Abstract
Background
The Edmonton Symptom Assessment Scale (ESAS) is widely used for symptom assessment in the clinical and research settings. We used the sensitivity-specificity approach to identify the minimal clinically important difference (MCID) for improvement and deterioration for each of the 10 ESAS symptoms.
Methods
This multicenter, prospective, longitudinal study enrolled advanced cancer patients. ESAS was measured at first clinic visit and a second visit 3 weeks later. For each symptom, we assessed Patient's Global Impression (“better”, “about the same”, or “worse”) at the second visit as the external criterion, and determined the MCID based on the optimal cutoff in receiver-operating characteristic (ROC) curve. We conducted sensitivity analysis by estimating MCIDs using other approaches.
Results
Among the 796 participants, the median duration between the 2 study visits was 21 days (interquartile range 18-28 days). The area under the ROC curve varied between 0.70-0.87, suggesting good responsiveness. For all 10 symptoms, the optimal cutoff was ≥1 point for improvement and ≤−1 point for deterioration, with sensitivities of 59%-85% and specificities of 69%-85%. Using other approaches, the MCIDs varied between 0.8 and 2.2 for improvement and between −0.8 and −2.3 for deterioration in within-patient analysis, between 1.2 and 1.6 with the ½ standard deviation approach, and between 1.3 and 1.7 with the standard error of measurement approach.
Conclusions
ESAS was responsive to change. The optimal cutoffs were ≥1 point for improvement and ≤−1 point for deterioration for each of the 10 symptoms. Our findings have implications for sample size calculations and response determination.
Keywords: Neoplasms, outcome measures, pain, sample size, sensitivity and specificity, symptom assessment
INTRODUCTION
Patients with advanced cancer frequently experience significant symptom burden throughout the disease trajectory [1]. Routine standardized symptom assessment employing patient-reported outcomes represents the cornerstone of personalized symptom management [2]. A number of symptom batteries have been developed, with the Edmonton Symptom Assessment Scale (ESAS) being one of the most widely used questionnaires in clinical practice and research [3]. ESAS has been translated and adopted for symptom screening in many countries in North America, South America, Europe, Asia and Africa, and has been validated in different oncology and palliative care settings [4-11].
One critical aspect related to the symptom assessment using ESAS involves identifying what constitutes a minimal clinically important difference (MCID) [12]. In the acute pain setting, the MCID for a single 0-10 numeric rating scale has been studied [13]; however, the MCID of the 10 ESAS symptoms has not been systematically assessed in a prospective fashion [14]. A better understanding of the MCID of ESAS has important implications for symptom response determination and sample size calculation. In this multicenter prospective study, we determined the MCID for each of the 10 ESAS symptoms in patients with advanced cancer using the sensitivity-specificity anchor-based approach.
METHODS
Participants
This is an international longitudinal observational study. The inclusion criteria included the following: (1) diagnosis of advanced cancer, defined as locally advanced, recurrent or metastatic disease, (2) 18 years of age or greater, (3) seen at an outpatient clinic at one of the 6 participating centers, and (4) scheduled to return to clinic 14 to 34 days after the first study visit for a second ESAS questionnaire. Patients with delirium (Memorial Delirium Assessment Scale [MDAS] of 13 or greater) were excluded. The institution review boards at all participating centers approved the study. All participants provided written informed consent.
Participating centers included MD Anderson Cancer Center in Houston; United States, King Hussein Cancer Center in Amman, Jordan; Barretos Cancer Hospital in Barretos, Brazil; Pontificia Universidad Catolica de Chile in Santigo, Chile, Kangdong Sacred Heart hospital in Seoul, Republic of Korea, and Tata Memorial Center in Mumbai, India. All 6 institutions were tertiary care hospitals with access to comprehensive cancer treatments and supportive care. The centers in Houston, Jordan, Brazil and India are part of the Sister Institution Research Network, a multi-national cancer research cooperative. All participants were enrolled from the palliative care outpatient clinics at consultation with the following exceptions: a minority of Brazilian patients were enrolled at an outpatient palliative care follow up visit, US patients were consented during their first followup clinic visit because all assessments for the first study visit were routinely collected at consultation, and Korean patients were enrolled from oncology clinics. These minor variations in inception cohort provided us with a more diverse patient population to determine MCID and increased its generalizability.
Data collection
Data collection occurred between December 8, 2011 and April 30, 2014. We collected baseline patient characteristics, including age, sex, race, education level, cancer diagnosis, CAGE questionnaire [15] and MDAS [16] during the first study visit. For the purpose of this study, we considered all Brazilians and Chileans to be of Hispanic ethnicity. We also assessed ESAS and Karnofsky performance status during both the first and second visits, and Patient's Global Impression Scale (PGI) at the second visit. The site principal investigators all visited Houston to learn about the study procedures. To ensure data is collected in an accurate fashion, the study PI had regular teleconference with the research team at each site 1-2 times per month to provide training and longitudinal monitoring.
ESAS assesses the average intensity of 10 symptoms (pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, feelings of well-being and sleep) over the past 24 hours, each with an 11-point numerical rating scale that ranges from 0 (no symptom) to 10 (worst intensity) [3]. It has been translated into the languages in respective countries and by MAPI Research trust (i.e. English, Arabic, Portugese, Spanish, Korean and Hindi) and validated both linguistically and psychometrically [5, 8, 11, 17, 18].
PGI is a validated global rating of change scale used to evaluate subjective patients’ response at the second visit [19, 20]. Patients were asked to answer the question for each of the 10 ESAS symptoms: “How is your symptom over the last 24 hours compared to your last visit?” for each of the 10 ESAS symptoms (“better”, “about the same”, “worse”). If the patient answered “better”, they were asked “how much better?” (“much better”, “better”, “a little better”). Alternatively, if the patient answered “worse”, they were asked “how much worse?” (“much worse”, “worse”, “a little worse”). PGI has been commonly used as a secondary outcome in a large number of pain studies and also used in several studies as an anchor for establishing clinical importance levels [21, 22].
Statistical analysis
Our sample size calculation revealed that it would require 777 patients to test the dual null hypotheses H0: true positive fraction <0.70 or false positive fraction >0.25 and H1: true positive fraction ≥0.84 and false positive fraction ≤0.10 with 80% power at 0.5% significance (5% divided by 10 symptoms to account for multiple testing), assuming at least 20% of patients will be better according to PGI. The final sample was 796 because this study required patients to complete both study visits, and that by the time we reached our target sample some remaining patients from each study site had already completed the first study visit and thus followed to completion.
We summarized the patient characteristics with descriptive statistics, including means, standard deviations, medians, interquartile ranges, and 95% confidence intervals. We compared the changes in ESAS scores between the first and second visits using paired t-tests.
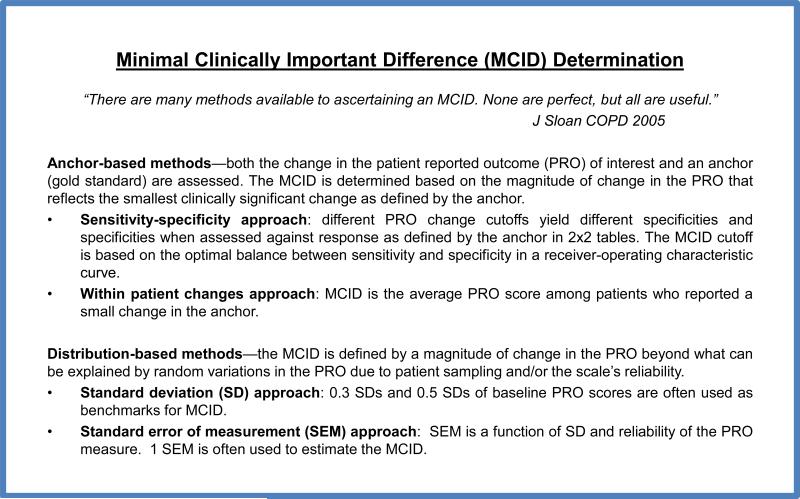
Figure 1 outlines some commonly used approaches to identify MCID. For our primary analysis, we used the PGI as an external criterion against which ESAS changes were anchored and calibrated. We determined the MCID using sensitivity-specificity approach for both improvement (PGI “better” vs. PGI “about the same” and “worse”) and deterioration (PGI “worse” vs. PGI “better” and “about the same”). We plotted the receiver-operating characteristic (ROC) curves with true positive rate (sensitivity) on the y-axis and false positive rate (1 – specificity) on the x-axis. We then calculated the area under the curve (AUC), and determined the optimal cutoff for improvement and deterioration for each symptom based on the Youden J's index. The top left approach was also applied as a confirmatory measure [23].
Figure 1.
Minimal Clinically Important Difference Determination.
We also conducted sensitivity analyses to estimate MCID using other commonly described anchor-based and distribution based approaches [24]. Specifically, we determined the within-patient changes by computing the average ESAS change for the PGI categories “a little better” and “a little worse” because these categories represented the smallest perceived change. 0.3 and 0.5 SD are often considered to be close approximates of MCID.[25, 26] We also examined the standard error of measurement (SEM), which represents the variation in the scores due to the unreliability of the scale using the following formula, SEM = SD × (1-reliability)1/2 [27].
The Statistical Analysis Software 9.3 (SAS Institute Inc., Cary, NC, USA) software was used for statistical analysis. Statistically significance was declared when the P-value is <0.05.
RESULTS
Patient characteristics
The baseline demographics are shown in Table 1. The average age was 57 (range 19-85), 380 (48%) were female, and 229 (29%) were Caucasian, 190 (24%) had gastrointestinal cancers, and a 692 (87%) had metastatic disease. The median duration between the two study visits was 21 days (interquartile range 18-28 days).
Table 1.
Patient characteristics
| Brazil N=131 (%)* |
Chile N=71 (%)* |
India N=44 (%)* |
Jordan N=182 (%)* |
Korea N=68 (%)* |
USA N=300(%)* |
Total N=796 (%)* |
|
|---|---|---|---|---|---|---|---|
| Age, average (range) | 58 (26-81) | 60 (28-85) | 51 (24-73) | 55 (19-84) | 59 (37-81) | 58 (21-85) | 57 (19-85) |
| Female | 61 (47) | 44 (62) | 26 (59) | 86 (47) | 19 (28) | 144 (48) | 380 (48) |
| Race | |||||||
| Caucasian | 0 | 0 | 0 | 0 | 0 | 229(76) | 229 (29) |
| Black | 0 | 0 | 0 | 0 | 0 | 37 (12) | 37 (5) |
| Hispanic | 131 (100) | 71 (100) | 0 | 0 | 0 | 22 (7) | 224 (28) |
| Asian | 0 | 0 | 44 (100) | 0 | 68 (100) | 11 (4) | 123 (15) |
| Other | 0 | 0 | 0 | 182 (100) | 0 | 1 (0) | 183 (24) |
| Marital status | |||||||
| Single | 16 (12) | 16 (23) | 2 (5) | 19 (10) | 5 (7) | 45 (15) | 103 (13) |
| Married | 85 (65) | 40 (56) | 38 (86) | 139 (76) | 54 (79) | 200 (67) | 556 (70) |
| Divorced | 30 (23) | 15 (21) | 4 (9) | 24 (13) | 9 (13) | 53 (18) | 135 (17) |
| Education | |||||||
| Illiterate | 0 | 0 | 6 (14) | 0 | 0 | 0 | 6 (1) |
| High school or less | 114 (87) | 21 (30) | 32 (73) | 111 (61) | 52 (76) | 77 (26) | 407 (51) |
| Some college up to Bachelor's | 16 (12) | 47 (66) | 5 (11) | 58 (32) | 15 (22) | 173 (58) | 314 (39) |
| Advanced degree | 1 (1) | 3 (4) | 1 (2) | 13 (7) | 1 (2) | 50 (17) | 69 (9) |
| Cancer | |||||||
| Breast | 26 (20) | 11 (15) | 6 (14) | 40 (22) | 3 (4) | 48 (16) | 134 (17) |
| Gastrointestinal | 25 (19) | 26 (37) | 7 (16) | 31 (17) | 33 (49) | 68 (23) | 190 (24) |
| Genitourinary | 30 (23) | 6 (8) | 2 (5) | 14 (8) | 2 (3) | 25 (8) | 79 (10) |
| Gynecological | 16 (12) | 6 (8) | 15 (34) | 7 (4) | 1 (1) | 20 (7) | 65 (8) |
| Head and neck | 5 (4) | 0 | 7 (16) | 18 (10) | 7 (10) | 40 (13) | 77 (10) |
| Hematological | 4 (3) | 8 (11) | 1 (2) | 10 (5) | 6 (9) | 8 (3) | 37 (5) |
| Other | 10 (8) | 6 (8) | 3 (7) | 25 (14) | 3 (4) | 40 (13) | 86 (11) |
| Respiratory | 15 (11) | 8 (11) | 3 (7) | 37 (20) | 13 (19) | 51 (17) | 128 (16) |
| Stage | |||||||
| Advanced | 0 | 0 | 0 | 8 (4) | 4 (6) | 8 (3) | 20 (3) |
| Locally advanced | 23 (18) | 8 (11) | 6 (14) | 9 (5) | 4 (6) | 34 (11) | 84 (11) |
| Metastatic | 108 (82) | 63 (89) | 38 (86) | 165 (91) | 60 (88) | 258 (86) | 692 (87) |
| CAGE positive | 38 (29) | 6 (8) | 6 (14) | 7 (4) | 10 (15) | 43 (14) | 110 (14) |
| MDAS, average (SD) | 2 (1) | 2 (2) | 2 (1) | 3 (2) | 2 (2) | 1 (1) | 2.0 (1.7) |
| KPS, average (SD) | 71 (13) | 78 (13) | 77 (6) | 68 (14) | 77 (11) | NA | 72 (13) |
| Duration between visits, median (Q1, Q3) | 23 (21, 26) | 25 (21, 32) | 17.5 (14, 22) | 21 (15, 28) | 21 (14.5, 25) | 22 (18, 28) | 21 (18, 28) |
unless otherwise specified
ESAS Intensity
Table 2 shows the ESAS intensity at first and second clinic visit. The symptoms of highest average intensity were fatigue (4.9/10), pain (4.5/10) and poor well being (4.4/10). Pain, fatigue, depression, anxiety, poor well being, dyspnea and poor sleep all had statistically significant improvement in symptom intensity.
Table 2.
Changes in Edmonton Symptom Assessment Scale
| ESAS, average (SD) | Global symptom assessment | |||||||
|---|---|---|---|---|---|---|---|---|
| First clinic visit | Second clinic visit | Change* | Percentage change from first visit (%)* | P-value† | Better N (%) | Same N (%) | Worse N (%) | |
| Pain | 4.55 (3) | 3.79 (3) | 0.76 (3.01) | 17 | <0.0001 | 377 (47) | 251 (32) | 167 (21) |
| Fatigue | 4.93 (2.82) | 4.47 (2.92) | 0.46 (2.95) | 9 | <0.0001 | 293 (37) | 297 (37) | 206 (26) |
| Nausea | 1.72 (2.68) | 1.59 (2.53) | 0.14 (2.91) | 8 | 0.10 | 209 (26) | 460 (58) | 125 (16) |
| Depression | 2.55 (2.87) | 2.38 (2.83) | 0.18 (2.58) | 7 | 0.02 | 169 (21) | 494 (62) | 132 (17) |
| Anxiety | 3.18 (3.11) | 2.82 (2.9) | 0.36 (2.78) | 11 | 0.0002 | 191 (24) | 472 (59) | 131 (16) |
| Drowsiness | 3.31 (2.99) | 3.26 (2.88) | 0.05 (3.08) | 2 | 0.31 | 217 (27) | 410 (52) | 169 (21) |
| Poor appetite | 4.01 (3.03) | 3.85 (3.06) | 0.15 (3.16) | 4 | 0.09 | 267 (34) | 353 (44) | 176 (22) |
| Poor well being | 4.41 (2.76) | 4.12 (2.74) | 0.29 (2.92) | 7 | 0.002 | 293 (37) | 328 (41) | 174 (22) |
| Dyspnea | 2.51 (2.89) | 2.23 (2.81) | 0.27 (2.42) | 11 | 0.0008 | 155 (19) | 533 (67) | 108 (14) |
| Poor sleep | 4.08 (3.06) | 3.46 (2.85) | 0.62 (3.13) | 15 | <0.0001 | 258 (32) | 410 (52) | 128 (16) |
improvement is indicated by a positive value
paired t-test was used to determine the difference in ESAS scores between first and second study visit
Patients reported if their perceived symptom change at the second visit relative to the first visit (Table 2). In PGI, 377 (47%), 293 (38%), and 293 (38%) felt that their pain, fatigue and poor well being improved, respectively. Dyspnea had the smallest proportion of patients reporting an improvement (19%).
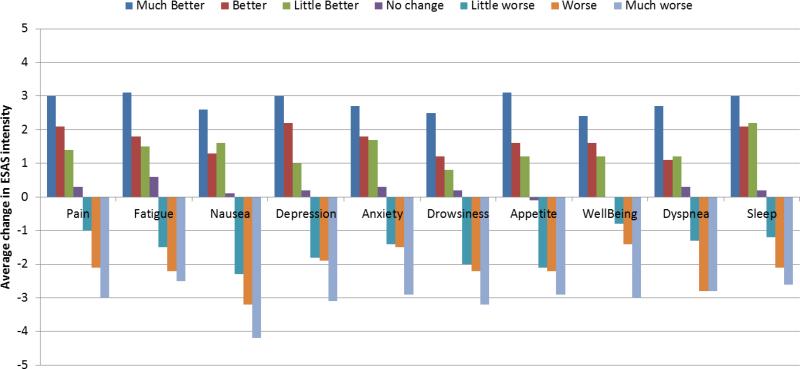
We plotted the average change in ESAS intensity by PGI categories which demonstrates a gradient effect (Figure 2).
Figure 2.
Average change in ESAS intensity between the first and second study visit by PGI category (n=796)
Determination of MCID
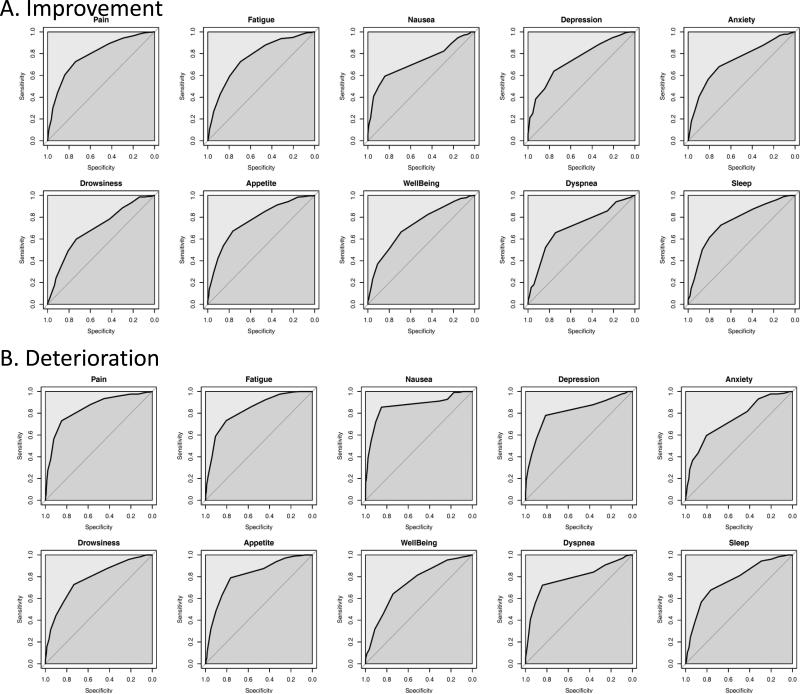
Figure 3 shows the ROC curves for each of the 10 ESAS symptom. Table 3 illustrates the ROC curve AUC and optimal cutoffs. The AUC varied between 0.70-0.87, suggesting good discrimination for ESAS. For all 10 symptoms, the optimal cutoffs were ≥1 point for improvement and ≤−1 point for deterioration based on both the Youden's J Index and top left methods. The sensitivities ranged between 59% and 85%, and specificities ranged between 69% and 85%.
Figure 3.
Receiver-operating characteristic curves for (A) Improvement and (B) Deterioration for the 10 Edmonton Symptom Assessment Scale Symptoms. The area under the curve ranged between 0.7 to 0.86, suggesting good discrimination.
Table 3.
Minimal Clinically Important Differences based on the Sensitivity-Specificity Approach
| Improvement | Deterioration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | Cutoff* | Sensitivity | Specificity | Youden's J† | Top left‡ | AUCψ | Cutoff* | Sensitivity | Specificity | Youden's J† | Top left‡ | AUCψ |
| Pain | ≥+1 | 0.727 | 0.739 | 0.715 | 0.378 | 0.788 | ≤−1 | 0.731 | 0.849 | 0.579 | 0.309 | 0.843 |
| ≥+2 | 0.605 | 0.840 | 0.444 | 0.426 | ≤−2 | 0.563 | 0.922 | 0.485 | 0.444 | |||
| ≥+3 | 0.443 | 0.907 | 0.350 | 0.565 | ≤−3 | 0.377 | 0.951 | 0.328 | 0.625 | |||
| Fatigue | ≥+1 | 0.727 | 0.694 | 0.421 | 0.410 | 0.766 | ≤−1 | 0.733 | 0.805 | 0.538 | 0.331 | 0.832 |
| ≥+2 | 0.594 | 0.795 | 0.389 | 0.455 | ≤−2 | 0.587 | 0.907 | 0.494 | 0.423 | |||
| ≥+3 | 0.430 | 0.883 | 0.313 | 0.582 | ≤−3 | 0.398 | 0.939 | 0.337 | 0.605 | |||
| Nausea | ≥+1 | 0.593 | 0.841 | 0.434 | 0.437 | 0.728 | ≤−1 | 0.856 | 0.851 | 0.707 | 0.208 | 0.868 |
| ≥+2 | 0.502 | 0.897 | 0.400 | 0.508 | ≤−2 | 0.720 | 0.904 | 0.624 | 0.296 | |||
| ≥+3 | 0.407 | 0.947 | 0.354 | 0.596 | ≤−3 | 0.544 | 0.948 | 0.492 | 0.459 | |||
| Depression | ≥+1 | 0.639 | 0.758 | 0.397 | 0.434 | 0.745 | ≤−1 | 0.780 | 0.813 | 0.593 | 0.289 | 0.812 |
| ≥+2 | 0.479 | 0.843 | 0.322 | 0.544 | ≤−2 | 0.561 | 0.900 | 0.461 | 0.451 | |||
| ≥+3 | 0.385 | 0.928 | 0.313 | 0.620 | ≤−3 | 0.417 | 0.944 | 0.361 | 0.586 | |||
| Anxiety | ≥+1 | 0.681 | 0.711 | 0.392 | 0.431 | 0.733 | ≤−1 | 0.595 | 0.805 | 0.401 | 0.449 | 0.746 |
| ≥+2 | 0.565 | 0.812 | 0.378 | 0.473 | ≤−2 | 0.435 | 0.882 | 0.317 | 0.577 | |||
| ≥+3 | 0.403 | 0.899 | 0.302 | 0.605 | ≤−3 | 0.366 | 0.935 | 0.301 | 0.637 | |||
| Drowsiness | ≥+1 | 0.599 | 0.732 | 0.331 | 0.482 | 0.696 | ≤−1 | 0.728 | 0.733 | 0.461 | 0.381 | 0.778 |
| ≥+2 | 0.488 | 0.810 | 0.298 | 0.546 | ≤−2 | 0.562 | 0.834 | 0.396 | 0.468 | |||
| ≥+3 | 0.341 | 0.877 | 0.218 | 0.670 | ≤−3 | 0.444 | 0.901 | 0.345 | 0.565 | |||
| Poor appetite | ≥+1 | 0.673 | 0.765 | 0.438 | 0.403 | 0.771 | ≤−1 | 0.790 | 0.765 | 0.555 | 0.315 | 0.812 |
| ≥+2 | 0.538 | 0.854 | 0.391 | 0.485 | ≤−2 | 0.631 | 0.844 | 0.475 | 0.401 | |||
| ≥+3 | 0.421 | 0.905 | 0.326 | 0.587 | ≤−3 | 0.483 | 0.903 | 0.386 | 0.526 | |||
| Poor well being | ≥+1 | 0.664 | 0.689 | 0.354 | 0.457 | 0.725 | ≤−1 | 0.642 | 0.743 | 0.384 | 0.441 | 0.738 |
| ≥+2 | 0.510 | 0.800 | 0.310 | 0.529 | ≤−2 | 0.462 | 0.832 | 0.294 | 0.563 | |||
| ≥+3 | 0.370 | 0.910 | 0.280 | 0.637 | ≤−3 | 0.318 | 0.913 | 0.231 | 0.688 | |||
| Dyspnea | ≥+1 | 0.658 | 0.743 | 0.401 | 0.428 | 0.712 | ≤−1 | 0.722 | 0.842 | 0.564 | 0.320 | 0.789 |
| ≥+2 | 0.523 | 0.836 | 0.359 | 0.505 | ≤−2 | 0.556 | 0.910 | 0.465 | 0.453 | |||
| ≥+3 | 0.323 | 0.900 | 0.223 | 0.685 | ≤−3 | 0.407 | 0.955 | 0.362 | 0.594 | |||
| Poor sleep | ≥+1 | 0.728 | 0.693 | 0.420 | 0.411 | 0.759 | ≤−1 | 0.677 | 0.765 | 0.442 | 0.400 | 0.765 |
| ≥+2 | 0.611 | 0.803 | 0.414 | 0.436 | ≤−2 | 0.567 | 0.853 | 0.420 | 0.457 | |||
| ≥+3 | 0.502 | 0.868 | 0.370 | 0.515 | ≤−3 | 0.370 | 0.918 | 0.288 | 0.635 | |||
Both Youden's J method and top left method provided the same optimal cutoff. Improvement is indicated by a positive value, and deterioration is indicated by a negative value.
The cutoff value is selected based on the largest Youden's J value, which represents the point on the ROC curve that represents the largest vertical distance from the ROC curve to the diagonal line of equality. The optimal cutoff is highlighted in bold.
The cutoff value is chosen based on the smallest top left value, which is on the point on the ROC curve that represents the shortest distance to the top left corner of the graph (where sensitivity = 100% and specificity = 100%). The optimal cutoff is highlighted in bold.
The area under the curve for each receiver-operating characteristic curve is shown (i.e. not cutoff specific). AUC is an indicator of the ability of the scale to discriminate change. The AUCs were between 0.7 to 0.87 for the ROC curves, which suggest good discrimination.
Abbreviations: AUC, area under the receiver-operating characteristic curve; ROC, receiver-operating characteristic curve
We conducted sensitivity analyses by estimating MCID using other commonly applied approaches are shown in eTable 1. Based on the within-patient approach, the MCIDs were 0.8-2.2 for improvement and −0.8 to −2.3 for deterioration. Using the distribution approach, 0.5 standard deviation revealed MCIDs of 1.2-1.6 points, which was similar to one standard error of measurement (1.3-1.7 point).
Response determination
eTable 2 illustrates the proportion of patients in our cohort with symptom response in the followup visit (defined as change of ≥+1), which varied between 27% (nausea) and 48% (pain). In contrast, between 23% (dyspnea) and 37% (drowsiness) of patients experienced symptom deteriorate, defined as change of ≤−1.
DISCUSSION
This study is the largest study to date to identify MCID of ESAS, and is the only prospective study specifically powered to address this important question. We found that ESAS had moderate to high responsiveness to change. In sensitivity-specific analysis, a ≥1 point change was identified as the “universal” cutoff for both improvement and deterioration for all 10 symptoms. The other anchor based and distribution based approaches also showed an MCID between 1-2 for a majority of the symptoms. Our findings have implications for response determination and sample size calculations in symptom research.
Interestingly, we found that a 1 point cutoff was applicable to all 10 symptoms and for both improvement and deterioration. In a post-hoc analysis, Bush et al. reported that an average change in ESAS Well being of 1.25 points corresponded with FACT-G change of ≥5 points, which is consistent with our findings.[28] In another secondary analysis, Reddy et al. assessed the MCID for ESAS-fatigue between baseline and day 8 in 194 cancer patients enrolled onto 2 double blind randomized controlled trials.[29] The anchor was the global benefit score, in which a score of at least 4/7 (moderately important, consistently beneficial) was considered a response. The optimal cutoff for improvement in ESAS-fatigue was identified as 4 points or more, which had a sensitivity of 66% and a specificity of 72%. The discrepancy between their findings and ours can be explained by different anchors and patient populations, and that fact that they were looking for what constituted a “≥moderate” instead of a “≥minimal” improvement.
More recently, Bedard et al. conducted a retrospective analysis of 276 cancer patients and identified the MCID using the between-patient change method for 8 ESAS symptoms with ESAS Wellbeing category change as an anchor.[14, 26] They reported that a decrease of 1.2 and 1.1 points in ESAS pain and depression, respectively, constituted clinically relevant improvement, and an increase of 1.4, 1.8, 1.1, 1.1, and 1.4 points in pain, tiredness, depression, anxiety, and appetite loss respectively, were the MCIDs for deterioration. Thus, the findings from this study were generally consistent with our prospective study despite the different methodologies.
A commonly cited MCID cutoff for pain 0-10 numeric rating scale is a ≥2/10 point or 33% decrease.[13] Importantly, this MCID was derived from data that assessed the change in pain intensity “now” measured 30 minutes from baseline, and the external criterion was the need for additional rescue opioid. This is in contrast to our study that assesses average ESAS symptom intensity over the past 24 hours between baseline and 3 weeks later. Thus, different timeframe anchors yielded different MCIDs.
In the research setting, MCID is an important determinant of sample size and whether we declare an intervention's effect clinically meaningful or not. A larger cutoff would set a higher bar for the intervention but require a smaller sample size, and vice versa. We calculated that to detect a ≥1 point improvement with 80% power and alpha 5% would require 284 patients using a two sample t-test, assuming a standard deviation of 3 points. In contrast, a 2 point cutoff would require only 72 patients. Given that the median sample size of supportive oncology randomized trials was only 70, they could potentially be underpowered if ESAS was the primary endpoint.
This study has a number of limitations. First, our MCIDs were derived from ambulatory cancer patients seen predominately in the palliative care setting with 2 visits approximately 3 weeks apart. Further prospective studies are required to determine if the MCID of ESAS varies with different patient populations, settings, and time intervals, and whether early symptom response is associated with other clinical outcomes such as quality of life improvement, cancer treatment response and survival.. Second, the sensitivity and specificity cutoffs for our MCIDs were less than 80%. A moderate sensitivity means that some patients who actually experienced a symptom change may have a lower ESAS change of <1 point (i.e. false negative), while a moderate specificity means that some patients who did not feel that their symptoms have changed in a meaningful manner may have ESAS change of ≥1 point (i.e. false positive). Thus, MCID cutoffs are more appropriate for group averages than individual response determination. Third, we used PGI as the gold standard, which has face validity and was easy to understand by patients [30, 31]. However, other external criteria such as quality of life questionnaires and functional scales may also be useful.
In summary, ESAS was responsive to change and that the optimal cutoff for improvement/deterioration was ≥1 point for all 10 symptoms. Further studies should examine if this cutoff remains relevant in other patient populations, settings and different time frames. Findings from this study may facilitate the design and interpretation of symptom control studies employing ESAS as the primary outcome.
Supplementary Material
Precis.
When assessing symptom response, how much of a change in a 0-10 point numeric rating scale is considered clinically significant? In this multicenter, prospective, longitudinal study involving 796 patients with advanced cancer, we found that the minimal clinically important difference was universally a 1 point difference for both improvement and deterioration for each of the 10 symptoms in the Edmonton Symptom Assessment Scale, one of the most widely used symptom assessment batteries in oncology.
Acknowledgements
We thank all the patients who participated in this study. We also thank Swati Bansal, Dr. Odai Khamash, Dr. Abdelrahman Alhawamdeh, Natalia Campacci, Camila Souza Crovador, Won Ji Yuen, Dr. Sarika Sane, and Dr. Mrunal Marathe for their assistance with data collection.
Funding: This research is supported by the Sister Institution Network Fund from the University of Texas MD Anderson Cancer Center, King Hussein Cancer Center, Barretos Cancer Hospital and Tata Memorial Center. EB is supported in part by National Institutes of Health grants R01NR010162-01A1, R01CA122292-01, and R01CA124481-01. DH is supported in part by an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research, MRSG-14-1418-01-CCE.
Footnotes
Disclosure: The authors have declared no conflicts of interest.
References
- 1.Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–8. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 2.Hui D, Bruera E. A personalized approach to assessing and managing pain in patients with cancer. J Clin Oncol. 2014;32:1640–6. doi: 10.1200/JCO.2013.52.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 4.Philip J, Smith WB, Craft P, et al. Concurrent validity of the modified Edmonton Symptom Assessment System with the Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer. 1998;6:539–41. doi: 10.1007/s005200050212. [DOI] [PubMed] [Google Scholar]
- 5.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–71. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Stromgren AS, Goldschmidt D, Groenvold M, et al. Self-assessment in cancer patients referred to palliative care: a study of feasibility and symptom epidemiology. Cancer. 2002;94:512–20. doi: 10.1002/cncr.10222. [DOI] [PubMed] [Google Scholar]
- 7.Moro C, Brunelli C, Miccinesi G, et al. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30–37. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 8.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991--2006). Palliat Med. 2008;22:111–22. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 9.Barbera L, Seow H, Howell D, et al. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer. 2010;116:5767–76. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 10.Dudgeon D, King S, Howell D, et al. Cancer Care Ontario's experience with implementation of routine physical and psychological symptom distress screening. Psychooncology. 2012;21:357–64. doi: 10.1002/pon.1918. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psychooncology. 2012;21:977–85. doi: 10.1002/pon.1996. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 13.Farrar JT, Portenoy RK, Berlin JA, et al. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–94. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 14.Hui D, Bruera E. Minimal clinically important differences in the edmonton symptom assessment system: the anchor is key. J Pain Symptom Manage. 2013;45:e4–5. doi: 10.1016/j.jpainsymman.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–07. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 16.Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13:128–37. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 17.Carvajal A, Centeno C, Watson R, et al. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47:1863–72. doi: 10.1016/j.ejca.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Kwon JH, Nam SH, Koh S, et al. Validation of the Edmonton Symptom Assessment System in Korean patients with cancer. J Pain Symptom Manage. 2013;46:947–56. doi: 10.1016/j.jpainsymman.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer D, Stewart AL, Bloch DA, et al. Capturing the patient's view of change as a clinical outcome measure. JAMA. 1999;282:1157–62. doi: 10.1001/jama.282.12.1157. [DOI] [PubMed] [Google Scholar]
- 20.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. The Journal of manual & manipulative therapy. 2009;17:163–70. doi: 10.1179/jmt.2009.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauridsen HH, Hartvigsen J, Manniche C, et al. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord. 2006;7:82. doi: 10.1186/1471-2474-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrar JT, Pritchett YL, Robinson M, et al. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 2010;11:109–18. doi: 10.1016/j.jpain.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 24.Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7:541–6. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 26.Bedard G, Zeng L, Zhang L, et al. Minimal Clinically Important Differences in the Edmonton Symptom Assessment System in Patients With Advanced Cancer. J Pain Symptom Manage. 2012 doi: 10.1016/j.jpainsymman.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Wyrwich KW, Wolinsky FD. Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract. 2000;6:39–49. doi: 10.1046/j.1365-2753.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 28.Bush SH, Parsons HA, Palmer JL, et al. Single- vs. multiple-item instruments in the assessment of quality of life in patients with advanced cancer. J Pain Symptom Manage. 2010;39:564–71. doi: 10.1016/j.jpainsymman.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Reddy S, Bruera E, Pace E, et al. Clinically important improvement in the intensity of fatigue in patients with advanced cancer. J Palliat Med. 2007;10:1068–75. doi: 10.1089/jpm.2007.0007. [DOI] [PubMed] [Google Scholar]
- 30.Lydick E, Epstein RS. Interpretation of quality of life changes. Qual Life Res. 1993;2:221–6. doi: 10.1007/BF00435226. [DOI] [PubMed] [Google Scholar]
- 31.Juniper EF, Guyatt GH, Willan A, et al. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–7. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.