,754) | Esophageal eosinophiliaa (n = 14,381) | |
|---|---|---|
| Demographic characteristic | ||
| Age (yrs) mean ± SD (IQR) | 53.8 ±17.1 (43.6-66.2) | 45.0 ±16.2 (33.5-55.8) |
| Male n (%) | 31,297 (34.9) | 9,215 (64.1) |
| Clinical symptoms/EGD indications – n (%) | ||
| Dysphagia/odynophagia | 23,098 (25.7) | 7,725 (53.7) |
| Heartburn | 45,595 (50.8) | 5,935 (41.3) |
| Chest pain | 5,438 (6.1) | 478 (3.3) |
| Abdominal pain/dyspepsia | 36,108 (40.2) | 3,671 (25.5) |
| Nausea/vomiting | 7,524 (8.4) | 837 (5.8) |
| Weight loss | 2,739 (3.1) | 227 (1.6) |
| Suspected EoE | 18,212 (20.3) | 6,580 (45.8) |
| Histological features | ||
| Maximum eosinophil count, mean ± SD (IQR) | 0 ± 0.1 (0.0-0.0) | 37.0 ± 24.1 (20.0-50.0) |
| Eosinophil microabscesses n (%) | 0 (0.0) | 3,402 (23.7) |
| Population density by zip code unitb | ||
| Population density persons/mi2 - mean ± SD (IQR) | 4,457.2 ±12,642.0 (414.4-3,304.8) | 3,103.4 ±9,110.0 (360.2-2,951.6) |
| Population reporting white race - mean % ± SD (IQR) | 76.9 ±19.6 (70.0-90.0) | 79.6 ±16.6 (70.0-90.0) |
| Zip code level data | ||
| Distance traveled (miles) for endoscopyc mean ± SD (IQR) | 16.1 ±78.9 (4.0-13.6) | 18.4 ±92.6 (4.3-14.5) |
Patients with esophageal eosinophilia on esophageal biopsy with a maximum count of ≥ 15 eos/hpf
Obtained from linkage to 2010 U.S. Census data
Figure 1.
Residential locations of patients receiving upper endoscopy with esophageal biopsy from 2009 to 2012 in the U.S. Note that exact locations have been altered slightly to preserve confidentiality.
When patients residing in the most populous locations (highest quintile – 3,745-144,333 persons/sq. mile) were compared to patients residing in the least populous locations (lowest quintile - 0-251 persons/sq. mile), there were differences in some patient characteristics (Table 2). Patients residing in rural environments were slightly older and more commonly had dysphagia. The distance traveled to endoscopy center was also greater for these patients. Patients residing in highly populated locations were more likely to have abdominal pain/dyspepsia.
Table 2.
Characteristics of high vs low population density cases of esophageal eosinophilia (≥ 15 eos/hpf)
| Rural cases (n = 2,905) | Urban cases (n = 2,667) | p | |
|---|---|---|---|
| Characteristic | |||
| Age (yrs) mean ± SD (IQR) | 45.8 ±16.2 (34.5-56.8) | 43.1 ±16.3 (30.9-54.5) | <0.001 |
| Male n (%) | 1860 (64.0) | 1708 (64.0) | 0.99 |
| Clinical symptoms/EGD indications n (%) | |||
| Dysphagia/odynophagia | 1714 (59.0) | 1254 (47.0) | <0.001 |
| Heartburn | 1200 (41.3) | 1136 (42.6) | 0.33 |
| Chest pain | 99 (3.4) | 87 (3.3) | 0.76 |
| Abdominal pain/dyspepsia | 720 (24.8) | 781 (29.3) | <0.001 |
| Nausea/vomiting | 169 (5.8) | 157 (5.9) | 0.91 |
| Weight loss | 50 (1.7) | 41 (1.5) | 0.59 |
| Histological features | |||
| Maximum eosinophil count, mean ± SD (IQR) | 37 ±24 (20-50) | 36 ±24 (20-49) | 0.61 |
| Eosinophil microabscesses | 671 (23.6) | 630 (23.1) | 0.64 |
| Population density by zip code unitb | |||
| Population density persons/mi2 - mean ± SD (IQR) | 105.9 ± 70.3 (44.8-159.9) | 11,693.4 ± 18,901.0 (4,440.5-7,716.1) | <0.001 |
| Population reporting white race - mean % ± SD (IQR) | 86.4 ± 13.1(81.3-95.6) | 69.0 ± 19.3(60.2-82.9) | <0.001 |
| Zip code level data | |||
| Distance traveled for endoscopy (miles) mean ± SD (IQR)c | 35.4 ±127.8 (11.5-30.3) | 13.1 ±96.4 (2.8-8.9) | <0.001 |
a Low represents lowest quintile of population distribution, high represents highest quintile.
Obtained from linkage to 2010 U.S. Census
Population density was inversely associated with both esophageal eosinophilia and our case definitions of eosinophilic esophagitis. Specifically, as population density decreased, the odds of disease were significantly increased (p for trend <0.001) (Tables 3 and 4). For example, for eosinophilic esophagitis defined as the presence of ≥15 eos/hpf with dysphagia, the odds of disease were 59 percent more (aOR 1.59, 95% CI: 1.45, 1.76) among those at the lowest population density as compared to patients residing in the highest population density (Table 3).
Table 3.
Association between population density and case definitions of increasing specificity for eosinophilic esophagitis
| Esophageal eosinophiliaa with dysphagia (n=7,128)e |
Esophageal eosinophilia and exclusion of competing conditionsb (n=3,905)e |
Esophageal eosinophilia, exclusions of competing conditions, and eosinophilic microabscesses (n=1,337)e |
||||
|---|---|---|---|---|---|---|
| Population densityc | OR (95% CI) | aORd (95% CI) | OR (95% CI) | aORd (95% CI) | OR (95% CI) | aORd (95% CI) |
| 0-251 | 1.63 (1.50, 1.76) | 1.59 (1.45, 1.76) | 1.48 (1.33, 1.65) | 1.41 (1.24, 1.60) | 1.53 (1.27, 1.83) | 1.41 (1.14, 1.76) |
| 252-820 | 1.57 (1.45, 1.70) | 1.48 (1.35, 1.61) | 1.48 (1.33, 1.64) | 1.37 (1.21, 1.54) | 1.65 (1.38, 1.97) | 1.54 (1.26, 1.88) |
| 821-1,937 | 1.35 (1.25, 1.47) | 1.26 (1.16, 1.37) | 1.28 (1.15, 1.42) | 1.17 (1.05, 1.31) | 1.45 (1.22, 1.75) | 1.34 (1.11, 1.62) |
| 1,938-3,744 | 1.34 (1.23, 1.45) | 1.26 (1.15, 1.37) | 1.31 (1.18, 1.46) | 1.21 (1.09, 1.35) | 1.37 (1.14, 1.64) | 1.25 (1.04, 1.51) |
| 3,745-144,333 | Referent | Referent | Referent | Referent | Referent | Referent |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.016 | <0.001 |
≥15 eos/hpf
Competing conditions for esophageal eosinophilia included reflux/heartburn symptoms, reflux esophagitis, Barrett's esophagus on biopsy, inflammatory bowel disease, and eosinophilic gastroenteritis
Persons per square mile
Adjusted for age, sex, distance patient traveled to endoscopy site, and the proportion in the population in each zip code that reported White race
Number of patients meeting case group definition
Table 4.
Association between population density and increasing levels of esophageal eosinophilia
| ≥ 15 eos/hpf (n=14,381)c |
≥ 50 eos/hpf (n=3,783)c |
≥ 100 eos/hpf (n=823)c |
||||
|---|---|---|---|---|---|---|
| Population densitya | OR (95% CI) | aORb (95% CI) | OR (95% CI) | aORb (95% CI) | OR (95% CI) | aORb (95% CI) |
| 1-251 | 1.28 (1.21, 1.36) | 1.27 (1.18, 1.36) | 1.38 (1.24, 1.54) | 1.45 (1.27, 1.65) | 1.36 (1.08, 1.72) | 1.51 (1.14, 1.99) |
| 252-820 | 1.27 (1.20, 1.34) | 1.22 (1.15, 1.30) | 1.36 (1.22, 1.51) | 1.41 (1.25, 1.58) | 1.39 (1.11, 1.75) | 1.57 (1.22, 2.03) |
| 821-1,937 | 1.20 (1.13, 1.27) | 1.15 (1.08, 1.22) | 1.34 (1.20, 1.48) | 1.33 (1.19, 1.49) | 1.52 (1.23, 1.89) | 1.60 (1.26, 2.02) |
| 1,938-3,744 | 1.20 (1.13, 1.27) | 1.15 (1.08, 1.22) | 1.28 (1.15, 1.42) | 1.23 (1.10, 1.38) | 1.41 (1.12, 1.76) | 1.39 (1.10, 1.75) |
| 3,745-144,333 | Referent | Referent | Referent | Referent | Referent | Referent |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.016 | 0.003 |
Persons per square mile
Adjusted for age, sex, distance patient traveled to endoscopy site, and the proportion in the population in each zip code that reported white race
Number of patients meeting case group definition
Furthermore, with higher levels of esophageal eosinophilia, the observed inverse relationship strengthened (Table 4). For example, those with esophageal eosinophilia ≥15 eos/hpf had 27 percent higher odds of disease (aOR 1.27; 95% CI: 1.18-1.36) among the lowest compared to the highest quintile of population density, but those with esophageal eosinophilia ≥100 eos/hpf had 51 percent higher odds (aOR 1.51; 95% CI: 1.14-1.99).
Mapping the predicted odds, from multivariate analyses, for esophageal eosinophilia (≥15 eos/hpf) as a function of population density for each zip code unit in the Unites States, identified areas of disease clustering in the western and central portions of the country (shaded in red). The extreme eastern portion of the country indicated relatively lower odds of disease (shaded in blue) (Figure 2). The above estimates from the primarily analyses did not change substantially after also adjusting for climate zone (Supplemental Table 1).
Figure 2.
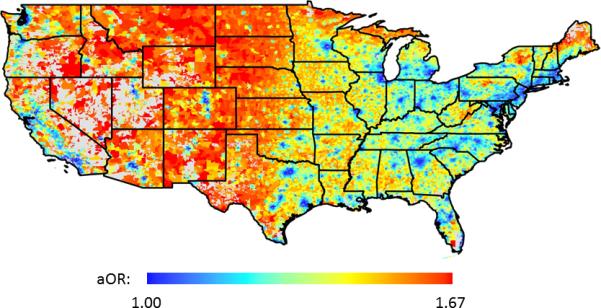
Predicted adjusted OR for eosinophilic esophagitis in each U.S. zip code. Increased odds are colored red, and decreased odds are colored blue. Zip codes with missing population density data are displayed in grey.
Sensitivity analyses
While there was a minor increase in the proportion of normal biopsies in the highest population density quintile, sensitivity analyses did not appreciably change the primary results (Supplemental Table 1). There was no evidence of an association between EGD volume and population density (Spearman's rho = −0.003, p=0.11). Results were also robust after evaluating for the potential for confounding by EGD frequency (Supplemental Table 1). In addition, the observed trend was unchanged when using reflux esophagitis as the comparator population.
Discussion
While the allergen-mediated pathogenesis of EoE is starting to be elucidated (3, 4), the specific risk factors that may predispose to or trigger the disease are poorly understood. EoE is becoming increasingly more common (15, 16, 29), but the relative rarity of the disease creates challenges for studying etiologic mechanisms as most studies may be either underpowered or subject to the biases inherent to hospital-based studies arising from a single center. In the present study we used a large, national pathology database to assess the relationship between population density, an exposure chosen to reflect urban or rural status, and the prevalence of esophageal eosinophilia, both as an eosinophil cell count alone and incorporating clinical findings to approximate a case definition of EoE. The results indicate a strong inverse association between population density and development of esophageal eosinophilia or eosinophilic esophagitis; those living in rural areas with a low population density had higher odds of having disease.
How can this result be interpreted in the context of what is known about EoE and other atopic diseases? With the exception of one prior physician survey which found that cases of EoE were more common in some rural areas (6), a recent study detecting no difference in the frequency of EoE diagnosis in urban or rural settings using a 1,000 person/square mile cut-point for rural versus urban status (30), and an abstract from a single center showing more EoE cases from rural areas than urban areas (23), this association has not been previously described in the EoE literature. In some respects, this finding is counterintuitive. Subjects in less populous areas have to travel further to obtain care, so one might expect a priori that diagnosed cases would be less common in rural areas, even if the true population prevalence was not associated with rural status. Additionally, the hygiene hypothesis (31-34) might suggest that the chances of developing an atopic disease in a rural setting would be less, because increased exposure to environmental pathogens and allergens may cause increased immune tolerance compared to what is encountered in urban settings. However, the epidemiologic evidence to support this supposition is mixed (35-37), and definitions of urban/rural status are not always consistent (38).
Our finding is intriguing, however, as it can be used to generate hypotheses about environmental triggers that may offer an improved understanding of the etiologic mechanisms for disease development. Population density may serve as a proxy measure for other exposures of interest. For example, less populous regions may have higher exposures to agricultural application of pesticides or herbicides, higher density of livestock, higher exposure to plant-based allergens (39), or differences in exposure to particulate matter (size and species) (40-43). For example, particulate matter (PM) in rural areas is more likely to be coarse (e.g. PM aerodynamic size between 2.5 and 10 micrometers), which typically arises from soil and road dust, whereas finer particulate matter (PM ≤2.5) is typically associated with industrial areas and areas of higher population density (40). Coarse PM has been associated with respiratory and cardiovascular disease and can induce oxidative stress and inflammation(41-43). It is unknown whether PM or other environmental exposures could explain geographical differences observed in EoE, but this is something that could be studied both in animal models and further epidemiologic studies.
Another area for further exploration is assessing whether the associations observed are evident in both the pediatric and adult populations. In the present study, only 3% of the biopsies were obtained from individuals <18 years of age. We attempted to evaluate whether the association between population density and EoE persisted in the pediatric population and found that the magnitude of the estimates were unchanged, but that the confidence intervals were wide, reflecting a lack of power to determine age-based differences in these data.
It is important to acknowledge possible limitations in this study. First is the potential for misclassification of disease status. Because the data source was a pathologic database, clinical information is limited. Additionally, while we know these patients had esophageal eosinophilia, we are unable to know whether they met consensus diagnostic guidelines for EoE (2) or if PPI-responsive esophageal eosinophilia was excluded (2, 44). For this reason, we specially constructed the study to assess the association of esophageal eosinophilia with population density, but performed additional analyses examining not only increasingly higher eosinophil counts, but a number of different EoE case definitions that approximate the clinical diagnosis. Any misclassification that did occur is likely to be non-differential with respect to population density and thus could bias estimates toward the null. Moreover, the increasing magnitude of estimates with increasingly stringent case definitions supports the robustness of study results despite the potential for misclassification.
A related limitation is that the data include patient zip code of residence only and therefore there is a potential for misclassification of population density as well. The population density of residence represented an average across the zip code of residence. This may or may not accurately reflect the population density of the patient's resident address. However, in this situation as well, any misclassification of exposure status is also likely to be non-differential and could bias estimates toward the null.
Other limitations include the possibility of confounding. We were only able to use zip code level data to characterize race and race could be a confounder in the association between factors related to geographic location and esophageal eosinophilia. However, inclusion of zip code level race in the adjusted analyses did not substantively change the estimates. Our data also included the patient residence as reported at the time of endoscopy. For prevalent EoE cases, the zip code of residence at the time of endoscopy may not reflect the zip code at time of disease onset. This could contribute to non-differential, mischaracterization of geographic density and thus an attenuation of study estimates. Additionally, although Miraca Life Sciences provides pathology services to a wide range of outpatient endoscopy and surgical centers across the United States, the results of this study may not be generalizable to all patients undergoing endoscopy. Finally, it is not possible to draw conclusions about causality from this association.
Despite these limitations, the study has substantial strengths. We used a large data source with cases from throughout the country. This allowed analysis of one of the largest cohorts of patients with esophageal eosinophilia yet presented in the literature, with a well-matched control group of patients with normal esophageal biopsies. The granularity of the pathologic data, in combination with the breadth of geographic data, makes this a unique resource to investigate questions that cannot be effectively answered at the single, or even multi-center, level. We also conducted analyses for both esophageal eosinophilia and for EoE case definitions. That our results were consistent across all case definitions, and were stronger with increasingly stringent definitions, lend credence to the results. The large number of subjects included in this study also allowed us to control for multiple potential confounders, including distance traveled to the endoscopy center, a proxy measure of access to health care services. Results were also robust to several sensitivity analyses which evaluated the potential for confounding by both endoscopy center volume and patient-related factors. There was also no evidence to support confounding by climate zone. To our knowledge, this is the first study in the EoE literature that has used GIS analysis techniques.
In conclusion, using carefully characterized histologic data collected from outpatient endoscopy centers from throughout the United States, in combination with GIS and epidemiologic methods, we found that population density is strongly and inversely associated with both esophageal eosinophilia and EoE, with these conditions being more common in rural areas. In addition, this association was robust to sensitivity analyses exploring varying case definitions. Environmental exposures in rural areas may be key in the pathogenesis of EoE. Exploration of these factors may yield an improved understanding of the mechanisms for development of EoE.
Supplementary Material
Study highlights.
What is current knowledge?
Eosinophilic esophagitis (EoE) is an immune-mediated disease, but risk factors for the development of EoE have yet to be fully elucidated.
The risk of other atopic diseases differs in rural and urban environments.
Geographic differences in development of EoE may provide etiologic clues for EoE pathogenesis, but the relationship between population density and EoE is unknown.
What is new here?
Population density was strongly and inversely associated with both esophageal eosinophilia and EoE, with these conditions being more common in rural areas.
With higher levels of esophageal eosinophilia, the observed inverse relationship strengthened.
A similar dose-response trend was observed across EoE case definitions with odds of EoE reduced in areas of high population density.
Acknowledgments
Financial support:
This study was funded, in part, by NIH Award K23 DK090073 (ESD).
The study sponsors had no role in the study design, collection, analysis, or interpretation of the data.
Abbreviations
- EoE
eosinophilic esophagitis
- PPI
proton-pump inhibitors
Footnotes
Guarantor of the article: Evan Dellon
Specific author contributions
Jensen: Data analysis, data interpretation, manuscript drafting, critical revision; approved final draft
Hoffman: Database construction, data analysis, data interpretation, critical revision; approved final draft
Shaheen: Data interpretation, critical revision; approved final draft
Genta: Project conception, data collection and database construction, data interpretation, critical revision, supervision; approved final draft
Dellon: Project conception, study design, obtained funding, data interpretation, manuscript drafting, critical revision, supervision; approved final draft
Potential competing interests
Robert Genta is an employee of Miraca Life Sciences. None of the other authors have conflicts of interest related to this manuscript.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). The American journal of gastroenterology. 2013;108:679–92. doi: 10.1038/ajg.2013.71. quiz 693. [DOI] [PubMed] [Google Scholar]
- 3.Liacouras CA. Clinical presentation and treatment of pediatric patients with eosinophilic esophagitis. Gastroenterol Hepatol (N Y) 2011;7:264–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology. 2009;137:1238–49. doi: 10.1053/j.gastro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spergel JM. Eosinophilic esophagitis in adults and children: evidence for a food allergy component in many patients. Curr Opin Allergy Clin Immunol. 2007;7:274–8. doi: 10.1097/ACI.0b013e32813aee4a. [DOI] [PubMed] [Google Scholar]
- 6.Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonis PA. Putting the puzzle together: epidemiological and clinical clues in the etiology of eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:41–52. doi: 10.1016/j.iac.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penfield JD, Lang DM, Goldblum JR, et al. The Role of Allergy Evaluation in Adults With Eosinophilic Esophagitis. J Clin Gastroenterol. 2010;44:22–7. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 10.Al-Subu A, Bevins L, Yulia D, et al. The accuracy of endoscopic features in eosinophilic esophagitis: the experience in children from rural West Virginia. Journal of clinical gastroenterology. 2012;46:e83–6. doi: 10.1097/MCG.0b013e3182471054. [DOI] [PubMed] [Google Scholar]
- 11.Elitsur Y, Aswani R, Lund V, et al. Seasonal distribution and eosinophilic esophagitis: the experience in children living in rural communities. Journal of clinical gastroenterology. 2013;47:287–8. doi: 10.1097/MCG.0b013e31826df861. [DOI] [PubMed] [Google Scholar]
- 12.Akei HS, Mishra A, Blanchard C, et al. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129:985–94. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Mishra A, Hogan SP, Brandt EB, et al. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf WA, Jerath MR, Dellon ES. De-novo onset of eosinophilic esophagitis after large volume allergen exposures. Journal of gastrointestinal and liver diseases : JGLD. 2013;22:205–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128:1349–1350. e5. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mate-Jimenez J, Munoz S, Vicent D, et al. Incidence and prevalence of ulcerative colitis and Crohn's disease in urban and rural areas of Spain from 1981 to 1988. Journal of clinical gastroenterology. 1994;18:27–31. doi: 10.1097/00004836-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Soon I, Molodecky N, Rabi D, et al. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterology. 2012;12:51. doi: 10.1186/1471-230X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotgiu S, Pugliatti M, Sotgiu A, et al. Does the “hygiene hypothesis” provide an explanation for the high prevalence of multiple sclerosis in Sardinia? Autoimmunity. 2003;36:257–60. doi: 10.1080/0891693031000151607. [DOI] [PubMed] [Google Scholar]
- 20.Schram ME, Tedja AM, Spijker R, et al. Is there a rural/urban gradient in the prevalence of eczema? A systematic review. British Journal of Dermatology. 2010;162:964–973. doi: 10.1111/j.1365-2133.2010.09689.x. [DOI] [PubMed] [Google Scholar]
- 21.Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011;141:1586–92. doi: 10.1053/j.gastro.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107:698–706. doi: 10.1038/ajg.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellon ES, Irani AM, Hill MR, et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634–42. doi: 10.1111/apt.12413. [DOI] [PubMed] [Google Scholar]
- 24.Gill R, Durst P, Rewalt M, et al. Eosinophilic Esophagitis Disease in Children from West Virginia: A Review of the Last Decade (1995-2004). Am J Gastroenterol. 2007;102:2281–5. doi: 10.1111/j.1572-0241.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 25.Hurrell JM, Sonnenberg A, Genta RM. Seasonal patterns in eosinophilic esophagitis: An analysis by month of diagnosis and month of birth. Am J Gastroenterol. 2011;106(Suppl 2):S2, Ab 5. [Google Scholar]
- 26.Kapel RC, Miller JK, Torres C, et al. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–21. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Bureau USDoC-USC. U.S. Department of Commerce Census Bureau Zip code tabulation areas. 2008 [cited; Available from: http://www.census.gov/geo/reference/zctas.html.
- 28.Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59–71. viii–ix. doi: 10.1016/j.giec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 30.Lee YJ, Redd M, Bayman L, et al. Comparison of clinical features in patients with eosinophilic esophagitis living in an urban and rural environment. Dis Esophagus. 2014 doi: 10.1111/dote.12164. [DOI] [PubMed] [Google Scholar]
- 31.Klement E, Lysy J, Hoshen M, et al. Childhood hygiene is associated with the risk for inflammatory bowel disease: a population-based study. The American journal of gastroenterology. 2008;103:1775–1782. doi: 10.1111/j.1572-0241.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs O, Genuneit J, Latzin P, et al. Farming environments and childhood atopy, wheeze, lung function, and exhaled nitric oxide. The Journal of allergy and clinical immunology. 2012;130:382–8. e6. doi: 10.1016/j.jaci.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 33.Okada H, Kuhn C, Feillet H, et al. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ege MJ, Mayer M, Normand A-C, et al. Exposure to Environmental Microorganisms and Childhood Asthma. New England Journal of Medicine. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 35.Rennie DC, Lawson JA, Senthilselvan A, et al. Domestic endotoxin exposure and asthma in children: epidemiological studies. Frontiers in bioscience. 2012;4:56–73. doi: 10.2741/e360. [DOI] [PubMed] [Google Scholar]
- 36.Limb M. Scientists debunk idea that rise in allergic diseases is due to homes becoming “too clean”. BMJ. 2012:345. doi: 10.1136/bmj.e6673. [DOI] [PubMed] [Google Scholar]
- 37.Genuneit J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with meta-analysis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2012;23:509–18. doi: 10.1111/j.1399-3038.2012.01312.x. [DOI] [PubMed] [Google Scholar]
- 38.Lim A. How are 'urban' and 'rural' defined in publications regarding asthma and related diseases? Allergologia et immunopathologia. 2013 doi: 10.1016/j.aller.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 39.van Rhijn BD, Smout AJ, Bredenoord AJ. [Eosinophilic oesophagitis: a frequently missed cause of dysphagia]. Ned Tijdschr Geneeskd. 2012;156:A4716. [PubMed] [Google Scholar]
- 40.Li R, Wiedinmyer C, Hannigan MP. Contrast and correlations between coarse and fine particulate matter in the United States. The Science of the total environment. 2013;456-457:346–58. doi: 10.1016/j.scitotenv.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 41.James K, Farrell RE, Siciliano SD. Comparison of human exposure pathways in an urban brownfield: reduced risk from paving roads. Environmental toxicology and chemistry / SETAC. 2012;31:2423–30. doi: 10.1002/etc.1952. [DOI] [PubMed] [Google Scholar]
- 42.Malig BJ, Green S, Basu R, et al. Coarse particles and respiratory emergency department visits in california. American Journal of Epidemiology. 2013;178:58–69. doi: 10.1093/aje/kws451. [DOI] [PubMed] [Google Scholar]
- 43.Willers SM, Eriksson C, Gidhagen L, et al. Fine and coarse particulate air pollution in relation to respiratory health in Sweden. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2013 doi: 10.1183/09031936.00088212. [DOI] [PubMed] [Google Scholar]
- 44.Molina-Infante J, Hernandez-Alonso M, Vinagre-Rodriguez G, et al. Proton pump inhibitors therapy for esophageal eosinophilia: simply following consensus guidelines. J Gastroenterol. 2011;46:712–3. doi: 10.1007/s00535-011-0388-8. author reply 714-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.