Abstract
Background
Increased arterial stiffness predicts morbidity and mortality, independently of other cardiovascular risk factors, and glycemic control impairments are related to higher vascular stiffness. The aim of this study was to evaluate the association between HbA1c levels and increased arterial stiffness in a Brazilian rural population.
Methods
For this study were selected 1675 individuals (both genders and aged over 18 years) resident in the municipality of Baependi, a city located in the Southeast of Brazil. HbA1c levels were determined by HPLC. Pulse wave velocity (PWV) was measured with a non-invasive automatic device (Complior).
Results
HbA1c levels were associated with an increased PWV. This was more relevant for the third tertile of age. In addition, logistic regression multivariate model including age, blood pressure, gender, BMI and fasting glucose showed that the elevation of a single unit percentage of HbA1c represented an increase of 54 % in the odds of increased arterial stiffness [OR 1.54 (95 % CI 1.01–2.17)]. Both, HbA1c and fasting glucose showed higher discriminatory power in the risk assessment for increased arterial stiffness in the non-diabetic when compared to the diabetic group (AUC of HbA1c = 0.71 vs 0.57, p = 0.02; AUC of fasting glucose = 0.66 vs 0.45, p = 0.0007, respectively).
Conclusion
Our findings indicate that a increase in HbA1c levels is associated with increased arterial stiffness and that both, HbA1c and fasting glucose, presented higher discriminatory power in the risk assessment for increased arterial stiffness in the non-diabetic group as compared to diabetic individuals.
Keywords: HbA1c, Arterial stiffness, Glycemic control
Background
Increased central arterial stiffness is an important determinant of cardiovascular disease (CVD) risk [1]. Epidemiological studies reported that increased arterial stiffness predicts morbidity and mortality, independently of other cardiovascular risk factors [1, 2]. Arterial stiffness measured through carotid-femoral pulse wave velocity (PWV)—a gold standard method—has been associated with measures of subclinical CVD [3]. Moreover, several clinical studies have shown that the arterial stiffness is strongly associated with age, blood pressure [4] and various pathological processes such as hypertension, metabolic syndrome, chronic renal disease, and diabetes [5–8].
Glycated hemoglobin (HbA1c) is a marker of glycemic control that reflects the average blood glucose level over a period of 2–3 months. In addition, the American Diabetes Association published clinical guidelines in which HbA1c level was recommended as a diagnostic test for diabetes [9]. The association between HbA1c levels and coronary heart disease risk has been demonstrated previously [10] and more recently, some studies have shown the association between HbA1c levels and arterial stiffness, measured by PWV, in individuals with and without diabetes [11, 12] and in hemodialysis [13]. However, studies demonstrating the impact of HbA1c levels in the risk of increased arterial stiffness in the general population are sparse.
Based on this scenario and on the clinical applicability of measurements of HbA1c and arterial stiffness, the aim of this study was to evaluate the association between HbA1c levels and increased arterial stiffness in a Brazilian rural population.
Methods
Study population
The Baependi Heart Study is a genetic epidemiological study of cardiovascular disease risk factors, with a longitudinal design whose methodology has been previously described [14]. For this study we carried out a cross-sectional analysis of data collected in the second visit of the protocol (between 2010 and 2013). In this study we selected 1675 individuals (both genders and aged 18–102 years) distributed in 109 families resident in the municipality of Baependi, a city located in the Southeast of Brazil. The study protocol was approved by the ethics committee of the Hospital das Clínicas, University of São Paulo, Brazil, and each subject provided informed written consent before participation.
Anthropometrical Investigations
Anthropometric parameters were measured according to a standard protocol [14]. Height was measured in centimeters and weight in kilograms using a calibrated digital balance. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2).
Blood pressure measurements
Blood pressure was measured using a standard digital sphygmomanometer (OMRON, Brazil) on the left arm after 5 min rest, in the sitting position. Systolic (SBP) and diastolic blood pressures (DBP) were calculated from three readings (mean value of all measurements), with a minimal interval of 3 min [14]. Pulse pressure (PP) was calculated as difference between SBP and DBP. The mean blood pressure (MBP) was calculated as DBP plus one-third PP. Hypertension was defined as mean SBP ≥140 mmHg and/or DBP ≥90 mmHg and/or antihypertensive drug use [15].
Glucose and HbA1c measurements
Fasting glucose were evaluated by standard techniques in 12-h fasting blood samples [16]. HbA1c levels were determined by high-performance liquid chromatography (HPLC) (National Glycohemoglobin Standardization Program, USA). Diabetes mellitus was diagnosed by the presence of fasting glucose ≥126 mg/dL, HbA1c ≥6.5 %, or antidiabetic drug use.
PWV determination
Carotid–femoral PWV was measured noninvasively by an experienced observer using the Complior SP® (Artech Medical, Pantin, France). PWV was measurement after each participant had rested for 10–15 min in a supine position. One measurement was made for each participant using as quality control values of tolerance inferior to 5 %. The carotid artery and femoral artery pressure waveforms were recorded by simultaneous assessment of the pulse waves in the right common carotid and femoral arteries as described [17]. The PWV values were obtained based on the direct carotid–femoral distance, the values were then standardized to the ‘real’ carotid–femoral distance by multiplying by 0.8 according to recent recommendations [18]. Because this correction, increased arterial stiffness was defined as PWV ≥10 m/s [18].
Statistical analysis
Categorical variables were presented as percentage, while continuous variables were presented as mean ± SD. Since there is a well established cutoff for PWV in the literature, we carried out univariate and multivariate logistic regression analysis to determinate the association between HbA1c and increased arterial stiffness, setting PWV >10 m/s as dependent variable, and age, MBP, gender, BMI and glucose fasting as predictor variables. Test for linear trend (Jonckheere–Terpstra test) was performed by assigning median value for each tertile and treated as continuous variables. The receiver operating characteristic (ROC) curve was built and the area under ROC curve (AUC) was used to measure the discriminatory power in the risk assessment for increased arterial stiffness. Areas under the ROC curves between the markers were compared using a parametric method, with GraphROC for Windows software [19]. Pearson correlation coefficients were used to estimate associations between PWV and their confusion variables. Statistical analyses were carried out using SPSS (version 20) software (Chicago, IL, USA), with the level of significance set at 5 %.
Results
Demographic data of the study sample
Of the 1675 individuals, 151 (9.0 %) had increased arterial stiffness. The distribution of gender between normal (PWV <10 m/s) and increased (PWV ≥10 m/s) arterial stiffness groups was similar. Hypertension and diabetes frequencies were higher in increased arterial stiffness individuals (Table 1). In addition, demographic and clinical data such as age, BMI, SBP, DBP, MBP, PP, PWV, glucose and HbA1c also were higher in increased arterial stiffness individuals (Table 1).
Table 1.
Characteristics of subjects stratified by increased arterial stiffness
| Characteristics | PWV <10 m/s | PWV ≥10 m/s | p value |
|---|---|---|---|
| n | 1524 | 151 | ***** |
| Age (years) | 42.4 ± 15.3 | 67.4 ± 10.0 | <0.001 |
| Gender, male (%) | 40.1 | 49.7 | 0.06 |
| Diabetes (%) | 5.0 | 30.5 | <0.001 |
| Hypertension (%) | 32.9 | 84.1 | <0.001 |
| BMI (kg/m2) | 25.7 ± 4.8 | 29.2 ± 29.1 | <0.001 |
| SBP (mmHg) | 123.7 ± 16.7 | 142.9 ± 17.2 | <0.001 |
| DBP (mmHg) | 75.8 ± 10.1 | 78.3 ± 10.5 | 0.005 |
| PP (mmHg) | 47.9 ± 16.3 | 64.8 ± 14.6 | <0.001 |
| MBP (mmHg) | 91.4 ± 10.3 | 101.7 ± 12.4 | <0.001 |
| PWV (m/s) | 7.4 ± 1.1 | 11.7 ± 1.6 | <0.001 |
| Fasting glucose (mg/dL) | 91.1 ± 17.5 | 103.5 ± 26.1 | <0.001 |
| HbA1c (%) | 5.6 ± 0.7 | 6.3 ± 1.1 | <0.001 |
Hypertension: systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or use of anti-hypertension drugs
Diabetes: fasting glucose ≥126 mg/dL and/or use of hypoglycemic drugs
Increased arterial stiffness: pulse wave velocity (PWV) ≥10 m/s
Continuous data are expressed as mean ± standard deviation
Categorical data are expressed as percentage
BMI body mass index, PWV pulse wave velocity, SBP systolic blood pressure, DBP diastolic blood pressure, MBP mean blood pressure, PP pulse pressure, HbA1c glycated hemoglobin
Correlations between PWV and covariables
Pearson correlation coefficients are summarized in Table 2. Age and MBP were the variables that showed the highest correlations with PWV (r = 0.68 and r = 0.46, respectively). In addition, among the variables associated with glycemic control, HbA1c showed a higher correlation with PWV when compared to fasting glucose (r = 0.34 vs r = 0.31, respectively).
Table 2.
Correlation matrix between age, BMI, fasting glucose, MBP, HbA1c and PWV
| Variables | Age | BMI | Fasting glucose | MBP | HbA1c | PWV |
|---|---|---|---|---|---|---|
| Age | 1 | 0.19* | 0.34* | 0.33* | 0.44* | 0.68* |
| BMI | 0.19* | 1 | 0.24* | 0.23* | 0.17* | 0.08* |
| Fasting glucose | 0.34* | 0.24* | 1 | 0.15* | 0.69* | 0.31* |
| MBP | 0.33* | 0.23* | 0.15* | 1 | 0.16* | 0.46* |
| HbA1c | 0.44* | 0.17* | 0.69* | 0.16* | 1 | 0.35* |
| PWV | 0.68* | 0.08* | 0.31* | 0.46* | 0.35* | 1 |
The level of significance was * p ≤ 0.001
PWV pulse wave velocity, BMI body mass index, MBP mean blood pressure, HbA1c glycated hemoglobin
Test for linear trend and association between HbA1c and increased arterial stiffness
HbA1c levels were associated with an increasing trend of PWV in a dose-dependent fashion. This was more pronounced in the third tertile of age (p for trend = 0.02) (Fig. 1). In this analysis, the difference in mean PWV values between the highest and the lowest tertiles of age was 2.44 m/s.
Fig. 1.
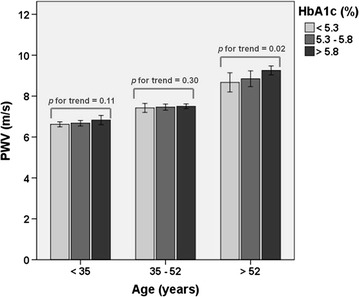
Test for linear trend stratified by age tertile—HbA1c levels were associated with an increasing trend of PWV in a dose-dependent fashion (p for trend <0.05)
The univariate logistic regression analysis showed that the elevation of a single unit percentage of HbA1c represented an increase of 119 % in the odds of increased arterial stiffness [OR 2.19 (95 % CI 1.81–2.65)]. In the multivariate model including age, MBP, gender, BMI and fasting glucose, the elevation of a single unit percentage of HbA1c represented an increase of 54 % in the odds of increased arterial stiffness [OR 1.54 (95 % CI 1.01–2.17)] (Table 3).
Table 3.
Association between HbA1c and increased arterial stiffness by logistic regression univariate and multivariate analysis in a Brazilian rural population
| Variable | Increased arterial stiffness | |
|---|---|---|
| OR (95 % CI), p value | ||
| Univariate | Multivariate | |
| HbA1c | 2.19 (1.81–2.65), <0.001 | 1.54 (1.01–2.17), 0.01 |
| Age | 1.13 (1.11–1.15), <0.001 | 1.14 (1.11–1.16), <0.001 |
| MBP | 1.08 (1.07–1.10), <0.001 | 1.07 (1.05–1.09), <0.001 |
| BMI | 1.02 (1.01–11.04), 0.04 | 1.01 (0.99–1.04), 0.28 |
| Fasting glucose | 1.02 (1.02–1.03), <0.001 | 0.99 (0.98–1.01), 0.54 |
| Gender (female)a | 1.47 (1.05–2.05), 0.03 | 1.01 (0.63–1.63), 0.95 |
Increased arterial stiffness: pulse wave velocity (PWV) ≥10 m/s
Multivariate model: HbA1c, age, MBP, BMI, fasting glucose and gender
aFemale group as reference
Area under ROC curve of HbA1c and fasting glucose in the diabetic and non-diabetic group
In both, diabetic and non-diabetic groups, the discriminatory powers of HbA1c and fasting glucose in the risk assessment for increased arterial stiffness were not different (AUC = 0.71 vs 0.66, p = 0.29; AUC = 0.57 vs 0.45, p = 0.13, respectively). However, the AUC of HbA1c and AUC of fasting glucose were higher in the non-diabetic when compared to the diabetic group (AUC of HbA1c = 0.71 vs 0.57, p = 0.02; AUC of fasting glucose = 0.66 vs 0.45, p = 0.0007, respectively) (Fig. 2).
Fig. 2.
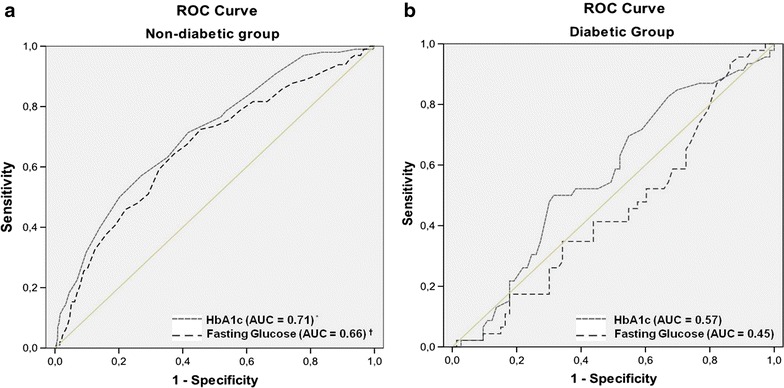
Area under the ROC curve (AUC)—a (non-diabetic group) and b (diabetic group). Comparison of discriminatory powers of HbA1c and fasting glucose in the risk assessment for increased between diabetic and non-diabetic group. *AUC of HbA1c in the non-diabetic vs the diabetic group (p = 0.02). †AUC of fasting glucose in the non- diabetic vs the diabetic group (p = 0.0007)
Discussion
The main finding of our study was the association between HbA1c with arterial stiffness in a Brazilian rural population in which the elevation of a single unit percentage of HbA1c represented an increase of 54 % in the odds of increased arterial stiffness even after adjusting for confounding variables such as age, MBP, BMI, gender and fasting glucose. In addition, the HbA1c presented higher discriminatory power in the risk assessment for increased arterial stiffness in the non-diabetic group.
Several studies have shown that HbA1c predicts cardiovascular risk in people with [20] and without diabetes [12]. Regarding the association of HbA1c with arterial stiffness, Teoh et al. [21], studying 860 individuals from the Edinburgh Type 2 Diabetes Study, showed that PWV was associated with increasing HbA1c, only in the group with mean HbA1c at least 7.6 %. Matsumae et al. [13], studying 242 hemodialysis patients with and without diabetes, showed that HbA1c level was an independent determinant of arterial stiffness, measured by carotid-femoral PWV, in both group. Corroborating such results, Liang et al. [12], studying 5098 Chinese individuals without diabetes, showed that HbA1c levels were significantly associated with an increasing trend of carotid-femoral PWV in a dose-dependent fashion. In our study, HbA1c also was associated to carotid-femoral PWV in which the elevation of a single unit percentage of HbA1c represented an increase 54 % in the odds of increased arterial stiffness even after adjusting for confounding variables such as age, MBP, BMI, gender and fasting glucose. In addition, our study also showed that HbA1c levels were associated with an increasing trend of PWV in a dose-dependent manner in individuals over 52 years (third tertile of age). However, unlike the studies presented above, our sample is composed of individuals from the general population with a more heterogeneous composition of clinical conditions (healthy, diabetic, hypertensive, obese, etc.).
The use of HbA1c as glycemic control and cardiovascular risk marker is widely accepted in diabetic patients [20] and its clinical application in individuals without diabetes has never had great relevance. However, several studies have demonstrated the role of HbA1c in the cardiovascular risk assessment of non-diabetic individuals [10, 12], which extends discussion about the use of HbA1c as a cardiovascular risk marker in individuals from the general population. In our study, HbA1c showed higher nominally discriminatory power in the risk assessment for increased arterial stiffness in the non-diabetic when compared to the diabetic group (AUC of HbA1c in the non-diabetic higher than diabetic group). These data demonstrated that HbA1c can be an important determinant in the risk assessment for increased arterial stiffness in non-diabetics individuals.
The association between excessive glucose exposure, assessed by HbA1c, and arterial stiffness may be explained by several mechanisms such as elevated formation of advanced glycation end products (AGEs) [22], which result in endothelial dysfunction due to the increased generation of reactive oxygen species (ROS), reduced bioavailability of nitric oxide (NO) and induction of inflammation [22–24]. All these mechanisms may contribute to development of cardiovascular disease.
Numerous studies have shown the superiority the HbA1c in relation to fasting glucose as a cardiovascular risk predictor variable [12, 24]. In our study, multivariate logistic regression analysis showed that HbA1c was associated to an increased arterial stiffness odds even after adjustment for fasting glucose in the general population. In addition, despite the discriminatory power in the risk assessment for increased arterial stiffness of either indices (HbA1c and fasting glucose) being similar in both groups (diabetics and non-diabetics), the behavior of glucose is different when dealing with non-diabetic as compared to diabetic individuals (Fig. 2).
Our study has two drawbacks. First, it is a cross-sectional analysis. Therefore, a causal relationship between arterial stiffness and HbA1c could not be established. Second, regarding measurement of HbA1c level, some factors such as kidney failure and uremia could influence the analysis [25]. However, as our study is based in a sample from the general population with low mean age, we think that these factors are not enough to modify our results due to the low prevalence of these two conditions in our cohort.
In summary, our findings indicate that the increase of HbA1c levels are associated with increased arterial stiffness and that both, HbA1c and fasting glucose, presented higher discriminatory power in the risk assessment for increased arterial stiffness in the non-diabetic group. Therefore, we suggest that elevation of HbA1c levels, even within the normal range, could trigger vascular dysfunction in individuals of Brazilian rural population.
Authors’ contributions
ROA and CAMJ participated in the design of the study, performed the statistical analysis and drafted the manuscript. RFL, CMO, ARVRH and VACH contributed to acquisition of data and its interpretation. LAB and JEK contributed to conception and design of the study. ACP conceived of the study, participated in its design, coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This research was supported by Grants from São Paulo Research Foundation (FAPESP). This is work was supported by Hospital Samaritano Society (Grant 25000.180.664/2011-35), through Ministry of Health to Support Program Institutional Development of the Unified Health System (SUS-PROADI).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviations
- PWV
pulse wave velocity
- AUC
area under ROC curve
- CVD
cardiovascular disease
- HbA1c
glycated hemoglobin
- BMI
body mass index
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- MBP
mean blood pressure
- HPLC
high-performance liquid chromatography
- ROC
receiver operating characteristic
- AGEs
advanced glycation end products
- ROS
reactive oxygen species
- NO
nitric oxide
- PP
pulse pressure
Contributor Information
Rafael de Oliveira Alvim, Email: alvimfaefid@ig.com.br.
Carlos Alberto Mourao-Junior, Email: camouraojr@gmail.com.
Camila Maciel de Oliveira, Email: camilamacieloliveira@gmail.com.
Rerisson de Faria Lima, Email: rerissonf@hotmail.com.
Andréa Roseli Vançan Russo Horimoto, Email: andreavrh@usp.br.
Valéria Aparecida Costa Hong, Email: hong.valeria@gmail.com.
Luiz Aparecido Bortolotto, Email: luiz.bortolotto@incor.usp.br.
José Eduardo Krieger, Email: krieger@incor.usp.br.
Alexandre Costa Pereira, Phone: (55 11) 2661-5929, Email: alexandre.pereira@incor.usp.br.
References
- 1.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Zoungas S, Asmar RP. Arterial stiffness and cardiovascular outcome. Clin Exp Pharmacol Physiol. 2007;34(7):647–651. doi: 10.1111/j.1440-1681.2007.04654.x. [DOI] [PubMed] [Google Scholar]
- 4.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15(12):1101–1108. doi: 10.1016/S0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 5.De Angelis L, Millasseau SC, Smith A, Viberti G, Jones RH, Ritter JM, et al. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension. 2004;44(1):67–71. doi: 10.1161/01.HYP.0000130482.81883.fd. [DOI] [PubMed] [Google Scholar]
- 6.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987–992. doi: 10.1161/01.CIR.103.7.987. [DOI] [PubMed] [Google Scholar]
- 7.Stewart AD, Jiang B, Millasseau SC, Ritter JM, Chowienczyk PJ. Acute reduction of blood pressure by nitroglycerin does not normalize large artery stiffness in essential hypertension. Hypertension. 2006;48(3):404–410. doi: 10.1161/01.HYP.0000237669.64066.c5. [DOI] [PubMed] [Google Scholar]
- 8.Whincup PH, Gilg JA, Donald AE, Katterhorn M, Oliver C, Cook DG, et al. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112(12):1789–1797. doi: 10.1161/CIRCULATIONAHA.104.532663. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med. 2005;165(16):1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Huang Y, Li X, Xu M, Bi Y, Zhang Y, et al. Association of arterial stiffness with HbA1c in 1,000 type 2 diabetic patients with or without hypertension. Endocrine. 2009;36(2):262–267. doi: 10.1007/s12020-009-9221-z. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Zhou N, Teng F, Zou C, Xue Y, Yang M, et al. Hemoglobin A1c levels and aortic arterial stiffness: the Cardiometabolic risk in Chinese (CRC) study. PLoS One. 2012;7(8):e38485. doi: 10.1371/journal.pone.0038485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumae T, Abe Y, Murakami G, Ueda K, Saito T. Effects of glucose metabolism on aortic pulse wave velocity in hemodialysis patients with and without diabetes. Hypertens Res. 2008;31(7):1365–1372. doi: 10.1291/hypres.31.1365. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira CM, Pereira AC, de Andrade M, Soler JM, Krieger JE. Heritability of cardiovascular risk factors in a Brazilian population: Baependi Heart Study. BMC Med Genet. 2008;9:32. doi: 10.1186/1471-2350-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Pereira AC, Sposito AC, Mota GF, Cunha RS, Herkenhoff FL, Mill JG, et al. Endothelial nitric oxide synthase gene variant modulates the relationship between serum cholesterol levels and blood pressure in the general population: new evidence for a direct effect of lipids in arterial blood pressure. Atherosclerosis. 2006;184(1):193–200. doi: 10.1016/j.atherosclerosis.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26(3):485–490. doi: 10.1161/01.HYP.26.3.485. [DOI] [PubMed] [Google Scholar]
- 18.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 19.Kairisto V, Poola A. Software for illustrative presentation of basic clinical characteristics of laboratory tests–GraphROC for Windows. Scand J Clin Lab Invest Suppl. 1995;222:43–60. doi: 10.3109/00365519509088450. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 21.Teoh WL, Price JF, Williamson RM, Payne RA, Van Look LA, Reynolds RM, et al. Metabolic parameters associated with arterial stiffness in older adults with Type 2 diabetes: the Edinburgh Type 2 diabetes study. J Hypertens. 2013;31(5):1010–1017. doi: 10.1097/HJH.0b013e32835f7ecf. [DOI] [PubMed] [Google Scholar]
- 22.Shirwany NA, Zou MH. Arterial stiffness: a brief review. Acta Pharmacol Sin. 2010;31(10):1267–1276. doi: 10.1038/aps.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, et al. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4(3):228–237. doi: 10.1007/s11883-002-0024-4. [DOI] [PubMed] [Google Scholar]
- 24.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–472. [PubMed] [Google Scholar]


