Abstract
Thirty percent of obese individuals are metabolically healthy and were noted have increased peripheral obesity. Adipose tissue is the primary source of adiponectin, an adipokine with insulin-sensitizing and anti-inflammatory properties. Lower adiponectin levels are observed in individuals with obesity and those at risk for cardiovascular disease. Conversely, higher levels are noted in some obese individuals who are metabolically healthy. Our objective was to determine whether abdominal adiposity distribution, rather than BMI status, influences plasma adiponectin level. Four-hundred and twenty-four subjects (female: 255) of Northern European ancestry were recruited from “Take Off Pounds Sensibly” (TOPS) weight loss club members. Demographics, anthropometrics, and dual X-ray absorptiometry of the whole body and CT scan of the abdomen were performed to obtain total body fat content and to quantify subcutaneous adipose tissue and visceral adipose tissue respectively. Laboratory measurements included fasting plasma glucose, insulin, lipid panel, and adiponectin. Age- and gender-adjusted correlation analyses showed that adiponectin levels were negatively correlated with body mass index, waist circumference, triglycerides, total fat mass, and visceral adipose tissue. A positive correlation was noted with HDL-cholesterol and fat free mass (p<0.05). Subcutaneous adipose tissue -to-visceral adipose tissue ratios were also significantly associated with adiponectin (r=0.13, p = 0.001). Further, the best positive predictors for plasma adiponectin were found to be subcutaneous adipose tissue -to-visceral adipose tissue ratios and gender by regression analyses (P<0.01). Abdominal adiposity distribution is an important predictor of plasma adiponectin and obese individuals with higher subcutaneous adipose tissue -to-visceral adipose tissue ratios may have higher adiponectin levels.
Introduction
Obesity is associated with insulin resistance, metabolic syndrome, and type 2 diabetes mellitus, and thus many obese individuals are at increased cardiovascular disease risk(1, 2). However, not all obese individuals are at increased risk for metabolic abnormalities noted above(3). Individuals with centripetal distribution of adiposity (visceral adiposity) are at a higher cardiovascular disease risk compared to individuals with peripheral adiposity distribution(3).
Adipose tissue, a dynamic endocrine organ, is a source of a number of adipocytokines and is responsible for a myriad of actions that may explain the metabolic risks attributed to adiposity(4). Adiponectin is one such adipokine derived exclusively from white adipose tissue and has been shown to have insulin-sensitizing, anti-inflammatory, and anti-apoptotic effects on a number of different cell types(5, 6). It is largely considered to have protective actions against obesity-related metabolic risks and lower adiponectin levels are considered a risk factor for type 2 diabetes mellitus and cardiovascular disease(7-9). Even though adipose tissue is the sole source of adiponectin, adiponectin levels are lower in individuals with higher body mass index, particularly visceral adiposity, suggesting a non-linear relationship with adipose tissue mass(10, 11).
Several studies have shown that females have higher levels of adiponectin than males(6, 8, 12), which may be the result of differences in body fat distribution between genders(8, 12). In addition, newly described metabolically healthy obese phenotype individuals were recently shown to have paradoxical hyperadiponectinemia(12, 13) with favorable metabolic risk profiles, suggesting that adiposity distribution may contribute to adiponectin levels and hence the cardiovascular risk of obesity. In the current study, we explore the relationship of plasma adiponectin level with total adiposity and abdominal adiposity distribution (subcutaneous vs. visceral).
Methods
Subjects
Four-hundred and twenty-four Caucasian subjects (male: 169, female: 255) were recruited from “Take Off Pounds Sensibly” (TOPS) weight loss club membership as has been previously described(14, 15). These subjects were part of a family-based study and recruitment criteria consisted of having at least 2 obese siblings (body mass index ≥ 30 kg/m2) and at least one non-obese sibling and/or parent (body mass index ≤ 27 kg/m2)(14, 15). Subjects with a history of type 1 diabetes mellitus, cancer, renal or hepatic disease, active coronary artery disease, substance abuse, corticosteroids, thyroid medications above the replacement dose, or history of weight loss of more than 10% of body weight in the preceding 12 months were excluded from the study. All procedures were approved by the Medical College of Wisconsin's Institutional Review Board and conform to the relevant ethical guidelines for human research.
Measurements
Weight, height, and blood pressure were measured using standardized methods. Waist circumference was measured at the level of the navel, and hip circumference was measured at the widest point of the buttock region. BMI and waist-to-hip ratio were calculated. Subjects were fasting at the time of laboratory measurements. Plasma glucose was measured in triplicate using a Glucose Analyzer II (Beckman Instruments, Brea, CA) with a glucose oxidase method. Plasma insulin was measured using a double-antibody, equilibrium RIA (Linco Research, St. Louis) specific to human insulin. The homeostasis model assessment (HOMA) method was used for calculation of insulin resistance (HOMA-IR)(16) in patients without type 2 diabetes mellitus (n=387). Plasma triglycerides were measured using a glycerylphosphate oxidase method (Stanbio Laboratory, Inc., San Antonio, TX). High-density lipoprotein-cholesterol (HDL-C) was measured using phosphotungstic acid/MgC12 precipitation (Roche-Boehringer, Indianapolis, IN). Low-density lipoprotein-cholesterol (LDL-C) was directly measured with an enzymatic selective protection method (Sigma Diagnostics, St. Louis, MO). Plasma adiponectin and leptin were determined using a double antibody equilibrium radioimmunoassay (Millipore Corporation, Billerica, MA)(17). Tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) were measured using Quantikine human ELISA kits from R&D systems (Minneapolis, MN)(18).
In addition, biological phenotypes were measured following standard published protocol to obtain total fat mass as well as lean mass in kilograms and percentage using dual-emission X-ray Absorptiometry (DXA)(19). Total abdominal, visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) depot sizes were estimated using the average of three computerized tomography (CT) sections at the fourth lumbar vertebra(20). CT data are expressed as cross-sectional areas of tissue in grams or cm2. The total adipose tissue area, total soft tissue area, and the mean attenuation of soft tissue on each cross-sectional image are determined. The subcutaneous and intra-abdominal adipose tissue areas are differentiated by encircling the abdominal muscular wall. The number of volume elements in the scan containing fat is determined by thresholding techniques(21, 22). Computer software delineated tissue areas, from which quantitative estimates of the amounts of adipose tissue, muscle, or bone can be estimated(23). The reproducibility of CT measurements was high; the coefficient of variation for our laboratory with a single observer over consecutive days is 1.75%. Although CT scanning requires a relatively high radiation dose, this has been minimized by limiting the scanning to three slices of 3.0 mm thickness.
Statistical analyses
In the univariate analysis, descriptive statistics (mean ± standard deviation) were calculated and a two-sample t-test was performed to compare female and male subjects on the overall basic characteristics. For the multivariate analysis, SAT to VAT ratio was transformed using a base-2 logarithmic transformation. Changes in these transformed values should be interpreted as multiplicative effects: for example, an increase of one in the transformed value corresponds to a two-fold change in the original scale. Partial correlation analysis was performed to investigate the relationship between adiponectin and each anthropometric characteristics controlling for age and gender in the overall data and adjusting for age in the gender separated data. Regression analysis with step-wise model selection method was performed to identify the significant predictors of adiponectin using the overall sample and gender separated samples. The significance level was set at 0.05 for all the analyses. All data analyses were carried out using the Statistical Analysis System, version 9.2 (SAS institute, Cary, NC, USA).
Results
The baseline anthropometric and metabolic characteristics are summarized in Table 1. Females had higher body mass index, percent fat mass, SAT, and higher SAT-to-VAT ratios compared to males. Males had a higher waist circumference, waist-to-hip ratio, and percent fat free mass. However, VAT area was similar between males and females. Males also had higher systolic blood pressure, diastolic blood pressure, LDL-C, and lower HDL–C levels. Insulin resistance, indicated by HOMA-IR, was not different between males and females (1.8 vs. 1.7) without diabetes mellitus. Females had higher plasma adiponectin levels.
Table 1.
Distributions of basic characteristics in Caucasian subjects (mean ± standard deviation)
| Variable | Total (n=424) | Females (n=255) | Males (n=169) |
|---|---|---|---|
| Age (years) | 42.6 ± 15.9 | 43.1 ± 15.6 | 41.8 ± 6.4 |
| Systolic blood pressure (mmHg) | 125.1 ± 17.7 | 123.0 ± 19.0 | 128.2 ± 15.1** |
| Diastolic blood pressure (mmHg) | 76.5 ± 11.9 | 75.3 ± 11.1 | 78.3 ± 12.8** |
| Anthropometric measurements | |||
| BMI (kg/m2) | 32.5 ± 8.9 | 33.7 ± 9.4 | 30.8 ± 7.8* |
| Waist circumference (cm) | 98.3 ± 18.4 | 96.6 ± 19.3 | 101.0 ± 16.6* |
| Waist to hip ratio (WHR) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1*** |
| Total fat (%) | 38.1 ± 11.0 | 43.9 ± 7.9 | 29.4 ± 9.1*** |
| Total fat free mass (%) | 62.1 ± 11.2 | 56.4 ± 8.4 | 70.7 ± 9.2*** |
| Abdominal adipose tissue area | |||
| Subcutaneous adipose tissue area (cm2) | 84.4 ± 48.2 | 92.9 ± 49.1 | 71.6 ± 44.1*** |
| Visceral adipose tissue area (cm2) | 48.1 ± 30.4 | 42.4 ± 29.1 | 56.8 ± 30.5 |
| Total abdominal adipose tissue area (cm2) | 486.5 ± 250.0 | 496.6 ± 255.3 | 471.1 ± 241.7 |
| SAT-to-VAT ratio | 2.2 ± 1.4 | 2.7 ± 1.5 | 1.4 ± 0.7*** |
| Laboratory measurements | |||
| Total cholesterol (mmol/L) | 4.94 ± 1.07 | 4.90 ± 1.02 | 5.00 ± 1.13 |
| HDL-cholesterol (mmol/L) | 1.06 ± 0.40 | 1.12 ± 0.45 | 0.98 ± 0.27*** |
| Triglycerides (mmol/L) | 1.31 ± 1.67 | 1.19 ± 0.79 | 1.50 ± 2.46 |
| LDL-cholesterol (mmol/L) | 3.36 ± 1.15 | 3.06 ± 0.95 | 3.51 ± 1.38* |
| Adiponectin (μg/mL)† | 8.5 ± 4.5 | 9.6 ± 4.9 | 6.8 ± 3.0*** |
| Interleukin-1β (pg/mL)† | 0.5 ± 1.0 | 0.5 ± 1.0 | 0.4 ± 1.0 |
| Interleukin -6 (pg/mL)† | 4.8 ± 9.0 | 5.8 ± 10.0 | 3.4 ± 7.1** |
| Tumor Necrosis Factor-α (pg/mL)† | 3.9 ± 2.7 | 3.8 ± 2.6 | 4.2 ± 2.8 |
| Among subjects without diabetes mellitus | |||
| Insulin resistance index (HOMA-IR) | 1.8 ± 1.1 | 1.8 ± 1.2 | 1.7 ± 1.0 |
P ≤ 0.05
p ≤ 0.01
p ≤ 0.001
SI units not available
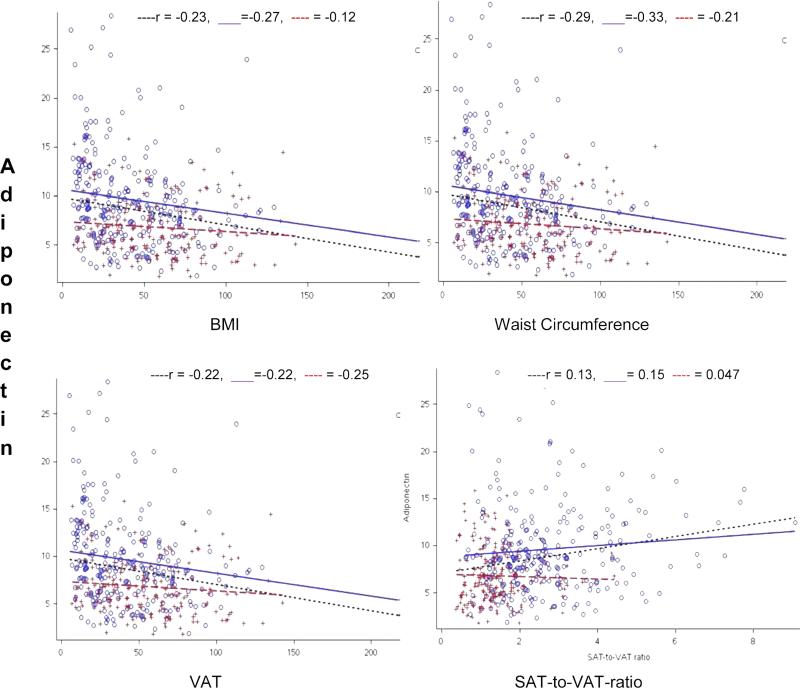
Overall adiponectin correlated positively with age (r=0.106, p = 0.03). When adjusted for age and gender, adiponectin was negatively correlated with body mass index, waist circumference, waist-to-hip ratio, percent total fat, total abdominal fat area, and unadjusted SAT and VAT areas, while a higher percentage of fat free mass was associated with higher adiponectin levels (Table 2). When SAT was adjusted for VAT (SAT-to-VAT ratio), it was positively correlated with adiponectin (Figure 1). Additionally, in the overall sample adiponectin was inversely related to total and LDL-C and triglycerides, and positively related to HDL-C, however, there was no relationship with any of the inflammatory markers, HOMA-IR, or blood pressure (Table 2).
Table 2.
Age and gender-adjusted partial correlation coefficients of adiponectin with anthropometric and biochemical characteristics
| Variable | Overall | Females | Males |
|---|---|---|---|
| BMI (kg/m2) | −0.23*** | −0.27*** | −0.12 |
| Waist circumference (cm) | −0.29*** | −0.33*** | −0.21** |
| Waist to hip ratio | −0.20*** | −0.20*** | −0.22*** |
| Total fat (%) | −0.20*** | −0.24** | −0.14 |
| Total fat free mass (%) | 0.24*** | 0.29*** | 0.17* |
| Subcutaneous adipose tissue area (cm2) | −0.20*** | −0.23*** | −0.13 |
| Visceral adipose tissue area (cm2) | −0.22*** | −0.22*** | −0.25*** |
| Total abdominal adipose tissue area (cm2) | −0.23*** | −0.25*** | −0.19* |
| SAT-to-VAT ratio | 0.13*** | 0.15* | 0.047 |
| SBP (mm Hg) | −0.04 | −0.03 | −0.06 |
| DBP (mm Hg) | −0.09* | −0.098 | −0.098 |
| Total cholesterol (mmol/L) | −0.12** | −0.10 | −0.18* |
| HDL-cholesterol (mmol/L) | 0.21*** | 0.23*** | 0.13 |
| LDL-cholesterol (mmol/L) | −0.11* | −0.07 | −0.19* |
| Triglycerides (mmol/L) | −0.11** | −0.21*** | −0.092 |
| Interleukin-1β (pg/mL) | −0.05 | −0.06 | −0.03 |
| Interleukin -6 | −0.002 | −0.005 | 0.16* |
| Tumor Necrosis Factor-α (pg/mL) | 0.027 | 0.056 | −0.033 |
P ≤ 0.05
p ≤ 0.01
p ≤ 0.001
Figure 1.
Regression plots showing the relationship between adiponectin and adiposity measurements
Trend lines depicting relationship of adiposity measurements with adiponectin ....... All subjects trend line ○ Females + Males ------ Male trend line — Female trend line
When a sub-group analysis was performed by gender, adiponectin showed similar relationships with adiposity measurements and metabolic parameters in females as the overall sample, including a positive association with SAT-to-VAT ratios (Figure 1). Similarly, in males adiponectin was negatively associated with waist circumference, waist-to-hip ratio, total abdominal fat, and VAT area. In contrast to females, males did not show any correlation between adiponectin and body mass index, percent body fat mass and SAT-to-VAT ratios (Table 2). Adiponectin in males was also not significantly related to HDL-C or triglycerides.
In a step-wise regression analyses to determine significant predictors of adiponectin in the overall sample (Table 3), age, gender, waist circumference, and SAT-to-VAT ratios were significant in predicting adiponectin levels. Twenty-one percent of the variability in adiponectin levels was explained by the model. SAT-to-VAT ratios, age, and gender were the two strongest positive predictors of adiponectin in the overall sample. SAT-to-VAT ratio remained significant even after adjustment for age, gender, and waist circumference. In gender-specific analyses, age, waist circumference, and SAT-to-VAT ratios were significant adiponectin predictors for both males and females (Table 3).
Table 3.
Stepwise Regression Analysis for Predictors of Adiponectin
| Variable | Estimate ± SE | P value | Estimate ± SE | P value | Estimate ± SE | P value |
|---|---|---|---|---|---|---|
| Total | Overall sample | Females | Males | |||
| Gender (female vs. male) | 1.30 ± 0.52 | 0.01 | ||||
| Age (years) | 0.08 ± 0.02 | <0.0001 | 0.098 ± 0.02 | <0.0001 | 0.06 ± 0.02 | 0.0008 |
| Waist Circumference | −0.08 ± 0.01 | <0.0001 | −0.09 ± 0.02 | <0.0001 | −0.053 ± 0.015 | 0.0008 |
| Log SAT-to-VAT ratio | 1.00 ± 0.32 | 0.0015 | 1.00 ± 0.48 | 0.04 | 0.850 ± 0.39 | 0.03 |
Discussion
The association of adiponectin levels with adiposity in various body compartments and waist to hip ratio has been previously proposed(11, 24). However, this is the first study that demonstrates a clear association between higher abdominal SAT (after adjustment for VAT) as measured unambiguously by the CT scan and plasma adiponectin levels, after adjustment for body mass index, waist circumference, and VAT. In addition, regression analyses showed that gender and SAT-to-VAT ratios were best positive determinants of plasma adiponectin levels. These findings highlight the potential role of abdominal adiposity distribution, rather than visceral adiposity, in regulating adiponectin levels. Furthermore, we confirm findings from previous studies showing that females have higher overall adiposity, higher SAT, and adiponectin levels(24)
There is a large inter-individual variation in the size and expandability of various adipose tissue depots, such as subcutaneous (e.g. abdominal, thigh, and gluteal regions) and visceral (e.g. omental, mesenteric, and perinephric) depots in humans. It has been suggested that the association of VAT with inflammatory cytokines (e.g. interleukins and TNF-α) contributes to the development of insulin resistance and cardiovascular disease(25). In contrast, individuals with peripheral adiposity have a predominantly subcutaneous accumulation of adipose tissue in the femoral-gluteal region and seem to be less susceptible to metabolic complications(26-28). Each standard deviation (SD) increase in SAT mass decreases the odds of insulin resistance by 48%, whereas a SD increase in VAT mass increases the odds of insulin resistance by 80%(29). These data suggest that SAT may be protective against the development of metabolic abnormalities and cardiovascular disease.
Adiponectin is a pleiotropic hormone that has emerged as a unique marker of metabolic protection offered by adipose tissue(30), with lower levels seen in individuals with unfavorable metabolic conditions such as insulin resistance and visceral obesity(10, 31, 32). However, Turer et al. showed that adiponectin levels were higher with increasing lower extremity adiposity and decreasing truncal adiposity(11), thus raising a possibility of independent association with SAT. Matoshima et al. studied secretion of adiponectin invitro from paired samples of isolated human omental and subcutaneous adipocytes(33). They noted that secretion of adiponectin from omental cells was generally higher than subcutaneous adipocytes, however showed a strong negative correlation with body mass index. They concluded that reduced secretion of adiponectin from omental adipose tissue depot might account for the decline in plasma adiponectin levels observed in obesity. In contrast, secretion from the subcutaneous adipocytes was unrelated to body mass index, which may explain the positive correlation of adiponectin levels with SAT.
The current study also highlights the role of gender in determining adiponectin levels. Female subjects not only had higher adiponectin levels, they also showed a more robust relationship to SAT. In addition, gender was one of best predictors of adiponectin levels in multiple regression analyses. Adiponectin has been shown to negatively correlate with testosterone levels in both males and females(34, 35). Testosterone therapy in aging men has been reported to decrease SAT and adiponectin levels(36, 37). It is possible that whatever protective effect might be offered by adiposity distribution is obtunded by high levels of androgens in males. It is unclear whether testosterone exerts it effects by changing adiposity distribution or via direct effects on adiponectin secretion(38-41).
Since adipose tissue depots differ in the strength of their association with the adverse metabolic consequences of obesity, and the secretion of adiponectin seems to differ based on the location of the adipose tissue, it is plausible that adiponectin levels can partly explain the relative metabolic protection offered by SAT. Accordingly, Doumatey et al. showed that metabolically healthy obese African Americans had higher adiponectin levels compared to their unhealthy counterparts. Our study sample size was inadequate to perform this evaluation.
Our study has several important limitations. The cross-sectional nature of our study design precludes establishing a causal relationship between SAT area and adiponectin levels. We also acknowledge that we did not control for exercise, menopausal/hormone replacement therapy status of the individuals. In addition, the time from enrollment into TOPS weight loss program was not recorded, hence the lifestyle changes that subjects may have made may have influenced the adiponectin levels, and those were not accounted for. However, the data were collected in a fasting state when subjects were at a stable weight for 6 months. Our study population is entirely Caucasian; hence, the results should be interpreted with caution when considering other ethnicities and races. In addition, it is not known whether the protective effect of peripheral fat distribution can be extrapolated to relatively higher adiponectin levels in these individuals. We were unable to show the connection between the higher subcutaneous adiposity, adiponectin levels, and metabolic protection due to inadequate sample size. Larger studies are necessary to clarify this relationship. Our study focuses on abdominal adiposity distribution, and hence may not be generalizable to total body subcutaneous adipose tissue area. However, WHR, a crude measure of central versus peripheral obesity, was negatively correlated with adiponectin (Table 2), giving credence to our conclusion that higher peripheral adiposity (higher hip circumference) is associated with higher adiponectin levels. In addition, SAT-to-VAT ratios correlated positively with hip circumference (r= 0.20, p <0.0001 respectively) and negatively with WHR (r= −0.425, p <0.0001), suggesting higher abdominal SAT also means higher general peripheral adiposity.
Apart from its association with obesity and metabolic syndrome, lower levels of adiponectin have been associated with atherosclerosis, liver steatosis, and some malignancies(42, 43). Despite a number of studies showing these associations, it is not known how adiponectin exerts its protective effects. Adiponectin activates 2 seven-transmembrane receptors, adiponectin receptor 1 (adipoR1) and adiponectin receptor 2 (adipoR2), and stimulation of either receptor leads to regulation of metabolic effects through activation of metabolic effects through a number of pathways including AMPK and MAPK(44, 45). Knockout (KO) of each receptor resulted in negative effects on metabolism. AdipoR1 KO had specifically increased adiposity and decreased glucose tolerance and adipoR2 KO mice were resistant to diet-induced obesity(46, 47). Adiponectin has been shown to reduce mRNA levels of VCAM-1 and other adhesion molecules, class A scavenger receptor, suppress several of TNF-alpha mediated pro-inflammatory actions, and inhibit transformation of macrophages to foam cells(42, 48-52). Hyperadiponectinemia was shown to exert cardioprotective effects via AMPK- and cyclooxygenase-2 (COX-2) dependent mechanisms(53, 54). Since the molecular targets of adiponectin are not fully known, it is not clear whether adiponectin is a direct regulator or a marker of another parameter that varies with adiposity compartments. It is not known why and at which point VAT production of adiponectin is reduced in obese subjects, while SAT production of adiponectin remains unchanged. We believe that retained adiponectin secretion from SAT leads to higher adiponectin levels in individuals with higher SAT-to-VAT ratio, perhaps resulting better metabolic effects in these individuals. Moreover, recent studies have questioned the previous associations of adiponectin with metabolic abnormalities and cardiovascular disease(55, 56).
In summary, we have shown that higher SAT is, when controlled for VAT and total adiposity, associated with increased adiponectin levels. This higher SAT-to-VAT ratio may mediate relative protection from type 2 diabetes mellitus and cardiovascular disease experienced by some obese individuals. Larger prospective studies with clearly defined metabolic parameters are necessary to further explore the protective effects of SAT and adiponectin.
Background.
Thirty percent of obese individuals are metabolically healthy. Studies have suggested that these obese individuals have predominantly peripheral obesity. Adiponectin, an adipokine, has insulin-sensitizing and anti-inflammatory properties. Adiponectin levels are lower in obese individuals with central adiposity. However, obese individuals who are metabolically healthy have been shown to have paradoxically higher adiponectin levels.
Translational significance.
In this study, we show that higher subcutaneous adipose tissue volume (as seen in individuals with peripheral obesity) may be associated with higher plasma adiponectin levels. This suggests higher subcutaneous adipose tissue volume may be protective by translating into higher circulating adiponectin levels.
Acknowledgements
Dr. Ahmed Kissebah, the principal investigator behind the body of this work, was fully involved in all aspects of the TOPS Family Study up to his death on 17 May 2012. We are saddened by his loss and deeply respectful of his long and distinguished career in obesity research. Dr. Srividya Kidambi, is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Acknowledgement also includes the contribution of the staff and local membership of TOPS Club, Inc. in the recruitment and ascertainment process and finally to all the members and their families who volunteered for this study.
Funding:
This work was supported by grants from the NIH (DK071895-03 and DK65598-01) for Dr. Kissebah and by TOPS (Take Off Pounds Sensibly) Club, Inc.
List of abbreviations
- TOPS
take off pounds sensibly
- HOMA
homeostasis model assessment
- IR
insulin resistance
- TNF-α
tumor necrosis factor –alpha
- IL
interleukin
- DXA
dual-emission X-ray Absorptiometry
- VAT
visceral adipose tissue
- SAT
subcutaneous adipose tissue
- CT
computerized tomography
- SD
standard deviation
- HDL-C
high-density lipoprotein-cholesterol
- LDL-C
low-density lipoprotein-cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
-
1)All authors have no financial or personal relationship with organizations that could potentially be perceived as influencing the described research
-
2)All authors have read the journal's policy on disclosure of potential conflicts of interest.
-
3)All authors have read the journal's authorship agreement.
References
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 3.Lebovitz HE. The relationship of obesity to the metabolic syndrome. Int J Clin Pract Suppl. 2003:18–27. [PubMed] [Google Scholar]
- 4.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–32. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 5.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–26. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 6.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. American Journal of Clinical Nutrition. 2010;91:258S–61S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okauchi Y, Kishida K, Funahashi T, et al. Changes in serum adiponectin concentrations correlate with changes in BMI, waist circumference, and estimated visceral fat area in middle-aged general population. Diabetes care. 2009;32:e122. doi: 10.2337/dc09-1130. [DOI] [PubMed] [Google Scholar]
- 8.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 9.Cook JR, Semple RK. Hypoadiponectinemia--cause or consequence of human “insulin resistance”? Journal of Clinical Endocrinology & Metabolism. 2010;95:1544–54. doi: 10.1210/jc.2009-2286. [DOI] [PubMed] [Google Scholar]
- 10.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 11.Turer AT, Khera A, Ayers CR, et al. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–24. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doumatey AP, Bentley AR, Zhou J, Huang H, Adeyemo A, Rotimi CN. Paradoxical Hyperadiponectinemia is Associated With the Metabolically Healthy Obese (MHO) Phenotype in African Americans. J Endocrinol Metab. 2012;2:51–65. doi: 10.4021/jem95W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 14.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 15.Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14478–83. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Comuzzie AG, Tejero ME, Funahashi T, et al. The genes influencing adiponectin levels also influence risk factors for metabolic syndrome and type 2 diabetes. Hum Biol. 2007;79:191–200. doi: 10.1353/hub.2007.0029. [DOI] [PubMed] [Google Scholar]
- 18.dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. Journal of reproductive immunology. 2005;66:175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svendsen OL, Haarbo J, Heitmann BL, Gotfredsen A, Christiansen C. Measurement of body fat in elderly subjects by dual-energy x-ray absorptiometry, bioelectrical impedance, and anthropometry. American Journal of Clinical Nutrition. 1991;53:1117–23. doi: 10.1093/ajcn/53.5.1117. [DOI] [PubMed] [Google Scholar]
- 20.Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of body fat topography to insulin sensitivity and metabolic profiles in premenopausal women. Metabolism: Clinical & Experimental. 1984;33:68–75. doi: 10.1016/0026-0495(84)90164-1. [DOI] [PubMed] [Google Scholar]
- 21.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–7. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 22.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 23.Nagy PG WC. Knowledge-based segmentation of abdominal CT images using actlve contour deformable models. Med Phys. 1996:6. [Google Scholar]
- 24.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 25.Usui C, Asaka M, Kawano H, et al. Visceral fat is a strong predictor of insulin resistance regardless of cardiorespiratory fitness in non-diabetic people. J Nutr Sci Vitaminol (Tokyo) 2010;56:109–16. doi: 10.3177/jnsv.56.109. [DOI] [PubMed] [Google Scholar]
- 26.Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res. 1997;5:93–9. doi: 10.1002/j.1550-8528.1997.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 27.Snijder MB, Dekker JM, Visser M, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–7. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 28.Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–31. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–60. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 31.Hara K, Horikoshi M, Yamauchi T, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes care. 2006;29:1357–62. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 32.Zhu N, Pankow JS, Ballantyne CM, et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab. 2010;95:5097–104. doi: 10.1210/jc.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motoshima H, Wu X, Sinha MK, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–7. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 34.Matsui S, Yasui T, Tani A, et al. Association of circulating adiponectin with testosterone in women during the menopausal transition. Maturitas. 2012;73:255–60. doi: 10.1016/j.maturitas.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Cui J, Wu X, Andrel J, Falkner B. Relationships of Total Adiponectin and Molecular Weight Fractions of Adiponectin With Free Testosterone in African Men and Premenopausal Women. The Journal of Clinical Hypertension. 2010;12:957–63. doi: 10.1111/j.1751-7176.2010.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frederiksen L, Hojlund K, Hougaard DM, et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. European Journal of Endocrinology. 2012;166:469–76. doi: 10.1530/EJE-11-0565. [DOI] [PubMed] [Google Scholar]
- 37.Page ST, Herbst KL, Amory JK, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- 38.Seftel AD. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Urol. 2005;174:1045–6. [PubMed] [Google Scholar]
- 39.Xu A, Chan KW, Hoo RLC, et al. Testosterone Selectively Reduces the High Molecular Weight Form of Adiponectin by Inhibiting Its Secretion from Adipocytes. Journal of Biological Chemistry. 2005;280:18073–80. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 40.Woo JG, Dolan LM, Daniels SR, Goodman E, Martin LJ. Adolescent sex differences in adiponectin are conditional on pubertal development and adiposity. Obes Res. 2005;13:2095–101. doi: 10.1038/oby.2005.260. [DOI] [PubMed] [Google Scholar]
- 41.De Maddalena C, Vodo S, Petroni A, Aloisi AM. Impact of testosterone on body fat composition. Journal of Cellular Physiology. 2012;227:3744–8. doi: 10.1002/jcp.24096. [DOI] [PubMed] [Google Scholar]
- 42.Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis. 2013;227:216–21. doi: 10.1016/j.atherosclerosis.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 43.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–69. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 44.Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19:1926–32. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 46.Bjursell M, Ahnmark A, Bohlooly YM, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–93. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–70. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto Y, Arita Y, Nishida M, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 50.Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–8. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi M, Shibata R, Takahashi H, et al. Association of adiponectin with carotid arteriosclerosis in predialysis chronic kidney disease. Am J Nephrol. 2011;34:249–55. doi: 10.1159/000330178. [DOI] [PubMed] [Google Scholar]
- 52.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 53.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemiareperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao G, Li W, Guo R, et al. Serum total adiponectin level and the risk of cardiovascular disease in general population: a meta-analysis of 17 prospective studies. Atherosclerosis. 2013;228:29–35. doi: 10.1016/j.atherosclerosis.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Yaghootkar H, Lamina C, Scott RA, et al. Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes. 2013;62:3589–98. doi: 10.2337/db13-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]