Abstract
Background
This study sought to compare the prevalence and modifying factors of normoalbuminuric (NA) versus albuminuric (ALB) CKD in the U.S. diabetic and nondiabetic populations.
Methods
NHANES 2001–2008 included 2798 diabetic and 15,743 nondiabetic participants. Age-specific prevalence of NA-CKD and ALB-CKD was calculated within each diabetes stratum and then stratified again according to gender, ethnicity, mean arterial pressure ≥105mmHg and HbA1c≥7%. Multivariate regression analyses were performed to determine odds ratios and 95% CI for NA-CKD.
Results
Prevalence of NA-CKD rose with age, with an overall mean of 9.7% in diabetic and 4.3% in nondiabetic participants. NA-CKD was less prevalent in diabetic men, OR=0.58 (95% CI: 0.39, 0.87). In comparison with whites, blacks and ‘other’ ethnic groups had an OR for NA-CKD of 0.44 (95% CI 0.29, 0.68) and 0.57 (95% CI: 0.34, 0.96), respectively. Poorly controlled blood pressure and glycemia resulted in a decreased OR for NA-CKD (OR=0.25, 95% CI: 0.13, 0.50) and (0.48, 95% CI: 0.31, 0.74), respectively. Similar results were obtained for nondiabetic participants.
Conclusions
NA-CKD is more common in people with diabetes, women, non-Hispanic whites, and in the setting of well controlled blood pressure and glycemia.
Keywords: Diabetes, Chronic Kidney Disease, Normoalbuminuria
1. Introduction
The prevalence of chronic kidney disease (CKD) among people with diabetes is the same today as it was twenty years ago (de Boer, Rue, Hall, Heagerty, Weiss, and Himmelfarb, 2011). This is despite more aggressive glycemic and blood pressure control and widespread use of inhibitors of the renin–angiotensin–aldosterone system (RAAS). Historically, albuminuria has been central to the diagnosis and clinical management of diabetic kidney disease. Over the last decade, it has been noted that nonalbuminuric chronic kidney disease (NA-CKD) is common, ranging between 25% and 50% among people with diabetes and estimated glomerular filtration rate (eGFR) less than 60ml/min/1.73m2 (Kramer, Nguyen, Curhan, and Hsu, 2003; Molitch et al., 2010; Penno et al., 2011; Retnakaran, Cull, Thorne, Adler, and Holman, 2006; Thomas et al., 2009). Nonalbuminuric diabetic kidney disease has been found to be increasingly prevalent with advanced age and female gender (Caramori, Fioretto, & Mauer, 2003; Penno et al., 2011; Thomas et al., 2009).
The pathogenesis of diabetic NA-CKD remains to be elucidated. In normoalbuminuric patients with type 1 diabetes and reduced GFR, the presence of relatively advanced classical diabetic nephropathy lesions could explain the GFR reduction (Lane, Steffes, and Mauer, 1992; Shideman, Buselmeier, Mauer, and Kjellstrand, 1972). However, this is less well studied in type 2 diabetes. Hypothesized etiologies of NA-CKD include renal hypertensive and vascular diseases, age related renal senescence and masking of albuminuria by RAAS inhibitors (Kramer et al., 2003; Macisaac and Jerums, 2011; MacIsaac, Tsalamandris, Panagiotopoulos, Smith, McNeil, and Jerums, 2004). Also hypothesized is a self-selected low protein diet in type 1 diabetic females with normoalbuminuria and low eGFR (Lane et al., 1992). The possibility that NA-CKD in people with diabetes may not be a consequence of hyperglycemia is supported by the facts that hemoglobin A1c (HbA1c) tends to be lower as is the frequency and severity of other microvascular complications, when compared to those diabetic persons with albuminuric chronic kidney disease (ALB-CKD) (Kramer et al., 2003; MacIsaac et al., 2004; Penno et al., 2011; Rigalleau et al., 2007; Thomas et al., 2009; Yokoyama, Sone, Oishi, Kawai, Fukumoto, and Kobayashi, 2009). However, a large population-based study has demonstrated a similar reduction in the incidence of decreased eGFR<60 ml/min/1.73 m2 for every 1% decrease in HbA1c, regardless of preexisting albuminuria or retinopathy (Bash, Selvin, Steffes, Coresh, and Astor, 2008). Moreover, renal biopsy studies have shown significant pathologic changes consistent with diabetic glomerulopathy in type 1 diabetes and NA-CKD, this primarily occurring in women (Caramori, Fioretto, and Mauer, 2003). Data are conflicting with respect to blood pressure differences between NA-CKD and ALB-CKD in diabetes (Penno et al., 2011; Rigalleau et al., 2007; Thomas et al., 2009; Yokoyama et al., 2009). Investigation of the risk factors for NA-CKD in the U.S. population has been limited, and more specifically, the impact of race and ethnicity has not been explored. Our goal was to determine the U.S. age-specific prevalence of NA-CKD in diabetic and nondiabetic populations and to describe differences in their sociodemographic, ethnic and clinical characteristics.
2. Materials and methods
2.1. Study population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative sample of the total US civilian, non-institutionalized population. It uses a stratified multistage probability design with planned oversampling of certain age and racial/ethnic groups (Centers for Disease Control and Prevention National Center for Health Statistics). Eligibility for inclusion in the analysis included: age 20years or older, complete sociodemographic information and available serum creatinine and urine albumin:creatinine ratio (UACR). The NHANES protocol was approved by a human subjects review board and written, informed consent was obtained from all participants (Centers for Disease Control and Prevention National Center for Health Statistics).
2.2. Covariate definitions and measurements
Diabetes was defined as at least one of the following: 1) self-report of being previously diagnosed by a physician (except during pregnancy), 2) a fasting glucose greater than 126mg/dl or 3) HbA1c greater than or equal to 6.5% (“International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes, 2009; Standards of medical care in diabetes–2010, 2010).
A single, random urine collection was obtained for UACR. Urinary albumin was quantified using solid-phase fluorescent immunoassay. Urinary creatinine was measured with the Jaffe rate reaction. Albuminuria was defined as a UACR of at least 30mg/g (“K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification”, 2002). Sensitivity analyses were performed also using gender-specific cut-points of ≥17mg/g for men and ≥25mg/g for women. Serum creatinine was measured using the modified kinetic Jaffe reaction Creatinine values from 2005–2006 were calibrated as previously described (Centers for Disease Control and Prevention National Center for Health Statistics; Stevens et al., 2007). GFR was estimated using the CKD-EPI equation (Levey et al., 2009). Chronic kidney disease was defined as an eGFR<60ml/min/1.73m2 (“K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification”, 2002).
Age was defined as the age at the time of the examination. Race and ethnicity were self-reported and categorized as Non-Hispanic White, Non-Hispanic Black, and for the purpose of this study, all others were grouped as ‘Other’. Cardiovascular disease history was self-reported and included history of congestive heart failure, coronary heart disease, heart attack, angina or stroke. Medications were assessed by an in-person interview. Blood pressure was defined as the mean of three separate readings. BMI was calculated from the weight and height measurements taken during the physical examination. HbA1c was measured by a high performance liquid chromatographic assay.
2.3. Statistical methods
All analyses were performed using Stata (version 11), or SPSS (version 16) statistical software. NHANES is a complex probability sample, so appropriate sample weights were used to estimate mean values and standard errors (Centers for Disease Control and Prevention National Center for Health Statistics). To combine data from four 2-yearcycles of the continuous NHANES (2001–2008), we created new sample weights. Age-specific prevalence of NA-CKD and ALB-CKD was calculated and stratified according to diabetes status, gender, ethnicity, uncontrolled diabetes (defined as an HbA1c≥7.0) and uncontrolled hypertension (defined as a mean arterial pressure (MAP) ≥105). Multivariate logistic regression analyses were performed to better understand the relationship between NA-CKD and diabetes status, gender, ethnicity, MAP and HbA1C. Odds ratios and 95% confidence intervals are reported, adjusted for age, gender, ethnicity, and MAP except when each was the variable of interest.
3. Results
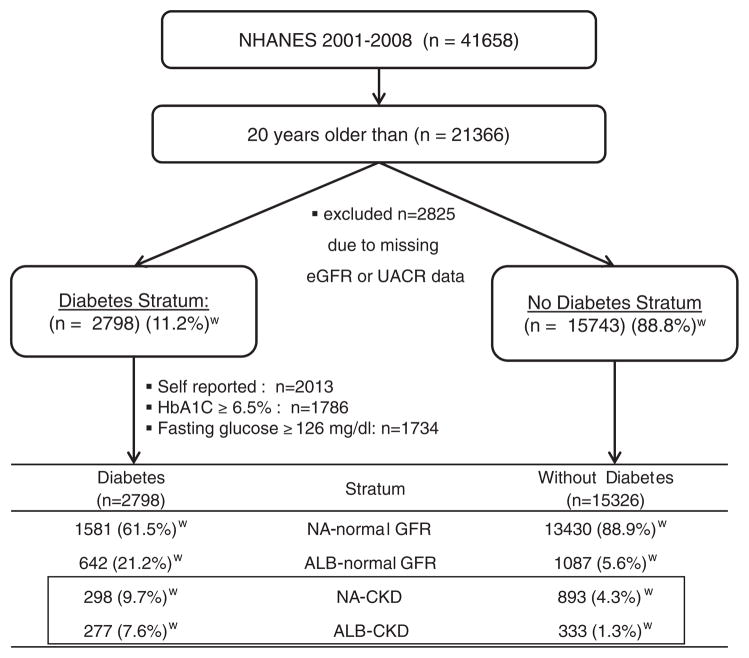
The participants meeting selection criteria are depicted in Fig. 1. A total of 15,743 persons without diabetes and 2798 persons with diabetes met inclusion criteria. Only 64 participants with diabetes were under the age of 30 and 254 were under the age of 40 (data not shown), making the vast majority of diabetic participants type 2. Abnormalities in UACR (≥30mg/g) or eGFR (<60ml/min/1.73m2) were present in 11.1% (95% CI 9.3–12.1) of persons without diabetes versus 38.5% (95% CI 36.5–40.5) of those with diabetes. NA-CKD was present in 4.3% of subjects without diabetes versus 9.7% with diabetes. Confining the analysis to those with decreased eGFR (<60ml/min/1.73m2), NA-CKD was present in 77.3% (95% CI 74.6–80) and 56.3% (95% CI 51.7–60.9) in the non-diabetic and diabetic strata, respectively.
Fig. 1.
Algorithm and participant numbers meeting selection criteria for study of chronic kidney disease (eGFR<60ml/min/1.73m2) with albuminuria (ALB) ≥30mg/g and normoalbuminuria (NA) <30mg/g in NHANES 2001–2008.
The clinical characteristics of participants with NA-CKD versus ALB-CKD stratified by diabetes status are displayed in Table 1. Treatment with RAAS blockers was more common in individuals with diabetes, but did not differ between NA-CKD and ALB-CKD. Poorly controlled hypertension (MAP≥105) was more common in the ALB-CKD groups, regardless of diabetes status. Estimated GFR was lower on average in the ALB-CKD groups, regardless of diabetes status. History of cardiovascular disease was highest in the diabetes stratum with ALB-CKD (58%, 95% CI 50%–65%), and lowest in the stratum without diabetes and with NA-CKD (30%, 95% CI 26%–35%). Those with diabetes and NA-CKD or without diabetes but with ALB-CKD were in between, and similar to one another 43% (34%–52%) and 39% (32%–46%), respectively.
Table 1.
Demographic and clinical characteristics of participants of NHANES 2001–2008 with CKD according to diabetes and albuminuric status.
| Characteristics | Diabetes Mellitus
|
Without Diabetes Mellitus
|
||
|---|---|---|---|---|
| Normoalbuminuric CKD
|
Albuminuric CKD
|
Normoalbuminuric CKD
|
Albuminuric CKD
|
|
| mean (95% CI) | mean (95% CI) | mean (95% CI) | mean (95% CI) | |
| N=298 | N=277 | 893 | 333 | |
| Age, years | 71 (69–73) | 72 (70–74) | 71 (70–72) | 73 (70–74) |
| gender, % female | 58 (52–65) | 52 (46–59) | 65 (61–70) | 52 (44–60)a |
| Ethnicity, % | ||||
| Non-Hispanic White | 78 (73–83) | 71 (65–78) | 92 (89–94)b | 79 (74–85)a |
| Non-Hispanic Black | 13 (9–17) | 16 (10–21) | 4 (3–5)c | 12 (8–17)a |
| Other | 9 (6–12) | 13 (7–19) | 5 (3–6)c | 8 (4–12) |
| Systolic Blood Pressure (mmHg) | 133 (130–136) | 146 (142–151)b | 133 (131–135) | 146 (143–149)a |
| Diastolic Blood Pressure (mmHg) | 61 (59–64) | 65 (62–68) | 66 (65–68)b | 67 (63–70) |
| Low Density Lipoprotein (mg/dl) | 109 (103–115) | 98 (89–107) | 116 (111–121) | 109 (101–117) |
| High Density Lipoprotein (mg/dl) | 51 (49–53) | 49 (47–51) | 56 (54–57)b | 54 (52–57) c |
| Body mass index, % ≥30 | 50 (43–56) | 50 (43–57) | 26 (23–29)b | 27 (21–33)c |
| Mean arterial pressure, % ≥105mmHg | 8 (5–12) | 19 (13–24)b | 9 (7–11) | 27 (21–32)a |
| History of cardiovascular disease, % | 38 (31–45) | 56 (49–63)b | 27 (24–31) | 39 (33–46)ac |
| History of antihypertensive treatment, % | 97 (94–99) | 97 (95–99) | 87 (83–91)b | 91 (88–95) |
| RAAS inhibitors | 44 (37–51) | 53 (47–60) | 28 (25–32)b | 35 (28–41)c |
| Beta blockers | 39 (32–46) | 46 (39–54) | 29 (26–33) | 35 (28–42) |
| Diuretics | 36 (30–43) | 43 (35–51) | 27 (24–30) | 35 (27–42) |
| Estimated GFR, ml/min/1.73m2 | 49 (48–50) | 42 (40–44)b | 50 (50–51) | 44 (43–45)a |
CKD=chronic kidney disease defined as eGFR<60ml/min/1.73m2; RAAS=renin–angiotensin–aldosterone system; GFR=glomerular filtration rate
Nonoverlapping confidence intervals with the nondiabetic normoalbuminuric CKD group.
Nonoverlapping confidence intervals with the diabetic normoalbuminuric CKD group.
Nonoverlapping confidence intervals with the diabetic albuminuric CKD group.
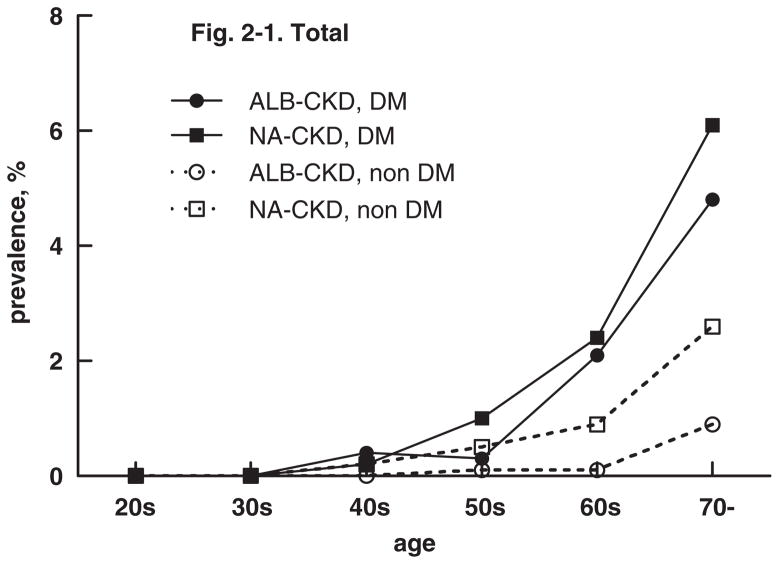
There was a clear relationship between age and any CKD as shown in Fig. 2. In persons with diabetes, NA-CKD and ALB-CKD parallel one another, with a steep rise in both starting in the 5th decade. In persons without diabetes, the prevalence of NA-CKD antedates that of ALB-CKD from age 40 onward, but took an even sharper rise at age 60.
Fig. 2.
Age-specific prevalence of chronic kidney disease (CKD) defined as eGFR <60ml/min/1.73m2 according to diabetes status and albuminuria (ALB) ≥30mg/g or normoalbuminuria (NA) <30mg/g on a random urine specimen from NHANES 2001–2008.
NA-CKD was less common in males, especially in the non-diabetic stratum. Multivariate regression analyses controlling for age, ethnicity and poorly controlled HTN showed that the effect of male gender on the prevalence of NA-CKD was similar regardless of diabetes status, with an OR=0.58 (95% CI 0.39–0.87) for the diabetic stratum and 0.60 (95% CI 0.41–0.88) for the non-diabetic stratum (Table 2).
Table 2.
Odds Ratios (OR) for Normoalbuminuric (NA) versus Albuminuric (ALB) Chronic Kidney disease (eGFR <60ml/min/1.73m2) according to gender, ethnicity, uncontrolled hypertension and uncontrolled diabetes.
| Variables | Diabetes OR (95% CI) | No Diabetes OR (95% CI) |
|---|---|---|
| Gender | ||
| Female (reference) | 1.00 | 1.00 |
| Male | 0.58 (0.39–0.87) | 0.60 (0.41–0.88) |
| Ethnicity | ||
| Non-Hispanic Whites (reference) | 1.00 | 1.00 |
| Non-Hispanic Blacks | 0.89 (0.53–1.51) | 0.44 (0.29–0.68) |
| Latino & Others | 0.67 (0.33–1.36) | 0.57 (0.34–0.96) |
| Mean Arterial Pressure | ||
| <105mmHg (reference) | 1.00 | 1.00 |
| ≥105mmHg | 0.25 (0.13–0.50) | 0.30 (0.20–0.46) |
| HbA1c | ||
| <7.0 % (reference) | 1.00 | 1.00 |
| ≥7.0 % | 0.48 (0.31–0.74) | N/A |
In the diabetic stratum, there is a similar prevalence of NA-CKD and ALB-CKD, regardless of ethnicity. Conversely, in the diabetic stratum, non-Hispanic Whites have a greater preponderance of NA-CKD whereas non-Hispanic Blacks have a greater predominance of ALB-CKD (Table 1). Multivariate regression analyses controlling for age, gender and poorly controlled HTN revealed that in comparison to non-Hispanic Whites, the OR for NA-CKD was significantly lower in the non-diabetic, non-Hispanic Black and ‘Other’ ethnic groups, OR=0.44 (95% CI 0.29–0.68) and 0.57 (95% CI 0.34–0.96), respectively (Table 2).
The prevalence of poorly controlled hypertension, defined as a MAP≥105mmHg, was significantly lower in those with NA-CKD, regardless of diabetes status (Table 1). In comparison with persons with a MAP<105mmHg, multivariate regression analyses adjusting for age, gender and ethnicity yielded an OR for NA-CKD of 0.25 (95% CI: 0.13–0.50) in the stratum with diabetes and 0.30 (95% CI 0.20–0.46) in the stratum without diabetes (Table 2).
In the cohort with diabetes, poor glycemic control (defined as an HbA1c≥7.0%) was also associated with a decreased prevalence of NA-CKD. In multivariate regression analyses controlling for age, gender, ethnicity and poorly controlled hypertension, the OR for NA-CKD was 0.48 (95% CI 0.31–0.74) (Table 2). Persons with diabetes and near normal HbA1c (<6.0%) still had higher prevalence rates of NA-CKD than those without diabetes (data not shown).
Sensitivity analyses using gender-specific cut-points for UACR did not significantly alter the results.
4. Discussion
In this study of NHANES 2001–2008, we found that at least half of the U.S. population with predominantly type 2 diabetes and eGFR<60ml/min/1.73m2 has normal urinary albumin excretion. While the proportion of individuals with NA-CKD is greater in the population with diabetes than without; NA-CKD accounts for a greater preponderance of CKD in the non-diabetic population. Women and non-Hispanic Whites account for the majority of NA-CKD. The proportion of individuals with diabetes who have NA-CKD decreases with worsening glycemic control and poorly controlled hypertension. Regardless of diabetes status, the majority of individuals with a MAP>105mmHg have albuminuric CKD.
It may be that diabetes contributes to the development of NA-CKD via pathways which may, at least in part, be separate from hyperglycemia and elevated blood pressure. This hypothesis is supported by our finding that in the diabetic population with low and even ‘normal’ HbA1c levels, NA-CKD prevalence is still higher than in the non-diabetic population. Likewise, others have found that HbA1c levels and the prevalence of other microvascular complications are lower in those type 2 diabetic persons with NA-CKD (Kramer et al., 2003; MacIsaac et al., 2004; Penno et al., 2011; Rigalleau et al., 2007; Thomas et al., 2009; Yokoyama et al., 2009). The finding that the prevalence of NA-CKD is significantly lower in those with poorly controlled hypertension, also supports the hypothesis that hypertension is less likely to be a key mediator of NA-CKD. Additionally, there is a well-documented association of hypertension and albuminuria, especially in the setting of diabetes (Bakris et al., 2003; Schrier, Estacio, Esler, and Mehler, 2002). However, a threshold effect of blood pressure on albuminuria and/or on the nature of the renal lesions mediated by hypertension cannot be ruled out. Potential pathogenic alternatives to the underlying mechanisms of NA-CKD include accelerated vascular disease, renal aging and diabetic nephropathy injury pathways which are, at least in part, independent of hyperglycemia and HTN.
If NA-CKD in type 2 diabetes is not the entirely the direct result of hyperglycemia or hypertension, these persons may have distinct nephropathologic findings compared with ALB-CKD. Biopsy studies have never been undertaken to specifically compare whether NA-CKD and ALB-CKD are distinct versus overlapping phenotypes of kidney disease in people with type 2 diabetes. Research kidney biopsies of type 1 diabetic patients with NA and decreased measured GFR (mGFR) <90ml/min/1.73m2 identified more severe diabetic glomerular changes than in type 1 diabetic patients with NA and normal mGFR (Caramori et al., 2003; Lane et al., 1992). Compared to type 1 diabetes, the pathology in people with type 2 diabetes is far more varied, and for any given level of albuminuria, classical diabetic nephropathy lesions are less severe in type 2 diabetic patients (Fioretto et al., 1996). Type 2 diabetic patients may have normal or near normal renal structure despite proteinuria or may have vascular, tubulointerstitial and/or global glomerulosclerotic lesions out of proportion to classical diabetic nephropathy lesions. Only about one-third have typical diabetic nephropathy lesions. This suggests either more complex pathogenetic processes and/or different responses of aging in renal tissues to the diabetic milieu. The importance of elucidating the underlying pathologic changes in NA versus ALB-CKD is to determine whether there may be distinct molecular mechanisms that should be targeted for therapy according to these two disease phenotypes.
NA-CKD in diabetes has been noted to carry a more benign clinical course with respect to GFR loss when compared to CKD and macroalbuminuria, but is similar to that of CKD with microalbuminuria (Molitch et al., 2010; Rigalleau et al., 2007). Regardless of diabetes status, NA-CKD also appears to carry a decreased risk of cardiovascular events when compared with ALB-CKD (Brantsma, Bakker, Hillege, de Zeeuw, de Jong, and Gansevoort, 2008; Hemmelgarn et al., 2010; Penno et al., 2011; Thomas et al., 2009). Undoubtedly, macroalbuminuria carries a very poor prognosis. From a population perspective, however, the burden of disease carried by NA-CKD is substantial. Moreover, given that CKD in the presence of microalbuminuria is similar in rate of progression to NA-CKD, these two entities, combined, account for the overwhelming majority of CKD in the U.S. population. Studies of the long-term natural history and prognosis of these persons are sorely needed. However, even if the risk for end-stage kidney disease is lower than for ALB-CKD, the healthcare costs, morbidity and mortality from cardiovascular and renal events from NA-CKD are still likely to be high (Ito et al., 2010; Penno et al., 2011). Moreover, with the rising incidence of diabetes (especially type 2) among youth, the duration of diabetes will correspondingly increase, resulting in more widespread microvascular complications (Liese et al., 2006).
Limitations to the present study include its exclusion of institutionalized individuals who likely have higher rates of diabetic complications, exclusion of a substantial number of individuals who did not have available a serum creatinine or UACR. UACR was measured using a single random urine collection which tends to be less robust in classification of albuminuria than first morning urine specimen, although this would favor under diagnosis of normoalbuminuria. Also, a single urine specimen is more prone to misclassification of the albuminuria status compared to the stricter standard of at least two of three consecutive samples in the same albuminuria range. We did not include duration of diabetes in multivariate analyses because this information was missing in the majority of participants. Strengths of the study include the ethnically diverse, population-based national sample. We used unbiased diagnostic criteria for diabetes, including those who were previously undiagnosed.
Our analyses of a comprehensive sample of the U.S. population underscore the large population burden of NA-CKD. We have elucidated several modifying factors of NA-CKD versus ALB-CKD among people with and without diabetes. These findings point to the possibility that these are distinct phenotypes, and suggest that further study of the underlying pathology should be undertaken.
Footnotes
Conflict of Interest: none.
Note: A portion of this work was presented in poster format at the American Society of Nephrology 2011 Annual Meeting, Philadelphia, Pennsylvania, November 8–13, 2011.
References
- Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, et al. Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAAL study. Archives of Internal Medicine. 2003;163(13):1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Archives of Internal Medicine. 2008;168(22):2440–2447. doi: 10.1001/archinte.168.22.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT. Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: The importance of urinary albumin excretion. Nephrology, Dialysis, Transplantation. 2008;23(12):3851–3858. doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]
- Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes. 2003;52(4):1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National Center for Health Statistics. National Health and Nutrition Examination Survey 2001–2002, 2003–2004, 2005–2006, and 2007–2008 documentaion files. 2011 from http://www.cdc.gov/nchs/nhanes.htm.
- de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA: The Journal of the American Medical Association. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39(12):1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA: The Journal of the American Medical Association. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Takeuchi Y, Ishida H, Antoku S, Abe M, Mifune M, et al. High frequencies of diabetic micro- and macroangiopathies in patients with type 2 diabetes mellitus with decreased estimated glomerular filtration rate and normoalbuminuria. Nephrology, Dialysis, Transplantation. 2010;25(4):1161–1167. doi: 10.1093/ndt/gfp579. [DOI] [PubMed] [Google Scholar]
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA: The Journal of the American Medical Association. 2003;289(24):3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- Lane PH, Steffes MW, Mauer SM. Glomerular structure in IDDM women with low glomerular filtration rate and normal urinary albumin excretion. Diabetes. 1992;41(5):581–586. doi: 10.2337/diab.41.5.581. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese AD, D’Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, et al. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Current Opinion in Nephrology and Hypertension. 2011;20(3):246–257. doi: 10.1097/MNH.0b013e3283456546. [DOI] [PubMed] [Google Scholar]
- MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27(1):195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, de Boer IH, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33(7):1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. Journal of Hypertension. 2011;29(9):1802–1809. doi: 10.1097/HJH.0b013e3283495cd6. [DOI] [PubMed] [Google Scholar]
- Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- Rigalleau V, Lasseur C, Raffaitin C, Beauvieux MC, Barthe N, Chauveau P, et al. Normoalbuminuric renal-insufficient diabetic patients: A lower-risk group. Diabetes Care. 2007;30(8):2034–2039. doi: 10.2337/dc07-0140. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney International. 2002;61(3):1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- Shideman JR, Buselmeier TJ, Mauer M, Kjellstrand CM. The Mini-D, a versatile dialyser for paediatric dialysis. Proceedings of the European Dialysis and Transplant Association. 1972;9:628–631. [PubMed] [Google Scholar]
- Standards of medical care in diabetes—. (2010) Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. American Journal of Kidney Diseases. 2007;50(1):21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Macisaac RJ, Jerums G, Weekes A, Moran J, Shaw JE, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11) Diabetes Care. 2009;32(8):1497–1502. doi: 10.2337/dc08-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi M. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: The Japan Diabetes Clinical Data Management study (JDDM15) Nephrology, Dialysis, Transplantation. 2009;24(4):1212–1219. doi: 10.1093/ndt/gfn603. [DOI] [PubMed] [Google Scholar]