Abstract
Neonatal exposure to (+)-methamphetamine (Meth) results in long-term behavioural abnormalities but its developmental mechanisms are unknown. In a series of experiments, rats were treated from post-natal days (PD) 11–20 (stage that approximates human development from the second to third trimester) with Meth or saline and assessed using locomotor activity as the readout following pharmacological challenge doses with dopamine, serotonin and glutamate agonists or antagonists during adulthood. Exposure to Meth early in life resulted in an exaggerated adult locomotor hyperactivity response to the dopamine D1 agonist SKF-82958 at multiple doses, a high dose only under-response activating effect of the D2 agonist quinpirole, and an exaggerated under-response to the activating effect of the N-methyl-D-aspartic acid (NMDA) receptor antagonist, MK-801. No change in locomotor response was seen following challenge with the 5-HT releaser p-chloroamphetamine or the 5-HT2/3 receptor agonist, quipazine. These are the first data to show that PD 11-20 Meth exposure induces long-lasting alterations to dopamine D1, D2 and glutamate NMDA receptor function and may suggest how developmental Meth exposure leads to many of its long-term adverse effects.
Keywords: Development, locomotor activity, methamphetamine, MK-801, p-chloroamphetamine, quinpirole, quipazine, SKF82958
Introduction
The majority of methamphetamine (Meth) users are of reproductive age (Kuczkowski, 2007; Substance Abuse & Mental Health Services Administration, 2009). Since approximately half are women and some are pregnant, the likelihood is high that some children are exposed in utero to Meth, yet the consequences of such exposures are largely unknown. A recent study found that, among pregnant women seeking treatment in 2006, nearly one in four (24%) reported Meth as their primary drug of abuse, up from 8% in 1994 (Terplan et al. 2009). Effects in exposed children documented thus far include reduced birth weight, height and head circumference (Chomchai et al. 2004; Dixon & Bejar, 1989; Little et al. 1988; Smith et al. 2008) and withdrawal symptoms shortly after birth (Chomchai et al. 2004; Dixon, 1989; Oro & Dixon, 1987). Later changes include growth reduction (Smith et al. 2003), neuroanatomical changes shown with magnetic resonance imaging (Chang et al. 2004; Cloak et al. 2009), elevated physiological stress (Smith et al. 2008) and learning and memory deficits (Chang et al. 2009; Struthers & Hansen, 1992).
We developed a preclinical model of mid- to late-prenatal exposure that shows related findings. Developmental Meth exposure in rats also causes weight reductions, elevated physiological stress and learning and memory deficits (Grace et al. 2008; Vorhees et al. 1994, 2007, 2008, 2009; Williams et al. 2002, 2003a, b, c). The most sensitive exposure period for these effects is post-natal days (PD) 11–20, an interval that corresponds to late second to third trimester in humans based on neurogenesis rates across species (Clancy et al. 2001, 2007a, b; Rice & Barone, 2000). However, the mechanisms that lead to cognitive deficits remain unknown.
A number of neurotransmitters and their receptors have been shown to be altered by Meth. For instance, the cholinergic system is altered (increasedM1mAChR number) in mice with developmental Meth-induced novel object and novel place recognition deficits (Siegel et al. 2010). Histamine and its receptors are also altered by Meth use and is involved in the cognitive deficits following both developmental and adult exposure to the drug (Acevedo & Raber, 2011; Noda et al. 2010). The GABAergic (Zhu et al. 2006) and norepinephrine (Graham et al. 2008) systems are also vulnerable to Meth toxicity. However, research has focused primarily upon dopamine (DA), serotonin (5-HT) and glutamate in adult animals. For instance, adult Meth exposure affects all three of these systems in both rodents and humans (Cadet & Krasnova, 2009). These same molecules influence the development of neurons and associated neurocircuitry at early stages of ontogeny (Thompson et al. 2009). We demonstrated that Meth administration from PD 11–20 produces long-term reductions in striatal DA and D2-like receptors (Crawford et al. 2003). DA receptors are involved in neuronal cell cycle progression (Ohtani et al. 2003), GABAergic migration (Crandall et al. 2007) and dendritic growth (Song et al. 2002) during development. While 5-HT is also reduced in neostriatum and entorhinal cortex following developmental Meth exposure (Grace et al. 2010) and 5-HT receptor levels are decreased in adult rats exposed to Meth (McCabe et al. 1987a), it is not clear if 5-HT receptors are affected by developmental Meth exposure, although others have shown that gestational Meth exposure does not affect some 5-HT receptor subtypes in male progeny (Cabrera et al. 1993). Since 5-HT is neurotrophic during development, (Whitaker-Azmitia et al. 1996), it is a candidate for influencing long-term outcome. This is supported by the finding that neonatal 5-HT depletion using the tryptophan hydroxylase inhibitor p-chlorophenylalanine results in behavioural and neurochemical abnormalities in the offspring (Mazer et al. 1997). Glutamatergic receptors, particularly the N-methyl-D-aspartic acid (NMDA) subtype, are important in the plasticity and structure of the developing brain (Scheetz & Constantine-Paton, 1994). NMDA receptors have been implicated in the maturation of cortical circuitry (Grutzendler et al. 2002) as well as the stabilization of synaptic connections (Parrish et al. 2007) and contribute to Meth neurotoxicity in mice (Sonsalla et al. 1998). As with 5-HT receptors, little is known about how developmental Meth exposure alters NMDA levels or function. However, studies by Slamberova and colleagues have demonstrated that early exposure to Meth results in increased sensitivity to NMDA-induced seizures later in life (Slamberova & Rokyta, 2005a, b ; Slamberova et al. 2009), thus implicating Meth-induced alterations to this receptor system.
Based on such evidence, we hypothesized that developmental Meth treatment induces alterations in DA, 5-HT and glutamatergic receptor function. The purpose of the experiments was to test this using locomotor activity as the outcome following drug challenge with selective agonists and/or antagonists for a subset of the receptors previously implicated in the effects of Meth in adults.
Materials and method
Animals
Male and nulliparous female (175–200 g) Sprague– Dawley (IGS) rats (Charles River Laboratories, USA), were bred in-house after at least 1 wk of acclimatization in the vivarium (AAALAC-accredited). The animal facility is controlled for temperature (20 ± 1 °C) and humidity (50 ± 10%) and is maintained on a 14:10 h light–dark cycle (lights on 06:00 hours). Throughout the study, rats had access to food and filtered water ad libitum. Presence of a sperm plug was designated embryonic day (ED) 0 and on ED 1 females were transferred to polycarbonate cages (46 × 24 × 20 cm) containing woodchip bedding. Day of birth was designated PD 0 and on PD 1 litters were culled to 12 pups, although if a litter contained <12, up to two pups from another litter born the same day were fostered to attain the appropriate litter size. Each litter contained at least six males as males were retained preferentially for this study. All animal procedures were approved by the Institutional Animal Care and Use Committee.
Developmental drug treatment
Four daily injections (every 2 h) of (+)-Meth HCl (10 mg/kg, expressed as the freebase, NIDA, >95% pure) were administered from PD 11–20 to half the males in each litter (range 3–6), while the remaining half received saline (Sal). Injections were administered s.c. This dose is similar to those used by some chronic users (Melega et al. 2007) when scaled to take into account species differences in size and metabolic rate between humans and rats (Mordenti & Chappell, 1989). Using Mordenti & Chapell’s formula 48, a PD 11 rat weighing 25 g and receiving a dose of 10 mg/kg Meth would be equivalent to an adult woman taking a dose of 58 mg Meth, or~1 mg/kg (assuming a human body weight of 60 kg). This is within the range of human addiction (Melega et al. 2007). In rodents, it has been demonstrated that maternal and fetal blood levels of Meth are similar (White et al. 2009) and in pregnant ewes Meth reaches an initially higher peak in maternal than in fetal plasma (Burchfield et al. 1991) but by 1 and 2 h post-treatment, foetal plasma Meth concentrations exceed maternal levels. Given this, direct treatment of pups is a reasonable approximation of human third trimester-equivalent exposure, given that the equivalent states of brain development in the rat occur postnatal (Clancy et al. 2007a, b). All drugs were delivered in a volume of 3 ml/kg normal Sal. Animals were weaned on PD 28 and housed in pairs.
Locomotor activity
Animals underwent locomotor activity testing at PD 60–70. Each rat was tested only once. On the day of testing, rats were weighed and placed in the locomotor chambers (41 cm × 41 cm; AccuScan Electronics, USA) for 1 h to habituate them to the test environment. Rats were removed, administered one of the pharmacological challenge drugs and returned to the test chamber for an additional 3 h. One challenge drug at one dose level was assigned to each rat within a litter. To establish dose-effect curves, three doses were utilized, such that one PD 11–20 Sal- and one Meth-treated pair from each litter were administered one of the three different doses per drug (Table 1).
Table 1.
Pharmacological challenges used for measurements of locomotor activity
| Drug challenge | Target receptor | Doses used part A (mg/kg) | Doses used part B (mg/kg) |
|---|---|---|---|
| MK-801 | Glutamate: NMDA receptor antagonist | 0.1, 0.2, 0.3 | 0.15, 0.20, 0.25 |
| SKF-82958 | Dopamine: D1 receptor agonist | 0.1, 1.0, 2.0 | 0.5, 1.0, 1.5 |
| Quinpirole | Dopamine: D2 receptor agonist | 0.5, 1.0, 1.5 | 1.5, 2.0, 3.0 |
| Quipazine | Serotonin : 5-HT2 receptor agonist | 0.1, 0.3, 0.5 | n.a. |
| p-Chloroamphetamine | Serotonin : 5-HT releasing compound | n.a. | 2.5, 3.75, 5.0 |
NMDA, N-Methyl-D-aspartic acid.
Drug challenges (part A)
The following challenges were used: (1) MK-801, a glutamatergic NMDA receptor antagonist, at doses of 0.1, 0.2 or 0.3 mg/kg (Bubenikova-Valesova et al. 2007; Jacobs et al. 2000; Su et al. 2007); (2) SKF-82958, a DA D1 receptor agonist, at doses of 0.1, 1.0 or 2.0 mg/kg (Maneuf et al. 1997); (3) quinpirole, a DA D2/3 receptor agonist, at doses of 0.5, 1.0 or 1.5 mg/kg (Stuchlik et al. 2007); or (4) quipazine, a non-selective serotonin 5-HT2/3 receptor agonist, was given of 0.1, 0.3 or 0.5 mg/kg (Antri et al. 2005; Ichiyama et al. 2008). All drugs were obtained from Sigma-Aldrich (USA). Following each challenge, animals were placed back in the locomotor chambers and activity was recorded for an additional 3 h. Dependent measures analysed were horizontal and regional (central vs. peripheral) distance travelled and were analysed in 10-min intervals. However, since no differential patterns were found between central and peripheral distance vs. total horizontal distance, regional data are not presented. Chambers were cleaned with 70% ethanol between subjects. At least 16 rats were used per challenge dose (n=16 per treatment × challenge × dose).
Drug challenges (part B)
Once basic patterns of effects were established in part A, effective doses of those showing effects were refined in part B and, in the case of 5-HT, a different drug was tested since no effect of quipazine was found in part A. New litters were treated with Meth or Sal from PD 11–20 as above and locomotor activity tested as in part A. The challenge drugs were: (1) MK-801 at doses of 0.15, 0.2 or 0.25 mg/kg; (2) SKF-82958 at adjusted doses of 0.5, 1.0 (same) or 1.5 mg/kg; (3) quinpirole at adjusted doses of 1.5, 2.0 (same) or 3.0 mg/kg; or (4) p-chloroamphetamine (PCA), a 5-HT releaser, at doses of 2.5, 3.75 or 5.0 mg/kg (Callaway et al. 1993; Sugita et al. 1994) (Table 1). As before, at least 16 rats were used for each treatment × challenge × dose group with no more than one rat per group per challenge dose level taken from any given litter to control for litter effects.
Data analyses
Data were analysed using mixed linear factorial analysis of variance (ANOVA; SAS v. 9.2 ; SAS Institute, USA). Pre-challenge and post-challenge data were analysed separately for each drug at each dose. In order to account for litter effects, litter was a block factor in a completely randomized block design with treatment as the fixed factor within blocks and interval as the repeated measure factor. Significant interactions were further analysed using slice-effect ANOVAs by interval. Where significant treatment effects were found in part A, these were used to make predictions about direction of change in part B. In these cases, analyses used pre-planned comparison methods (for MK-801 and SKF-82958). Degrees of freedom were by the Kenward–Roger method. Significance was set at p ≤ 0.05 ; data are presented as least square (LS) means and LS S.E.M.
Results
Quipazine: pre-challenge activity
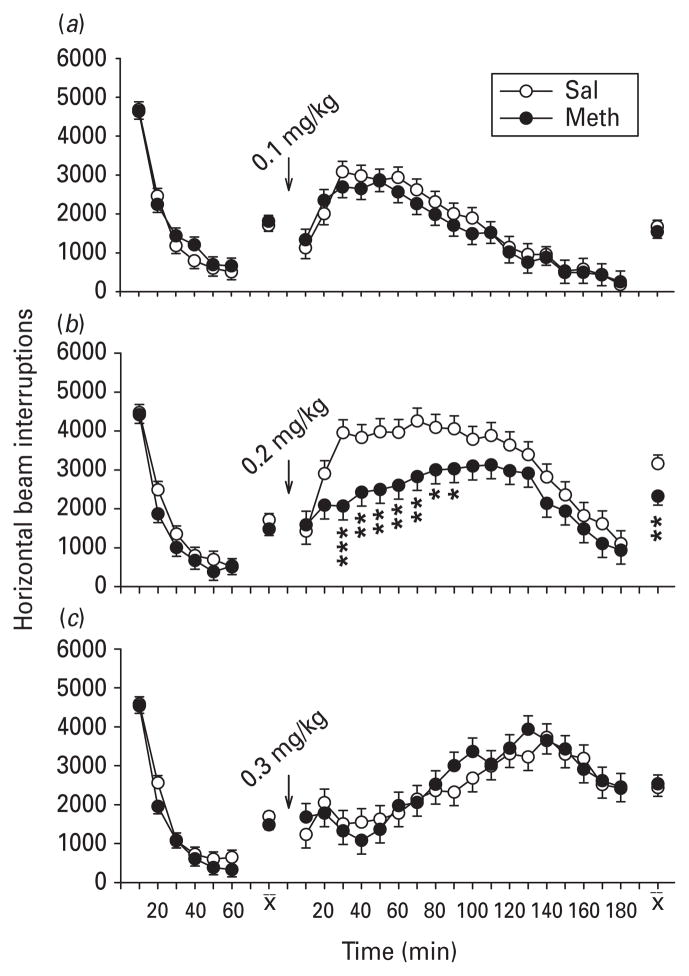
Prior to challenge in the 0.1 mg/kg dosage group, there was a significant treatment × interval interaction during the habituation period (F5,114=3.87, p<0.001), such that Meth-treated rats were hypoactive compared to Sal-treated rats, but only during the second interval ; there were no group differences by the last pre-challenge interval (Fig. 1 a). No differences in activity were noted for the 0.3 mg/kg dose (Fig. 1 b) or the 0.5 mg/kg dose between Meth and Sal treatments (Fig. 1 c).
Fig. 1.
Locomotor activity and quipazine: activity (least square mean ± S.E.M.) before (1 h) and after (3 h) quipazine challenge. (a) 0.1, (b) 0.3 and (c) 0.5 mg/kg quipazine treatment groups. Quipazine was administered s.c. to adult rats treated on post-natal days 11–20 with methamphetamine (Meth) or saline (Sal). A significant interaction (treatment × interval) was found pre-challenge with the low dose condition at 1 interval (20 min) in which the Meth group was less active than the Sal group (a). Post-challenge, no differential response to quipazine was found at any dose level. n=15–16 per group per challenge dose level. *** p<0.001 vs. Sal.
Quipazine: post-challenge activity
Following quipazine, all groups showed an increase in activity that lasted <1 h. There were no significant differential responses to quipazine at all doses between the Meth- and Sal-treated groups.
PCA: pre-challenge activity
To further evaluate possible serotonergic involvement, PCA was used (Fig. 2). No differences were noted at the low dose (2.5 mg/kg; Fig. 2 a). Prior to challenge at the mid-dose (3.75 mg/kg), Meth-treated animals were less active than Sal-treated animals (Fig. 2 b), Treatment (F1,45.4=5.24, p<0.05). At the high dose (Fig. 2 c ; 5 mg/kg), there was a treatment × interval interaction (F5,151=2.77, p<0.05). The effect was confined to interval 6.
Fig. 2.
Locomotor activity and p-chloroamphetamine (PCA): activity (least square mean ± S.E.M.) before and after PCA challenge. There were no significant pre-challenge group differences in the low or high PCA conditions, but there was a main effect of treatment in the mid-dose condition in which the methamphetamine (Meth)-treated group was less active than the saline (Sal)-treated group but the effect was no longer present during the final habituation interval prior to PCA administration. In the high dose pre-challenge animals there was a significant interaction with the Meth-treated animals showing less activity than Sal-treated animals at the last interval. Post-challenge PCA had no differential effect on activity because of Meth vs. Sal treatment on post-natal days 11–20. (a) 2.5, (b) 3.75 and (c) 5.0 mg/kg PCA given s.c. n=20–22 per group per dose level. * p<0.05 vs. Sal.
PCA: post-challenge activity
At the low and mid doses, PCA induced no increase in activity, but it did prevent continuation of the down-ward habituation curve that normally appears if no drug or Sal is given. At the highest dose, both groups showed slight, albeit significant, hyperactivity that developed gradually after ~90 min. At the high dose, the Meth-treated group showed a slightly larger increase than the Sal-treated group, but the difference was not significant.
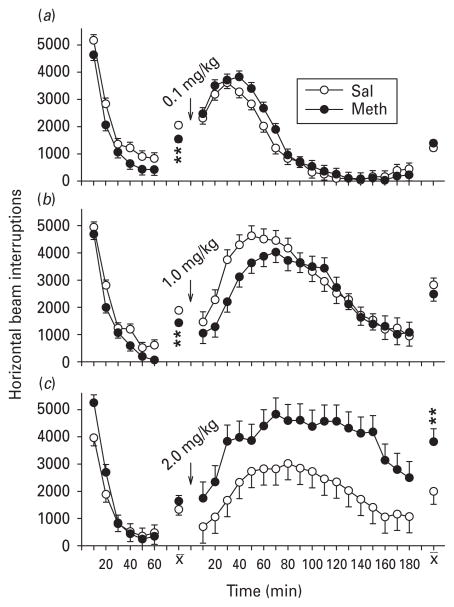
MK-801: pre-challenge activity (part A)
No significant differences were observed in activity during the pre-challenge habituation between Meth- and Sal-treated groups, i.e. all groups showed comparable initial exploration followed by decline as habituation to the novel environment progressed.
MK-801: post-challenge activity (part A)
MK-801 stimulated locomotor activity in all groups at all doses. No significant differential changes in locomotion were detected following administration of the low dose (0.1 mg/kg) between Meth- and Sal-treated groups (Fig. 3 a). A significant main effect of treatment was found following administration of MK-801 at the mid-level dose (0.2 mg/kg; Fig. 3 b), with the Meth-treated group showing significantly reduced hyperactivity (F1,40.8=8.72, p<0.01) compared to the Sal-treated group. A treatment × interval interaction (F17,550=1.98, p ≤ 0.01) was also obtained, with Meth-treated rats exhibiting reduced hyperactivity compared to Sal-treated rats from 20–90 min after MK-801. A treatment × interval interaction was also observed following treatment with the highest dose of MK-801 [0.3 mg/kg (F17,547=1.91, p<0.05)] ; however, no interval slice-effect ANOVA for any one interval was significant (Fig. 3 c). The interaction appears to have occurred because the Meth-treated group slightly under- responded to MK-801 at 40 min and modestly over-responded at 90, 100 and 130 min, creating a cross-over of the two curves.
Fig. 3.
Locomotor activity and MK-801 (part A): activity (least square mean ± S.E.M.) before and after MK-801 challenge. Pre-challenge, there were no significant group differences as a function of post-natal days 11–20 methamphetamine (Meth) vs. saline (Sal) treatment in the different dose groups. (a) 0.1, (b) 0.2 and (c) 0.3 mg/kg MK-801 given s.c. A significant treatment main effect and treatment × interval interaction were uncovered at the 0.2 mg/kg challenge dose and a complex treatment × interval interaction at the 0.3 mg/kg challenge dose that is not marked as the curves crossed one another and no slice × interval analyses of variance were significant for this condition. n=17–19 per group per challenge dose level. * p<0.05, ** p<0.01, *** p<0.001 vs. Sal.
MK-801: pre-challenge activity (part B)
To refine the effective doses of MK-801, smaller incremental doses were used in part B (Fig. 4) together with a repeat of the mid dose. There was a significant difference between treatment groups in the pre-challenge phase in those groups assigned to the MK- 801 mid dose condition (F1,31.4=7.6, p<0.01) ; this effect, although significant, was attributable to intervals 3 and 4. There were no group differences during intervals 5 and 6 by direct t test (Fig. 4 b). No differences were observed in the groups assigned to the lower (Fig. 4 a) or higher (Fig. 4 c) dose.
Fig. 4.
Locomotor activity and MK-801 (part B) : activity (least square mean ± S.E.M.) before and after MK-801 challenge. Pre-challenge, there were no significant group differences as a function of post-natal days 11–20 methamphetamine (Meth) vs. saline (Sal) treatment in the low or high dose conditions, but there was at the mid-dose condition because the Meth-treated group was more active than the Sal-treated group but the effect was no longer present during the final two habituation intervals prior to MK-801 administration. (a) 0.15, (b) 0.2 and (c) 0.25 mg/kg MK-801 given s.c. Developmental Meth treatment resulted in a large number of significant changes due to treatment and treatment × interval following MK-801 challenge in which the Meth-treated groups showed attenuated responses to MK-801 compared to Sal-treated groups. n=14–16 per group per challenge dose level. * p<0.05, ** p<0.01, *** p<0.001 vs. Sal.
MK-801: post-challenge activity (part B)
All doses of MK-801 induced hyperactivity. Pre-planned comparisons predicting reduced hyperactivity following MK-801 in Meth-treated animals were used and significant main effects of treatment were found at all three challenge dose levels (p<0.05 to p<0.001). Following the low dose (0.15 mg/kg), Meth-treated rats were less hyperactive than their Sal-treated counterparts from 20 to 70 min post-challenge (Fig. 4 a). Similar findings were observed following the mid-level dose (0.2 mg/kg), in which Meth-treated rats were significantly less hyperactive from 10 to 150 min post-challenge except at 110–120 min (Fig. 4 b). Following the high dose (0.25 mg/kg), the Meth-treated group was also significantly less hyperactive from 30 to 120 min post-challenge (Fig. 4 c) but as the hyperactivity in the Meth-treated group increased, the under-response in the Meth-treated group appeared to reach a ceiling that caused the magnitude of the difference to be compressed toward the Sal-treated group.
Quinpirole: pre-challenge activity (part A)
During the habituation period, there were no significant differences between the Meth-treated and Sal-treated groups (Fig. 5a–c) ; all groups showed typical exploratory and habituation curves.
Fig. 5.
Locomotor activity and quinpirole (part A): activity (least square mean ± S.E.M.) before and after quinpirole. Pre-challenge, there were no significant group differences as a function of post-natal days (PD) 11–20 methamphetamine (Meth) vs. saline (Sal) treatment. Post-challenge, there were no significant differential effects of quinpirole challenge as a function of PD 11–20 Meth vs. Sal treatment. (a) 0.5, (b) 1.0 and (c) 1.5 mg/kg of quinpirole given s.c. n=17–19 per group per challenge dose level.
Quinpirole: post-challenge activity (part A)
Following quinpirole challenge, all groups showed D2-type hyperactivity with slow onset. No significant effect of treatment or treatment × interval was found at any dose of quinpirole. However, there appeared to be a trend toward over-response at the highest dose in the Meth-treated group; therefore, different doses were used in part B.
Quinpirole: pre-challenge activity (part B)
There were no significant group differences during the pre-challenge habituation interval at any of the doses (Fig. 6a–c).
Fig. 6.
Locomotor activity and quinpirole (part B) : activity (least square mean ± S.E.M.) before and after quinpirole. Pre-challenge, there were no significant group differences as a function of post-natal days 11–20 methamphetamine (Meth) vs. saline (Sal) treatment. Post-challenge, a main effect of treatment was revealed in the Meth-treated group following the moderate dose of quinpirole challenge only in which the Meth-treated group under-responded to the D2 agonist compared to the Sal-treated group. (a) 1.5, (b) 2.0 and (c) 3.0 mg/kg quinpirole given s.c. n=12–15 per group per challenge dose level. * p<0.05 vs. Sal.
Quinpirole: post-challenge activity (part B)
Following quinpirole, the drug again induced slow-onset hyperactivity in all groups. The low dose of quinpirole (1.5 mg/kg, which was the same as the high dose in part A) had no significant differential effect upon activity in Meth-treated vs. Sal-treated groups (Fig. 6 a) as before. At the next higher dose (2.0 mg/kg), however, there was a significant main effect of treatment (F1,30=4.06, p ≤ 0.05), whereby the Meth-treated group was less hyperactive compared to their Sal-treated counterparts (Fig. 6 b). The highest dose of quinpirole (3.0 mg/kg) produced no significant treatment-related main effect or interactions (Fig. 6 c). This appeared to be attributable to the fact that, at this dose, quinpirole no longer induced as much stimulation and instead showed signs of partial antagonism.
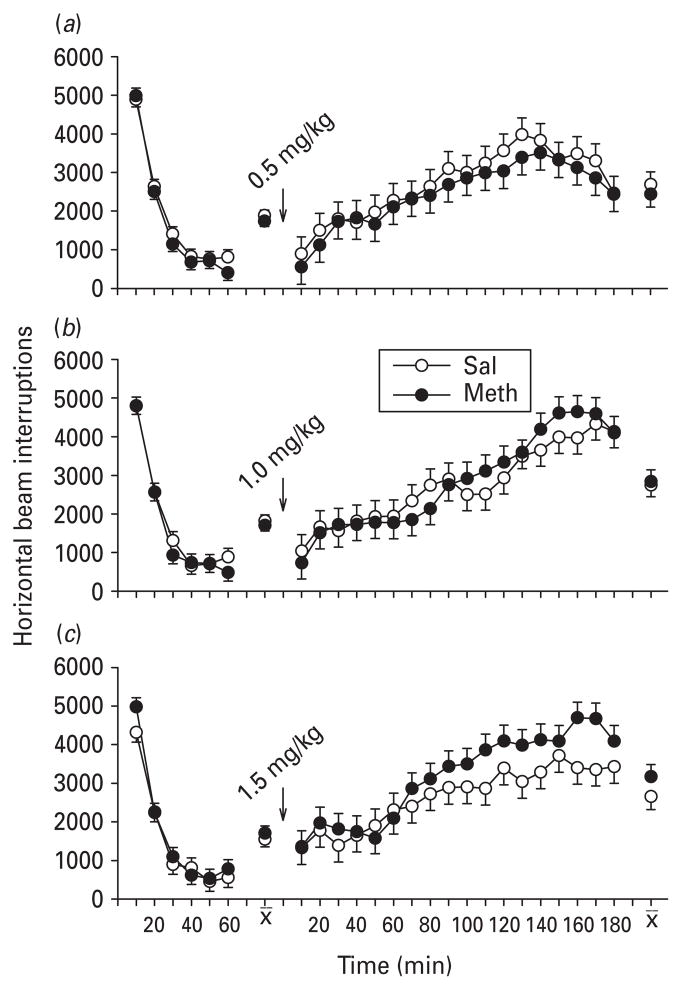
SKF-82928: pre-challenge activity (part A)
During habituation, there was a significant effect of treatment in the groups assigned to low and mid SKF-82958 [0.1 mg/kg (F1,30=9.89, p<0.01) ; Fig. 7 a] and [1.0 mg/kg (F1,32.4=8.01, p<0.01) ; Fig. 7 b] ; in both cases the Meth-treated groups showed hypoactivity compared to Sal-treated groups. This was not seen in the high SKF-82958 challenge dose (Fig. 7 c).
Fig. 7.
Locomotor activity and SKF-82958 (D1 agonist ; part A): activity (least square mean ± S.E.M.) before and after SKF-82958. Pre-challenge group differences were found in the low and mid-dose SKF-82958 challenge between the post-natal days 11–20 methamphetamine (Meth)-treated vs. saline (Sal)-treated groups. In both cases, the Meth-treated group was significantly less active, especially during the last 30 min of the 1 h habituation phase. SKF-82958 induced hyperactivity in all groups; however, neither of the two conditions (low and moderate) that showed pre-challenge differences exhibited any differential responses to the SKF-82958 challenge. By contrast, at the highest challenge dose, where the groups showed no pre-challenge differences, SKF-82958 induced a significantly exaggerated hyperactive response in the Meth-treated group compared to the Sal-treated group. (a) 0.1, (b) 1.0 and (c) 2.0 mg/kg of SKF-82958 given s.c. n=16–17 per group per challenge dose level. ** p<0.01 vs. Sal.
SKF-82928: post-challenge activity (part A)
Post-challenge, all groups at all doses showed marked hyperactivity in response to the drug. No differential effects of prior Meth treatment on SKF-82958-induced hyperactivity were apparent at the low (Fig. 7 a) or intermediate (Fig. 7 b) dose levels. At the highest dose of SKF-82958, an exaggerated hyperactivity in the Meth-treated group compared to the Sal-treated group was found; main effect of treatment: F1,31.6= 7.28, p ≤ 0.01 (Fig. 7 c). Based on these findings, in part B a narrower range of doses was used.
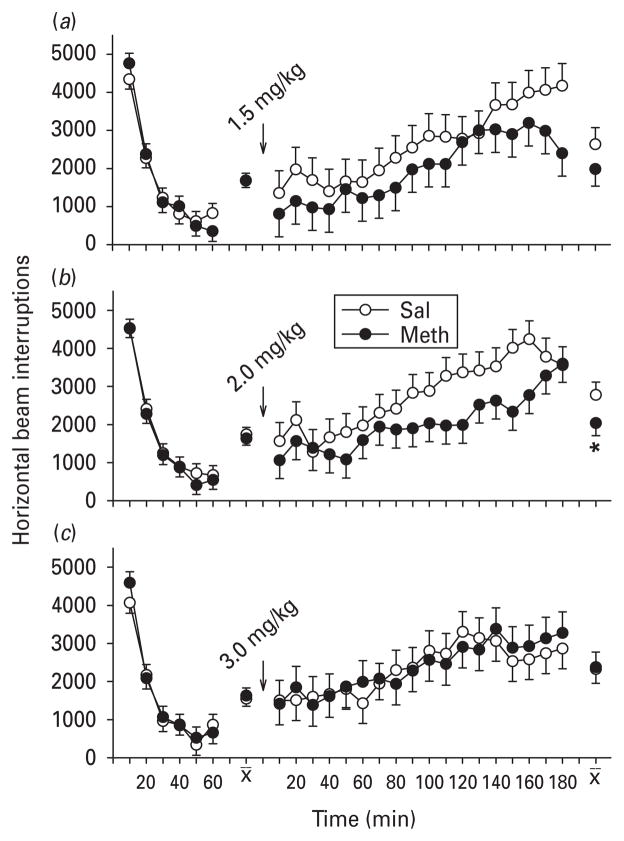
SKF-82928: pre-challenge activity (part B)
The 1.0 mg/kg dose of SKF-82958 was retained and a lower dose (but higher than the low dose from part A of 0.5 mg/kg) and a dose higher than the mid dose (1.5 mg/kg) were chosen, with the latter not as high as in part A (Fig. 8). There were no significant group differences during the pre-challenge habituation period.
Fig. 8.
Locomotor activity and SKF-82958 (D1 agonist ; part B) : activity (least square mean ± S.E.M.) before and after SKF-82958. Pre-challenge, there were no significant group differences in any of the groups for any challenge conditions. Post-challenge, significant interval-by-interval effects from post-natal days 11–20 methamphetamine (Meth) vs. saline (Sal)-treatment occurred at the mid and high dose challenge conditions. At these doses, SKF-82958 induced exaggerated hyperactivity in the Meth-treated group compared to the Sal-treated group. (a) 0.5, (b) 1.0 and (c) 1.5 mg/kg SKF-82958 given s.c. n=15–17 per group per challenge dose level. * p<0.05, ** p<0.01 vs. Sal.
SKF-82928: post-challenge activity (part B)
Post-challenge, all groups at all doses showed marked hyperactivity in response to the drug. Pre-planned comparisons were used to predict that Meth-treated animals would have an increased response to the drug. No significant differential effects of prior Meth treatment were seen following the low dose of SKF-82958 (0.5 mg/kg; Fig. 8a). Significant differences in the degree of hyperactivity were seen 40–100 min following the mid-level dose (1.0 mg/kg), in which Meth-treated rats were more hyperactive, as predicted from part A, compared to Sal-treated rats (Fig. 8 b). Likewise, Meth exposure resulted in significantly increased hyperactivity from 100 to 160 min post-challenge after the highest dose (1.5 mg/kg), showing a shift to the right of the dose–response curve (Fig. 8 c).
Discussion
The results show that developmental exposure to Meth results in long-term alterations in receptor function as determined by locomotor activity using selective pharmacological agents. Neither of the serotonergic agents (quipazine or PCA) induced significant changes in activity in adult rats following developmental exposure to Meth or Sal. This could be because 5-HT receptors are unchanged or, given that there are at least seven families of 5-HT receptors with multiple subtypes within each family (Pytliak et al. 2011), we may not have tested for the affected receptor. Alternatively, 5-HT receptor dysfunction may not be unmasked with locomotor tests, but may be apparent with other types of behavioural tests. We have shown that developmental Meth exposure (PD 11–20) results in sharp reductions in 5-HT and its metabolite 5-HIAA during and shortly after treatment (Schaefer et al. 2008) and, while partial recovery occurs, long-term reductions remain (Grace et al. 2010). Thus, neither quipazine, an agonist of 5-HT2/3 receptor subtypes, nor PCA, a 5-HT releaser, had a significant effect on locomotor activity relative to previous Meth treatment. However, as previously mentioned, there are a number of 5-HT receptor subtypes and we only tested one such group directly. Thus, additional experiments specific for the other 5-HT receptor classes are necessary before a conclusive statement can be made upon long-term 5-HT receptor dysfunction following developmental Meth exposure. These and other data indicate that developmental Meth treatment does not result in long-term alterations in 5-HT2/3 receptor or serotonergic metabolism.
Quinpirole, a D2 agonist, induced less hyperactivity in Meth-treated rats, but only at one dose level. By contrast, the D1 agonist SKF-82958 resulted in increased hyperactivity following developmental Meth treatment at several doses. DA is integral to the mechanism of action of Meth in adults (Hyman et al. 2006) and is the major neurotransmitter affected by neurotoxic Meth exposure (O’Callaghan & Miller, 2002; Wilson et al. 1996). Clinical and preclinical data have shown that adult Meth exposure can induce long-lasting decreases in levels of the DA transporter (Fleckenstein et al. 1997; Johanson et al. 2006; Kokoshka et al. 1998; McCann et al. 1998; Sekine et al. 2001; Volkow et al. 2001b; Wilson et al. 1996), the vesicular monoamine transporter and DA (Friedman et al. 1998; Ricaurte et al. 1980; Wagner et al. 1979, 1980; Wilson et al. 1996).
It has been proposed that DA receptors play an integral role in Meth-induced addiction and neurotoxicity (Chapman et al. 2001; Self, 1998; Wang & McGinty, 1996). One clinical study found that Meth users had elevated D1 protein levels, but only in the nucleus accumbens, following post-mortem examination (Worsley et al. 2000). Despite this increase, a follow-up study indicated that D1 receptor functionality was decreased in Meth users (Tong et al. 2003), suggesting that receptor abundance does not correlate with receptor function. Additionally, adult chronic Meth users exhibit lower levels of D2 (and D3) receptor availability, a phenomenon that has been linked to impulsive behaviour (Lee et al. 2009; Volkow et al. 2001a), while others have shown that this decrease is non-significant (Worsley et al. 2000). Animal studies have revealed that, following a behavioural sensitization paradigm, there is no change in the number of D1 or D2 receptors (Suzuki et al. 1997), while others noted that D1 and D2 receptor levels were decreased 18 h following a neurotoxic regimen of Meth (5 × 15 mg/kg every 6 h; McCabe et al. 1987b). However, normal receptor levels were attained between 7 and 21 d post-treatment, indicating that receptor number, not affinity, had changed transiently.
Less is known about the receptor alterations following early Meth exposure, as the aforementioned data are gleaned from adult rodent studies and short-term changes in DA levels are not reported to occur during PD 11–20 drug administration (Schaefer et al. 2006, 2008). Interestingly, the findings in the present study indicate that the D1 and D2 receptors show lasting functional changes following developmental Meth treatment although we did not directly assay receptor number or affinity. Moreover, it is not clear how early Meth exposure induces long-term DA receptor changes. Only one other study has looked at long-lasting DA receptor changes resulting from developmental Meth exposure. We demonstrated that this same post-natal Meth exposure resulted in decreases in dorsostriatal D2 receptor binding and protein kinase A (PKA) activity (a modulator of the D1 receptor) when examined in adulthood (PD 90; Crawford et al. 2003). We had posited that this reduction in PKA activity was attributable to D1 receptor desensitization. Since Meth-treated rats later challenged with SKF-82958 exhibited greater activity than Sal-treated rats, it does not appear likely that the developmental Meth treatment results in long-term D1 receptor desensitization ; in fact the opposite appears more likely. This is based on the assumption that striatal D1 receptors are affected, as this brain region is highly innervated with DA receptors and plays a dominant role in locomotor activity (Holschneider & Maarek, 2008; Kalivas et al. 1999). In addition, it is known that activation of D1 receptors in other brain regions such as the medial prefrontal cortex and orbitofrontal cortex also play a role in motor inhibition (Diaz et al. 2004; Heijtz et al. 2007; Pellis et al. 2006). Therefore, we suggest that early Meth treatment sensitizes DA D1 receptors while desensitizing D2 receptors (Crawford et al. 2003; Schaefer et al. 2006, 2008; Williams et al. 2003a). Whether the smaller D2 receptor change was a direct effect of Meth exposure or an indirect effect via the Meth-induced change in D1 receptor sensitivity cannot be determined from the present data.
This study further demonstrated that early Meth-treated rats showed marked changes following adult exposure to the NMDA receptor antagonist, MK-801, resulting in decreased activation relative to Sal-treated controls. In adult animals, Meth causes the release of extracellular glutamate (Nash & Yamamoto, 1992), which can contribute to Meth-induced neurotoxicity via excitotoxicity and reactive nitrogen species (Cadet & Brannock, 1998; Dawson et al. 1993; Garthwaite, 1991; Imam et al. 2001). NMDA itself is able to decrease DA uptake in vitro and DA levels in vivo and Meth potentiates the latter effect when co-administered (Sonsalla et al. 1998). Others have found that administration of MK-801 attenuates the DA depleting effects of Meth and the inhibitory effect of Meth on tyrosine hydroxylase (Miller & O’Callaghan, 1995; Sonsalla et al. 1989). It is known that glutamatergic and dopaminergic systems interact and this association is significant in Meth-induced neurotoxicity. NMDA receptors are localized on DA nerve terminals (Krebs et al. 1991) and NMDA receptors regulate D1 receptors via physical interaction, such that activation of the NMDA receptor results in an up-regulation of D1 receptors (Pei et al. 2004). Yamamoto and colleagues found that Meth increases glutamate release via a D1-mediated mechanism in adult rats (Mark et al. 2004). While it is not known whether a similar mechanism occurs during developmental Meth treatment, it is clear that developmental treatment has multiple effects on dopaminergic and glutamatergic systems.
In addition to the above, age of exposure is pivotal to understanding the effects we observed. The treatment period encompassed critical stages of cortical and limbic development, roughly equivalent to late second to third trimester brain development in humans (Clancy et al. 2007a, b; Rice & Barone, 2000). DA receptor mRNA is highly expressed embryonically, indicating a role in neurogenesis; however, DA receptors do not appear to be functionally active until PD 14–21 (Schambra et al. 1994). Likewise, while NMDA receptors are expressed pre-natally, they are not functionally active until after birth, with receptor binding and expression peaking between PD 7 and PD 20 (Insel et al. 1990; Luo et al. 1996; Monyer et al. 1994). During these stages, NMDA receptors are most susceptible to toxic insult (Haberny et al. 2002; Scheetz & Constantine-Paton, 1994). By contrast, expression of 5-HT receptors, including 5-HT2/3 receptors, peaks during the embryonic period and maximal ligand binding occurs during the late embryonic–early post-natal period (e.g. ED 17–PD 13 for 5-HT2 subtypes; Bell et al. 1992; Johnson & Heinemann, 1995; Miquel et al. 1995; Roth et al. 1991; Wu et al. 1999). As such, the dosing regimen used here (PD 11–20) aligns with vulnerable periods of DA and NMDA receptor development more than with that of 5-HT receptors. One in vitro study noted that the activation of NMDA receptors produced more toxicity in brain slices from younger rats (PD 21 ± 2) than from adults relative to other glutamate receptor subtypes (Sanganahalli et al. 2006). We have demonstrated that PD 11–20 Meth exposure results in enduring deficits in Cincinnati water maze (CWM) learning (Grace et al. 2010; Vorhees et al. 2008, 2009). This maze assesses route-based egocentric learning, a form of navigation that relies on the use of self-movement cues and signposts to determine location within a given space (Byrne, 1982; di Fiore & Suarez, 2007). As such, it is possible that the CWM is mediated via the neostriatum, particularly the caudate nucleus (Cook & Kesner, 1988), a region rich in DA projections. It is plausible that Meth exposure during this stage permanently altered DA and glutamate receptors critical for the formation or integration of route-based information.
In sum, the results identified several long-term receptor changes in the DA and glutamate systems important for locomotion following post-natal Meth treatment. The changes observed in Meth-treated offspring in their adult functional response to a D1 receptor agonist was opposite to that after a D2 receptor agonist or an NMDA receptor antagonist. Since this exposure also causes later cognitive deficits, the functional changes seen here may be indirectly related to the cognitive effects, but further experiments will be required to test this connection since locomotor behaviour only was assessed here.
Acknowledgments
We thank Mary Moran, Lindsey Burns, Brian Hoffman, Emily Hautman and Holly Johnson for technical assistance. Supported by NIH grants DA006733 (C.V.V.), ES015689 (M.T.W.) and training grant T32 ES007051 (D.L.G., R.M.A.K., A.A.B., C.E.G., T.L.S.).
Footnotes
Statement of Interest
None.
References
- Acevedo SF, Raber J. Histamine-dependent behavioral response to methamphetamine in 12-month-old male mice. Brain Research. 2011;1393:23–30. doi: 10.1016/j.brainres.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neuroscience Letters. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Bell J, III, Zhang XN, Whitaker-Azmitia PM. 5-HT3 receptor-active drugs alter development of spinal serotonergic innervation: lack of effect of other serotonergic agents. Brain Research. 1992;571:293–297. doi: 10.1016/0006-8993(92)90667-x. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Votava M, Palenicek T, Horacek J. The opposite effect of a low and a high dose of serotonin-1A agonist on behavior induced by MK-801. Neuropharmacology. 2007;52:1071–1078. doi: 10.1016/j.neuropharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Burchfield DJ, Lucas VW, Abrams RM, Miller RL, et al. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. Journal of the American Medical Association. 1991;265:1968–1973. [PubMed] [Google Scholar]
- Byrne RW. Geographical knowledge and orientation. In: Ellis AW, editor. Normality and Pathology in Cognitive Functions. London: Academic Press; 1982. pp. 239–264. [Google Scholar]
- Cabrera TM, Levy AD, Li Q, van de Kar LD, et al. Prenatal methamphetamine attenuates serotonin mediated renin secretion in male and female rat progeny: evidence for selective long-term dysfunction of serotonin pathways in brain. Synapse. 1993;15:198–208. doi: 10.1002/syn.890150305. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochemistry International. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. International Review of Neurobiology. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Nichols DE, Geyer MA. Suppression of behavioral activity by norfenfluramine and related drugs in rats is not mediated by serotonin release. Psychopharmacology (Berlin) 1993;111:169–178. doi: 10.1007/BF02245519. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, et al. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Research : Neuroimaging. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. Journal of Pharmacology and Experimental Therapeutics. 2001;296:520–527. [PubMed] [Google Scholar]
- Chomchai C, Na MN, Watanarungsan P, Yossuck P, et al. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, et al. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, et al. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Kesner RP. Caudate nucleus and memory for egocentric localization. Behavioral and Neural Biology. 1988;49:332–343. doi: 10.1016/s0163-1047(88)90338-x. [DOI] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, Sims JR, et al. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. Journal of Neuroscience. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Newman ER, McDougall SA, et al. Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2003;48:131–137. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, Bartley DA, Uhl GR, et al. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. Journal of Neuroscience. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Fiore A, Suarez SA. Route-based travel and shared routes in sympatric spider and woolly monkeys: cognitive and evolutionary implications. Animal Cognition. 2007;10:317–329. doi: 10.1007/s10071-006-0067-y. [DOI] [PubMed] [Google Scholar]
- Diaz HR, Scott L, Forssberg H. Alteration of dopamine D1 receptor-mediated motor inhibition and stimulation during development in rats is associated with distinct patterns of c-fos mRNA expression in the frontal–striatal circuitry. European Journal of Neuroscience. 2004;19:945–956. doi: 10.1111/j.0953-816x.2004.03154.x. [DOI] [PubMed] [Google Scholar]
- Dixon SD. Effects of transplacental exposure to cocaine and methamphetamine on the neonate. Western Journal of Medicine. 1989;150:436–442. [PMC free article] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. Journal of Pediatrics. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Gibb JW, Hanson GR. A rapid and reversible change in dopamine transporters induced by methamphetamine. European Journal of Pharmacology. 1997;323:R9–R10. doi: 10.1016/s0014-2999(97)00148-9. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacology, Biochemistry and Behavior. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signaling in the nervous system. Trends in Neuroscience. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Gudelsky GA, Williams MT, et al. Neonatal methamphetamine-induced corticosterone release in rats is inhibited by adrenal autotransplantation without altering the effect of the drug on hippocampal serotonin. Neurotoxicology and Teratology. 2010;32:356–361. doi: 10.1016/j.ntt.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Skelton MR, et al. (+)-Methamphetamine increases corticosterone in plasma and BDNF in brain more than forced swim or isolation in neonatal rats. Synapse. 2008;62:110–121. doi: 10.1002/syn.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Noailles PA, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. Journal of Neurochemistry. 2008;105:1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, et al. Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicological Sciences. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Kolb B, Forssberg H. Motor inhibitory role of dopamine D1 receptors : implications for ADHD. Physiology and Behavior. 2007;92:155–160. doi: 10.1016/j.physbeh.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM. Brain maps on the go: functional imaging during motor challenge in animals. Methods. 2008;45:255–261. doi: 10.1016/j.ymeth.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction : the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko Y, Jindrich DL, Zhong H, et al. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neuroscience Letters. 2008;438:281–285. doi: 10.1016/j.neulet.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, el-Yazal J, Newport GD, Itzhak Y, et al. Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Annals of the New York Academy of Science. 2001;939:366–380. doi: 10.1111/j.1749-6632.2001.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain – I. N-Methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Jacobs PS, Taylor BM, Bardgett ME. Maturation of locomotor and Fos responses to the NMDA antagonists, PCP and MK-801. Brain Research, Developmental Brain Research. 2000;122:91–95. doi: 10.1016/s0165-3806(00)00059-6. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berlin) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Johnson DS, Heinemann SF. Embryonic expression of the 5-HT3 receptor subunit, 5-HT3R-A, in the rat : an in situ hybridization study. Molecular and Cellular Neuroscience. 1995;6:122–138. doi: 10.1006/mcne.1995.1012. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Annals of the New York Academy of Science. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. European Journal of Pharmacology. 1998;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Desce WJ, Kemel M, Gauchy C, et al. Glutamatergic control of dopamine release in the rat striatum : evidence of presynaptic N-methyl-D-aspartate receptors on dopaminergic nerve terminals. Journal of Neurochemistry. 1991;56:81–85. doi: 10.1111/j.1471-4159.1991.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Kuczkowski KM. The effects of drug abuse on pregnancy. Current Opinion in Obstetrics and Gynecology. 2007;19:578–585. doi: 10.1097/GCO.0b013e3282f1bf17. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC. Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstetrics and Gynecology. 1988;72:541–544. [PubMed] [Google Scholar]
- Luo J, Bosy TZ, Wang Y, Yasuda RP, et al. Ontogeny of NMDA R1 subunit protein expression in five regions of rat brain. Brain Research, Developmental Brain Research. 1996;92:10–17. doi: 10.1016/0165-3806(95)00191-3. [DOI] [PubMed] [Google Scholar]
- McCabe RT, Gibb JW, Wamsley JK, Hanson GR. Autoradiographic analysis of muscarinic cholinergic and serotonergic receptor alterations following methamphetamine treatment. Brain Research Bulletin. 1987a;19:551–557. doi: 10.1016/0361-9230(87)90072-4. [DOI] [PubMed] [Google Scholar]
- McCabe RT, Hanson GR, Dawson TM, Wamsley JK, et al. Methamphetamine-induced reduction in D1 and D2 dopamine receptors as evidenced by autoradiography: comparison with tyrosine hydroxylase activity. Neuroscience. 1987b;23:253–261. doi: 10.1016/0306-4522(87)90287-9. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, et al. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users : evidence from positron emission tomography studies with [11C]WIN-35,428. Journal of Neuroscience. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Crossman AR, Brotchie JM. The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Experimental Neurology. 1997;148:265–270. doi: 10.1006/exnr.1997.6645. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. Journal of Neuroscience. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, et al. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat : a possible model of neurodevelopmental disorders with cognitive deficits. Brain Research. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Molecular Neurobiology. 1995;11:177–192. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Gingrich JA, Nosjean A, et al. Developmental changes in the differential expression of two serotonin 5-HT3 receptor splice variants in the rat. Journal of Neurochemistry. 1995;65:475–483. doi: 10.1046/j.1471-4159.1995.65020475.x. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, et al. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chappell W. The use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokinetics in New Drug Development. New York: Pergamon Press; 1989. pp. 42–96. [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release : comparison to 3,4-methylenedioxymethamphetamine. Brain Research. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Noda Y, Mouri A, Ando Y, Waki Y, et al. Galantamine ameliorates the impairment of recognition memory in mice repeatedly treated with methamphetamine: involvement of allosteric potentiation of nicotinic acetylcholine receptors and dopaminergic-ERK1/2 systems. International Journal of Neuropsychopharmacology. 2010;13:1343–1354. doi: 10.1017/S1461145710000222. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxic effects of substituted amphetamines in rats and mice: Challenges to the current dogma. In: Massaro EJ, editor. Handbook of Neurotoxicity. Totowa, NJ: Humana Press; 2002. pp. 269–301. [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. Journal of Neuroscience. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. Journal of Pediatrics. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annual Review of Neuroscience. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- Pei L, Lee FJ, Moszczynska A, Vukusic B, et al. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. Journal of Neuroscience. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM, Hastings E, Shimizu T, Kamitakahara H, et al. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and nonplayful social contexts. Behavioral Neuroscience. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- Pytliak M, Vargova V, Mechirova V, Felsoci M. Serotonin receptors – from molecular biology to clinical applications. Physiological Research. 2011;60:15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain : a regional study. Brain Research. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Hamblin MW, Ciaranello RD. Developmental regulation of 5-HT2 and 5-HT1c mRNA and receptor levels. Brain Research, Developmental Brain Research. 1991;58:51–58. doi: 10.1016/0165-3806(91)90236-c. [DOI] [PubMed] [Google Scholar]
- Sanganahalli BG, Joshi PG, Joshi NB. NMDA and non-NMDA receptors stimulation causes differential oxidative stress in rat cortical slices. Neurochemistry International. 2006;49:475–480. doi: 10.1016/j.neuint.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, et al. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−) 3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−)methylphenidate administration in the neonatal rat. Journal of Neurochemistry. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, et al. Short- and long-term effects of (+)-methamphetamine and (+/−)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. Journal of Neurochemistry. 2008;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra UB, Duncan GE, Breese GR, Fornaretto MG, et al. Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Scheetz AJ, Constantine-Paton M. Modulation of NMDA receptor function : implications for vertebrate neural development. FASEB Journal. 1994;8:745–752. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. American Journal of Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Self DW. Neural substrates of drug craving and relapse in drug addiction. Annals of Medicine. 1998;30:379–389. doi: 10.3109/07853899809029938. [DOI] [PubMed] [Google Scholar]
- Siegel JA, Craytor MJ, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behavioural Pharmacology. 2010;21:602–614. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R, Rokyta R. Occurrence of bicuculline-, NMDA- and kainic acid-induced seizures in prenatally methamphetamine-exposed adult male rats. Naunyn Schmiedebergs Archives of Pharmacology. 2005a;372:236–241. doi: 10.1007/s00210-005-0016-3. [DOI] [PubMed] [Google Scholar]
- Slamberova R, Rokyta R. Seizure susceptibility in prenatally methamphetamine-exposed adult female rats. Brain Research. 2005b;1060:193–197. doi: 10.1016/j.brainres.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Slamberova R, Schutova B, Matejovska I, Bernaskova K, et al. Effects of a single postnatal methamphetamine administration on NMDA-induced seizures are sex- and prenatal exposure-specific. Naunyn Schmiedebergs Archives of Pharmacology. 2009;380:109–114. doi: 10.1007/s00210-009-0427-7. [DOI] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, et al. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. Journal of Developmental and Behavioral Pediatrics. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicology and Teratology. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZM, Undie AS, Koh PO, Fang YY, et al. D1 dopamine receptor regulation of microtubule-associated protein-2 phosphorylation in developing cerebral cortical neurons. Journal of Neuroscience. 2002;22:6092–6105. doi: 10.1523/JNEUROSCI.22-14-06092.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Albers DS, Zeevalk GD. Role of glutamate in neurodegeneration of dopamine neurons in several animal models of Parkinsonism. Amino Acids. 1998;14:69–74. doi: 10.1007/BF01345245. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. Journal of Developmental and Behavioral Pediatrics. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Rehakova L, Rambousek L, Svoboda J, et al. Manipulation of D2 receptors with quinpirole and sulpiride affects locomotor activity before spatial behavior of rats in an active place avoidance task. Neuroscience Research. 2007;58:133–139. doi: 10.1016/j.neures.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Su YA, Si TM, Zhou DF, Guo CM, et al. Risperidone attenuates MK-801-induced hyperlocomotion in mice via the blockade of serotonin 5-HT 2A/2C receptors. European Journal of Pharmacology. 2007;564:123–130. doi: 10.1016/j.ejphar.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health : National Findings. Rockville: US Department of Health and Human Services; 2009. [Google Scholar]
- Sugita R, Terada K, Sekiya Y, Sawa Y, et al. Effect of p-chloroamphetamine administration on monoamine metabolism in the rat nucleus accumbens and locomotor activity : studies with intracerebral dialysis in freely moving rats. Brain Research. 1994;658:255–258. doi: 10.1016/s0006-8993(09)90033-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Shishido T, Watanabe Y, Abe H, et al. Changes of behavior and monoamine metabolites in the rat brain after repeated methamphetamine administration : effects of duration of repeated administration. Progress in Neuropsychopharmacology and Biological Psychiatry. 1997;21:359–369. doi: 10.1016/s0278-5846(97)00006-7. [DOI] [PubMed] [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstetrics and Gynecology. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nature Reviews Neuroscience. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Ross BM, Schmunk GA, Peretti FJ, et al. Decreased striatal dopamine D1 receptor-stimulated adenylyl cyclase activity in human methamphetamine users. American Journal of Psychiatry. 2003;160:896–903. doi: 10.1176/appi.ajp.160.5.896. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, et al. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. American Journal of Psychiatry. 2001b;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, et al. Methamphetamine exposure during early postnatal development in rats : I. Acoustic startle augmentation and spatial learning deficits. Psychopharmacology. 1994;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, et al. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats : effects of dose and rearing conditions. International Journal of Developmental Neuroscience. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Grace CE, Schaefer TL, et al. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. International Journal of Developmental Neuroscience. 2009;27:289–298. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behavavioural Pharmacology. 2007;18:549–562. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, et al. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Research. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Seiden LS, Schuster CR. Methamphetamine induced changes in brain catecholamines in rats and guinea pigs. Drug and Alcohol Dependence. 1979;4:435–438. doi: 10.1016/0376-8716(79)90076-0. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. D1 and D2 receptor regulation of preproenkephalin and preprodynorphin mRNA in rat striatum following acute injection of amphetamine or methamphetamine. Synapse. 1996;22:114–122. doi: 10.1002/(SICI)1098-2396(199602)22:2<114::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behavioural Brain Research. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- White SJ, Laurenzana EM, Gentry WB, Hendrickson HP, et al. Vulnerability to (+)-methamphetamine effects and the relationship to drug disposition in pregnant rats during chronic infusion. Toxicological Sciences. 2009;111:27–36. doi: 10.1093/toxsci/kfp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, et al. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Developmental Brain Research. 2003a;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003b;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, et al. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003c;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, et al. Methamphetamine exposure from postnatal days 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Research. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature Medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Molecular Psychiatry. 2000;5:664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- Wu C, Dias P, Kumar S, Lauder JM, et al. Differential expression of serotonin 5-HT2 receptors during rat embryogenesis. Developmental Neuroscience. 1999;21:22–28. doi: 10.1159/000017362. [DOI] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]