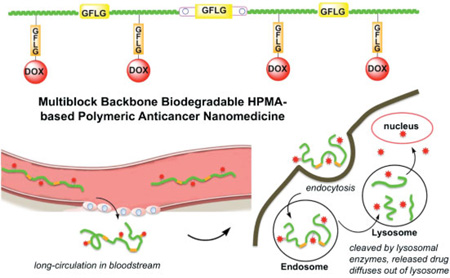
Abstract
Backbone degradable, linear, multiblock N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer–doxorubicin (DOX) conjugates are synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization followed by chain extension via thiol-ene click reaction. The examination of molecular-weight-dependent antitumor activity toward human ovarian A2780/AD carcinoma in nude mice reveals enhanced activity of multiblock, second-generation, higher molecular weight conjugates when compared with traditional HPMA copolymer–DOX conjugates. The examination of body weight changes during treatment indicates the absence of non-specific adverse effects.
Keywords: biodegradable copolymers, click reactions, doxorubicin, ovarian cancer, RAFT polymerization
Graphical abstract
1. Introduction
The concept of polymer–drug conjugates was developed to address the lack of specificity of low molecular weight drugs to malignant cells.[1] This approach is based on the lysosomotropism of polymer conjugates and suitable chemistry. The linker between polymer and drug should be stable during transport and the drug released in the lysosomal compartment of the target cell at a predetermined rate.[2] The first example of a synthetic polymer— low molecular weight drug conjugate to enter clinical trials was (N-(2-hydroxypropyl)methacrylamide) (HPMA) copolymer–doxorubicin (DOX) conjugate.[3] Its efficacy and reduced nonspecific toxicity for the treatment of ovarian cancer has been demonstrated. DOX possesses serious cardiotoxicity; its maximum tolerated dose (MTD) in humans is 60–80 mg · m−2, whereas the MTD of HPMA copolymer–DOX conjugate (in DOX equivalent) in humans was 320 mg · m−2 mainly due to the fact that accumulation and endocytosis are not very effective in heart tissue.[3] However, the molecular weight of currently used polymer–drug conjugates is suboptimal. Due to the nondegradable structure of the backbone, molecular weights have to be used that are below the renal threshold. This results in short circulation time and decreased accumulation of the conjugate in solid tumors via the enhanced permeability and retention (EPR) effect,[4–7] and suboptimal antitumor activity.
It is well established that higher molecular weight polymer–drug conjugates show enhanced tumor accumulation.[8,9] For example, the treatment of human ovarian xenografts in mice with branched HPMA copolymer–DOX conjugates indicated that the higher the molecular weight of the carrier, the higher the accumulation in solid tumor with concomitant increase in therapeutic efficacy.[9] Fortunately, recent developments in living radical polymerization and bioconjugation via click reactions permitted the design and synthesis of a new generation of anticancer nanomedicines based on high molecular weight, linear polymeric carriers containing enzymatically degradable bonds in the (linear) polymer backbone.[10–12] Compared with current anticancer drug-delivery systems, the distinct features of the new design include: (i) longer intravascular half-life and higher accumulation in tumor tissue due to the EPR effect;[7,13,14] (ii) substantially augmented efficacy due to increased drug concentration in tumor tissue; and (iii) potential synergistic effect of combination of drugs[15] and multivalency effect[16,17] due to multiple drug and/or targeting moieties in multiblock copolymers.
Here, we present the first report on the synthesis of linear, backbone degradable HPMA copolymer–DOX (mP-DOX) conjugates and the evaluation of the relationship between the molecular weight of the carrier and antitumor efficacy on a human ovarian carcinoma A2780 animal model.
2. Experimental Section
2.1. Materials
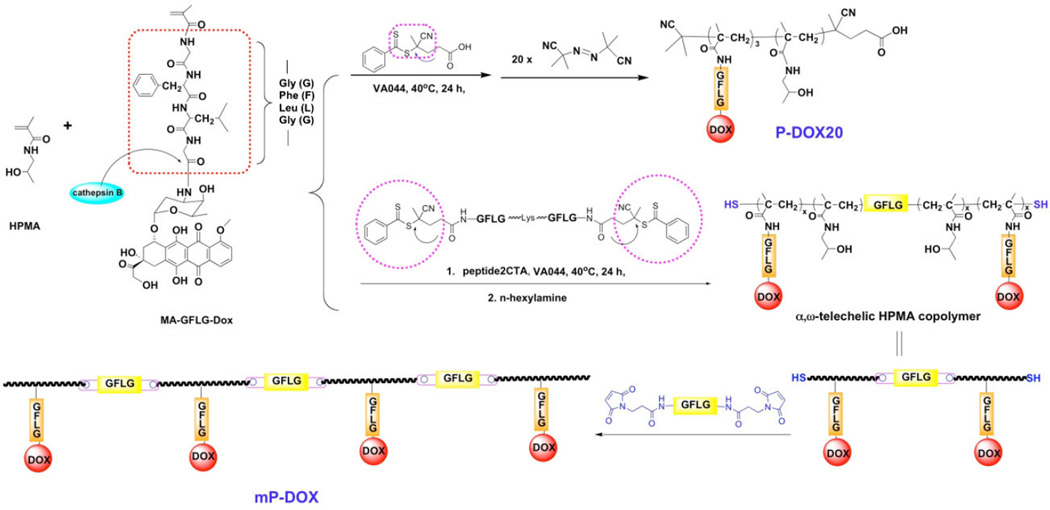
N-±-Fmoc protected amino acids, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and 2-Cl-trityl chloride resin (100–200 mesh, 1.27 mmol · g−1) were purchased from AAPPTec Biosciences (Louisville, KY, USA). 1-Hydroxybenzotriazole (HOBt) was from AK Scientific (Mountain View, CA, USA), 4-(N-maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester (SMCC) was purchased from Soltec Ventures (Beverly, MA, USA), 2,2′-azobis-(2-imidazolin-2-yl) propane dihydrochloride (VA-044) was from Wako Chemicals (Richmond, VA, USA), N,N′-diisopropylcarbodiimide (DIC) was from Fluka, and 2,2,2-trifluoroethanol (TFE), 2,2′-azobis(isobutyronitrile) (AIBN) and all other reagents and solvents were from Sigma–Aldrich (St. Louis, MO, USA). DOX was a kind gift from Meiji Seika Kaisha Ltd. (Tokyo, Japan). HPMA,[18] 4-cyanopentanoic acid dithiobenzoate,[19] and peptide2CTA (Nα,Ne-bis (4-cyano-4-(phenylcarbonothioylthio)pentanoylglycylphenylalanylleucylglycyl) lysine)[12] were synthesized according to literature. N-Methacryloylglycylphenylalanylleucylglycyl-doxorubicin (MA-GFLG-DOX) was prepared by the reaction N-methacryloylglycylphenylalanylleucylglycine 4-nitrophenyl ester (MA-GFLG-ONp) with DOX hydrochloride in dimethylformamide (DMF) in the presence of diisopropylethylamine according to a described procedure.[20]
2.2. Synthesis of Nonapeptide Containing Linker Nα,Nε-Bis(maleimidopropionylglycylphenylalanylleucylglycyl) lysine (P9MP2)
The maleimido linker containing an enzyme-sensitive peptide sequence was synthesized by solid phase peptide synthesis (SPPS) methodology and manual Fmoc/tBu strategy on 2-chlorotrityl chloride resin. HBTU was used as the coupling agent and 20% piperidine in DMF as the deprotection agent for Fmoc protected amino acids (Fmoc-AA-OH). Briefly, Fmoc protected amino acids, Fmoc-Lys(Fmoc)-OH, Fmoc-Gly-OH, Fmoc-Leu-OH, Fmoc-Phe-OH, and Fmoc-Gly-OH were coupled sequentially to the beads (60 mg beads, 0.02 mmol loading). After deprotection, 3-maleimidopropionic acid (three times excess) was coupled to the terminal glycyl residue in DMF. The peptide was isolated following cleavage from resin by 30% TFE in DCM for 2 h. Yield 20 mg (75%). ESI-MS (LTQ-FT, ThermoElectron, Waltham, MA, USA): m/z = 1197.3 [M + H]+, 599.3 [M + 2H]2+ (Figure S1 of Supporting Information). The 1H NMR spectrum of P9MP2 is shown in Figure S2 of Supporting Information.
2.3. Synthesis of Long-Circulating Backbone Degradable HPMA Copolymer-DOX Conjugates (mP-DOX)
2.3.1. Preparation of Extendable HPMA Copolymer–DOX Conjugate (P-DOX-e)
HPMA (825 mg, 96 mol-%), MA-GFLG-DOX (237 mg, 4 mol-%), peptide2CTA (7.5 mg), and initiator VA-044 (1.1 mg) were dissolved in methanol (4.5 mL). The polymerization solution in an ampoule was bubbled with N2 for 30 min, flame sealed and polymerized at 40 °C for 24 h. The polymer was purified by dissolution–precipitation method in methanol–acetone three times, washed with acetone two times, tetrahydrofuran and ether three times, and dried under reduced pressure at room temperature. P-DOX-e was applied to an LH-20 column eluted with methanol to remove unreacted monomer. The P-DOX-e (620 mg) was further purified using an XK50/100 column with acetate/acetonitrile (70/30; pH 6.5) as the mobile phase. The main fraction was concentrated by ultrafiltration (MWCO 10 kDa), dialyzed against DI water (MWCO 12–14 kDa) for 24 h, and freeze–dried. A conjugate (300 mg) with M̅w of 97.4 kDa and PDI of 1.19 was obtained.
2.3.2. Chain Extension and Fractionation
All solvents were bubbled with N2 for at least 30 min before use. P-DOX-e (97.4 kDa, 300 mg) was dissolved in methanol (3 mL) and n-hexylamine (300 µL) was added. The solution was stirred at room temperature for 10 min, then the telechelic dithiol P-DOX-e precipitated into ether, washed with ether three times and dried under reduced pressure at room temperature. Telechelic dithiol P-DOX-e (300 mg) and P9MP2 (4.5 mg) were dissolved in 1.5 mL DMF. The reaction mixture was stirred at room temperature for 24 h. After chain extension, the solution was diluted with methanol and precipitated into ether. The precipitate was re-dissolved in methanol, re-precipitated into ether, and washed with ether three times. The product was dried under reduced pressure at room temperature. The chain-extended conjugate was fractionated using an XK50 column and fractions were collected every 20 min. The salt in the fractions was removed by dialysis. The narrow polydispersity polymer fractions were obtained after freeze-drying. The FPLC profiles of fractions (mP-DOX conjugates) are shown in Figure S3 of Supporting Information and the UV–Vis spectrum of mP-DOX349 in Figure S4 of Supporting Information.
2.4. Synthesis of PolyHPMA/P-DOX with Low M̅w as Controls
2.4.1. PolyHPMA (Vehicle (PHPMA))
HPMA (212 mg), 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (3.7 mg) and initiator VA-044 (1.1 mg) were dissolved in methanol (0.85 mL). The polymerization method was similar to that described above. Following precipitation into aceton/ether mixture (1:1) the control polymer was dried under reduced pressure at room temperature, re-dissolved in water, and freeze-dried. Yield 105 mg.
2.4.2. Traditional HPMA Copolymer–DOX Conjugate (P-DOX20)
HPMA (165 mg, 96 mol-%), MA-GFLG-DOX (47 mg, 4 mol-%), 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (3.7 mg), and initiator VA-044 (1.1 mg) were dissolved in methanol (0.85 mL). The polymerization method was similar to the description above. Yield 81 mg.
Post-Polymerization Chain End Modification
AIBN was used to replace the dithiobenzoate end groups in P-DOX20. The conjugate, 10× excess of AIBN and a stirring bar were added into an ampoule. Oxygen was removed by vacuum-nitrogen charge for three times. Nitrogen bubbled methanol was injected into the ampoule. The concentration of the conjugate was 10 wt%. The ampoule was flame sealed and stirred in 70 °C oil bath for 2 h. After reaction, the conjugate was precipitated in acetone/ether (1:1), and purified by dissolution–precipitation in methanol–acetone/ether (1:1) three times. P-DOX20 was re-dissolved in methanol, further purified by an LH-20 column eluted with methanol. The solvent was removed by rotary-evaporation, the conjugate re-dissolved in water and freeze-dried.
2.5. Characterization of Copolymers
The average molecular weight and polydispersity index (PDI) of polymers were measured on an ÄKTA FPLC (fast protein liquid chromatography) system (GE Healthcare, formerly Amersham) equipped with miniDAWN TREOS and OptilabEX detectors (Wyatt Technology, Santa Barbara, CA, USA) using Superose 6 and/or Superose 12 HR10/30 columns with acetate buffer/acetonitrile (70:30, pH 6.5) as the mobile phase and flow rate 0.4 mL · min−1. For fractionation, XK50 column was used and flow rate was 2.5 mL · min−1. The amount of DOX incorporated into polymer conjugates was determined spectrophotometrically (Varian Cary 400 Bio UV–Visible spectrophotometer), using DOX molar extinction coefficient ε484 = 13 500 m−1 · cm−1 (water).
2.6. Animal Model and Evaluation of Efficacy
All animal studies were carried out in accordance with the University of Utah IACUC guidelines and approved protocols. The human ovarian carcinoma A2780/AD DOX resistant cells (5 × 106 cells in 100 µL medium mixed with 100 µL Matrigel Basement Membrane Matrix (BD Biosciences, San Jose, CA, USA)) were subcutaneously transplanted (injected) into the right flanks of female athymic nu/nu mice. Cells were cultured in RPMI 1640 medium supplemented with 10% FBS in 5% CO2 at 37 °C. Five groups of mice (n = 5) were evaluated: HPMA copolymer carrier (PHPMA; no drug, M̅w 20 kDa); “traditional” HPMA copolymer–DOX conjugate (P-DOX20; 20.3 kDa); backbone degradable multi-block HPMA copolymer–DOX conjugates, mP-DOX94, mP-DOX185, and mP-DOX349, respectively. When the tumors reached a size of about 1cm2 (between 13 and 17 d after inoculation; start of treatment was denoted as day 0), mice were treated intravenously three times (days 0, 7, and 14) with conjugates dissolved in sterile 0.9% NaCl solution at 8 mg · kg−1 DOX equivalent dose. A suppression of tumor growth was used as an indicator of antitumor activity of HPMA copolymer-bound DOX. Tumor size was determined by measurement using external calipers in two orthogonal dimensions every 2–3 d. The body weight of mice was monitored to determine the toxicity of the conjugates.
2.7. Statistical Analysis
All quantified data were presented as means ± SD (n = 5). Statistical analyses were done using one-way analysis of variance (ANOVA) and p values of <0.05 indicating statistically significant differences.
3. Results and Discussion
From the experimental evidence on the fate of macromolecular therapeutics in the organism,[21–25] it is evident that high molecular weight carriers accumulate to a higher extent in solid tumors. To extravasate from the leaky tumor vasculature into a solid tumor a concentration gradient needs to be sustained for extended time. However, increasing molecular weight of synthetic, non-degradable carriers impairs biocompatibility. Drawing on recent advances in living radical polymerization and click reactions we developed backbone degradable, long circulating HPMA-based carriers.[10–12] These carriers are composed of linear multiblock copolymers where synthetic HPMA copolymer blocks alternate with enzyme degradable oligopeptide sequences (Scheme 1). The backbone of these new polymer drug carriers is degradable by cathepsin B,[12] papain,[12] and in vivo.[26] Concomitantly, the drug (DOX) was released from the side chain termini.[12]
Scheme 1.
Synthesis of multiblock HPMA copolymers.
Here, we aimed to evaluate the relationship between molecular weight of degradable multiblock HPMA copolymer–DOX conjugates and their efficacy to treat A2780/AD human ovarian carcinoma xenografts in nudemice. To this end we synthesized telechelic copolymers of HPMA with MA-GFLG-DOX via reversible addition–fragmentation chain transfer (RAFT) polymerization using a bifunctional peptide2CTA as the RAFT chain transfer agent. By post-polymerization chain-end modification (aminolysis) the terminal dithiobenzoate groups were converted into thiol groups. The thiol telechelic HPMA copolymer–DOX conjugate macromolecules were chain extended with a maleimide terminated nonapeptide (GFLGKGFLG)-containing linker (P9MP2). Finally, the chain extended multiblock HPMA copolymer conjugate was fractionated on an XK50 column to produce three fractions of varying molecular weight and narrow polydispersity (PDI). The fractions and the controls are characterized in Table 1.
Table 1.
Characterization of HPMA copolymer–DOX conjugates and vehicle.
| Sample | M̅w [kDa] | PDI | DOX [wt%] |
|---|---|---|---|
| PHPMA | 20.0 | 1.03 | 0 |
| P-DOX20 | 20.3 | 1.05 | 8.70 |
| mP-DOX94 | 93.5 | 1.14 | 7.69 |
| mP-DOX185 | 184.8 | 1.11 | 7.75 |
| mP-DOX349 | 348.5 | 1.13 | 8.26 |
PHPMA, homopolymer of HPMA; P, HPMA copolymer; mP, multiblock HPMA copolymer.
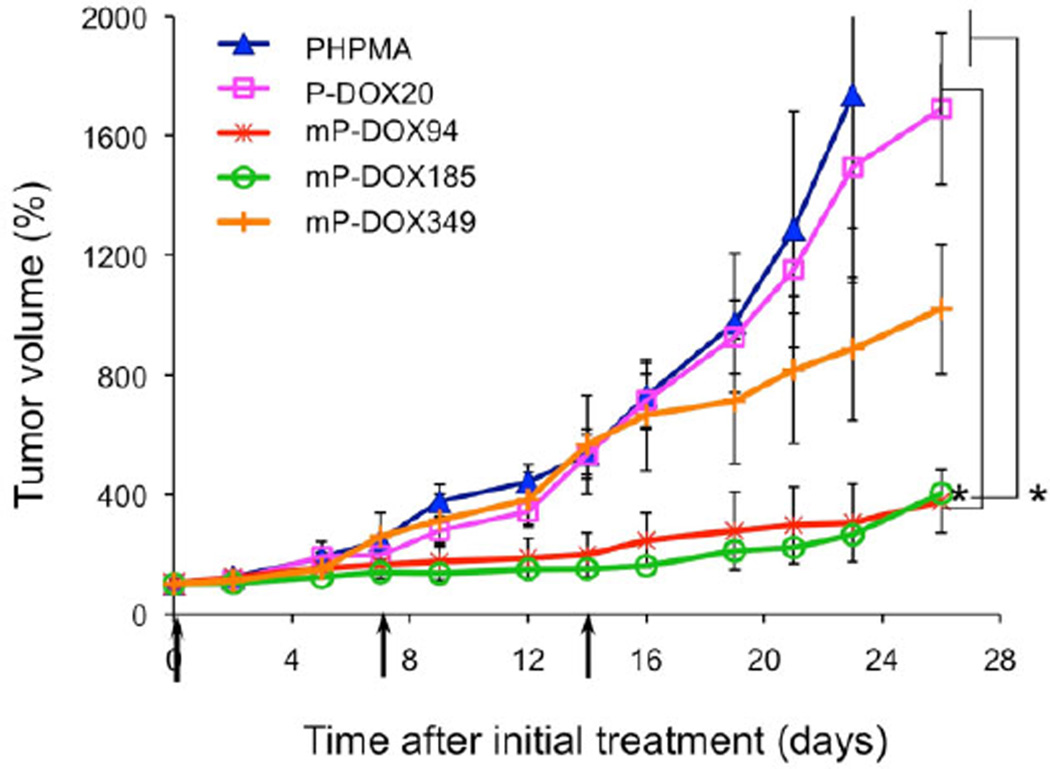
Five samples were used in the in vivo study: HPMA copolymer (PHPMA; control; 20 kDa, no drug); first generation HPMA copolymer–DOX conjugate (P-DOX20; 20.3 kDa); and three multiblock biodegradable HPMA copolymer–DOX conjugates [mP-DOX94 (93.5 kDa), mP-DOX185 (184.8 kDa), and mP-DOX349 (348.5 kDa), respectively]. The results of the treatment of ovarian tumor xenografts (Figure 1) clearly indicate the advantage of the multiblock conjugates; mP-DOX94 and mP-DOX185 inhibited tumor growth significantly more than the first generation conjugate (P-DOX20). The conjugate mP-DOX349 performed better than the first generation conjugate (P-DOX20), but the inhibition of tumor growth was lower than with conjugates mP-DOX94 and mPDOX185. This reflects the limits of molecular weight of conjugates that contain hydrophobic substituents (drugs) at side chain termini. Hydrophobic interactions result in conformation changes into compact coils as we determined previously by fluorescence resonance energy transfer (FRET),[27] fluorescence spectroscopy,[28,29] and quantum yield of singlet oxygen formation[30] techniques. These effects result in decreased solubility in aqueous environment, decrease of rate of enzymatic drug release, or decrease in quantum yield of singlet oxygen formation (when photosensitizers are used). Another possible factor is the size of the P-DOX349 conjugate. The size should not restrict extravasation and due to long-circulation time a high accumulation of the conjugate in tumor is expected. But the size might prevent diffusion into the tumor mass and restrict the conjugate to the tumor periphery.[31]
Figure 1.
Antitumor effect of multiblock HPMA copolymer–DOX conjugates (mP-DOX94, mP-DOX185, and mP-DOX349) on the growth of human ovarian A2780/AD tumors in nude mice compared to first generation HPMA copolymer–DOX conjugate (P-DOX20) and vehicle (pHPMA). Data represent mean ± s.d. *p<0.05.
The fact the conjugate P-DOX94 was as effective as the conjugate mP-DOX185 is very important for the potential scale-up of the synthesis. The design and synthesis of RAFT chain transfer agent peptide2CTA that possesses two active dithiobenzoate groups connected via an enzymically degradable oligopeptide sequence permits the synthesis of diblock copolymers with a degradable sequence connecting the blocks. Consequently, it is possible to synthesize a diblock HPMA copolymer–drug conjugate of mol. wt. ≈100 kDa in one step that will degrade into two ≈50 kDa fragments with molecular weight below the renal threshold.[12] This would avoid the need for fractionation, enormously simplifying large scale synthesis.
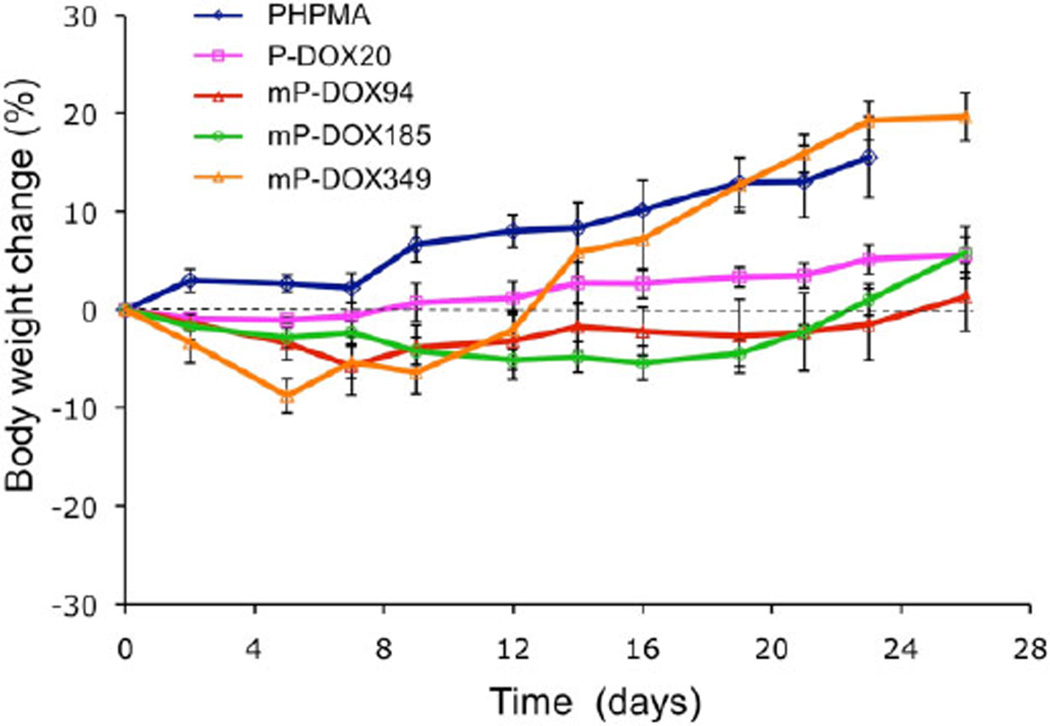
To demonstrate the biocompatibility of the carrier and conjugates we used HPMA homopolymer (PHPMA) as control and measured body weight in all animal groups during the experiment. The changes of body weight were acceptable (Figure 2). The larger increase of bodyweight for groups administered vehicle (PHPMA) and the highest molecular weight (mP-DOX349) are partially due to the weight of the tumor. The data indicate the absence of nonspecific toxic effects.
Figure 2.
Body weight changes of nude mice during treatment of A2780/AD tumors with multiblock HPMA copolymer–DOX conjugates (mP-DOX94, mP-DOX185, and mP-DOX349), first generation HPMA copolymer–DOX conjugate (P-DOX20), and vehicle (pHPMA). Data represent mean ± s.d.
The results clearly indicate that polymer–anticancer conjugates have a great potential as anticancer nanomedicines. Their translation into the clinics is the ultimate goal.[5,32,33]
4. Conclusion
New linear multiblock backbone biodegradable HPMA copolymer–DOX conjugates of varying molecular weight were synthesized and their molecular-weight-dependent activity toward A2780/AD human ovarian carcinoma xenografts in nude mice was determined.
All three multiblock conjugates possessed higher antitumor activity than the first generation conjugate. An optimal molecular weight appears to be operative; increasing the molecular weight further does not result in higher efficacy.
The results bode well for the development of efficient second-generation anticancer nanomedicines.
Supplementary Material
Acknowledgements
We thank Dr. Pavla Kopečková for valuable discussions. The research was supported in part by NIH grants CA51578, CA132831, CA156933 (to J.K.) and the University of Utah Research Foundation. We also acknowledge support of funds in conjunction with grant P30 CA042014 awarded to the Huntsman Cancer Institute, University of Utah
Footnotes
Supporting Information for this article is available from the Wiley Online Library or from the author.
Contributor Information
Huaizhong Pan, Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, Utah 84112, USA.
Monika Sima, Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, Utah 84112, USA.
Jiyuan Yang, Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, Utah 84112, USA.
Jindřich Kopeček, Email: jindrich.kopecek@utah.edu, Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, Utah 84112, USA; Department of Bioengineering, University of Utah, Salt Lake City, Utah 84112, USA.
References
- 1.Kopeček J. Polim. Med. 1977;7:191. [PubMed] [Google Scholar]
- 2.Kopeček J, Kopečková P. Adv. Drug Delivery Rev. 2010;62:122. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigeiro E, Cassidy J. Clin. Cancer Res. 1999;5:83. [PubMed] [Google Scholar]
- 4.Lammers T, Kühnlein R, Kissel M, Šubr V, Etrych T, Pola R, Pechar M, Ulbrich K, Storm G, Huber P, Peschke P. J. Controlled Release. 2005;110:103. doi: 10.1016/j.jconrel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Kopeček J. Mol. Pharm. 2010;7:922. doi: 10.1021/mp1001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopeček J, Kopečková P. In: Drug Delivery in Oncology, Vol. 2. Kratz F, Senter P, Steinhagen H, editors. Ch. 17. Wiley-VCH; Weinheim, Germany; 2012. pp. 485–512. [Google Scholar]
- 7.Fang J, Nakamura H, Maeda H. Adv. Drug Delivery Rev. 2011;63:136. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Seymour LW, Duncan R, Strohalm J, Kopeček J. J. Biomed. Mater. Res. 1987;21:1341. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- 9.Shiah J-G, Dvořák M, Kopečková P, Sun Y, Peterson CM, Kopeček J. Eur. J. Cancer. 2001;37:131. doi: 10.1016/s0959-8049(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Luo K, Pan H, Kopečková P, Kopeček J. React. Funct. Polym. 2011;71:294. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo K, Yang J, Kopečková P, Kopeček J. Macromolecules. 2011;44:2481. doi: 10.1021/ma102574e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan H, Yang J, Kopečková P, Kopeček J. Biomacromolecules. 2011;12:247. doi: 10.1021/bm101254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumura Y, Maeda H. Cancer Res. 1886;46:6387. [PubMed] [Google Scholar]
- 14.Maeda H. Bioconjugate Chem. 2010;21:797. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 15.Hongrapipat J, Kopečková P, Liu J, Prakongpan S, Kopeček J. Mol. Pharm. 2008;5:696. doi: 10.1021/mp800006e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu T-W, Yang J, Kopeček J. Biomaterials. 2012;33:7174. doi: 10.1016/j.biomaterials.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RN, Kopečková P, Kopeček J. Biomacromolecules. 2012;13:727. doi: 10.1021/bm201656k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopeček J, Bažilová H. Eur. Polym. J. 1973;9:7. [Google Scholar]
- 19.Mitsukami Y, Donovan MS, Lowe AB, McCormick CL. Macromolecules. 2001;34:2248. [Google Scholar]
- 20.Ulbrich K, Šubr V, Strohalm J, Plocová D, Jelínková M, Říhová B. J. Controlled Release. 2000;64:63. doi: 10.1016/s0168-3659(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand N, Leroux J-C. J. Controlled Release. 2012;161:152. doi: 10.1016/j.jconrel.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 22.Shiah J-G, Sun Y, Peterson CM, Kopeček J. J. Controlled Release. 1999;6:145. doi: 10.1016/s0168-3659(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 23.Etrych T, Šubr V, Strohalm J, Šírová M, Říhová B, Ulbrich K. J. Controlled Release. 2012;164:346. doi: 10.1016/j.jconrel.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Maeda H, Takeshita J, Kanamaru R. Int. J. Protein Pept. Res. 1979;14:81. doi: 10.1111/j.1399-3011.1979.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 25.Konno T, Maeda H, Iwai K, Tashiro S, Maki S, Morinaga T, Mochinaga M, Hiraoka T, Yokoyama I. Eur. J. Cancer Clin. Oncol. 1983;19:1053. doi: 10.1016/0277-5379(83)90028-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Luo K, Yang J, Sima M, Sun Y, Janát-Amsbury MM, Kopeček J. J. Controlled Release. 2013 doi: 10.1016/j.jconrel.2012.12.009. accepted (Ms. Ref. No. JCR-D-12-01077R1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding H, Kopečková P, Kopeček J. J. Drug Targeting. 2007;15:465. doi: 10.1080/10611860701500016. [DOI] [PubMed] [Google Scholar]
- 28.Shiah J-G, Koňák Č, Spikes JD, Kopeček J. J. Phys. Chem. B. 1997;101:6803. [Google Scholar]
- 29.Gu Z-W, Omelyanenko V, Kopečková P, Kopeček J, Koňák Č. Macromolecules. 1995;28:8475. [Google Scholar]
- 30.Gu Z-W, Spikes JD, Kopečková P, Kopeček J. Collect. Czech. Chem. Commun. 1993;58:2321. [Google Scholar]
- 31.Zhou Y, Kopeček J. J. Drug Targeting [Google Scholar]
- 32.Duncan R. Nat. Rev. Drug Discovery. 2003;2:347. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 33.Kopeček J. Adv. Drug Delivery Rev. 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.