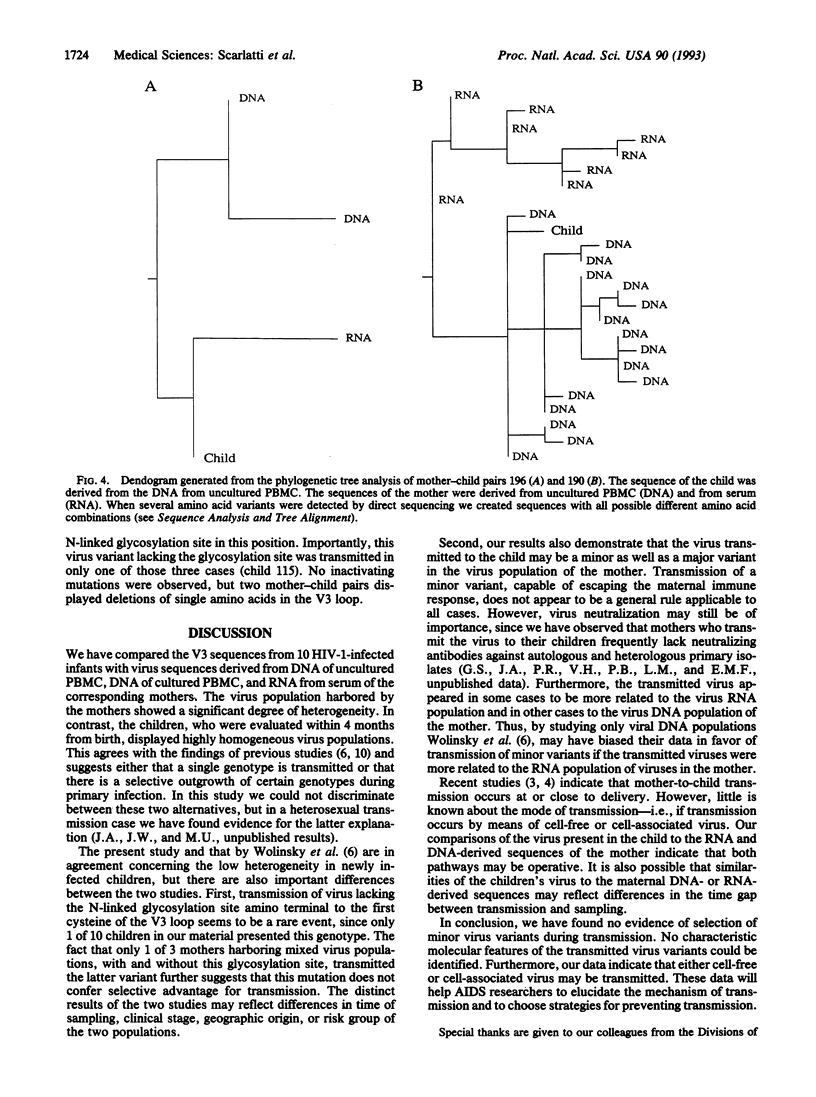
Abstract
We have compared the variable region 3 sequences from 10 human immunodeficiency virus type 1 (HIV-1)-infected infants to virus sequences from the corresponding mothers. The sequences were derived from DNA of uncultured peripheral blood mononuclear cells (PBMC), DNA of cultured PBMC, and RNA from serum collected at or shortly after delivery. The infected infants, in contrast to the mothers, harbored homogeneous virus populations. Comparison of sequences from the children and clones derived from DNA of the corresponding mothers showed that the transmitted virus represented either a minor or a major virus population of the mother. In contrast to an earlier study, we found no evidence of selection of minor virus variants during transmission. Furthermore, the transmitted virus variant did not show any characteristic molecular features. In some cases the transmitted virus was more related to the virus RNA population of the mother and in other cases it was more related to the virus DNA population. This suggests that either cell-free or cell-associated virus may be transmitted. These data will help AIDS researchers to understand the mechanism of transmission and to plan strategies for prevention of transmission.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Fenyö E. M. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 in clinical specimens by polymerase chain reaction with nested primers. J Clin Microbiol. 1990 Jul;28(7):1560–1564. doi: 10.1128/jcm.28.7.1560-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Chiodi F., Keys B., Albert J., Hagberg L., Lundeberg J., Uhlén M., Fenyö E. M., Norkrans G. Human immunodeficiency virus type 1 is present in the cerebrospinal fluid of a majority of infected individuals. J Clin Microbiol. 1992 Jul;30(7):1768–1771. doi: 10.1128/jcm.30.7.1768-1771.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnst A., Lindgren S., Dictor M., Johansson B., Sönnerborg A., Czajkowski J., Sundin G., Bohlin A. B. HIV in pregnant women and their offspring: evidence for late transmission. Lancet. 1991 Jul 27;338(8761):203–207. doi: 10.1016/0140-6736(91)90347-r. [DOI] [PubMed] [Google Scholar]
- Goedert J. J., Duliège A. M., Amos C. I., Felton S., Biggar R. J. High risk of HIV-1 infection for first-born twins. The International Registry of HIV-exposed Twins. Lancet. 1991 Dec 14;338(8781):1471–1475. doi: 10.1016/0140-6736(91)92297-f. [DOI] [PubMed] [Google Scholar]
- Hein J. Unified approach to alignment and phylogenies. Methods Enzymol. 1990;183:626–645. doi: 10.1016/0076-6879(90)83041-7. [DOI] [PubMed] [Google Scholar]
- Hultman T., Murby M., Ståhl S., Hornes E., Uhlén M. Solid phase in vitro mutagenesis using plasmid DNA template. Nucleic Acids Res. 1990 Sep 11;18(17):5107–5112. doi: 10.1093/nar/18.17.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Ryder R. W., Nsa W., Hassig S. E., Behets F., Rayfield M., Ekungola B., Nelson A. M., Mulenda U., Francis H., Mwandagalirwa K. Perinatal transmission of the human immunodeficiency virus type 1 to infants of seropositive women in Zaire. N Engl J Med. 1989 Jun 22;320(25):1637–1642. doi: 10.1056/NEJM198906223202501. [DOI] [PubMed] [Google Scholar]
- Scarlatti G., Lombardi V., Plebani A., Principi N., Vegni C., Ferraris G., Bucceri A., Fenyö E. M., Wigzell H., Rossi P. Polymerase chain reaction, virus isolation and antigen assay in HIV-1-antibody-positive mothers and their children. AIDS. 1991 Oct;5(10):1173–1178. doi: 10.1097/00002030-199110000-00003. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Wahlberg J., Albert J., Lundeberg J., Von Gegerfelt A., Broliden K., Utter G., Fenyö E. M., Uhlén M. Analysis of the V3 loop in neutralization-resistant human immunodeficiency virus type 1 variants by direct solid-phase DNA sequencing. AIDS Res Hum Retroviruses. 1991 Dec;7(12):983–990. doi: 10.1089/aid.1991.7.983. [DOI] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992 Jul;189(1):103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- Wolinsky S. M., Wike C. M., Korber B. T., Hutto C., Parks W. P., Rosenblum L. L., Kunstman K. J., Furtado M. R., Muñoz J. L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992 Feb 28;255(5048):1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]



