Abstract
We previously showed that developmental 3,4-methylenedioxymethamphetamine (MDMA) treatment induces long-term spatial and egocentric learning and memory deficits and serotonin (5-HT) reductions. During brain development, 5-HT is a neurotrophic factor influencing neurogenesis, synaptogenesis, migration, and target field organization. MDMA (10 mg/kg×4/d at 2 h intervals) given on post-natal day (PD) 11–20 in rats (a period of limbic system development that approximates human third trimester brain development) induces 50% reductions in 5-HT during treatment and 20% reductions when assessed as adults. To determine whether the 5-HT reduction is responsible for the cognitive deficits, we used citalopram (Cit) pretreatment to inhibit the effects of MDMA on 5-HT reuptake in a companion study. Cit attenuated MDMA-induced 5-HT reductions by 50% (Schaefer et al., 2012). Here we tested whether Cit (5 or 7.5 mg/kg×2/d) pretreatment attenuates the cognitive effects of MDMA. Within each litter, different offspring were treated on PD11–20 with saline (Sal)+MDMA, Cit+MDMA, Cit+Sal or Sal+Sal. Neither spatial nor egocentric learning/memory was improved by Cit pretreatment. Unexpectedly, Cit+Sal (at both doses) produced spatial and egocentric learning deficits as severe as those caused by Sal+MDMA. These are the first data showing cognitive deficits resulting from developmental exposure to a selective serotonin reuptake inhibitor. These data indicate the need for further research on the long-term safety of antidepressants during pregnancy.
Keywords: Citalopram, cognition, development, MDMA, serotonin
Introduction
Many drugs that target the serotonin (5-HT) system are taken during pregnancy (Plessinger, 1998; Ho et al., 2001; Oberlander et al., 2007) but their long-term effects are largely unknown. In the adult brain 5-HT is involved in cognition, anxiety, sleep, aggression and sexual function (Whitaker-Azmitia et al., 1996). Developmentally, 5-HT is the first neurotransmitter expressed (Lauder and Bloom, 1974; Lauder and Krebs, 1978; Lauder et al., 1981). At early stages, 5-HT has neurotrophic effects, regulating growth and development of target regions, modulates its own development and is involved in cell division, differentiation, migration and synaptogenesis (Lauder, 1993; Whitaker-Azmitia et al., 1996; Azmitia, 2001; Vitalis and Parnavelas, 2003).
We showed that post-natal day (PD) 11–20 exposure to 3,4-methylenedioxymethamphetamine (MDMA) produces reductions in hippocampal, neostriatal and entorhinal cortex 5-HT (Williams et al., 2005; Schaefer et al., 2006, 2008, 2012). It also results in egocentric and allocentric learning and memory deficits (Vorhees et al., 2004, 2007a; Skelton et al., 2006). Many areas of the brain that are serotonergically innervated, including the entorhinal cortex (Bobillier et al., 1975; Pazos and Palacios, 1985; Pazos et al., 1985; Fuhs and Touretzky, 2006; McNaughton et al., 2006; Sargolini et al., 2006; Witter and Moser, 2006), neostriatum (Cook and Kesner, 1988; Brown and Molliver, 2000) and hippocampus (Morris et al., 1982; Jacobs and Azmitia, 1992), play roles in egocentric and allocentric learning and memory. For example, hippocampal granule cells proliferate well into the third trimester in humans, a stage equivalent in rodents to the pre-weaning period (Bayer et al., 1993; Clancy et al., 2007a, b). Recently, a regression-based algorithm has been developed to compare brain development across species (Clancy et al., 2007b). The comparisons are based primarily on rates of neurogenesis. Using this method in which gestation is counted as 21.5 d in rats and 270 d in humans (38.6 wk), PD 11 rat brain development approximates human cortical development on gestation day 181.6 (26 wk, i.e. the very beginning of third trimester) and human limbic development gestation day 132.3 (19 wk, i.e. mid-second trimester). The algorithm does not translate beyond rat PD 13.3, which is equivalent to human cortical development on gestation day 196.1 (28 wk, or early third trimester) and human limbic development on day 142.7 (20.4 wk or slightly beyond mid-second trimester); however, by inference the rat equivalent of human brain development at birth for some cortical regions and most limbic regions would be well beyond PD 13.3 in the rat and another review places it at PD 19 (Bayer et al., 1993).
We hypothesized that preventing MDMA-induced 5-HT reductions by pretreatment with the selective serotonin reuptake inhibitor (SSRI), citalopram (Cit), would interfere with MDMA binding to the serotonin transporter (SERT) and ameliorate its adverse effects. Cit was chosen because it is the most selective SSRI with high affnity for SERT and has the least effect on cytochrome P450 catabolic enzymes crucial for MDMA metabolism (Hemeryck and Belpaire, 2002; De La Torre et al., 2004). In a companion study (Schaefer et al., 2012), we developed a Cit dosing regimen that attenuates MDMA-induced 5-HT reductions during treatment by 50% or more. The purpose of the present experiment was to use this Cit regimen to (1) determine if it was neuroprotective against MDMA-induced learning and memory deficits and (2) determine the effects of Cit in the absence of MDMA since the effects of SSRIs on cognitive development are largely unknown, despite studies of their effects on non-cognitive behaviour (Casper et al., 2003; Hendrick et al., 2003; Maciag et al., 2006a, b, c; Homberg et al., 2010; Croen et al., 2011; Darling et al., 2011; Rodriguez-Porcel et al., 2011; Zhang et al., 2011).
Method and materials
Animals and housing
Nulliparous female and male Sprague–Dawley CD rats (International Genetic Standard) were obtained from Charles River Laboratories (USA) and bred in-house. Animals were housed in 22±1 °C rooms at 50±10% humidity on 14/10 h light/dark cycle (lights on 06.00 hours). Cages (46×24×20 cm) contained woodchip bedding, ad libitum NIH-07 diet and filtered water and were equipped with stainless steel enrichment enclosures (Vorhees et al., 2008a). The Institutional Animal Care and Use Committee approved the research protocol and the vivarium was accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Birth was designated PD 0; on PD 1, litters were culled to eight males. If a litter did not have eight males, up to two males were fostered from litters not used in the experiment born within 24 h. Pups were identified by ear punch on PD 7. We have shown no MDMA×sex interactions on learning and memory using this treatment regimen (Broening et al., 2001; Williams et al., 2003; Vorhees et al., 2004, 2007a; Skelton et al., 2006, 2009) and MDMA causes no sex differences on corticosterone or monoamines at these ages (Williams et al., 2005).
Drug treatment
As a prelude to the present experiment we developed the Cit-MDMA 5-HT attenuating dosing regimen (Schaefer et al., 2012). Utilizing it for the current study, 20 litters, in a split-litter design, were prepared with individual offspring in each litter receiving different treatment combinations. The dose of MDMA was 10 mg/kg (administered 4× daily with a 2 h interdose interval) and the doses of Cit were 5 or 7.5 mg/kg (administered twice daily, 30 min prior to the first and fourth MDMA dose). The following groups were created: Sal+Sal; Sal+MDMA; Cit5+MDMA; Cit7.5+MDMA; Cit5+Sal; Cit7.5+Sal with two animals/litter not tested to maintain the same litter size as in past experiments. Each drug was dissolved in physiological saline (Sal) in a volume of 3 ml/kg and s.c. injected. MDMA was obtained from the National Institute on Drug Abuse (Bethesda, USA) and Cit was obtained from Sigma-Aldrich (USA).
The MDMA dose was designed to mimic binge patterns seen in some ecstasy users. The dose of 10 mg/kg MDMA was based on data that show that a higher dose is required in rats to achieve the same plasma concentrations as in humans (Green et al., 2009, 2012). For example, 7 mg/kg MDMA in rats approximates a dose of 2.0 mg/kg in humans (Green et al., 2009). This dose may not be fully comparable to humans because humans exhibit auto-inhibition of metabolism (Green et al., 2009), an effect not found in rats. In addition, it has been suggested that MDMA neurotoxicity in adult rats is attributable to its metabolites and not the parent compound. Because of their more rapid metabolism, rats create metabolites more rapidly than humans and therefore may generate more neurotoxic intermediates (Green et al., 2012). How this may apply to the developing brain remains to be determined since several of the effects of MDMA are different during development. For example, MDMA induces hyperthermia and 5-HT and dopamine release in adult rats but in developing rats does not induce hyperthermia or dopamine release. In addition, the 5-HT release is transient during development whereas it is persistent in adults at equivalent doses (Broening et al., 1994, 1997). Hence, 10 mg/kg MDMA×4 at 2 h intervals is neurotoxic in adult rats where it also causes drug-induced hyperthermia (Malberg et al., 1996; Sabol and Seiden, 1998). This increase in body temperature does not emerge until adolescence (Meyer et al., 2008) at approximately PD 40 (Broening et al., 1995). Similar age-dependent neurotoxicity has also been found with the related substituted phenylethylamine, methamphetamine (Cappon et al., 1997), where pre-weaning rats do not show the same neurotoxic effects seen in adults but do show long-term cognitive deficits (Vorhees et al., 2000, 2007b, 2008b, 2009). Therefore, the doses used here cannot be directly compared to those used in adult rats.
Cit was given twice daily prior to the first and last MDMA doses in an effort to interfere with MDMA binding to SERT. The schedule of MDMA administration was based on the half-life of MDMA in rats at PD 11 (Williams et al., 2004). At this age the elimination half-life of a higher dose of 20 mg/kg MDMA is under 4 h (vs. 8–10 h in humans). The elimination half-life of Cit in rats is 3 h (vs. 35 h in humans) hence the times for Cit injections were chosen to interfere with all four doses of MDMA. It should be noted that a Cit dose of 5 mg/kg administered twice daily from PD 8–21 in rats was shown to produce plasma drug concentrations comparable to human therapeutic doses on the final day of exposure (Maciag et al., 2006b).
Behavioural methods
Straight channel
On PD 61, animals were tested in a 15×244×51 cm straight swimming channel with a platform submerged 1.5 cm below the surface at one end. Each rat received four trials. Rats were placed at one end facing the wall and timed to reach the platform (limit=2 min). These trials acclimatize rats to swimming, measure latency (speed) as an index of performance and teach them that escape is possible.
Cincinnati water maze
The Cincinnati water maze (CWM), a test of route-based egocentric learning, began on PD 62. It is a multiple-T maze (Vorhees, 1987, 1991, 2011) with nine branching Ts from the main channel (width=15 cm; walls=51 cm high and filled halfway with water). Water was changed twice weekly and equilibrated to room temperature overnight (21±1 °C). Testing was conducted in complete darkness using infrared LED emitters and camera above the maze connected to a closed circuit monitor in an adjacent room. Animals were tested in path-B of the maze (Vorhees, 1987) and allowed 5 min/trial to find the escape. Rats were given two trials daily for 18 d. Errors and latency were recorded as described (Vorhees et al., 2011), i.e. an error was digression into the stem or arm of a T, or re-entering the start area.
Morris water maze
Allocentric (spatial) learning and memory were assessed in the Morris water maze (MWM; Vorhees and Williams, 2006). Testing began on PD 80 in a 210 cm diameter×51 cm circular tank filled halfway with water. Animals were tested in phases: acquisition (platform in SW position); reversal (platform in NE position); shift (platform in NW position). Rats received four trials daily for 6 d followed by a 30 s probe trial on the seventh day of each phase with the platform removed. Each phase used a platform of a different size (10, 7 and 5 cm diameter, respectively). Following the hidden phases, the cued version was conducted. For this, the submerged platform had a plastic ball attached to a brass rod that protruded above the surface. Curtains were closed around the pool to minimize extra-maze cues and the animals were given four trials daily for 2 d with the platform and start positions changed for every trial such that spatial strategies were ineffective. A camera and tracking software were used to map performance (AnyMaze; Stoelting Instruments, USA). On platform trials, latency, cumulative distance, path length, swim speed and average heading error were analysed. On probe trials, average distance, site crossovers, percent time in the target quadrant, initial heading error and swim speed were analysed.
Statistical analysis
Body weights were analysed using mixed linear analysis of variance models (SAS version 9.2, Proc Mixed; SAS Institute, USA). Learning data were analysed using planned comparisons based on prior findings with MDMA or for Cit based on the null hypothesis of no improvement. The false discovery rate (FDR) method was used (Benjamini and Hochberg, 1995; Benjamini et al., 2001) for comparisons between the Sal+Sal group and each drug group. Significance was set at p≤0.05. Data are presented as least square (LS) mean±LS s.e.m.
Results
Straight channel
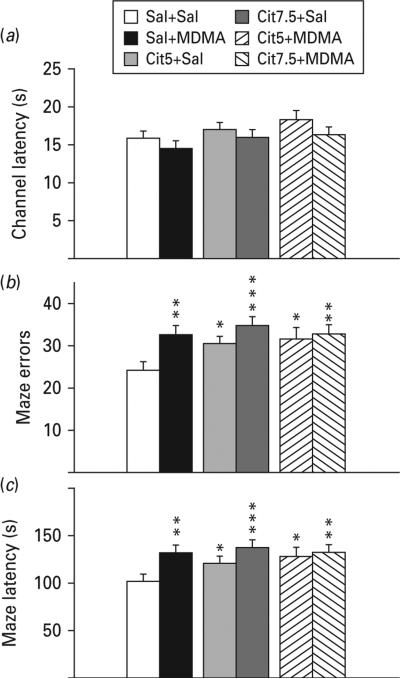
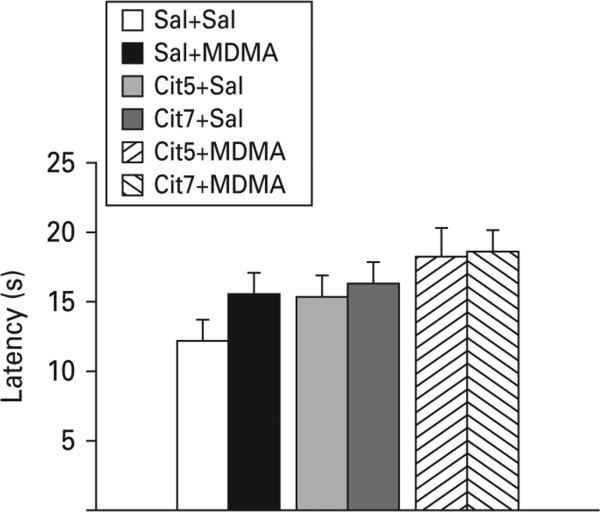
All groups had similar swimming latencies in the straight channel reflecting equal performance and motivation (Fig. 1a).
Fig. 1.
Straight swim channel and Cincinnati water maze (CWM). (a) Pre-maze trials in a 244 cm long straight swimming channel. There were no differences in latency to reach the platform during straight channel trials reflecting equivalent motoric ability and motivation to escape. (b) CWM errors of commission: number of incorrect stem, arm and start return errors in the maze. Errors were significantly increased in all groups compared to Sal+Sal. (c) CWM escape latency: time to find the escape platform in the maze. Latency was increased in all groups compared with Sal+Sal. Group comparisons were by planned false discovery rate (FDR) comparisons. * p<0.05, ** p<0.01, *** p<0.001 compared to Sal+Sal; n=16–27 per group (males) from 27 different litters; not more than one rat per group from any one litter was used to control for litter effects. Cit5, 5 mg/kg citalopram; Cit7.5, 7.5 mg/kg citalopram; MDMA, 3,4-methylenedioxymethamphetamine; Sal, saline.
Cincinnati water maze
The Sal+MDMA group made more errors and had longer latencies compared to the Sal+Sal group (Fig. 1b, c). In addition, errors and latencies were increased in both Cit+Sal groups and the Cit+MDMA groups showed similar impairments compared with the Sal+MDMA group indicating that pretreatment with Cit failed to attenuate the cognitive effects of MDMA.
Morris water maze
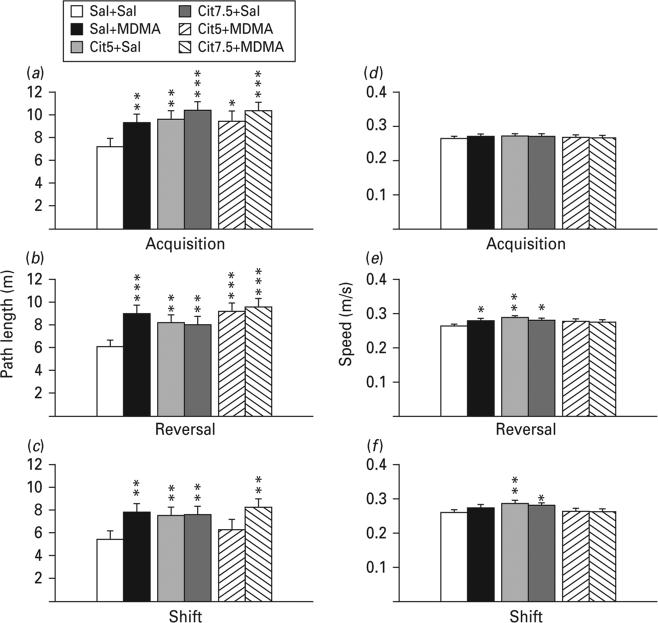
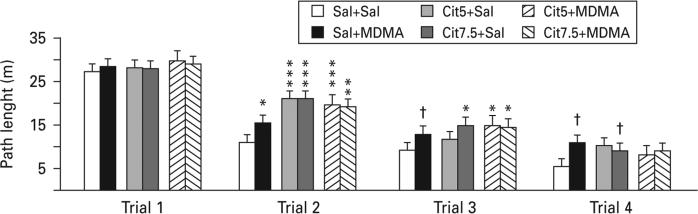
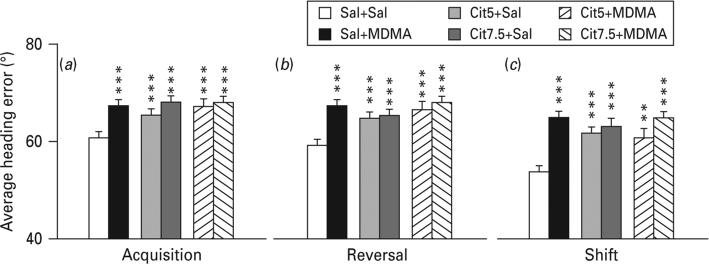
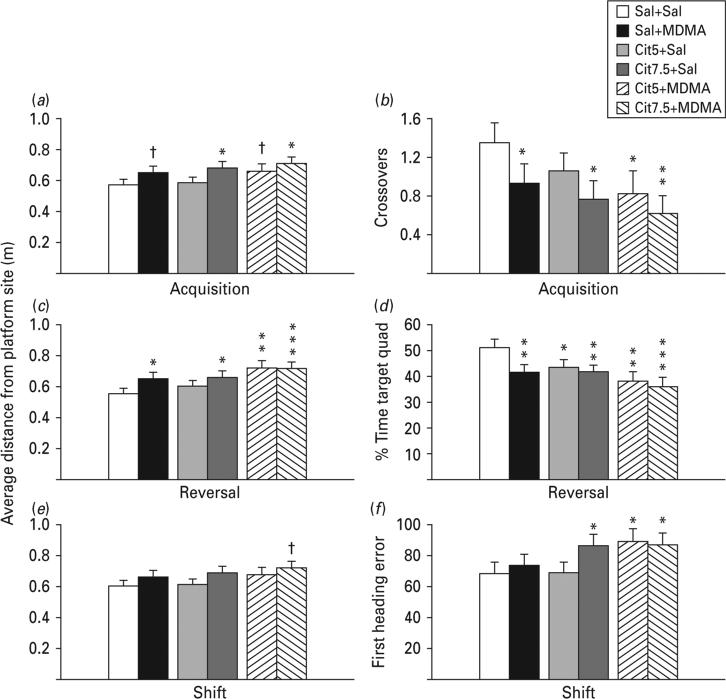
Latency, path length and cumulative distance all showed comparable effects; therefore path length is shown. During all three phases, all drug-treated groups showed significantly increased path lengths compared with the Sal+Sal group with the exception of the Cit5+MDMA group during shift learning (Fig. 2a–c). Swim speed was slightly increased in the Cit+Sal groups during reversal and shift and in the Sal+MDMA group during reversal (Fig. 2d–f). In order to determine if the drug-treated groups began the test equally, trials on day 1 were analysed separately. Path lengths were comparable among groups on trial 1, but by trial 2 group differences emerged (Fig. 3). In order to ensure that the deficit was spatial, average heading errors were analysed, i.e. the angle that each animal's swim path deviated from a straight line to the goal. This showed that all drug-treated groups were more off-course than Sal+Sal controls during all test phases (Fig. 4).
Fig. 2.
Morris water maze. (a, b, c) Path length (m) during each phase of hidden platform spatial learning. (d, e, f) Swim speed on the phases of panels a, b, c, respectively. (a) and (d) refer to the acquisition phase; (b) and (e) refer to the reversal phase; (c) and (f) refer to the shift phase. Path lengths were significantly increased in all groups compared with Sal+Sal with the exception of the Cit5+MDMA group during the shift phase. Speed was either not different or some drug-treated groups swam significantly faster than Sal+Sal as indicated. False discovery rate comparisons: * p<0.05, ** p<0.01, *** p<0.001 compared to Sal+Sal; n=16–27 per group (males) from 27 different litters; not more than one rat per group from any one litter was used to control for litter effects. Cit5, 5 mg/kg citalopram; Cit7.5, 7.5 mg/kg citalopram; MDMA, 3,4-methylenedioxymethamphetamine; Sal, saline.
Fig. 3.
Morris water maze acquisition day 1. There were not significant differences on path length on day-1 on trial-1 or trial-4. False discovery rate comparisons: † p<0.10 (trend), * p<0.05, ** p<0.01, *** p<0.001 compared to Sal+Sal; n=16–27 per group (males) from 27 different litters; not more than one rat per group from any one litter was used to control for litter effects. Cit5, 5 mg/kg citalopram; Cit7.5, 7.5 mg/kg citalopram; MDMA, 3,4-methylenedioxymethamphetamine; Sal, saline.
Fig. 4.
Morris water maze average heading error (degrees of deviation from lay line). All drug-treated groups were significantly farther off-course compared to Sal+Sal on all three phases of hidden platform learning. (a) Acquisition; (b) reversal; (c) shift. False discovery rate comparisons: ** p<0.01, *** p<0.001 compared to Sal+Sal; n=16–27 per group (males) from 27 different litters; not more than one rat per group from any one litter was used to control for litter effects. Cit5, 5 mg/kg citalopram; Cit7.5, 7.5 mg/kg citalopram; MDMA, 3,4-methylenedioxymethamphetamine; Sal, saline.
The probe trial given 24 h after the last training trial of each phase assessed reference memory. On the acquisition probe trial the Cit7.5+Sal and the Cit7.5+MDMA groups were farther from the platform site than Sal+Sal controls (Fig. 5a), indicating that the higher Cit dose had the greatest adverse effect on memory. In addition, the Sal+MDMA and Cit5+MDMA groups trended toward longer distances from the platform site. For acquisition, platform crossovers confirmed that drug-treated groups (except the Cit5+Sal group) crossed the site less often than the Sal+Sal group (Fig. 5b).
Fig. 5.
Morris water maze probe. (a, c, e) Average distance from the platform site (m) with the platform removed 24 h after the last platform trial of each phase. Retention deficits reflected by average distance to the site were evident after acquisition and reversal, but after shift these were diminished. Other measures of retention, however, revealed differences. (b) Acquisition probe platform site crossovers; (d) reversal percentage time in the target quadrant; (f) shift initial heading error. Initial heading error reflects directional deviation from a line to the goal in the first seconds after the start of the trial; three of the drug-treated experimental groups were significantly (~15°) more off-course than were Sal+Sal controls. False discovery rate comparisons: ° p<0.10 (trend), * p<0.05, ** p<0.01; *** p<0.001 compared to Sal+Sal. n=16–27 per group (males) from 27 different litters; not more than one rat per group from any one litter was used to control for litter effects. Cit5, 5 mg/kg citalopram; Cit7.5, 7.5 mg/kg citalopram; MDMA, 3,4-methylenedioxymethamphetamine; Sal, saline.
On reversal probe, all drug groups except the Cit5+Sal group had longer distances to the platform site compared with the Sal+Sal group (Fig. 5c). Crossovers were too infrequent to analyse because of task diffculty, but percentage time spent in the target quadrant showed that drug groups spent significantly lower percentages of their time in the goal quadrant (Fig. 5d) and did not differ among themselves.
On shift probe, no significant group differences were found for average distance (Fig. 5e), although a trend for greater distances in the Cit7.5+MDMA was seen. However, initial heading error, i.e. angle of error at the start of the trial, showed significant differences with the Cit7.5+Sal, Cit5+MDMA and Cit7.5+MDMA groups differing compared with the Sal+Sal group, but not the Sal+MDMA group (Fig. 5f).
During cued testing with a visible platform, no significant group differences were observed (p>0.27; Fig. 6).
Fig. 6.
Morris water maze cued trials. No significant differences in latency to reach the visibly cued platform were seen; n=16–27 per group (males) from 27 different litters; not more than one rat per group from any one litter was used to control for litter effects. Cit5, 5 mg/kg citalopram; Cit7.5, 7.5 mg/kg citalopram; MDMA, 3,4-methylenedioxymethamphetamine; Sal, saline.
Body weight
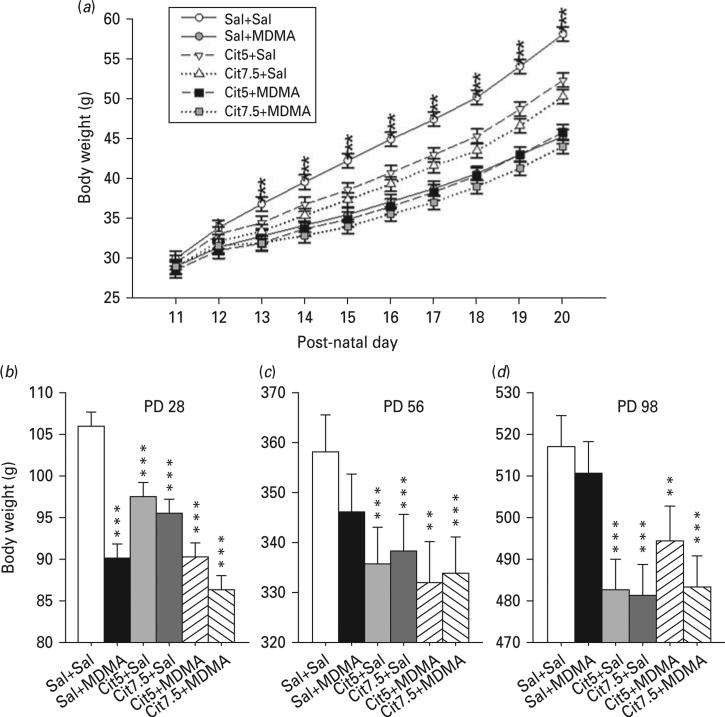
Body weight was affected by the drugs during treatment. There was a significant main effect of treatment (p<0.001) and a significant treatment×day interaction (p<0.001; Fig. 7). Further analyses showed that body weights were unchanged before the first treatment on PD 11. By PD 12, all treatment groups had reduced body weight except the Cit5+Sal group compared to the Sal+Sal group. On PD 13–20, all treatments caused a reduction in body weight gain compared with the Sal+Sal group. Analysis of body weight after the end of treatment but before weaning (PD 21 and PD 28), also showed an effect of treatment (p<0.001). All drug treatment groups showed a reduction in body weight compared with the Sal+Sal group (Fig. 7).
Fig. 7.
Body weights. During dosing (a), all groups had comparable weights on post- natal day (PD) 11, but by PD 12 all treated groups with the exception of the Cit5+Sal group weighed less than Sal+Sal. Throughout the remainder of dosing (PD 13–20), all drug-treated groups had significantly lower body weights compared to Sal+Sal. (b) Body weights on PD 21 and PD 28 remained significantly decreased by the drug treatments with all groups weighing less than Sal+Sal. Body weights taken from PD 35–98 were significantly affected by drug treatment with all groups that received Cit alone or in combination with 3,4-methylenedioxymethamphetamine (MDMA) weighing less than Sal+Sal. (c) Body weight at PD 56 just prior to the start of behavioural testing. (d) Body weight at PD 98 after completion of behavioural testing. (a): * p<0.05, all groups were different except Cit5+Sal vs. Sal+Sal; *** p<0.001, all drug treated groups compared to Sal+Sal. (b, c, d): ** p<0.01, compared to Sal+Sal; *** p<0.001 compared to Sal+Sal; n=16–27 per group (males) from 27 different litters; not more than one rat per group from any one litter was used to control for litter effects. Cit5, 5 mg/kg citalopram; Cit7.5, 7.5 mg/kg citalopram; Sal, saline.
Analysis of body weight after weaning (PD 35–98), showed a main effect of treatment (p<0.001), but no treatment×age interaction (Fig. 7c, d). Cit-treated groups weighed less than the Sal+Sal group whereas the weights of the Sal+MDMA-treated group recovered and were not different from the Sal+Sal group by PD 35.
Discussion
In the present study, Cit was used as a tool to test the hypothesis that Cit mediated attenuation of MDMA-induced 5-HT depletions would be neuroprotective against the developmental cognitive effects of MDMA. Exposure was during a period in rat brain development, which according to recent regression analyses of a large database of studies most closely resembles third trimester brain development in humans (Bayer et al., 1993; Clancy et al., 2001, 2007b). Previously, it has been suggested that rat brain development on PD 10 approximates human brain development at birth (Dobbing and Sands, 1979; Rodier, 1980; West and Pierce, 1987) a view that remains based on some markers (Rakhade and Jensen, 2009). However, alternate views have existed simultaneously suggesting that the rat brain at PD 15–20 is closer to human brain at birth (Bayer et al., 1993; Rice and Barone, 2000; Herlenius and Lagercrantz, 2004). Recently, cross-species comparisons have been improved by the use of a large database approach with best-fit regression analyses (Clancy et al., 2001, 2007a, b). The newer comparison suggests that rodent brain ontogeny extends significantly longer into post-natal development in relation to human brain development at birth than previous estimates suggested. Our model relied on these newer estimates and that was why we exposed on PD 11–20. Neither time window is exact because different regions of rat and human brain develop at different rates relative to one another and this includes not only neurogenesis, but migration, synaptogenesis, apoptosis, receptor development and synaptic pruning. Rather than there being a single window of comparability in rats to each stage of human in utero brain development, there are many windows each slightly different for each region, neurotransmitter and receptor type. While our exposure period being analogous to limbic third trimester development is not absolute, there is more than adequate data to support its relevance.
The PD 11–20 exposure window results in acute 5-HT reductions in the hippocampus, neostriatum and entorhinal cortex during drug treatment (Schaefer et al., 2012). We previously showed that pretreatment with Cit attenuates the short-term 5-HT reductions caused by MDMA by ≥50%. On this basis we predicted that it would also attenuate the long-term cognitive effects of MDMA. This prediction was supported by data showing a dose-dependent relationship between short-term 5-HT reductions and later learning deficits and by data with a related serotonergic drug, fenfluramine, that when given during the same exposure period induced larger 5-HT reductions than MDMA and greater cognitive deficits (Morford et al., 2002; Schaefer et al., 2006). Yet despite Cit effcacy at attenuating MDMA effects on 5-HT, it failed to moderate the effects of MDMA on learning and memory. In fact the two Cit-treated groups exhibited learning and memory deficits comparable with those induced by MDMA and in one case (acquisition probe), the group receiving the 7.5 mg/kg Cit dose was the most impaired making this finding arguably more significant than the effects of MDMA since antidepressants (ADs) are widely used during pregnancy.
Thus, the new observation arising unexpectedly from this experiment was that developmental exposure to Cit induces learning and memory impairments. These are the first data to find significant learning and memory deficits of this type following developmental SSRI exposure. This warrants further investigation because of its implications for human use during pregnancy which is increasing in this vulnerable population as well as in the general population. The doses of Cit (5 and 7.5 mg/kg) chosen here were those producing serum and brain drug and metabolite levels in neonatal rats similar to those observed in adult rats which in turn have been shown to be comparable to human therapeutic doses (Maciag et al., 2006b).
The present deficits observed in learning and memory cannot be accounted for by differences in swimming ability for several reasons: (1) in the CWM we found the same effects on errors and on latency to escape. Had rats swum slower but not been impaired, they would have had comparable numbers of errors, which they did not; (2) pre-maze straight channel swimming latencies did not differ across groups; (3) in the MWM we used path length and average heading error, both immune to latency differences, for comparison and found significant learning impairments in the drug treated groups; (4) several of the drug groups showed faster swimming speed in the MWM than controls, which if anything would be expected to facilitate finding the platform, increase reinforcement and result in faster learning, but the exact opposite was observed; drug-treated groups swam faster but learned slower; (5) MWM probe trial performance supports the interpretation that the drug-treated groups were impaired because they showed impaired retention for where they had previously found the platform in most phases of this test. Collectively, the two tests and all the indices of learning vs. performance support the view that the drug-induced deficits were cognitive and not linked to sensorimotor deficiencies.
Studies on infants and children after prenatal AD exposure have largely ignored cognitive ability and focused on emotional development and one new study reported a 2-fold increased association between prenatal SSRI exposure and autism spectrum disorder (ASD; Croen et al., 2011). While this has not been replicated, such a finding would be consistent with the present data since 62% of ASD children exhibit some degree of intellectual impairment (Centers for Disease Control and Prevention, 2012).
In addition to the finding that developmental Cit exposure reduces hippocampal 5-HT levels by approximately 25% into adulthood (Schaefer et al., 2012), others have reported lasting neurochemical changes in rats treated with Cit from PD 8–21 in which they observed reduced tryptophan hydroxylase immunoreactivity in the dorsal and medial raphe nuclei and reduced SERT immunoreactivity in the cortex (Maciag et al., 2006b). A second study similarly found reduced SERT immunoreactivity in the hippocampus after PD 8–21 Cit treatment (Weaver et al., 2010). New data indicate that E7-19, PD 1–7 or PD 7–21 exposure to Cit 10 mg/kg twice daily causes 5-HT immunoreactivity differences in fibre shape and density in the hippocampus, reduced tryptophan hydroxylase immunofluorescence labelling in the raphe and reduced oligodendrocyte myelin wrapping in the corpus callosum (Simpson et al., 2011). Together with our data, these findings suggest that the observed Cit-induced learning deficits are the result of developmental changes in 5-HT that persist well after developmental drug exposure has ended suggesting permanent changes to the serotonergic system and other structures.
Since MDMA and Cit both bind to SERT and interfere with 5-HT reuptake, it may be that this shared mechanism leads to a transient increase in 5-HT receptor stimulation followed by a long-term 5-HT reduction via feedback down-regulation of tryptophan hydroxylase by 5-HT pre-synaptic autoreceptors, especially 5-HT1A and 5-HT1D receptors, which are known to mediate this process in adult brain (McDevitt and Neumaier, 2011; Winterer et al., 2011). This might explain why Cit+Sal and Sal+MDMA result in comparable cognitive outcomes and long-term hippocampal 5-HT reductions after developmental exposure if one assumes the same mechanisms are at work. Even if Cit out-competes MDMA for SERT binding when given in succession (thereby limiting MDMA-induced 5-HT release and early depletions), synaptic 5-HT in both cases changes 5-HT receptor stimulation such that receptor function may be perturbed. While Cit has some ability to stimulate adult 5-HT release (Ceglia et al., 2004; Baumann et al., 2007) this effect is less pronounced than that caused by MDMA, which suggests that 5-HT release is not the critical factor in these drugs’ long-term cognitive effects. Developmental MDMA administration also increases 5-HT1A receptor sensitivity (Crawford et al., 2006) and this too could be a contributing factor. Collectively, such data suggest that additional study of the effects of serotonergic drugs on 5-HT receptor development will shed further light on this question.
It appears unlikely that the effects seen here are the product of MDMA-induced effects on dopamine because even though MDMA affects dopamine in adult brain it does not do so in developing brain at ages comparable to those used in the present experiment (Broening et al., 1994, 1997; Schaefer et al., 2006, 2008).
Both drugs were found to impact growth as reflected by body weight gain. It could be argued that this secondary effect impacted learning, but this is unlikely as we previously showed that the decrease in body weight induced by neonatal MDMA can be controlled for without changing the learning and memory deficits caused by MDMA by using weight-matched controls (Williams et al., 2003).
Limitations of the study include use of only two doses for Cit and one dose of MDMA and only one dosing schedule of the two drugs. It may be that different doses, different dose spacing, different Cit pretreatment intervals, or different exposure ages would have different long-term effects. In addition, we have no data on the cellular changes in these animals after testing which might provide correlational support for how the two drugs alter brain structure or protein expression that could be linked to the learning and memory deficits. In addition, both cognitive tests relied on swimming and it cannot be ruled out that motivation derived from escape from water might interact in some unknown way with the drug effects. Future experiments using appetitive motivation or novelty (as in novel object recognition) should be conducted to provide convergent evidence. Finally, it has been shown that swimming mazes induce larger hypothalamus–pituitary–adrenal axis activation as reflected by plasma corticosterone in mice than does the Barnes maze (Harrison et al., 2009) and we cannot rule out drug×stress interaction effects until tests using other types of motivation are evaluated.
The Cit findings raise concerns about the long-term safety of SSRIs after developmental exposure. The most recent NHANES data show that use of ADs is at an all-time high and rising (Pratt et al., 2011); moreover, the rate of AD use is 2.5 times higher in women (15.4%) than in men (6%). The Food and Drug Administration classifies all but one AD as category C, stating that no adequate data on prenatal use are available but that benefits may warrant use during pregnancy. The American Psychiatric Association, American Academy of Pediatrics and the American College of Obstetrics and Gynecology do not recommend discontinuation of AD use during pregnancy. The Center for Disease Control and Prevention finds that only one-third of those taking ADs have seen a mental health provider (Pratt et al., 2011) suggesting that these drugs are regarded as relatively safe, but this may not apply to the foetal brain. Another consideration is that 40% of those taking ADs take more than one at a time, a pattern trending upwards despite our poor understanding of how even one of these drugs affect brain development and later cognitive ability.
In conclusion, Cit attenuated MDMA-induced 5-HT reductions during brain development did not ameliorate the cognitive deficits associated with MDMA on either allocentric or egocentric learning or reference memory. Moreover, Cit treatment alone produced learning and memory impairments as severe as those caused by MDMA. The latter finding raises new concerns about the safety of SSRIs during pregnancy. Further studies are needed assessing the effects of exposure during other (earlier) stages of brain development, on comparing different SSRIs and comparing SSRIs to non-SSRIs and mixed reuptake inhibitors, in determining if there are thresholds below which de minimus risk to cognitive development can be shown and in assessing whether other cognitive domains (e.g. working memory, executive functions, etc.) not tested here are also at risk.
Acknowledgements
Supported by National Institutes of Health grants DA014269 (MTW) and DA21394 (CVV) and training grant ES007051 (TLS and MRS).
Footnotes
Statement of Interest
Dr Vorhees discloses that he has provided consultation to Eli Lilly and Company on research unrelated to the present work in the last 3 yr.
References
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc B. 1995;57:289–300. [Google Scholar]
- Bobillier P, Pettijean F, Salvert D, Ligier M, Seguin S. Differential projections of the nucleus raphe dorsalis and nucleus raphe centralis as revealed by autoradiography. Brain Res. 1975;85:205–210. doi: 10.1016/0006-8993(75)90071-2. [DOI] [PubMed] [Google Scholar]
- Broening HW, Bacon L, Slikker W., Jr. Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr. Age dependent sensitivity of rats to the long-term effects of the serotonin neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (ecstasy) induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broening HW, St. Clair C, Morford LL, Inman SL, Moran MS, Fukumura M, Vorhees CV. Developmental exposure to 3,4-methylenedioxymethamphetamine (MDMA) in rats produces persistent deficits in Cincinnati water maze performance. Neuroticol Teracol. 1997;19:246. [Google Scholar]
- Brown P, Molliver ME. Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J Neurosci. 2000;20:1952–1963. doi: 10.1523/JNEUROSCI.20-05-01952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon GD, Morford LL, Vorhees CV. Ontogeny of methamphetamine-induced neurotoxicity and associated hyperthermic response. Dev Brain Res. 1997;103:155–162. doi: 10.1016/s0165-3806(97)81791-9. [DOI] [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Acconcia S, Fracasso C, Colovic M, Caccia S, Invernizzi RW. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br J Pharmacol. 2004;142:469–478. doi: 10.1038/sj.bjp.0705800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Morbid Mort Week Rep. 2012;61:1–24. [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Cook D, Kesner RP. Caudate nucleus and memory for egocentric localization. Behav Neural Biol. 1988;49:332–343. doi: 10.1016/s0163-1047(88)90338-x. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Kohutek JL, Choi FY, Yoshida ST, McDougall SA, Vorhees CV. Neonatal 3,4-methylenedioxymethamphetamine (MDMA) exposure alters neuronal protein kinase A activity, serotonin and dopamine content, and [(35)S]GTPgammaS binding in adult rats. Brain Res. 2006;1077:178–186. doi: 10.1016/j.brainres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- Darling RD, Alzghoul L, Zhang J, Khatri N, Paul IA, Simpson KL, Lin RC. Perinatal citalopram exposure selectively increases locus ceruleus circuit function in male rats. J Neurosci. 2011;31:16709–16715. doi: 10.1523/JNEUROSCI.3736-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Cami J. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Fuhs MC, Touretzky DS. A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci. 2006;26:4266–4276. doi: 10.1523/JNEUROSCI.4353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Gabrielsson J, Marsden CA, Fone KC. MDMA: on the translation from rodent to human dosing. Psychopharmacology (Berl) 2009;204:375–378. doi: 10.1007/s00213-008-1453-8. [DOI] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KC. Lost in translation: preclinical studies on 3,4-methylenedioxymethamphetamine provide information on mechanisms of action, but do not allow accurate prediction of adverse events in humans. Br J Pharmacol. 2012;166:1523–1536. doi: 10.1111/j.1476-5381.2011.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drug Metab. 2002;3:13–37. doi: 10.2174/1389200023338017. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Smith LM, Suri R, Hwang S, Haynes D, Altshuler L. Birth outcomes after prenatal exposure to antidepressant medication. Am J Obstet Gynecol. 2003;188:812–815. doi: 10.1067/mob.2003.172. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use ecstasy (3,4-methylenedoxymethamphetamine). Neurotoxicol Teratol. 2001;23:561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010;31:60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974;155:469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Wallace JA, Krebs H. Roles for serotonin in neuroembryogenesis. Adv Exp Med Biol. 1981;133:477–506. doi: 10.1007/978-1-4684-3860-4_28. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Neumaier JF. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J Chem Neuroanat. 2011;41:234–246. doi: 10.1016/j.jchemneu.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Coppinger D, Paul IA. Evidence that the deficit in sexual behavior in adult rats neonatally exposed to citalopram is a consequence of 5-HT1 receptor stimulation during development. Brain Res. 2006a;1125:171–175. doi: 10.1016/j.brainres.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006b;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Williams L, Coppinger D, Paul IA. Neonatal citalopram exposure produces lasting changes in behavior which are reversed by adult imipramine treatment. Eur J Pharmacol. 2006c;532:265–269. doi: 10.1016/j.ejphar.2005.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Sabol KE, Seiden LS. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J Pharmacol Exp Ther. 1996;278:258–267. [PubMed] [Google Scholar]
- Meyer JS, Piper BJ, Vancollie VE. Development and characterization of a novel animal model of intermittent MDMA (“Ecstasy”) exposure during adolescence. Ann N Y Acad Sci. 2008;1139:151–163. doi: 10.1196/annals.1432.029. [DOI] [PubMed] [Google Scholar]
- Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with d-fenfluramine. Eur J Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med. 2007;161:22–29. doi: 10.1001/archpedi.161.1.22. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Plessinger MA. Prenatal exposure to amphetamines: risks and adverse outcomes in pregnancy. Obstet Gynecol Clin N Amer. 1998;25:119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005–2008. CDC NCHS Data Brief. 2011;76:1–8. [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Chronology of neuron development: animal studies and their clinical implications. Dev Med Child Neurol. 1980;22:525–545. doi: 10.1111/j.1469-8749.1980.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Porcel F, Green D, Khatri N, Harris SS, May WL, Lin RC, Paul IA. Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anat Rec (Hoboken) 2011;294:1726–1735. doi: 10.1002/ar.21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol KE, Seiden LS. Reserpine attenuates D-amphetamine and MDMA-induced transmitter release in vivo: a consideration of dose, core temperature and dopamine synthesis. Brain Res. 1998;806:69–78. doi: 10.1016/s0006-8993(98)00720-3. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−)methylphenidate administration in the neonatal rat. J Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Grace CE, Skelton MR, Graham DL, Gudelsky GA, Vorhees CV, Williams MT. Neonatal citalopram treatment inhibits the 5-HT depleting effects of MDMA exposure in rats. ACS Chem Neurosci. 2012;3:12–21. doi: 10.1021/cn2000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short- and long-term effects of (+)-methamphetamine and (+/−)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J Neurochem. 2008;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci USA. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Schaefer TL, Herring NR, Grace CE, Vorhees CV, Williams MT. Comparison of the developmental effects of 5-methoxy-N,N-diisopropyltryptamine (Foxy) to (+/−)-3,4-methylenedioxymethamphetamine (ecstasy) in rats. Psychopharmacology (Berl) 2009;204:287–297. doi: 10.1007/s00213-009-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Treatment with MDMA from P11-20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berl) 2006;189:307–318. doi: 10.1007/s00213-006-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev Neurosci. 2003;25:245–256. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, +/−methylenedioxymethamphetamine, (+)-amphetamine and +/−fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2011;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008a;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008b;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Williams MT. Developmental effects of +/−3,4-methylenedioxymethamphetamine on spatial vs. path integration learning: effects of dose distribution. Synapse. 2007a;61:488–499. doi: 10.1002/syn.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci. 2009;27:289–298. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav Pharmacol. 2007b;18:549–562. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KJ, Paul IA, Lin RC, Simpson KL. Neonatal exposure to citalopram selectively alters the expression of the serotonin transporter in the hippocampus: dose-dependent effects. Anat Rec (Hoboken) 2010;293:1920–1932. doi: 10.1002/ar.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Pierce DR. Perinatal alcohol exposure and neuronal damage. In: West JR, editor. Alcohol and brain development. Oxford UP; New York: 1987. pp. 120–157. [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Brown CA, Skelton MR, Vinks AA, Vorhees CV. Absorption and clearance of ±3,4-methylenedioxymethamphetamine from the plasma of neonatal rats. Neurotoxicol Teratol. 2004;26:849–856. doi: 10.1016/j.ntt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects, or injection stress. Brain Res. 2003;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees 3,4-Methylenedioxymethamphetamine administration on postnatal day 11 in rats increases pituitary-adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res. 2005;CV:1039:97–107. doi: 10.1016/j.brainres.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer J, Stempel AV, Dugladze T, Foldy C, Maziashvili N, Zivkovic AR, Priller J, Soltesz I, Gloveli T, Schmitz D. Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. J Neurosci. 2011;31:8464–8475. doi: 10.1523/JNEUROSCI.6382-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Darling RD, Paul IA, Simpson KL, Chen K, Shih JC, Lin RC. Altered expression of tyrosine hydroxylase in the locus coeruleus noradrenergic system in citalopram neonatally exposed rats and monoamine oxidase a knock out mice. Anat Rec (Hoboken) 2011;294:1685–1697. doi: 10.1002/ar.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]