Abstract
Neural function within the medial prefrontal cortex (mPFC) regulates normal cognition, attention and impulse control, implicating neuroregulatory abnormalities within this region in mental dysfunction related to schizophrenia, depression and drug abuse. Both serotonin -2A (5-HT2A) and -2C (5-HT2C) receptors are known to be important in neuropsychiatric drug action and are distributed throughout the mPFC. However, their interactive role in serotonergic cortical regulation is poorly understood. While the main signal transduction mechanism for both receptors is stimulation of phosphoinositide production, they can have opposite effects downstream. 5-HT2A versus 5-HT2C receptor activation oppositely regulates behavior and can oppositely affect neurochemical release within the mPFC. These distinct receptor effects could be caused by their differential cellular distribution within the cortex and/or other areas. It is known that both receptors are located on GABAergic and pyramidal cells within the mPFC, but it is not clear whether they are expressed on the same or different cells. The present work employed immunofluorescence with confocal microscopy to examine this in layers V-VI of the prelimbic mPFC. The majority of GABA cells in the deep prelimbic mPFC expressed 5-HT2C receptor immunoreactivity. Furthermore, most cells expressing 5-HT2C receptor immunoreactivity notably co-expressed 5-HT2A receptors. However, 27% of 5-HT2C receptor immunoreactive cells were not GABAergic, indicating that a population of prelimbic pyramidal projection cells could express the 5-HT2C receptor. Indeed, some cells with 5-HT2C and 5-HT2A receptor co-labeling had a pyramidal shape and were expressed in the typical layered fashion of pyramidal cells. This indirectly demonstrates that 5-HT2C and 5-HT2A receptors may be commonly co-expressed on GABAergic cells within the deep layers of the prelimbic mPFC and perhaps co-localized on a small population of local pyramidal projection cells. Thus a complex interplay of cortical 5-HT2A and 5-HT2C receptor mechanisms exists, which if altered, could modulate efferent brain systems implicated in mental illness.
Keywords: 5-HT, GABA, pyramidal, immunofluorescence, 5-HT2A receptor, 5-HT2C receptor
1. INTRODUCTION
The medial prefrontal cortex (mPFC) plays a critical executive role in working memory, attention and impulse control. Lesions of the PFC in animals (Goldman et al., 1971;Fritts et al., 1998; though see D'Esposito et al., 2006) and humans (Barbey et al., 2013;Tsuchida and Fellows, 2013) disrupt working memory. mPFC lesions also diminish the ability to attend to life-threatening or -enhancing environmental stimuli (Wilkins et al., 1987;Passetti et al., 2002;Ng et al., 2007;Lovstad et al., 2012) and to restrain behavior when needed (Perret, 1974;Muir et al., 1996;Quirk et al., 2000;Chudasama et al., 2003;although see, Eagle et al., 2008). It is thus not surprising that abnormalities in the mPFC have been associated with schizophrenia, depression and drug addiction; illnesses that are characterized with these cognitive and behavioral disturbances (Altman et al., 1996;Drevets, 2000;George et al., 2001;Brody et al., 2001;Stockmeier and Rajkowska, 2004;Mayberg et al., 2005;Lambe et al., 2007;Driesen et al., 2008;Kalivas, 2008;Covington et al., 2010;Li et al., 2011;Nocjar et al., 2012).
Serotonin, which interacts with at least 14 different receptor subtypes (Hoyer et al., 1994;Roth et al., 2000;Berger et al., 2009), is thought to play an important role in these psychological disorders (Roth and Meltzer, 1995;Kosten et al., 1998;Aghajanian and Marek, 2000;Manji et al., 2001;Nestler et al., 2002;Celada et al., 2004;Cunningham et al., 2013). The serotonin -2A and -2C receptor subtypes (5-HT2AR and 5-HT2CR, respectively) are widely dispersed throughout the mPFC, although density of 5-HT2ARs is higher (Leysen et al., 1982;Ashby et al., 1990;Mengod et al., 1990;Pompeiano et al., 1994;Lopez-Gimenez et al., 1997;Willins et al., 1997;Jakab and Goldman-Rakic, 1998;Clemett et al., 2000;Pandey et al., 2006;Liu et al., 2007;Yadav et al., 2011b). Both receptors are implicated in antipsychotic (Roth et al., 1992;Martin et al., 1998;Willins et al., 1999;Rauser et al., 2001;Bonaccorso et al., 2002), antidepressant (Cryan and Lucki, 2000;McMahon and Cunningham, 2001;Van Oekelen et al., 2003;Serretti et al., 2004;Millan, 2005;Opal et al., 2013) and addictive drug action (e.g. McMahon and Cunningham, 1999;Van Oekelen et al., 2003;Cunningham et al., 2013), with those localized to the mPFC purportedly playing a vital role (e.g. Aghajanian and Marek, 1999;Tarazi et al., 2002;Filip and Cunningham, 2003;Celada et al., 2004;Ramos et al., 2005;Huang et al., 2006;Pehek et al., 2006;Carli et al., 2006;Pockros et al., 2011;Opal et al., 2013). Impaired cortical 5-HT2AR and 5-HT2CR function could thus contribute to a variety of neuropsychiatric diseases, but how this might occur is unclear (Meltzer and Roth, 2013).
The main signal transduction mechanism for 5-HT2A and 5-HT2C receptors is stimulation of phosphoinositide production (Roth et al., 1984;Conn and Sanders-Bush, 1986;Sanders-Bush et al., 1988;Araneda and Andrade, 1991;Rick et al., 1995;Garcia et al., 2007), but the direct cellular excitation induced by their activation can produce opposite effects downstream. 5-HT2A versus 5-HT2C receptor activation oppositely affects dopamine release within the mPFC (for review see Di Matteo et al., 2001;Alex and Pehek, 2007). The receptors also oppositely regulate behavior controlled by the mPFC (Williams et al., 2002;Winstanley et al., 2004;Mirjana et al., 2004;Ramos et al., 2005;Bubar and Cunningham, 2006;Carli et al., 2006;Pentkowski and Neisewander, 2008;Jensen et al., 2010;Pockros et al., 2011;Cunningham et al., 2013). Although indicating that a differential cellular distribution of 5-HT2A and 5-HT2C receptors likely exists in the brain, it is not clear whether this occurs in the mPFC.
The two major cell types in the mPFC are GABA local circuit interneurons and glutamate-containing pyramidal projection neurons (Fuster, 1997;Gabbott et al., 1997), with local GABA release playing a crucial regulatory function over mPFC pyramidal output (see Eyles et al., 2002). 5-HT2AR and 5-HT2CR expression has been seen on both GABA and pyramidal cells within the prelimbic mPFC (Willins et al., 1997;Jakab and Goldman-Rakic, 1998;Jakab and Goldman-Rakic, 2000;Carr et al., 2002;Santana et al., 2004;Liu et al., 2007), but whether they are individually expressed or localized on the same cell is still not clear. The present work employed immunofluorescence with confocal microscopy to examine this in the prelimbic mPFC of rats.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
Six naive male Sprague-Dawley rats (Harlan, Indianapolis, USA) weighing 330–400 g were used. Rats were housed in pairs and maintained for at least 1-month after arrival in a temperature and humidity controlled rodent colony room with food and water available ad libitum. Rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, IP) and transcardially perfused with 250ml of phosphate-buffered saline (PBS; 8g NaCl, 1.44g Na2HPO4, 240mg KH2PO4, 200mg KCl in 1L dH2O, pH 7.40) and then with 500 ml of a 4% paraformaldehyde PBS solution. Brains were harvested, postfixed for 24 hours in 4% paraformaldehyde in PBS and then cryoprotected in a 30% sucrose PBS solution until they sank (~ 36–48 hrs). Brains were then rinsed in PBS, rapidly frozen on crushed dry ice and stored at −80°C until sectioning. 40 µm coronal sections containing the mPFC (bregma +3.76 to 3.20mm) were collected according to the rat brain atlas of Paxinos and Watson (2007) using a cryostat set to −23°C (Microm International, Germany). Alternating sections from the same animal were placed in two separate well-plates containing ice-cold PBS. One well-plate with its free-floating sections was used and processed in Experiment II and the other in Experiment III (See immunofluorescent microscopy). All animal procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local institutional animal care and use committee. All efforts were made to minimize the number of animals used and their suffering.
2.2 Antibodies
This study conducted three experiments using the primary antibodies listed in Table 1. The D-12 mouse monoclonal 5-HT2CR antibody (Santa Cruz Biotechnology) has been shown to selectively detect human (Anastasio et al., 2010) and rat 5-HT2CRs (Morabito et al., 2010) in prior western blot work. Experiment I extended this by conducting both western blot and confocal immunofluorescent assessments of D-12 5-HT2CR protein detection in PO1C cells that express rat 5-HT2CRs and in GF62 cells that only express rat 5-HT2ARs (Experiment Ia and Ib, respectively).
Table 1.
Primary antibodies employed within experiments.
| Antibody | 5-HT2CR (D-12) | 5-HT2AR | GAD-67 (H101) | Parvalbumin (PV25) |
|---|---|---|---|---|
| Experiment | Exp Ia &1b, Exp II Exp III | Exp III | Exp II | Exp II |
| Immunogen | Human 5-HT2CR C-terminus (374–458) | Rat 5-HT2AR N-terminus (22–41) | Human GAD-67 N-terminus (1–101) | Rat muscle Parvalbumin calcium binding protein |
| Manufacturer | Santa Cruz Biotechnology, Santa Cruz,CA Sc-17797 | Exp II:Neuromics, Edina, MN | Santa Cruz Biotechnology (Santa Cruz, CA) | Swant, Marly1, Switzerland |
| Catalog # | Sc-17797 | RA24288 | sc-5602 | PV25 |
| Host/clonality | Mouse monoclonal | Rabbit polyclonal | Rabbit polyclonal | Rabbit polyclonal |
| Dilution | 1:500 (Exp 1a) 1:100 (Exp Ib) 1:50 (Exp II; Exp III) |
1:100 | 1:50 | 1:2000 |
Experiment II used immunofluorescent confocal microscopy to determine whether D-12 also performed similar to other 5-HT2CR antibodies by detecting 5-HT2CR expression on GABAergic cells in the prelimbic mPFC (Liu et al., 2007). To localize GABA cell expression in the mPFC, the rabbit H101 anti-GAD-67 (glutamic acid decarboxylase isoform 67, Santa Cruz Biotechnology, California) and PV 25 anti-parvalbumin antibodies (Swant, Switzerland) were used as indicated in Table 1. GAD-67 is an enzyme involved in the synthesis of GABA, thus antibodies raised against the enzyme are useful in the identification of GABA-synthesizing cells in the brain. The H101 GAD-67 antibody has been shown to detect a similar number of GABA cells as other anti-GABA antibodies (Akema et al., 2005). Parvalbumin is a calcium-binding protein that is found in basket and chandelier subtype GABAergic cells (Conde et al., 1994;Gabbott et al., 1997) that directly modulate efferent signaling of cortical pyramidal neurons (Miles et al., 1996;Markram et al., 2004;Lewis et al., 2005). Specificity of the PV 25 anti-parvalbumin antibody has been validated in immunohistochemistry studies of cortical and muscle tissue from wild type versus parvalbumin knockout mice (Schwaller et al., 1999;Schwaller et al., 2004).
Experiment III used the same D-12 5-HT2CR antibody to determine whether cells that express 5-HT2CRs in the prelimbic mPFC also co-express 5-HT2AR s. As indicated in Table 1, the rabbit immunostar 5-HT2AR antibody from Neuromics was used. We and others have validated the receptor specificity of this antibody in western blot and immunohistochemistry studies of cortical tissue from 5-HT2AR knockout and wild-type mice (Magalhaes et al., 2010;Weber and Andrade, 2010;Yadav et al., 2011a). The antibody also sensitively detects changes in cortical 5-HT2AR levels (Yadav et al., 2011b).
Fluorescent-conjugated secondary antibodies from Invitrogen (Eugene, OR, USA) were used in all experiments to visualize primary antibody staining: Alexa Fluor 488 goat anti-mouse (fluoresces green), Alexa Fluor 594 goat anti-rabbit (fluoresces red).
2.3 Western Blots
To determine western blot D-12 5-HT2CR specificity in PO1C versus GF62 cells in Experiment Ia (see Antibodies above), PO1C and GF62 cells were pelleted by centrifugation and then pellets were lysed in 1 mL of Hepes buffer including CHAPS and protease inhibitors to prepare lysates. Lysates were normalized for protein content. Half of the lysates for each cell type were incubated with Wheat Germ Agglutinin/lectin beads for 2 hours at 4°C. SDS sample buffer was added to the lysates and beads, which were then incubated at 67°C for 5 minutes. Beads were then spun down and 30 µL from the top of each sample was loaded onto the gel. The protein was then transferred to a nitrocellulose membrane overnight, followed by one hour incubation in blocking buffer (tris buffered saline [TBS], 0.1% Tween, 5% milk) and 2 hours incubation in the primary antibody solution (1:500 D-12 goat-anti-mouse 5-HT2CR antibody, Santa Cruz, CA). The membranes were then washed several times, incubated in horseradish-peroxidase secondary antibody, and washed again several times. Finally, the membranes were incubated in western blot substrate and developed.
2.4 Immunofluorescent Microscopy
To further validate D-12 5-HT2CR specificity, immunofluorescent assessments were also conducted on cultured PO1C and GF62 cells in Experiment Ib (see Antibodies above). Cells were grown on coverslips, then permeabilized with 0.3% triton X-100 (in PBS) for 15 minutes, exposed to a PBS blocking buffer that contained 5% milk, 4% normal goat serum and 0.3% triton X-100 for at least one hour, and individually incubated in a solution containing the primary antibody (D-12 anti-5-HT2CR in blocking buffer, 1:100; see Table 1) for 2-hours at room temperature and then overnight at 4°C. They were then washed five times in 0.3% triton X-100, incubated at room temperature in secondary antibody (Alexa Fluor 488 goat anti-mouse, 1:200 in blocking buffer; see Antibodies) for one hour, and then washed four times in 0.3% triton X-100 and once in PBS. 5-HT2CR expression was then visualized using a Zeiss LSM Confocal Microscope with digital imaging software (Carl Zeiss, Thornwood, NY).
Dual immunofluorescent microscopy of rat brain tissue was conducted in Experiment II to determine whether D-12 performs like other 5-HT2CR antibodies by detecting 5-HT2CR expression on GABA cells in the prelimbic mPFC (Liu et al., 2007). Experiment III determined whether 5-HT2CR-IR cells within this region also expressed 5-HT2AR s. Free-floating rat brain sections (see Animals above) were allowed to equilibrate to room temperature on a gentle orbital shaker for 20min. Using room temperature solutions and continued gentle shaking, sections were permeabilized in 0.3% Triton X-100 in PBS for 1hour, incubated in 0.03% Triton X-100 blocking buffer (60µl Triton X-100, 1600µl normal goat serum and 2g non-fat dry milk in 40ml PBS) for 2-hours, and then incubated in blocking buffer containing two primary antibodies (Experiment II and III:D-12 and anti-GAD-67 or anti-parvalbumin antibodies, Experiment III: D-12 and 5-HT2AR antibodies; at the concentrations described in Table 1) for 2 hours and then for 72 hours at 4°C. While under continued gentle shaking, sections were allowed to return to room temperature for 20min, and then with room temperature solutions, were washed 3× in 0.03% Triton X-100 in PBS for 10min each, incubated in blocking buffer containing the secondary antibodies (Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit; 1:200 and 1:300 respectively in blocking buffer, see Antibodies) for 1hour protected from light, then washed 4× in 0.03% Triton X-100 and 1× in PBS for 10min each. Sections were then mounted onto slides with Vectashield Fluorescent Mounting Medium (Vector Laboratories), coverslipped, sealed with clear nail polish, air dried for 20min while protected from light and then stored at 4°C until viewed. Brain tissue expression of the two fluorescence tagged antibodies within an experiment (Experiment II and III:D-12 and anti-GAD-67 or anti-parvalbumin antibodies, Experiment III: D-12 and 5-HT2AR antibodies) was visualized and photographed as in our prior work (Nocjar et al., 2002; Burke et al., 2014) using a dual channel Zeiss LSM5 Confocal Microscope with digital imaging software (Carl Zeiss, Thornwood, NY). All digital photomicrographs were of single optical sections and analyzed in Adobe Photoshop CS6 (Adobe Systems Incorporated, San Jose, CA) using the count tool. Immunofluorescent labeling by each of the two antibodies was analyzed separately under either the red or green channel, with cells identified and counted under both channels indicating co-localized immunolabeling.
3. RESULTS
3.1 D-12 5-HT2C receptor immunoreactivity was selectively demonstrated in POIC versus GF62 cells
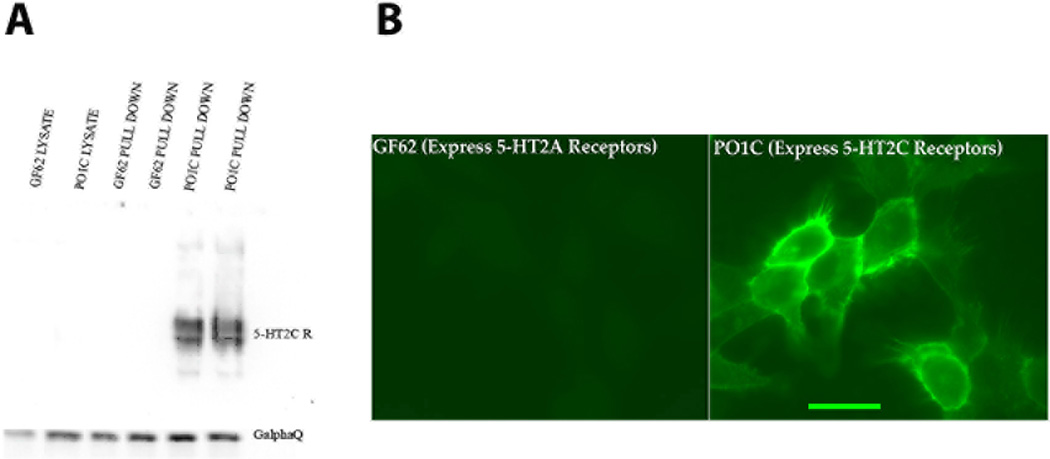
Experiments Ia and Ib were conducted to validate the receptor specificity of D-12 in POIC and GF62 cells that are known to differentially express the rat 5-HT2C and 5-HT2A receptors. As illustrated in Fig1A and 1B, POIC cells that express 5-HT2CRs consistently showed D-12 5-HT2CR antibody expression under Western Blot and immunofluorescent microscopy assessments. However, GF62 cells that only express 5-HT2ARs showed no D12 immunoreactivity in either test, indicating that D-12 is a 5-HT2CR specific antibody.
Fig1. Western blot and immunofluorescent microscopy (see Experimental Procedures) indicate that the D-12 5-HT2CR antibody used throughout this study is specific for the 5-HT2CR.
POIC cells that express 5-HT2CRs consistently showed D-12 5-HT2CR-antibody expression under both Western Blot (see A, black bars in histogram) and Immunohistochemical assessment (see B, confocal microscopy image of green cellular 5-HT2CR immunofluorescence). However, GF62 cells that only express 5-HT2AR s showed no D-12 antibody expression in either test (see A and B). Scale bar = 20 µm.
3.2 D-12 5-HT2C receptor immunoreactivity was expressed in GABA cells of the rat prelimbic mPFC
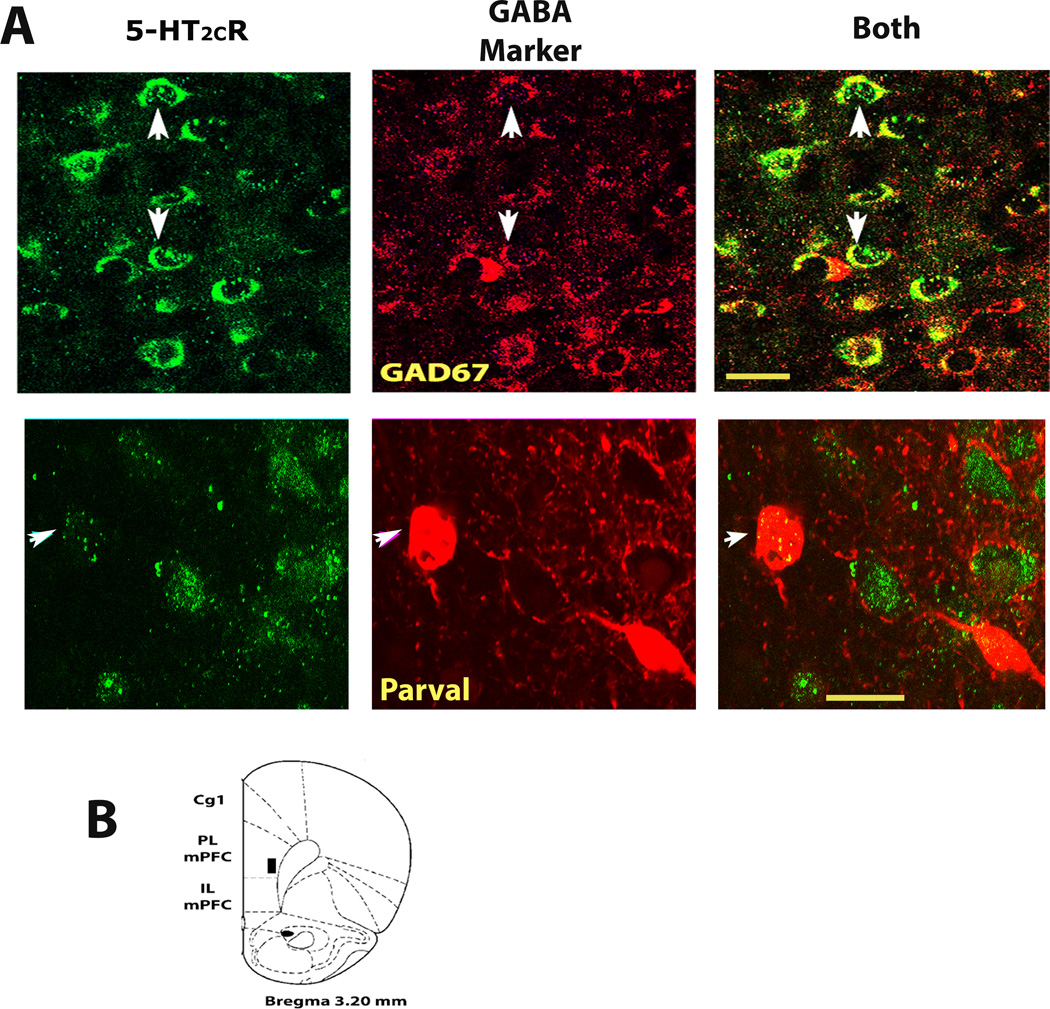
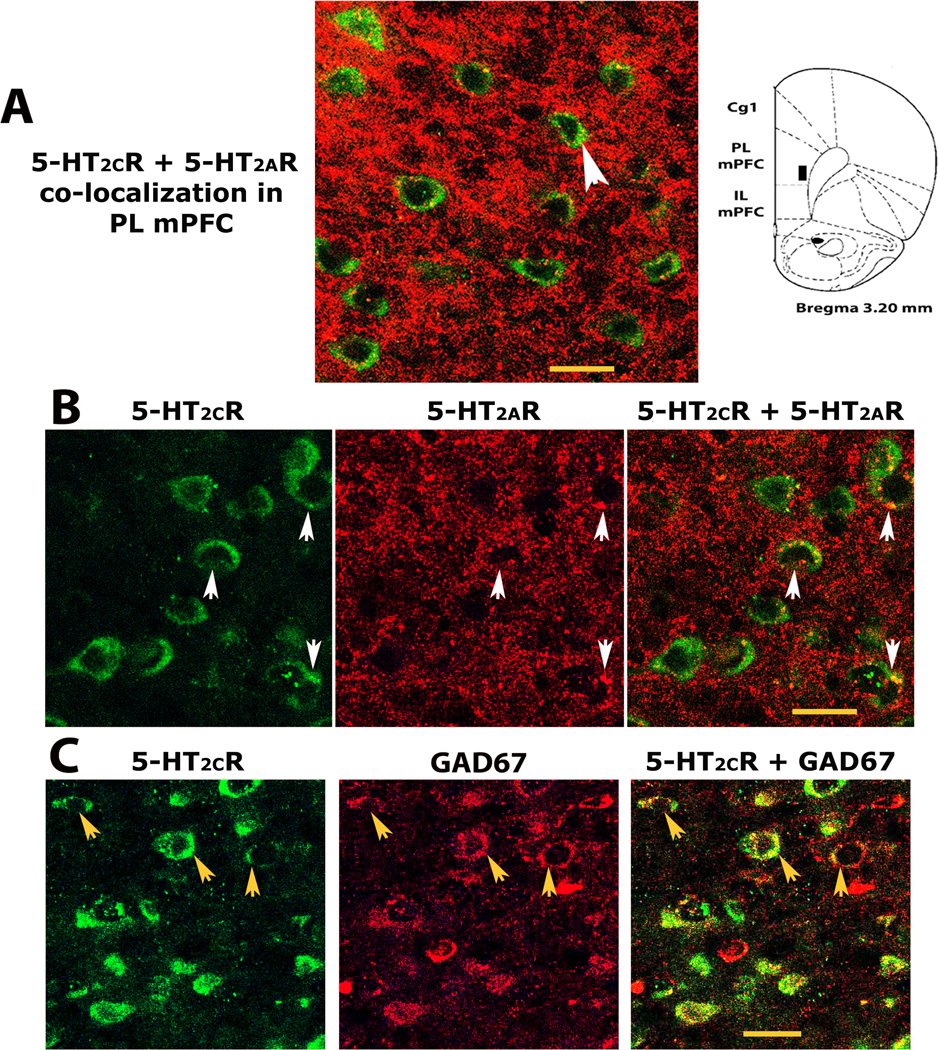
Experiment II was conducted to determine whether the D12 5-HT2CR antibody colabeled GABAergic cells like another 5-HT2CR antibody (Liu et al., 2007). Fig 2A shows D-12 antibody expression (green fluorescence) on GABA cells (red fluorescence) in the deep layers of the prelimbic mPFC (see prelimbic area assessed in B). As seen in A, D-12 detects 5-HT2CR expression within cell soma and their initial segment. Importantly, it detected 5-HT2CR expression in both GAD-67 and parvalbumin GABA cells in the mPFC (see white arrows in Fig 2A, top and bottom rows respectively) as previously reported using a different 5-HT2CR antibody (Liu et al., 2007). Because it acted similarly to another 5-HT2CR antibody and selectively identified cells that expressed the rat 5-HT2CR in the above experiment as in prior work (Morabito et al., 2010), the D-12 5-HT2CR antibody was used throughout the remaining study.
Fig2. GABA cells within the deep layers of the rat prelimbic medial prefrontal cortex (mPFC) express 5-HT2CRs.
A, confocal photomicrographs of mPFC tissue showing D-12 5-HT2CR antibody immunoreactivity (green florescence) and of cells expressing the GABA cell markers, GAD67 or parvalbumin (red immunostaining, top and bottom rows respectively). White arrows depict the identical cell across each row of photos. The left and middle photos show each antibody separately. Yellow staining in far right photos (under BOTH) illustrates 5-HT2CR and GABA cell co-localization. B, Cartoon representation of prelimbic mPFC region assessed in this experiment (see black box) according to the rat brain atlas of Paxinos and Watson (2007). 5-HT2CR, serotonin 2C receptor; GAD67, glutamic acid decarboxylase isoform 67; Parval, parvalbumin; PL mPFC, prelimbic subregion of medial prefrontal cortex. All confocal photomicrographs that were used to assess dual-immunolabeling in this report, including those presented in this figure through Fig 5, were of single optical sections (see experimental procedures, section 2.4). Scale bar = 20 µm.
3.3 5-HT2A and 5-HT2C receptors were expressed in a laminar overlapping fashion in the most rostral prelimbic mPFC and were co-expressed on cells in layer V
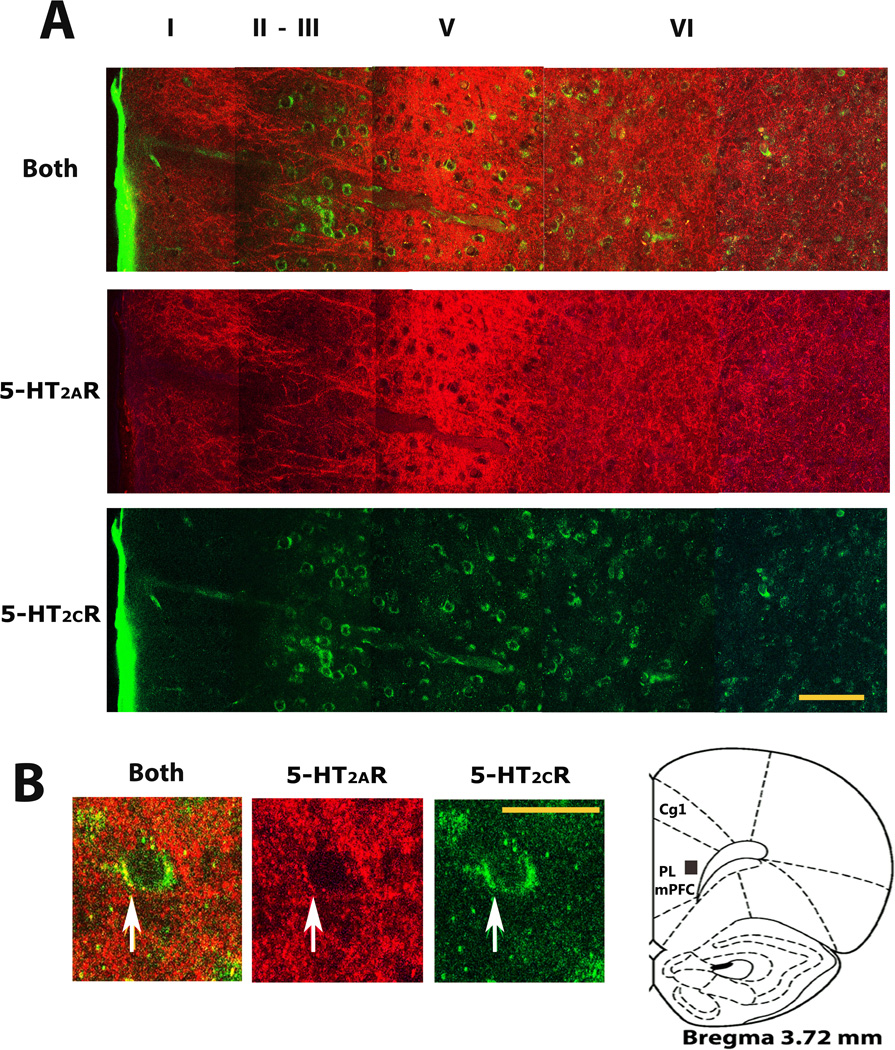
Experiment III was conducted to determine whether mPFC cells that expressed 5-HT2CRs also expressed 5-HT2ARs. Fig 3 shows a photomontage of 5-HT2AR and 5-HT2CR expression across the mediolateral extent of the most rostral level of the prelimbic mPFC (see AP location in the cartoon brain representation shown in B). As seen in A (middle row), a profuse laminar distribution of 5-HT2AR immunoreactivity was detected in the rat mPFC using the Immunostar 5-HT2AR antibody from Neuromics (see Table1). The strongest 5-HT2AR immunoreactivity was seen in layer V. Though quite profuse, the 5-HT2AR immunostaining produced by the antibody is typical for this cortical region (Weber and Andrade, 2010;Yadav et al., 2011b). Under higher magnification, a punctate 5-HT2AR expression could be seen on the soma and initial segment of cells in the region (see sample cell in B, center and left photo). Though impossible to identify in B due to dense 5-HT2AR staining in this layer, the middle panel in A clearly shows labeled neuronal processes in more superficial layers as identified previously with this antibody in the mouse prelimbic mPFC (Yadav et al., 2011a). The bottom row of photomicrographs in A shows that 5-HT2CR s in the identical mPFC tissue also had a laminar distribution; which is notably similar to that seen in an earlier report (Liu et al., 2007). However, this figure shows a population of 5-HT2CR -IR cells within superficial layers II–III which was negligible in this earlier report at a more posterior location of the prelimbic mPFC (Liu et al., 2007); perhaps due to the rapid anteroposterior decrease in 5-HT2CR expression demonstrated in the mPFC (Pompeiano et al., 1994). Most importantly, B shows 5-HT2CR and 5-HT2AR co-localization within the soma and initial segment of a sample cell in layer V of this rostral mPFC region (see black box in cartoon brain representation for approximate dorsoventral location of cell in subregion).
Fig3. 5-HT2AR and 5-HT2CR expression and cellular co-localization in the rostral prelimbic mPFC.
A, All three rows show the identical confocal photomontage collected across layers I to VI of the prelimbic mPFC (cartoon in B indicates the anteroposterior brain level assessed according to Paxinos and Watson, 2007). A distinct laminar 5-HT2AR immunoreactivity (see red, middle row), laminar 5-HT2CR immunoreactivity (see green, bottom row) and overlapping 5-HT2AR and 5-HT2CR expression (see green and red staining in top row) was seen. Note the distinct subpopulation of 5-HT2CR-IR cells amongst 5-HT2AR immunoreactive fibers in superficial layers II–III and another within the dense 5-HT2AR expression in layer V. B, High magnification confocal image of a sample cell in layer V showing 5HT-2A and -2C receptor co-localization. The middle and end photos show the cells 5-HT2AR (red) and 5-HT2CR (green) immunoreactivity, while the left photo shows its 5-HT2AR and 5-HT2CR co-expression (yellow staining). The black box in the brain cartoon shows where the cell was sampled (Paxinos and Watson, 2007). 5-HT2AR, serotonin 2A receptors; 5-HT2CR-IR, serotonin 2C receptor immunoreactivity; other abbreviations and confocal microscopy, see Fig 2. Scale bar = 100µm (A) and 20µm (B).
3.4 The majority of cells showing 5-HT2C receptor immunoreactivity in layer V-VI of the prelimbic mPFC also co-expressed 5-HT2A receptors
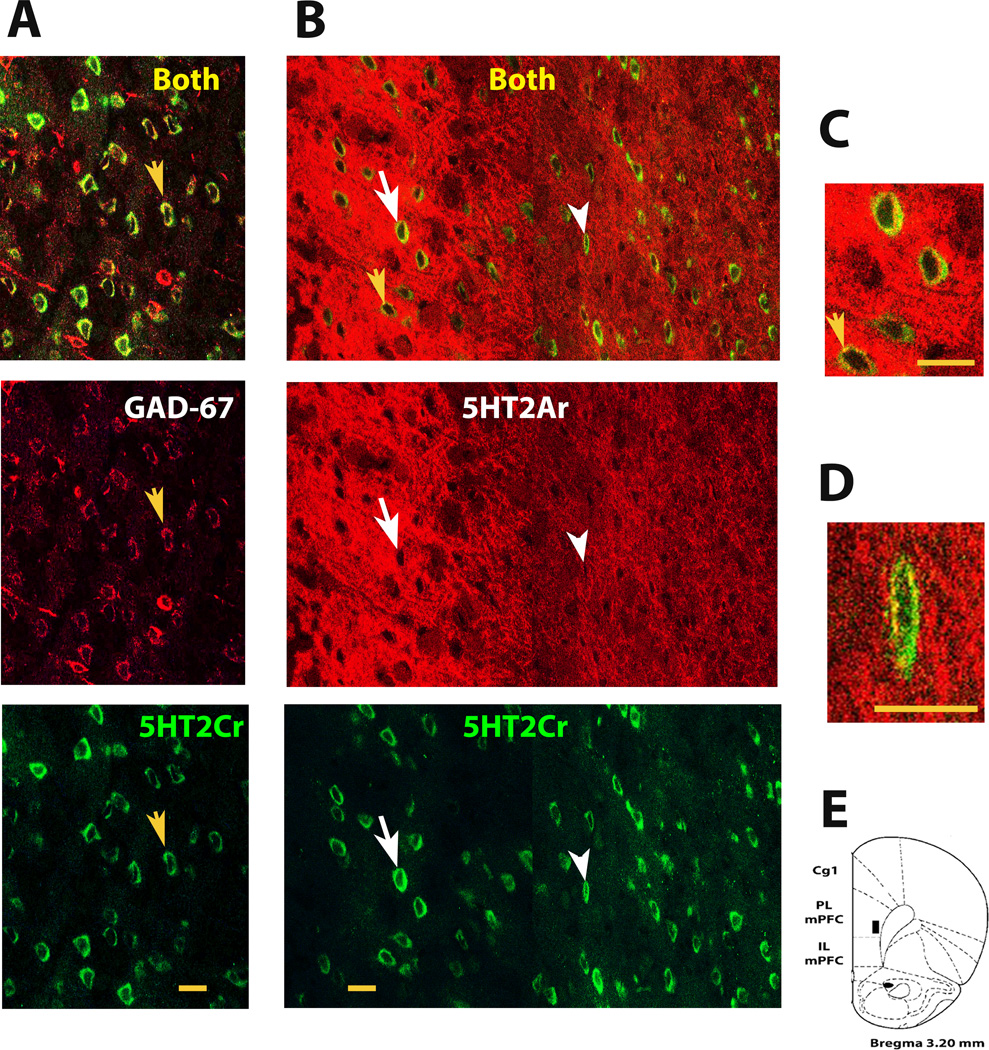
Fig 4 shows layer V 5-HT2C and 5-HT2A receptor expression at a more posterior level of the prelimbic mPFC in these same animals (see AP level in E). We first confirmed that 5-HT2CR-IR cells (green) in layer V co-expressed the GAD-67 GABA cell marker as we found in the rostral prelimbic mPFC in experiment II (see sample cell with yellow arrow in A). Photos in B confirmed that layer V also had the same profuse laminar 5-HT2AR expression (red) and cellular 5-HT2CR expression (green) as we found above in the deep layers of the more rostral prelimbic mPFC (see Fig 3). Note the typical strong 5-HT2AR immunoreactivity in layer Va that rapidly diminishes laterally in layer Vb (see Fig 3; Weber and Andrade, 2010). Most notably, many 5-HT2CR-IR cells in Layer V of the prelimbic mPFC were found to co-express 5-HT2ARs. The white arrow in B shows sample cells below it with 5-HT2CR and 5-HT2AR co-localization in layer Va [as delineated by Weber & Andrade (2010)]. These cells can be seen more clearly in C. The yellow arrow shows the same punctate 5-HT2AR expression encircling the cell's nucleus as we found above in the rostral prelimbic mPFC. The white arrowhead in B shows a representative cell with 5-HT2CR and 5-HT2AR co-localization in layer Vb [as delineated by Weber & Andrade (2010)]. This co-localization can be clearly seen in D. As indicated in Table2, nearly 70% of 5-HT2CR-IR cells in layers V–VI of this prelimbic mPFC region coexpressed 5-HT2ARs (brain cartoon blackened box indicates area assessed according to Paxinos and Watson (2007)).
Fig4. GABA cell 5-HT2CR expression and cellular 5-HT2CR and 5-HT2AR co-expression in Layer V at a more posterior level of the prelimbic mPFC.
A, confocal photomicrographs of prelimbic tissue showing GAD67-identified GABA cells with 5-HT2CR co-immunoreactivity (top photo, yellow and arrowed cells) in layer V at the anteroposterior level depicted in E. The middle and bottom photos show each antibody separately. White arrows depict the identical cell. B, confocal photomicrographs at the same level of the prelimbic mPFC showing 5-HT2CR-expressing cells with 5-HT2AR co-immunoreactivity in layer Va and layer Vb (see top photo, white arrow and arrowhead, respectively) as delineated by Weber & Andrade (2010). Cells in layer Va that are seen between the white and yellow arrows in the top photo were magnified in C to more clearly show their 5-HT2CR and 5-HT2AR co-expression (yellow staining). The cell in layer Vb (see white arrowhead in B) was magnified in D to more clearly show its 5HT-2C and -2A receptor co-expression. The middle and bottom photos show each antibody separately. White arrows depict the identical cells. Abbreviations and confocal microscopy, see Fig 2 & 3. Scale bar = 20 µm.
Table 2.
Immunohistochemical localization of 5-HT2ARs on 5-HT2CR-IR cells within the deep layers of the prelimbic mPFC (mean ± SEM) in experiment III
| Number of 5-HT2CR-IR cells |
Number of cells with 5-HT2CR + 5-HT2AR co-labeling |
% of 5-HT2CR-IR cells that express 5-HT2ARs |
 |
| 281 ± 6.2 | 189 ± 9.3 | 67.4 ± 1.81 |
3.5 Most cells that expressed 5-HT2C receptors in the deep layers of the prelimbic mPFC were GABAergic, suggesting that the majority of cells that co-express 5-HT2C and 5-HT2A receptors are likely GABA cells. The remaining cells with 5-HT2C receptors must be pyramidal, and we found some pyramidal-shaped cells with 5-HT2C and 5-HT2A receptor co-labeling
It is well known that layer V of the prelimbic mPFC contains a high number of pyramidal neurons with GABAergic cells dispersed throughout it. Thus we wanted to assess the approximate percentage of 5-HT2CR-IR cells that co-expressed GAD-67 in the region versus those that did not to determine the differential expression of these receptors on GABAergic versus pyramidal neurons in the region. Table 3 shows that approximately 73% of 5-HT2CR-IR cells in layers V-VI of the prelimbic mPFC are GABAergic (same prelimbic level assessed as in Table 2). Glutamate pyramidal projection cells are the only other cells in the region. Thus, the remaining 27% of 5-HT2CR-IR cells in the region are likely pyramidal cells.
Table 3.
Immunohistochemical validation of 5-HT2CR-IR on GAD67 labeled GABAergic cells within the deep layers of the prelimbic mPFC (mean ± SEM) in experiment II
| Number of 5-HT2CR-IR cells |
Number of cells with 5-HT2CR + GAD67 co-labeling |
% of 5-HT2CR-IR cells that express GAD67 |
Number of GAD67 cells |
% of GAD67 cells that express 5-HT2CRs |
| 285 ± 13.0 | 209 ± 9.0 | 73.3 ± 0.18 | 297 ± 9.5 | 70.2 ± 0.78 |
Fig 5 indicates that a portion of these pyramidal 5-HT2CR-IR cells might co-express 5-HT2ARs. As seen in A, some cells demonstrating 5-HT2CR and 5-HT2AR co-expression in layer V of the prelimbic mPFC have a pyramidal shape (see arrowed sample) and tightly layered distribution that is typical of glutamate pyramidal projection cells. B shows another tightly layered 5-HT2CR cell distribution with 5-HT2AR co-localization (see white arrows). C however, shows a more diffuse expression of 5-HT2CR-IR cells within the same region with GAD-67 GABAergic co-expression (see yellow arrows). Thus although the majority of cells that express 5-HT2C and 5-HT2A receptor co-localization in the mPFC are likely GABAergic, some could be pyramidal.
Fig5. Cells with a pyramidal shape and layered expression show 5-HT2AR and 5-HT2CR co-labeling in layer V of the prelimbic mPFC.
A. Immunofluorescent confocal images of potential pyramidal 5-HT2AR and 5-HT2CR co-labeling (see sample cell above arrowhead with green 5-HT2CR immunolabeling and yellow punctate staining around its nucleus indicative of 5-HT2AR co-expression). B. A confocal image showing a population of linearly expressed pyramidal-shaped cells in layer V of the prelimbic mPFC with green 5-HT2CR expression and punctate 5-HT2AR co-labeling around their nucleus (see arrows in the end photo). The first and middle photos show each antibody separately (see same arrowed cells). C. A comparison population of widely dispersed red GAD67-identified GABA cells in the same layer with 5-HT2CR co-expression (see yellowed cells with arrows in the end photo). The first and middle photos show each antibody separately (see same arrowed cells). Abbreviations and confocal microscopy, see Fig 2 & 3. Scale bar = 20 µm.
4. DISCUSSION
This study found that the majority of neurons expressing 5-HT2CR-IR in layers V–VI of the prelimbic mPFC also co-expressed the 5-HT2AR, demonstrating that a cellular subpopulation within the deep layers of the prelimbic mPFC could be directly co-regulated by 5-HT2C and 5-HT2A receptors. These cells are likely GABAergic for the most part since 73% of 5-HT2CR-IR cells in this region co-expressed the GABA cell marker GAD-67. Though 5-HT2CR and 5-HT2AR protein have each been detected previously on prelimbic GABAergic cells (Willins et al., 1997;Liu et al., 2007), this is the first demonstration that GABA cells may co-express both receptor proteins within the deep layers of the prelimbic cortex where GABAergic cells are known to provide a critical inhibitory control over efferent pyramidal projections from the mPFC (Eyles et al., 2002). Interestingly, a recent report found pyramidal shaped 5-HT2CR-IR cells in the prelimbic mPFC (Liu et al., 2007), and we found that 27% of 5-HT2CR -IR cells in the deep prelimbic mPFC were not GABAergic cells. Also, some cells with 5-HT2CR and 5-HT2AR co-labeling in this region had a pyramidal shape and tightly layered distribution that is typical of pyramidal cellular expression. This suggests that 5-HT2A and 5-HT2C receptors may also be co-localized on a small population of pyramidal cells in Layer V.
It is unlikely that the evidenced cellular 5-HT2CR and 5-HT2AR co-immunoreactivity was due to antibody non-specificity. Both antibodies employed are specific for their respective receptor. Though there has been specificity issues raised regarding some 5-HT2AR antibodies (Weber and Andrade, 2010), we used the Immunostar 5-HT2AR antibody that generates immunolabeling in wild-type but not 5-HT2AR knockout animals (Magalhaes et al., 2010;Weber and Andrade, 2010). A gradient anteroposterior distribution of cortical 5-HT2AR expression has also been identified with this antibody (Weber and Andrade, 2010) as seen in 5-HT2AR binding, mRNA and gene expression work (Blue et al., 1988;Pompeiano et al., 1994;Lopez-Gimenez et al., 1997). Specificity of the D12 5-HT2CR antibody employed has also been confirmed. Prior western blot work validated that D12 selectively induced immunolabeling in Chinese hamster ovary (CHO) cells that expressed the human 5-HT2CR but not in parental CHO cells that lack the receptor (Anastasio et al., 2010). Immunofluorescent microscopy in the current work also detected selective D-12 immunolabeling in POIC cells that express rat 5-HT2CRs, but not in GF62 cells that express 5-HT2ARs. The same findings were found with western blot replicating prior work (Morabito et al., 2010). Western blot D-12 assessments also sensitively detect increases and decreases in 5-HT2CR protein levels in brain tissue and mirror 5-HT2CR binding, function and behavioral assessments (Morabito et al., 2010; Abbas et al., 2009). Moreover, D12 co-labeled both GAD-67 and parvalbumin -identified GABAergic cells in the deep prelimbic mPFC in the current work as previously seen with another 5-HT2CR specific antibody (Liu et al., 2007;Anastasio et al., 2010), and genetic 5-HT2CR knockdown reduced D-12 5-HT2CR immunolabeling in mPFC tissue of rats (Anastasio et al., 2014).
We found a striking laminar distribution of both 5HT2 receptor proteins in the rat mPFC. 5-HT2AR immunoreactivity was extremely profuse in the deep cellular layers of the prelimbic mPFC, particularly in layer V. In superficial layers I-III, rather sparse 5-HT2AR dispersion progressed laterally to a highly localized expression on neural processes. This laminar expression is nearly identical to that reported in mouse mPFC with the same Immunostar 5-HT2AR antibody (Magalhaes et al., 2010;Weber and Andrade, 2010;Yadav et al., 2011a); it is not seen if an antibody lacks 5-HT2AR specificity (Weber and Andrade, 2010). Importantly, our laminar expression mirrors 5-HT2AR binding (Pazos et al., 1985;Blue et al., 1988;Mengod et al., 1990;Lopez-Gimenez et al., 1997;Marek et al., 2000) and Hrt2A gene expression at the mPFC level assessed here (Weber and Andrade, 2010). A nearly identical pattern of 5-HT2AR mRNA has also been reported in prior in situ hybridization studies (Pompeiano et al., 1994;Wright et al., 1995;Amargos-Bosch et al., 2004). Also, 5-HT2AR mRNA, binding, gene expression and Immunostar protein labeling have all demonstrated that 5-HT2AR expression is most profuse in the anterior mPFC (Blue et al., 1988;Pompeiano et al., 1994;Lopez-Gimenez et al., 1997;Weber and Andrade, 2010) where we found cellular 5-HT2AR and 5-HT2CR co-expression.
D-12 5-HT2CR-IR was expressed throughout the soma and initial segment of cells. 5-HT2CR protein levels were clearly low compared to 5-HT2AR protein within the region, supporting prior mRNA work (Pompeiano et al., 1994). Furthermore, 5-HT2CRs showed a distinct laminar distribution as seen in prior rodent receptor mRNA and protein work at a similar anteroposterior level of the mPFC (Pompeiano et al., 1994;Liu et al., 2007). Laminar 5-HT2CR binding in layer III and 5-HT2CR mRNA in layer V has also been seen in primate cortex (Pazos et al., 1987;Pasqualetti et al., 1999;Lopez-Gimenez et al., 2001). We did not assess high magnification receptor co-expression in more superficial layers of the mPFC. However, low magnification identified 5-HT2AR -expressing neural processes within layers II–III of the most rostral prelimbic mPFC as seen previously within the mPFC (Yadav et al., 2011a); and dispersed among these fibers was a distinct population of 5-HT2CR-IR cells. Most interesting however, was our demonstration that a laminar distribution of 5-HT2CR-IR cells within the deep layers of the prelimbic mPFC co-expressed 5-HT2ARs.
In fact, 67% of 5-HT2CR-IR cells in Layers V–VI of the prelimbic mPFC co-expressed 5-HT2AR s. Their co-expression was seen on round and fusiform shaped cells that were widely dispersed within these layers, suggestive of GABAergic expression, and on cells with a pyramidal shape and tight linear expression in layer V where large pyramidal somata are located (Bartos et al., 2007;Shepherd, 2009;Weber and Andrade, 2010), suggestive of pyramidal cell expression. However, most 5-HT2CR-IR cells in the region were found to be GABAergic. This indirectly demonstrates that 5-HT2CRs and 5-HT2ARs are likely co-expressed predominantly on GABA cells and perhaps on a small population of pyramidal cells within the deep layers of the prelimbic mPFC.
GABA interneurons, GABA long-range projection neurons and glutamate pyramidal projection cells are located within the mPFC (Fuster, 1997;Gabbott et al., 1997;Lee et al., 2014), but GABAergic cells are the major site of serotonin projection to the region (Smiley and Goldman-Rakic, 1996). 5-HT2AR transcript and protein has been evidenced in mPFC GABA cells (Willins et al., 1997;Weber and Andrade, 2010). GABAergic 5-HT2CR expression has also been seen, though it is region specific. In situ hybridization found few if any GABAergic cells that expressed 5-HT2CR mRNA in the secondary motor cortex which is located at the most dorsal extent of the mPFC (Puig et al., 2010). However, the current study replicated earlier evidence of 5-HT2CR protein expression in GAD67-identified GABA cells within the prelimbic mPFC, and we replicated this using a different 5-HT2CR antibody (Liu et al., 2007; current work). We also identified subcortical GABAergic 5-HT2CR protein expression with the antibody (Burke et al., 2014), directly supporting transcriptional evidence of GABAergic 5-HT2CR mRNA in the region (Eberle-Wang et al., 1997). Reverse transcription-PCR has also identified 5-HT2CR mRNA in GABA cells of the mPFC. In fact, Vysokanov and co-workers (1998) found GABAergic cells with 5-HT2CR and 5-HT2AR mRNA co-expression, directly supporting our 5-HT2 receptor protein co-expression. Their percentage of GABAergic cells with 5-HT2CR mRNA was lower than we report, but they may have assessed a more superficial layer or posterior mPFC region. 5-HT2CR mRNA shows a rapid anteroposterior decrease through the mPFC (Pompeiano et al., 1994). So does 5-HT2CR protein. We identified a distinct population of 5-HT2CR-expressing cells within the superficial layers of the most rostral prelimbic mPFC in the current study which was notably sparse at a more posterior level of the prelimbic mPFC in another report (Liu et al., 2007). Nonetheless, 50% of the GABAergic cells that expressed 5-HT2CR mRNA in the above report by Vysokanov also co-expressed 5-HT2AR mRNA; strikingly similar to the 67% of 5-HT2CR-IR cells that co-expressed the 5-HT2AR protein within the prelimbic mPFC in our study.
Support of pyramidal 5-HT2CR and 5-HT2AR co-expression also exists. Each receptor has been shown to exist on the apical dendrites of cortical pyramidal cells (Willins et al., 1997;Jakab and Goldman-Rakic, 1998;Cornea-Hebert et al., 1999;Clemett et al., 2000). We assessed 5-HT2 receptor co-expression in the deep layers of the prelimbic mPFC where large pyramidal cell bodies are located in layer V (see Weber and Andrade, 2010). Both 5-HT2AR protein (Willins et al., 1997;Weber and Andrade, 2010) and 5-HT2CR protein (Liu et al., 2007) are expressed in this layer, and on pyramidal shaped cells in this layer (Willins et al., 1997;Liu et al., 2007), as we report here. Transcriptional quantification also supports pyramidal 5-HT2AR expression in this layer (Vysokanov et al., 1998;Carr et al., 2002;Weber and Andrade, 2010), but is at odds regarding 5-HT2CRs. In situ hybridization work (Pasqualetti et al., 1999;Lopez-Gimenez et al., 2001) found 5-HT2CR mRNA in this layer, but not on pyramidal cells. However, two studies that used the highly visualized single cell reverse transcription-PCR technique did identify mRNA for the 5-HT2CR in mPFC layer V pyramidal cells (Vysokanov et al., 1998;Carr et al., 2002). Furthermore, they found that nearly all 5-HT2CR-expressing pyramidal shaped cells co-expressed 5-HT2AR mRNA (Carr et al., 2002), though only 28–53% of 5-HT2AR-expressing pyramidal cells co-expressed 5-HT2CR mRNA (Vysokanov et al., 1998;Carr et al., 2002). Though this evidence robustly supports our demonstration of prelimbic pyramidal-shaped cells with 5-HT2CR and 5-HT2AR protein co-expression, future immunohistochemical work that identifies pyramidal cells specifically with a glutamatergic cell marker could substantiate our findings.
How might 5HT-2C and -2A receptor co-expression on a cortical cell surface affect its function? Intra-mPFC administration of 5-HT2AR agonists enhance local pyramidal cell excitation in a dose-dependent manner (Ashby et al., 1990;Arvanov et al., 1999;Lambe and Aghajanian, 2007), while local 5-HT2CR agonism triggers GABA cell excitation and transmitter release that is thought to conversely inhibit mPFC pyramidal function (Mackowiak et al., 1999;Abi-Saab et al., 1999;Leggio et al., 2009;Zhang et al., 2010). Studies suggest that 5-HT2AR pyramidal excitation is due to the receptors preferential location on pyramidal neurons (Santana et al., 2004;Celada et al., 2013). Furthermore, we and others found a preferential GABAergic 5-HT2CR localization in the prelimbic mPFC (Liu et al., 2007; current work); providing a viable mechanism for the indirect 5-HT2CR inhibition of cortical pyramidal function (Ashby et al., 1990;Bergqvist et al., 1999;Eyles et al., 2002). However, findings in this report do not support this clear division of receptor function. Although 70% of GABA cells expressed 5-HT2CRs in the deep layers of the prelimbic mPFC, most 5-HT2CR-IR cells also co-expressed 5-HT2ARs. This indirectly suggests that prelimbic GABAergic cells largely express both receptors, and are most likely regulated by a balance in their function.
5HT-2C and -2A receptors share a high degree of homology (Roth et al., 1998) and activate many of the same second messenger signaling systems. Activation of either receptor triggers phosphoinositide and diacylglycerol production that in turn stimulates intracellular calcium release and ERK production under a similar time scale and responsivity to receptor density (Sanders-Bush et al., 1988;Araneda and Andrade, 1991;Stanford et al., 2005;Garcia et al., 2007;Seitz et al., 2012;Meltzer and Roth, 2013). Both 5HT2 receptors similarly activate phospholipase D and phospholipase A2 stimulation of arachidonic acid production (McGrew et al., 2002;Liu and Fanburg, 2008).
However, there are differences. 5-HT has higher affinity and potency at 5-HT2C versus 5-HT2A receptors (Berg et al., 2005). Agonist-directed recruitment of intracellular signaling differs between both G-protein-coupled receptors (Berg et al., 1998b). Agonist independent constitutive activation for at least some editing isoforms of 5-HT2CRs is stronger than that of 5-HT2ARs, which would differentially affect their sensitivity to ligand stimulation and recruitment of intracellular signaling pathways (Rauser et al., 2001;Shapiro et al., 2002;Berg et al., 2005). The surrounding ligand milieu more powerfully dampens serotonergic stimulation of 5-HT2CR intracellular pathways than 5-HT2AR signaling systems (Seitz et al., 2012). The 5-HT2CR is unique in that it undergoes RNA editing (Niswender et al., 1998;Abbas et al., 2010), which determines the receptor's trafficking, ligand response, constitutive activational status, and ability to couple to its G protein, trigger intracellular signal transduction pathways (Burns et al., 1997;Herrick-Davis et al., 1999;Berg et al., 2001;Hoyer et al., 2002;Marion et al., 2004;Berg et al., 2005;Millan et al., 2008;Werry et al., 2008;Labasque et al., 2010;Cordova-Sintjago et al., 2014) and affect behavior controlled by the mPFC (Anastasio et al., 2014). The conformation, sensitivity and trafficking of both 5-HT2 receptors dynamically change in response to inverse agonists, antagonists or endogenous 5-HT, but the constitutional status and edited state of the 5-HT2CR strongly determines how it changes, if at all (Willins et al., 1998;Porter et al., 1999;Berg et al., 1999;Gray and Roth, 2001;Van Oekelen et al., 2003;Devlin et al., 2004;Berg et al., 2005;Yadav et al., 2011b;Seitz et al., 2012;Lopez-Gimenez et al., 2013). These functional differences provide a physiological rationale for the dual expression of both 5-HT2 receptors on a cortical GABAergic or pyramidal neuron, even though the 5-HT2CR and 5-HT2AR are highly homologous and both activated by 5-HT.
Perhaps their dual functional state on GABAergic cells fine-tune serotonergic control of inhibitory function in the mPFC, an important mechanism in a region where too much versus too little neurotransmitter function detrimentally affects impulsivity, attention and working memory (Arnsten et al., 1994;Goldman-Rakic, 1995;Harrison et al., 1997;Zahrt et al., 1997;Granon et al., 2000;Dalley et al., 2002;Winstanley et al., 2004;Pezze et al., 2014). Evidence supports this hypothesis. Intra-mPFC infusion with the 5-HT -2A and -2C receptor (5-HT2A/2C R) agonist DOI causes GABA cell stimulation that is partially blocked by 5-HT2CR antagonists yet completely blocked by dual 5-HT2A/2CR antagonism (Zhang et al., 2010). GABA released by the infusion also inhibits local pyramidal excitability (Carr et al., 2002;Wang et al., 2009).
Serotonergic heteroreceptor co-expression within the mPFC is not new (see Celada et al., 2013). 5-HT1ARs and 5-HT2ARs are co-localized on the majority of pyramidal neurons in the region (Martin-Ruiz et al., 2001;Amargos-Bosch et al., 2004;Santana et al., 2004;Puig et al., 2010) and evidence of their opposing cross-talk has been reported (Araneda and Andrade, 1991;Berg et al., 1998a;Martin-Ruiz et al., 2001;Amargos-Bosch et al., 2004;Yuen et al., 2008). However, a recent report indicated that the inhibitory effect of 5-HT1AR s on NMDA-induced pyramidal excitation within the mPFC is reversed by local 5-HT2A/2CR co-activation (Yuen et al., 2008) and cross-talk between the 5-HT1AR and both 5-HT2 receptors has been evidenced (Berg et al., 1998a;Zhong et al., 2008). The current study and others support the existence of a pyramidal subpopulation with 5-HT2A/2CR co-expression within the mPFC (current study; Vysokanov et al., 1998;Carr et al., 2002). Though premature to suggest their involvement in this 5-HT1A heteroreceptor pyramidal control, it certainly deserves further exploration.
A broader anatomical understanding of serotonergic 5-HT2 receptor circuitry across sub-regions of the mPFC is needed. This study assessed the prelimbic mPFC where an optimal neurochemical balance is required for memory and attentional function (Arnsten et al., 1994;Bussey et al., 1997;Granon et al., 2000;Williams et al., 2002;Winstanley et al., 2003;Maddux and Holland, 2011). However, an optimal mPFC function is also required to control impulsivity (Harrison et al., 1997;Dalley et al., 2002;Winstanley et al., 2004), a behavior triggered by cortical 5-HT release (Dalley et al., 2002) and selectively regulated by the more ventral infralimbic mPFC (Chudasama et al., 2003). Infralimbic GABA and 5-HT2 receptor function can produce an impulsive inability to control ones behavior (Passetti et al., 2003;Carli et al., 2006;Murphy et al., 2012; see also Winstanley et al., 2004). Future infralimbic 5-HT2 receptor assessment could have particular relevance to addiction neurocircuitry. Impulsivity and cocaine-seeking are triggered in rats by an identical imbalance in cortical 5-HT2A/2CR function (Filip and Cunningham, 2003;Pockros et al., 2011;Cunningham et al., 2013;Anastasio et al., 2014; Fink et al., 2015).
In summary, this study identified a new cortical mechanism through which serotonin might fine-tune working memory and emotional control. 5-HT2CR-expressing cells in the deep layers of the prelimbic mPFC commonly co-expressed 5-HT2ARs. They also largely co-expressed GAD-67, with only 27% showing a non-GABAergic presumably pyramidal cell type. Thus this study indirectly demonstrates that 5-HT2C and 5-HT2A receptors may be commonly co-localized on GABA cells in the region and perhaps on a minor population of layer V pyramidal neurons. Importantly, it indicates that 5-HT2A/2CRs might perform a key direct interactive role in GABA's inhibitory control of pyramidal function within the prelimbic mPFC. It is not clear how 5HT2 receptors might interactively modulate intracellular signaling pathways within a cortical cell, but such knowledge could provide new molecular strategies in psychotherapeutic treatment for schizophrenia, depression and drug abuse.
Most GABA cells in Layer V of the rat prelimbic mPFC expressed 5-HT2C receptors
Likewise, most 5-HT2C receptor-expressing cells were GABAergic
Most 5-HT2C receptor-expressing cells also co-expressed 5-HT2A receptors
Acknowledgments
FUNDING AND DISCLOSURE. This work was supported by R01MH52220 and a Department of Veterans Affairs MERIT and Research Foundation award to EAP, and R01MH61887 to BLR. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit this work for publication.
Abbreviations
- GAD
Glutamic acid decarboxylase
- mPFC
medial prefrontal cortex
- 5-HT
Serotonin
- 5-HT2CR
Serotonin2c receptor
- 5-HT2CR-IR
Serotonin2c receptor immunoreactivity
- 5-HT2AR
Serotonin2a receptor
- 5-HT2A/2CRs
Serotonin2a and 2c receptors
- GABA
y-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflicts of interest.
Contributor Information
Christine Nocjar, Email: cxn18@case.edu.
Katherine D Alex, Email: ktlx11@yahoo.com.
Alex Sonneborn, Email: sonneborn.alex@gmail.com.
Atheir I Abbas, Email: atheirabbas@gmail.com.
Bryan L Roth, Email: bryan_roth@med.unc.edu.
Elizabeth A Pehek, Email: eap6@case.edu.
Reference List
- Abbas AI, Urban DJ, Jensen NH, Farrell MS, Kroeze WK, Mieczkowski P, Wang Z, Roth BL. Assessing serotonin receptor mRNA editing frequency by a novel ultra high-throughput sequencing method. Nucleic Acids Research. 2010;38:e118. doi: 10.1093/nar/gkq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AI, Yadav PN, Yao W-D, Arbuckle MI, Grant SGN, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Saab WM, Bubser M, Roth RH, Deutch AY. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology. 1999;20:92–96. doi: 10.1016/S0893-133X(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin, via 5HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Akema T, He D, Sugiyama H. Lipopolysaccharide increases gamma-amminobutyric acid synthesis in medial preoptic neurons in association with inhibition of steriod-induced luteinising hormone surge in female rats. J Neuroendocrinology. 2005;17:672–678. doi: 10.1111/j.1365-2826.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Everitt BJ, Glautier S, Markou A, Nutt D, Oretti R, Phillips GD, Robbins TW. The biological, social and clinical bases of drug addiction: commentary and debate. Psychopharmacology. 1996;125:285–345. doi: 10.1007/BF02246016. [DOI] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig M, Serrats J, Adell A, Celada P, et al. Co-expression and in vivo interaction of serotonin1a and serotonin2a receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, Watson CS, Cunningham KA. Serotonin 5-HT2C receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem. 2010;113:1504–1515. doi: 10.1111/j.1471-4159.2010.06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O'Neal RT, Fink LH, Li D, Green TA, Moeller FG, Cunningham CL. Functional status of the serotonin 5HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39:360–372. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Cai LX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Magro P, Roberts R, Wang RY. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A,2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci. 1999;11:2917–2934. doi: 10.1046/j.1460-9568.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Jiang LH, Kasser RJ, Wang RY. Electrophysiological characterization of 5-Hydroxytryptamine2 receptors in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 1990;252:171–178. [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–1205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gama oscillations in inhibitory interneuron networks. Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RBk, Clarke WP. RNA-editing of the 5HT(2C) receptor alters agonist-receptor-effector coupling specificity. Br J Pharmacol. 2001;134:386–392. doi: 10.1038/sj.bjp.0704255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Harvey JA, Spampinato U, Clarke WP. Physiological relevance of constitutive activity of 5HT2A and 5HT2C receptors. Trends Pharm Sci. 2005;26:625–630. doi: 10.1016/j.tips.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Clarke WP. Interactions between effectors linked to serotonin receptors. Ann N Y Acad Sci. 1998a;861:111–120. doi: 10.1111/j.1749-6632.1998.tb10181.x. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998b;54:94–104. [PubMed] [Google Scholar]
- Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP. Novel actions of inverse agonists on 5HT2C receptor systems. Mol Pharmacol. 1999;55:863–872. [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Ann Rev Medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist PBF, Dong J, Blier P. Effect of atypical antipsychotic drugs on 5-HT2 receptors in the rat orbito-frontal cortex: an in vivo electrophysiological study. Psychopharmacology. 1999;143:89–96. doi: 10.1007/s002130050923. [DOI] [PubMed] [Google Scholar]
- Blue ME, Yagaloff KA, Mamounas LA, Hartig PR, Molliver ME. Correspondence between 5-HT2 receptors and serotonergic axons in rat neocortex. Brain Res. 1988;453:315–328. doi: 10.1016/0006-8993(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Meltzer HY, Li Z, Dai J, Alboszta AR, Ichikawa J. SR46349-B, a 5-HT2A/2C receptor antagonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucelus accumbens. Neuropsychopharmacology. 2002;27:430–441. doi: 10.1016/S0893-133X(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Brody AL, Barsom MW, Bota RG, Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry. 2001;6:102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Burke M, Nocjar C, Sonneborn A, McCreary A, Pehek EA. Striatal Serotonin 2C receptors decrease nigrostriatal dopamine release by increasing GABA-A receptor tone in the substantia nigra. J Neurochem. 2014;131:432–443. doi: 10.1111/jnc.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi R, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front Integ Neuroscience. 2013;7:1–20. [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localization of the 5HT2C receptor protein in the rat CNS. Neurophar. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. Agonist-induced phosphoinositide hydrolysis in chloroid plexus. J Neurochem. 1986;47:1754–1760. doi: 10.1111/j.1471-4159.1986.tb13085.x. [DOI] [PubMed] [Google Scholar]
- Cordova-Sintjago T, Villa N, Fang L, Booth RG. Aromatic interactions impact ligand binding and function at serotonin 5HT2C G protein-coupled receptors: receptor homology modeling, ligand docking, and molecular dynamics results validated by experimental studies. Mol Phys. 2014;112:398–407. doi: 10.1080/00268976.2013.833656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurology. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Covington HEI, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, Deisseroth K, Nestler EJ. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neuroscience. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar JJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, Moeller FG. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci. 2013;4:110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. Is the prefrontal coretex necessary for delay task performance? Evidence from lesion and FMRI data. J Int Neuropsychol Soc. 2006;12:248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Devlin MG, Smith NJ, Ryan OM, Guida E, Sexton PM, Christopoulous A. Regulation of serotonin 5-HT2C receptors by chronic ligand exposure. Eur J Pharmacol. 2004;498:59–69. doi: 10.1016/j.ejphar.2004.07.102. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT2C receptors in the control of central dopamine function. Trends Pharm Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. 2000:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Driesen NR, Leung HC, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, Skudlarski P, Goldman-Rakic PS, Krystal JH. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64:1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet M-F. Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurology. 1997;384:233–247. [PubMed] [Google Scholar]
- Eyles DW, McGrath JJ, Reynolds GP. Neuronal calcium-binding proteins and schizophrenia. Schizophr Res. 2002;57:27–34. doi: 10.1016/s0920-9964(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. Hyperlocomotive and discriminative stimulus effects of cocaine are under the control of serotonin(2C) (5HT(2C)) receptors in rat prefrontal cortex. J Pharmacol Exp Ther. 2003;306:734–743. doi: 10.1124/jpet.102.045716. [DOI] [PubMed] [Google Scholar]
- Fink HL, Anastasio NC, Fox RG, Rice KC, Moeller FG, Cunningham KA. Individual differences in impulsive action reflect variation in the cortical serotonin 5-HT2A receptor system. Neuropsychopharmacology. doi: 10.1038/npp.2015.46. (in press) online Feb 10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritts ME, Asbury ET, Horton JE, Isaac WL. Medial prefrontal lesion deficit involving or sparing the prelimbic area in the rat. Physiol Behav. 1998;64:373–380. doi: 10.1016/s0031-9384(98)00096-1. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology and neuropsychology of the frontal lobe. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25,32, and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Vest B, Galkan TW. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J Comp Physiol Psychol. 1971;77:212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti E, Thomas KL, Dally J, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–452. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Niswender CM. Serotonin 5-HT21C receptor RNA editing alters receptor basal activity; implications for serotonergic signal transduction. J Neurochem. 1999;73:1711–1717. doi: 10.1046/j.1471-4159.1999.731711.x. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig GR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PA. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharm Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huang M, Ichiwaka J, Li Z, Dai J, Meltzer HY. Augmentation by citalopram of risperidone-induced monoamine release in rat prefrontal cortex. Psychopharmacology. 2006;185:274–281. doi: 10.1007/s00213-005-0206-1. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. PNAS. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jensen NH, Cremers TI, Scotty F. Therapeutic potential of 5HT2C receptor ligands. Scientific World Journal. 2010;10:1870–1885. doi: 10.1100/tsw.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Markou A, Koob GF. Depression and stimulant dependence: Neurobiology and pharmaco-therapy. J Nerv Ment Dis. 1998;186:737–745. doi: 10.1097/00005053-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Labasque M, Meffre J, Carrat G, Becamel D, Bockaert J, Marin P. Constitutive activity of serotonin2C receptors at G protein-independent signaling: modulation by RNA editing and antidepressants. Mol Pharmacol. 2010;78:818–826. doi: 10.1124/mol.110.066035. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Prefrontal cortical network activity: Opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience. 2007:900–910. doi: 10.1016/j.neuroscience.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Liu RJ, Aghajanian GK. Schizophrenia, hypocretin (orexin), and the thalamocortical activating system. Schizophrenia Bulletin. 2007;33:1284–1290. doi: 10.1093/schbul/sbm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Vogt D, Rubenstein JL, Sohal VS. A class of GABAergic neurons in the prefrontl cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J Neurosci. 2014;34:11519–11525. doi: 10.1523/JNEUROSCI.1157-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio GM, Cathala A, Moison Dj, Cunningham KA, Piazza PV, Spampinato U. Serotonin2C receptors in the medial prefrontal cortex facilitate cocaine-induced dopamine release in the rat nucleus accumbens. Neuropharmacology. 2009;56:507–513. doi: 10.1016/j.neuropharm.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Geerts R, Gommeren W, Verwimp M, Van Gompel P. Regional distribution of serotonin-2 receptor binding sites in the brain and effects of neuronal lesions. Arch Int Pharmacodyn. 1982;256:301–305. [PubMed] [Google Scholar]
- Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, Li X-Y, Aghajanian GK, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fanburg BL. Phospholipase D signaling in serotonin-induced mitogenesis of pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L471–L478. doi: 10.1152/ajplung.00071.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Regional distribution and cellular localization of 5-HT2C receptor mRNA in monkey brain: comparison with [3H] mesulergine bindingh sites and choline acetyltransferase mRNA. Synapse. 2001;42:12–26. doi: 10.1002/syn.1095. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]M100907. Naunyn-Schmiedeberg's Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G. Multiple conformations of the 5-HT2A and 5-HT2C receptors in rat brain: an autoradiographic study with [125I](±)DOI. Exp Brain Res. 2013;230:395–406. doi: 10.1007/s00221-013-3636-8. [DOI] [PubMed] [Google Scholar]
- Lovstad M, Funderud I, Meling T, Kramer UM, Voytek B, Due-Tonnessen P, Endestad T, Lindgren M, Knight RT, Solbakk AK. Anterior cingulate cortex and cognitive control: neuropsychological and electrophysiological findings in two patients with lesions to dorsomedial prefrontal cortex. Brain Cogn. 2012;80:237–249. doi: 10.1016/j.bandc.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak M, Chocyk A, Figal K, Czyrak A, Wedzony K. c-Fos proteins, induced by the serotonin receptor agonist DOI, are not expressed in 5HT2A positive cortical neurons. Brain Res Mol Brain Res. 1999;71:358–363. doi: 10.1016/s0169-328x(99)00195-3. [DOI] [PubMed] [Google Scholar]
- Maddux J-M, Holland PC. Effects of dorsal or ventral medial prefrontal cortical lesions on five-choice serial reaction time performance in rats. Beh Brain Res. 2011;221:63–74. doi: 10.1016/j.bbr.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Domps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin J-P, Anisman H, Ferguson SSG. CRF receptor 1 regulates anxiety behavior via sensitization of 5HT2 receptor signaling. Nature Neuroscience. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem. 2004;279:2945–2954. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory sytem. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martin P, Waters N, Schmidt CJ, Carlsson A, Carlsson ML. Rodent data and general hypothesis: antipsychotic action exerted through 5-HT2A receptor antagonism is dependent on increased serotonergic tone. J Neural Transm. 1998;105:365–396. doi: 10.1007/s007020050064. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep Brain stimulation for treatment-resistent depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McGrew L, Chang MS, Sanders-Bush E. Phospholipase D activation by endogenous 5-hydroxytryptamine 2C receptors is mediated by Galpha13 and pertussis toxin-insensitive Gbetagamma subunits. Mol Pharmacol. 2002;62:1339–1343. doi: 10.1124/mol.62.6.1339. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Cunningham KA. Attenuation of the locomotor stimulant and discriminative stimulus effects of cocaine in rats by the 5-HT2A antagonist MDL 100,907. Soc Neurosci Abstr. 1999;25:228.1. [Google Scholar]
- McMahon LR, Cunningham KA. Role of 5-HT2A and 5-HT2B/2C receptors in the behavioral interactions between serotonin and catecholamine reuptake inhibitors. Neuropsychopharmacology. 2001;24:319–329. doi: 10.1016/S0893-133X(00)00206-2. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Roth BL. Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-tageted drugs. J Clin Invest. 2013;123:4986–4991. doi: 10.1172/JCI70678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G, Pompeiano M, Palacios JM. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, la Cour CM. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharm Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Mirjana C, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, Kump DS, Hackey DL, Roth BL, Emeson RB. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis. 2010;39:169–180. doi: 10.1016/j.nbd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando ABP, Urcelay GP, Robinson ESJ, Mar AD, Theobald DEH, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Ng C-W, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. J Neurosci. 2007;27:12123–12131. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Sanders-Bush E, Emeson RB. Identification and characterization of RNA editing events within the 5-HT2C receptor. Ann N Y Acad Sci. 1998;861:38–48. doi: 10.1111/j.1749-6632.1998.tb10171.x. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Roth BL, Pehek P. Localization of 5-HT2A receptors on dopamine cells in subnucleii of the midbrain A10 cell group. Neuroscience. 2002;111:163–176. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J. The social-defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Opal MD, Klenotich SC, Morais M, Bessa J, Winkle J, Doukas D, Kay LJ, Sousa N, Dulawa SM. Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol Psychiatry. 2013;19:1106–1114. doi: 10.1038/mp.2013.144. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Faludi G, Sarosi A, Palkovits M. Regional distribution and relative abundance of serotonin(2c) receptors in human brain: effect of suicide. Neurochem Res. 2006;31:167–176. doi: 10.1007/s11064-005-9006-6. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Castagna M, Marazziti D, Cassano GB, Nardi I. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience. 1999;92:601–611. doi: 10.1016/s0306-4522(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, Robbins TW. Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology. 2003;165:136–145. doi: 10.1007/s00213-002-1227-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain: II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987;21:97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Neisewander JL. Stimulation of medial prefrontal cortex 5HT2C receptors attenuates cocaine-seeking behavior, but not cocaine self-administration. Neuropsychopharmacology. 2008;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual resposes in verbal categorical behavior. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Pezze M, McGarrity S, Mason R, Fone KC, Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J Neurosci. 2014;34:7931–7946. doi: 10.1523/JNEUROSCI.3450-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology. 2011;213:307–320. doi: 10.1007/s00213-010-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Molecular Brain Research. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Reell DFk, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5HT2B and 5HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosience. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Goni-Allo B, Aquirre N. Administration of SCH23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology. 2005;30:2180–2191. doi: 10.1038/sj.npp.1300735. [DOI] [PubMed] [Google Scholar]
- Rauser L, Savage JE, Meltzer HY, Roth BL. Inverse agonist actions of typical and atypicl antipsychotic drugs at the human 5-hydroxytryptamine2C receptor. J Pharmacol Exp Ther. 2001;299:83–89. [PubMed] [Google Scholar]
- Rick CE, Stanford IM, Lacey MG. Excitation of rat substantia nigra pars regiculate neurons by 5-hydroxytryptamine in vitro: Evidence for a direct action mediated by 5-hydroxytryptamine2C receptors. Neurosci. 1995;69:903–913. doi: 10.1016/0306-4522(95)00283-o. [DOI] [PubMed] [Google Scholar]
- Roth BL, Ciaranello RD, Meltzer HY. Binding of typical and atypical antipsychotic agents to transiently expressed 5-HT1C receptors. J Pharmacol Exp Ther. 1992;260:1361–1365. [PubMed] [Google Scholar]
- Roth BL, Lopez E, Patel S, Kroeze WK. The multiplicity of serotonin receptors: Uselessly diverse molecules or an embarrassment of riches? The Neuroscientist. 2000;6:252–262. [Google Scholar]
- Roth BL, Meltzer HY. The role of serotonin in schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, Ltd.; 1995. pp. 1215–1227. [Google Scholar]
- Roth BL, Nakaki T, Chuang D-M, Costa E. Aortic recognition sites for serotonin (5-HT) are coupled to phospholipase C and modulate phosphatidylinositol turnover. Neuropharmacology. 1984;23:1223–1225. doi: 10.1016/0028-3908(84)90244-2. [DOI] [PubMed] [Google Scholar]
- Roth BL, Willins D, Kristiansen K, Kroeze WK. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): Where structure meets function. Pharmacol Ther. 1998;79:231–257. doi: 10.1016/s0163-7258(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Burris KD, Knoth D. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolisis. J Pharmacol Exp Ther. 1988;246:924–928. [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Dick J, Dhoot G, Carroll S, Vrbova G, Nicotera P, Pette D, Wyss A, Bluethmann H, Hunziker W, Celio MR. Prolonged contraction-relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. Am J Physiol. 1999;276:C395–C403. doi: 10.1152/ajpcell.1999.276.2.C395. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, Potier M-C, Celio MR, Villa AEP. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neursci. 2004;25:650–663. doi: 10.1016/j.mcn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Seitz PK, Bremer NM, McGinnis AG, Cunningham KA, Watson CS. Quantitative changes in intracellular calcium and extracellular-regulated kinase activation measured in parallel in CHO cells stably expressing serotonin (5HT) 5HT2A or 5HT2C receptors. BMC Neuroscience. 2012;13:1–14. doi: 10.1186/1471-2202-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Artioli P, De Ronchi D. The 5-HT2C receptor as a target for mood disorders. Expert Opin Ther Targets. 2004;8:15–23. doi: 10.1517/14728222.8.1.15. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Kristiansen K, Weiner DM, Kroeze WK, Roth BL. Evidence for a model of agonist-induced activation of the 5-hydroxytryptamine 2A serotonin receptors that involves the disruption of a strong ionic interaction between helices 3 and 6. J Biol Chem. 2002;277:11441–11449. doi: 10.1074/jbc.M111675200. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG. Intracortical cartography in an agranular area. Front Neuroscience. 2009;3:337–343. doi: 10.3389/neuro.01.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J Comp Neurol. 1996;367:431–443. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Kantaria MA, Chahal HS, Loucif KC, Wilson CL. 5-Hydroxytryptamine induced excitation and inhibition in the sub-thalamic nucleus: action at 5-HT(2C), 5-HT(4), 5-HT(1A) receptors. Neuropharmacology. 2005;49:1228–1234. doi: 10.1016/j.neuropharm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Rajkowska G. Cellular abnormalities in depression: evidence from postmortem brain tissue. Dialogues Clin Neurosci. 2004;6:185–197. doi: 10.31887/DCNS.2004.6.2/cstockmeier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology. 2002;161:263–270. doi: 10.1007/s00213-002-1016-3. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are core component processes of executive function dissociable within the fontal lobes? Evidence from humans with focal prefrontal damage. Cortex. 2013;49:1790–1800. doi: 10.1016/j.cortex.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Van Oekelen D, Luyten WHML, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sciences. 2003;72:2429–2449. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Vysokanov A, Flores-Hernandez J, Surmeier DJ. mRNAs for clozapine-sensitive receptors co-localize in rat prefrontal cortex neurons. Neuroscience Letters. 1998;258:179–182. doi: 10.1016/s0304-3940(98)00882-9. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang QJ, Liu J, Ali U, Wu AH, Chen L, Gui ZH, Wang Y, Hui YP. In vivo effects of activation and blockade of 5-HT2A/2C receptors in the firing activity of pyramidal neurons of medial prefrontal cortex in a rodent model of Parkinson's disease. Exp Neurology. 2009;219:239–248. doi: 10.1016/j.expneurol.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Weber ET, Andrade R. Htr2a gene and 5HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neuroscience. 2010;4:36. doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Stewart GD, Crouch MF, Watts A, Sexton PM, Christopoulos A. Pharmacology of 5HT(2C) receptor-mediated ERK1/2 phosphorylation: agonist-specific activation pathways and the impact of RNA editing. Biochem Pharmacol. 2008;76:1276–1287. doi: 10.1016/j.bcp.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Wilkins AJl, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins D, Alsayegh L, Berry S, Backstrom J, Sanders-Bush E, Friedman L, Khan N, Roth B. Serotonergic antagonist effects on trafficking of serotonin 5-HT2A receptors in vitro and in vivo. Ann N Y Acad Sci. 1998;861:121–127. doi: 10.1111/j.1749-6632.1998.tb10182.x. [DOI] [PubMed] [Google Scholar]
- Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, Roth BL. Clozapine and other 5-Hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-Hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience. 1999;91:599–606. doi: 10.1016/s0306-4522(98)00653-8. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin 1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurology. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Yadav PN, Abbas AI, Farrell MS, Setola V, Sciaky N, Huang X-P, Kroeze WK, Crawford LK, Piel D, Keiser MJ, Irwin JJ, Schoichet BK, Deneris ES, Gingrich J, Beck SG, Roth BL. The presynaptic component of the serotonergic system is required for clozapine's efficacy. Neuropsychopharmacology. 2011a;36:638–651. doi: 10.1038/npp.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PN, Kroeze WK, Farrell MS, Roth BL. Antagonist functional selectivity, 5-HT2A serotonin receptor antagonists differentially regulate 5-HT2A receptor protein level in vivo. J Pharmacol Exp Ther. 2011b;339:99–105. doi: 10.1124/jpet.111.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of N-methyl-d-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008;283:17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neuroscience. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Wang S, Liu J, Ali U, Gui ZH, Wu ZH, Hui YP, Wang Y, Chen L. Unilateral lesion of the nigrostriatal pathway decreases the response of interneurons in medial prefrontal cortex to 5HT2A/2C receptor stimulation in the rat. Brain Res. 2010;1312:127–137. doi: 10.1016/j.brainres.2009.11.052. [DOI] [PubMed] [Google Scholar]
- Zhong P, Yuen EY, Yan Z. Modulation of neuronal excitability by serotonin-NMDA interactions in prefrontal cortex. Mol Cell Neuroscience. 2008;38:290–299. doi: 10.1016/j.mcn.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]