Abstract
Background
Increasing evidence supports a neurodevelopmental model for bipolar disorder (BD), with adolescence as a critical period in its development. Developmental abnormalities of anterior paralimbic and heteromodal frontal cortices, key structures in emotional regulation processes and central in BD, are implicated. However, few longitudinal studies have been conducted, limiting understanding of trajectory alterations in BD. In this study, we performed longitudinal neuroimaging of adolescents with and without BD and assessed volume changes over time, including changes in tissue overall and within gray and white matter. Larger decreases over time in anterior cortical volumes in the adolescents with BD were hypothesized. Gray matter decreases and white matter increases are typically observed during adolescence in anterior cortices. It was hypothesized that volume decreases over time in BD would reflect alterations in those processes, showing larger gray matter contraction and decreased white matter expansion.
Methods
Two high-resolution magnetic resonance imaging scans were obtained approximately two-years apart for 35 adolescents with BDI and 37 healthy adolescents. Differences over time between groups were investigated for volume overall and specifically for gray and white matter.
Results
Relative to healthy adolescents, adolescents with BDI showed greater volume contraction over time in a region including insula, and orbitofrontal, rostral and dorsolateral prefrontal cortices (P<.05, corrected), including greater gray matter contraction and decreased white matter expansion over time, in the BD compared to the healthy group.
Conclusions:
The findings support neurodevelopmental abnormalities during adolescence in BDI in anterior cortices, include altered developmental trajectories of anterior gray and white matter.
Keywords: Bipolar disorder, Magnetic resonance imaging, Longitudinal studies, Adolescent, Development, Prefrontal Cortex
Introduction
A unifying neuroanatomical model for the constellation of symptoms of bipolar disorder (BD) has been proposed to include limbic, anterior paralimbic and heteromodal prefrontal structures, including the amygdala, insula, orbitofrontal cortex (OFC), rostral prefrontal cortex (RPFC) and dorsolateral PFC (DLPFC) (1,2). These highly interconnected structures (3), with their additional connection sites such as the hypothalamus, comprise a system that adaptively regulates processes disrupted in BD, including emotions, impulses, appetitive drives and circadian rhythms (3). Increasing evidence supports a neurodevelopmental basis for BD (1,4). As acute BD episodes often emerge during adolescence (5), and the system matures during this time (6,7), altered neurodevelopmental trajectories in this system during adolescence are implicated in the development of the disorder (4).
Cross-sectional neuroimaging evidence supports morphological abnormalities in the amygdala-anterior paralimbic-heteromodal system in adults with BD. Decreased gray matter (GM) volume in adults with BD has been observed in the amygdala, insula and frontal cortices, including ventral prefrontal cortex (VPFC), RPFC and DLPFC (1,8). White matter (WM) abnormalities include decreases in volume and structural integrity in the system connections (9). Postmortem studies are consistent with neuroimaging studies, indicating decreases in amygdala volume and neurons (10) and in frontal neurons, glia and their markers, including oligodendrocytes and myelination markers (11,12).
Cross-sectional studies of children and adolescents with BD have also shown amygdala volume decreases (13–17). In contrast, cortical volume findings in youths are inconsistent, including decreases or no differences (16,18–23). While there are increasing reports of decreased WM integrity in adolescents with BD, as few studies have focused specifically on anterior paralimbic-heteromodal WM integrity, the extent of abnormalities in those regions in adolescents with BD is not clear (24–30).
During adolescence, typical PFC developmental changes include synaptic pruning and subtle GM volume decreases, and myelination and WM volume increases (6,31). If BD pathophysiology involves abnormalities in these processes, GM and WM trajectories may progressively diverge over adolescence. Some inconsistencies in anterior cortical findings in youths with BD could therefore be related to differences in the developmental times at which adolescents were studied (4,18). However, drawing conclusions about neurodevelopmental processes from cross-sectional studies must be done with caution. Longitudinal studies are needed to elucidate system trajectories in BD.
Preliminary longitudinal studies of brain morphology in small samples of adolescents/young adults with BD support altered trajectories in VPFC, RPFC and DLPFC. For example, Kalmar et al. (32) found more volume contraction in adolescents with BD, than healthy control (HC) adolescents, in VPFC and RPFC. Studying youths before and after BD onset, Gogtay et al. (33) found ventral anterior cingulate GM decreases. These studies did not, however, address possible differences in contributions of GM and WM that could help to reveal mechanisms underlying abnormal trajectories in BD. A study by Farrow et al. (34) showed anterior cingulate GM reductions and frontal WM increases in adolescents with BD studied over two-years; however, follow-up data was not available in the HC group to determine whether findings were disease-related. Thus, longitudinal studies of larger adolescent samples with BD, compared to HC adolescents, examining different contributions of local GM and WM changes, are needed.
The differential patterns of changes in frontotemporal GM and WM changes typical of adolescence have been detected in neuroimaging studies that demonstrate frontotemporal GM decreases and WM increases in healthy adolescents (35–40). Examining maturational trajectories for the two types of tissue is critical to disentangle the GM and WM trajectory alterations that contribute to BD. This is important as it could implicate particular GM and/or WM neurodevelopmental pathophysiological processes in the disorder. This could aid understanding of the pathophysiology underlying the development of symptoms. For example, detection of disruption of the maturational development within the GM regions and/or the WM of their projections, implicated in emotion processing and regulation, could help to elucidate the types of neurodevelopmental system abnormalities that contribute to the emergence of complex affective symptoms of BD and how they may change over time during adolescence. Identification of GM and/or WM abnormalities could also, importantly, influence methods for early detection, and targeted treatment and prevention strategies.
This investigation tested the hypothesis that anterior paralimbic and heteromodal cortical volume decreases over time are more pronounced in adolescents with BD, than HC adolescents, by comparing high-resolution structural magnetic resonance imaging (MRI) data in adolescents with and without BD scanned twice over approximately two years. To optimize the use of serial data and registration, images obtained at time 2 were registered pairwise to images at time 1 and local volume expansion and contraction were calculated (32). It was further hypothesized that anterior paralimbic and heteromodal trajectory abnormalities in BD would include GM decreases and WM increases over time resulting from disruptions in the healthy maturation changes that typically occur in these tissue types during adolescence.
Methods and Materials
Subjects
Thirty-five subjects with BDI were scanned at time 1 (T1) (48% female, age range 10–21 years, mean age 16.8±SD2.8 years) and re-scanned at time 2 (T2) (13–23 years, mean 19.1±2.9 years), after a mean interval of 2.4±0.65 years (range 1.42–3.72 years). Thirty-seven HC subjects without DSM-IV Axis I diagnoses, or first-degree relatives with an Axis I diagnosis, were scanned at T1 (54% female, 11–21 years, mean 16.2±2.7) and re-scanned at T2 (13–23 years, mean 18.3±2.9), after a mean interval of 2.1±0.45 years (range 1.51–3.55 years) (Table 1). The presence or absence of psychiatric disorders and mood state on the scanning day were confirmed by structured clinical interview: the Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime Version (41) for subjects ages ≤18 years, with subject and parent/guardian interviewed separately, and Structured Clinical Interview for DSM-IV Axis I Disorders (Version 2.0) (42) for subjects >18 years. Final DSM-IV consensus diagnoses were established by trained and reliable interviewers and a board-certified psychiatrist or mental health clinician with expertise in clinical and research interviews. Family history was assessed by the Family History Screen for Epidemiologic Studies (43). Exclusion criteria included neurological disorders, history of loss of consciousness ≥five minutes or contraindications to MRI scanning. Subjects had no significant medical illness other than treated hypothyroidism in two BD subjects. After explanation of the study, written informed consent was obtained from all subjects ≥18 years. For subjects <18 years, the parents or legal guardians provided written informed consent, and the subjects written assent. The study protocol was performed in accordance with the human investigation committee of the Yale School of Medicine.
Table 1.
Age, Scanning Interval and Gender Distribution of the Healthy Control and Bipolar Disorder Groups
| Healthy controls |
Bipolar disorder |
t value (df) |
P value | |
|---|---|---|---|---|
| Interval Mean [SD] | 2.1[.4] | 2.4[.7] | −1.86(70) | .07 |
| Age at time1 Mean [SD] | 16.2[2.7] | 16.8[2.8] | −.93(70) | .36 |
| Age at time2 Mean [SD] | 18.3[2.9] | 19.1[2.9] | −1.25(70) | .22 |
| §Female N(%) | 20(54) | 17(48) | .22(1) | .64 |
All results are from Independent sample t-tests except for § from Pearson Chi-square
Table 2 provides clinical information for the BD subjects at each time point, including information regarding medication, comorbidities, mood states, rapid-cycling, psychosis and hospitalizations. To improve generalizability, as an estimated 60% of individuals with BD also have a substance use disorder (44), subjects with histories of substance abuse or dependence were included. Subjects were in remission from substance abuse/dependence for >four months, except four BD subjects with current alcohol and/or marijuana abuse/dependence at either T1 (N=1) and/or T2 (N=3). Analyses were also performed excluding subjects with current or lifetime history of substance abuse/dependence.
Table 2.
Clinical Characteristics of the Group with Bipolar Disorder I (N=35)
| First scan | Second scan | |
|---|---|---|
| Medication | ||
| Medication N(%) | 33(94) | 26(74) |
| Antipsychotics N(%) | 21(60) | 18(51) |
| Anticonvulsants N(%) | 17(49) | 10(29) |
| Lithium carbonate N(%) | 8(23) | 6(17) |
| Antidepressants N(%) | 6(17) | 5(14) |
| Psychostimulants N(%) | 6(17) | 9(26) |
| Benzodiazepines N(%) | 4(11) | 4(11) |
| Sympatholytic medications N(%) | 1(3) | 1(3) |
| Hypnotics N(%) | 1(3) | - |
| Lifetime Comorbidities | ||
| Attention deficit hyperactivity disorder* N(%) | 11(31) | 11(31) |
| Conduct disorder* N(%) | 4(11) | 4(11) |
| Oppositional defiant disorder* N(%) | 3(9) | 4(11) |
| Separation anxiety disorder* N(%) | 1(3) | 1(3) |
| Tic disorder* N(%) | 1(3) | 1(3) |
| Simple phobia N(%) | 3(9) | 3(9) |
| Anorexia nervosa N(%) | 1(3) | 1(3) |
| Generalized anxiety disorder N(%) | 1(3) | 1(3) |
| Obsessive compulsive disorder N(%) | 1(3) | 1(3) |
| Panic disorder N(%) | 1(3) | 2(6) |
| Binge eating disorder N(%) | - | 1(3) |
| Post-traumatic stress disorder N(%) | - | 1(3) |
| Substance Use Disorders | ||
| Current substance abuse or dependence: marijuana / alcohol N(%) | 2(6) / 1(3) | 3(9) / 1(3) |
| History substance abuse or dependence: marijuana / alcohol /cocaine / polysubstance N(%) | 5(14) / 1(3) / 2(6) / 1(3) | 6(17) / 1(3) / 2(6) / 1(3) |
| Mood State at Scanning | ||
| Euthymic / elevated (hypomanic + manic + mixed) / depressed N(%) | 22(63) / 7(20) / 6(17) | 31(89) / 3(9) / 1(3) |
| Rapid-Cycling N(%) | 15(43) | 13(37) |
| Psychosis | ||
| Current N(%) | 1(3) | 1(3) |
| Lifetime N(%) | 16(46) | 17(49) |
| Hospitalizations | ||
| N(%) | 22(63) | 24(66) |
| Mean[SD] | 2.0[2.3] | 2.5[2.6] |
These disorders only assessed in subjects ≤18 years of age
MRI Acquisition and Processing
MRI scans at both time points were obtained using the same 3-Tesla Trio MR scanner (Siemens Erlangen, Germany). A three-dimensional magnetization-prepared rapid acquisition gradient echo T1-weighted sequence was used to acquire sagittal images (repetition time = 1500ms, echo time = 2.83ms, field of view = 256 × 256 mm2, matrix 256 × 256, slice thickness = 1.0mm without gap, 160 slices, two averages).
Brain volume changes were measured via the Yale BioImage Suite software package (http://www.bioimagesuite.org) running on a Linux workstation. A non-rigid registration algorithm, based on a free-form deformation model and normalized mutual information (45, 46), was used to compute the deformations required to establish dense correspondence between T1 and T2 scans for each subject. From these within-subject registered transformations, the degree of local expansion or contraction (percent change) was computed with Jacobian maps, based on the determinant of the Jacobian of the displacement field generated by each transformation, which will be referred to from here on as “Jacobian” for short (47). This analysis produced a Jacobian map with a resolution of 1×1×1 mm3 where each voxel had a value representing the local volume change required to map an individual subject’s T1 scan onto his/her follow-up scan: 1=no volume change, >1=T2 scan was larger than T1 scan, and <1=T1 scan was larger than T2 scan. Jacobian maps were checked to confirm transformations were free of singularities (i.e. |J| ≤0). The nonlinear registration algorithm was also applied to transform T1 scans for each subject into standard space by normalizing to the Montreal Neurological Institute (MNI) template. In a separate step, the individual Jacobian maps were warped to the space of the MNI template using the second registration. The Jacobian values were extracted in the region showing group differences in brain volume changes over time for further analyses.
To identify the relative contributions of abnormalities in the trajectories of GM and WM to the region of significant group differences, tissue segmentation into GM and WM was performed for each subject's T1 scan based on the signal intensities corresponding to the two types of matter (FSL; http://www.fmrib.ox.ac.uk/fsl). The region showing group difference in common space was then warped back to each subject’s segmented T1 scan. Finally, the Jacobian values were extracted separately from the GM or WM segmented subregions for further analyses.
Data Analysis
The Jacobian values at each voxel throughout the brain were subjected to two-sample t-tests using BioImage Suite to assess group differences between all HC and all BD subjects. The maps were thresholded at P<.05, two-tailed, controlling for the family-wise error rate (FWE) and clusters >50 contiguous voxels (mm3) using Statistical Parametric Mapping 8 (SPM8) (http://www.fil.ion.ucl.ac.uk/spm). For within group analysis, the mean Jacobian values within the cluster showing significant group differences in volume change over time, extracted from each participant’s normalized Jacobian maps, were subjected to an independent sample t-test for each group separately using SPSS (SPSS Inc, Chicago, IL, USA).
The mean Jacobian values for the GM and for the WM within the cluster showing significant group differences in volume change over time, extracted from each participant’s normalized Jacobian maps, were subjected to between-group analyses for each tissue type separately, using two-sample t-tests in SPSS, thresholded at P<.05, two-tailed. Similar to overall brain parenchyma, within group analyses were conducted in SPSS using the mean Jacobian values within the cluster of the region of difference for GM and WM, separately, extracted from each participant’s normalized Jacobian maps. In addition, the main analyses comparing BD and HC were adjusted for age at study entry using analysis of covariance.
Post hoc analyses were performed in SPSS using the mean signal extracted from each participant’s normalized Jacobian maps for overall difference and separately for GM and WM. Analyses of variance were used to explore potential main effects of age on Jacobian values, across all subjects and within HC or BD groups. Analysis of variance was used to assess for potential effects of gender across all subjects and two-samples t-tests were used to assess for gender effects within groups. Effects of clinical variables were also explored within the BD group at each time point with two-sample t-tests for medication (on/off), medication subclasses present for 5 or more subjects (on/off) (antipsychotics, anticonvulsants, lithium carbonate, antidepressants and psychostimulants), for the presence or absence of comorbidities present for 5 or more subjects including attention deficit hyperactivity disorder (ADHD), and substance abuse or dependence, separately for cannabis abuse/dependence, as well as for acute mood episode type [pairwise comparisons of elevated (hypomanic/manic/mixed), depressed, euthymic], rapid-cycling or lifetime psychosis.
Additional analyses were performed comparing all HC to all BD subjects without current substance use disorders, and all HC to all BD without lifetime substance use disorders.
Results
The BD and HC groups did not differ significantly in gender, age at T1 or T2, or time between scans (Table 1).
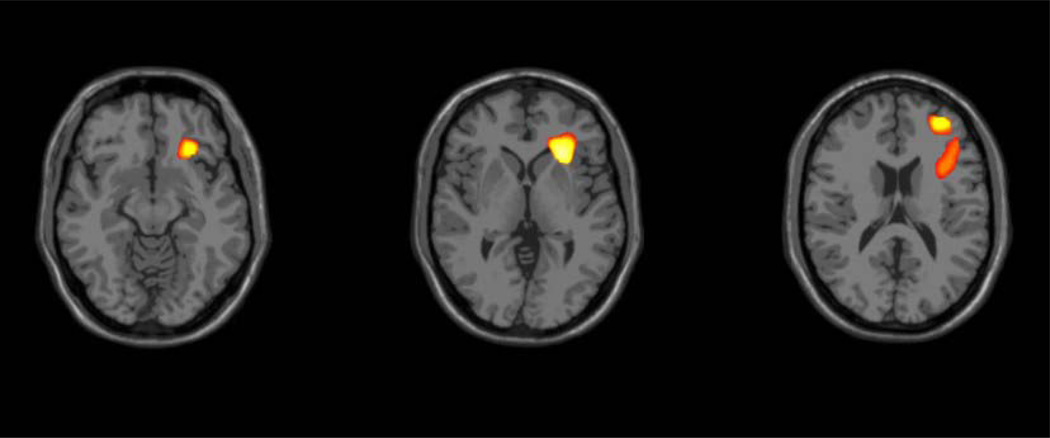
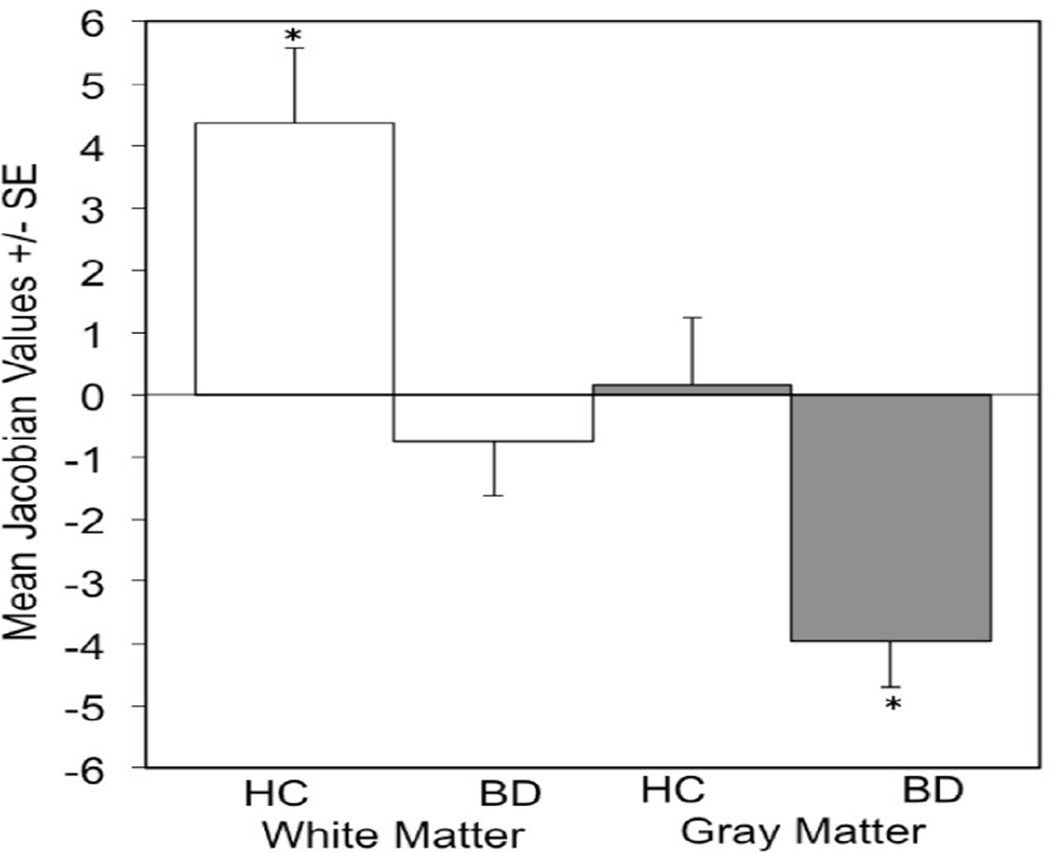
Significant differences in overall brain changes over time were observed between groups in one cluster within the right hemisphere, located in the region of OFC [Brodmann area (BA)47], and extending into the insula, inferior PFC (BA44/45), RPFC (BA10) and DLPFC (BA9) (with a local maxima at x=24mm, y=24mm, z=0mm; t70 = 1.99, P<.05, FWE-corrected) (Figure 1). Within this region, BD subjects showed tissue reduction over time (t34 = −2.97, P<.01) and HC subjects showed tissue enlargement over time (t36 = 2.18, P<.05). Group differences over time were observed in both GM (t70 = 3.07, P<.005) and WM (t70 = 3.42, P<.001) within this cluster. In GM, the BD group showed significant contraction over time (3.95% tissue reduction, t34 = −5.29, P<.001), whereas the HC group did not demonstrate significant change over time. In WM, the HC group showed significant expansion over time (4.36% tissue enlargement, t36 = 3.56, P<.005), whereas the BD group did not demonstrate significant change over time (Figure 2).
Figure 1. Volume Change in Bipolar Disorder Compared to Health During Adolescence.
The images (Montreal Neurological Institute z’s = −12, 0,16 mm) demonstrate the regions of right insula and frontal volume decreases over approximately two years in adolescents with bipolar disorder, compared to healthy adolescents (P<.05 two tailed, family-wise error corrected, cluster >50 voxels).
Figure 2. Gray and White Matter Changes in Bipolar Disorder Compared to Health During Adolescence.
The graphs show the percent changes in gray and white matter in the area of difference over approximately two years between the healthy control and bipolar disorder groups (*P<.005).
Significant group differences remained after controlling for age in overall tissue (p=.002, age covariate p=.19), GM (p=.005, age covariate p=.21) and WM (p=.002, age covariate p=.18). Similar results were found when controlling for inter-scan interval: overall tissue (p=.004, interval covariate p=.49), GM (p=.007, interval covariate p=.49) and WM (p=.002, interval covariate p=.50).
Post hoc analyses of the region showing significant differences, for total volume as well as for GM or WM, did not reveal any significant effects for demographic and clinical variables at T1 or T2. Findings were similar for overall, as well as GM and WM, volume reductions in the additional comparisons between the HC group and the BD group without current substance use disorder, and the HC group and the BD group without lifetime substance use disorder.
Discussion
This study demonstrates differences in longitudinal brain changes between adolescents with and without BD. Over approximately two years, adolescents with BD exhibited more volume decreases within right anterior paralimbic and heteromodal cortices than HC adolescents. In addition, diverging patterns of GM and WM development in the adolescents with BD, compared to HC adolescents, were observed. Specifically, whereas adolescents with BD showed greater GM contraction over time, they showed less WM expansion, compared to healthy adolescents.
The altered trajectory of insular and orbitofrontal cortices early in the course of BD is in line with a recent model of BD implicating a central role for the olfactocentric paralimbic cortices (OPC) during adolescence in development of the disorder (1,20). This is consistent with previous longitudinal studies supporting altered progression in anterior paralimbic regions in BD (32–34). However, other results have also been reported, including increases in parietal and precuneus volumes (48) and no significant differences (49). The discrepancies between these findings with present results may be related to differences in subject characteristics and/or imaging methodologies.
In addition to these GM abnormalities within the OPC, abnormalities in the underlying WM were also observed. Abnormalities included the area of the uncinate fasciculus (UF), a WM tract that courses through OPC and provides major amygdala-OFC connections (50). Abnormalities in the UF have been demonstrated previously in adults with BD (51–60). The observation of progressive WM abnormalities in BD in this study suggests that the disorder is associated with a disruption in the development of such WM connections to frontal cortex that typically occurs during adolescence.
The extension of the findings into more rostral, dorsal and lateral heteromodal frontal regions is consistent with previous cross-sectional and longitudinal studies (1,2,32,34). Similar reductions were not seen in a longitudinal study using a surface area analysis of GM (33). Thus, it is possible that mechanisms that contribute to altered trajectories in GM thickness and WM volume, but not GM surface area, are involved in the development of BD (61).
Group differences over time were not detected in the amygdala, consistent with studies suggesting amygdala abnormalities may already be established by adolescence in BD (4,13–17,62), while abnormalities in anterior cortices and frontotemporal WM connections continue to progress. Progressive differences in amygdala volumes after the first episode of mania in adolescents with BD have been reported (63), suggesting amygdala changes may be some of the first neural changes associated with BD onset. Adolescents in the current study were not all studied at initial onset. Further study of adolescents prior to and after the onset of BD is needed.
Longitudinal combined functional and structural neuroimaging studies in adolescents with BD are needed to elucidate the relationships between the abnormalities in morphological trajectories and functional abnormalities observed in adolescents in anterior paralimbic and heteromodal cortices (64–68). A small cross-sectional functional MRI study, during performance of a prepotent response inhibition task, provided preliminary evidence that HC adolescents exhibit increased rostroventral PFC responses with age, whereas adolescents with BD did not show similar age-related increases (69). This suggested that a pattern of functional abnormalities over time, similar to the structural abnormalities, may be present in BD. There could be differences in clinical presentation in youths and adults. Progressive paralimbic structural abnormalities could be reflected in progressive and/or differing character of impairments in regulating responses to emotional and motivational stimuli. Progressive heteromodal abnormalities could be reflected in progressive and/or differing character of impairments in top-down executive control of behavior, suggested by preliminary findings of changes with age in heteromodal responses to the need to inhibit maladaptive behavioral responses (69), as well as in additional anterior heteromodal processes such as working memory, problem solving and goal-directed behavior. However, this remains speculative given the paucity of longitudinal data in this area. An alternative interpretation of the results of this study is that the observed changes are reflecting compensatory mechanisms, rather than pathology. Longitudinal studies that investigate both brain structure and function, and associated negative and/or positive changes in symptoms and behaviors, are needed.
Findings reported here were lateralized to the right hemisphere. Lesion studies suggest that right frontal lesions are especially associated with manic symptoms (70,71). Consistent with right-lateralized abnormalities, studies have reported reduced right frontal GM volume (72,73) and right UF WM integrity (53,60). PFC functional abnormalities in mania in BD have also tended to be right-sided (74–77). These findings suggest that the right hemisphere abnormalities observed in this study might be related to increased risk for mania. However, as the number of prior manic episodes could not be reliably calculated in this study, especially for subjects with rapid-cycling, it was not possible to discern a relationship between the findings and vulnerability to elevated mood states. Future studies are warranted to clarify this issue.
While this study had a larger sample size compared to previous longitudinal studies in adolescents with BD, the sample size is modest and did not provide ability to definitively assess medication and comorbidity factors. Additionally, subjects were taking varying medications often in combination, and substance and other comorbidity histories also varied. The BD sample only included individuals with BD type I, increasing sample homogeneity but findings may not generalize to other BD subtypes. Pre-adult onset BD is associated with worse course and prognosis (78,79). All of the subjects in this study had onset by the teenage years. It is unclear whether findings will generalize to individuals with later onsets.
We previously suggested that progressive abnormalities in BD may advance from more caudal paralimbic regions to more rostral regions (20,32). While age-related changes were not observed in the present study, the sample included a wide age range and small numbers of subjects at each age. Age-related findings might become more evident with more strictly defined age groups and larger samples. Progressive abnormalities reported here, suggested to be related to the development of BD, could also be a result of disease progression, possibly due to neurotoxic effects associated with episodes. The inclusion of small samples at each age and only two longitudinal time points may have limited ability to model patterns of neurodevelopmental differences which may be differently influenced by different ages; longitudinal studies with larger samples including additional time points are warranted. The majority of subjects were taking psychotropic medications. Previous studies have shown that mood stabilizers such as lithium carbonate are associated with neurotrophic and neuroprotective effects and, in individuals with BD, with increases in GM volume in anterior paralimbic and heteromodal structures (18,80–83). Significant effects of medication were not observed in this study, although power may not have been sufficient to detect differences. Subjects reported varying medication combinations and medications were not studied systematically. Studying medication load would be ideal, however, reliability of reports of specific medications taken and amounts are not clear in BD populations. These factors are almost inevitable when studying individuals with BD, unless for example, studying never medicated individuals systematically over time in a controlled medication trial. Additionally, eleven BD subjects had either current or past substance abuse or dependence, which could have confounded results. However, comparisons of BD subjects with history of substance use disorders to those without did not show significant differences in the region of the main group differences, and analyses performed between HC subjects and BD subjects excluding those with current substance abuse/dependence demonstrated findings similar to those when including all BD subjects. Comorbidities also varied among the BD subjects. Differing effects were not detected for the presence of ADHD, although power was limited given the small number of subjects with it, and there were not sufficient numbers of subjects to examine potential effects of other comorbidities.
In summary, findings support greater GM reduction and diminished WM expansion in anterior paralimbic and heteromodal cortices in adolescents with BDI, compared to healthy adolescents, implicating anterior gray and white matter developmental abnormalities during adolescence in the disorder. Alterations in anterior GM and WM developmental mechanisms, such as neuronal pruning and/or myelination, may have contributed to differences in neural trajectories observed in adolescents with BD. Findings could aid elucidation of the developmental pathophysiology of BD and help inform strategies to halt progression in the disorder.
Acknowledgments
This work was funded by grants R01MH69747 (HB), R01MH070902 (HB), RC1MH088366 (HB), T32 2MH014276 (ETC), and K01MH086621 (FW) from the National Institute of Mental Health, RL1DA024856 from the National Institute of Drug Abuse, CTSA UL1RR0249139 from the NIH National Center for Research Resources (HB, FW). The study was also supported by the National Alliance for Research in Schizophrenia and Depression (HB, FW), American Foundation for Suicide Prevention (HB), International Bipolar Foundation (HB), Klingenstein Foundation (FW), Attias Family Foundation (HB) and Women’s Health Research at Yale (HB). The funding agencies had no role in the design of the study, collection or analysis of the data, or decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Blond BN, Fredericks CA, Blumberg HP. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord. 2012;14:340–355. doi: 10.1111/j.1399-5618.2012.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci. 2004;1021:376–383. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- 5.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 6.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- 8.Selvaraj S, Arnone D, Job D, Stanfield A, Farrow TF, Nugent AC, et al. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14:135–145. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- 9.Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533–554. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 12.Rajkowska G. Depression: what we can learn from postmortem studies. Neuroscientist. 2003;9:273–284. doi: 10.1177/1073858403252773. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 14.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 15.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 16.Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 17.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Kalmar JH, Womer FY, Edmiston EE, Chepenik LG, Chen R, et al. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain. 2011;134:2005–2012. doi: 10.1093/brain/awr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 22.Singh MK, Chang KD, Chen MC, Kelley RG, Garrett A, Mitsunaga MM, et al. Volumetric reductions in the subgenual anterior cingulate cortex in adolescents with bipolar I disorder. Bipolar Disord. 2012;14:585–596. doi: 10.1111/j.1399-5618.2012.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanches M, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Hatch JP, et al. Subgenual prefrontal cortex of child and adolescent bipolar patients: a morphometric magnetic resonance imaging study. Psychiatry Res. 2005;138:43–49. doi: 10.1016/j.pscychresns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163:322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 25.Caetano SC, Silveira CM, Kaur S, Nicoletti M, Hatch JP, Brambilla P, et al. Abnormal corpus callosum myelination in pediatric bipolar patients. J Affect Disord. 2008;108:297–301. doi: 10.1016/j.jad.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry. 2009;66:238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Kafantaris V, Kingsley P, Ardekani B, Saito E, Lencz T, Lim K, et al. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonenc A, Frazier JA, Crowley DJ, Moore CM. Combined diffusion tensor imaging and transverse relaxometry in early-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:1260–1268. doi: 10.1016/j.jaac.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena K, Tamm L, Walley A, Simmons A, Rollins N, Chia J, et al. A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. J Child Adolesc Psychopharmacol. 2012;22:112–119. doi: 10.1089/cap.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao W, Jiao Q, Qi R, Zhong Y, Lu D, Xiao Q, et al. Combined analyses of gray matter voxel-based morphometry and white matter tract-based spatial statistics in pediatric bipolar mania. J Affect Disord. 2013;150:70–76. doi: 10.1016/j.jad.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- 32.Kalmar JH, Wang F, Spencer L, Edmiston E, Lacadie CM, Martin A, et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 34.Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 35.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 36.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 37.Wu M, Lu LH, Lowes A, Yang S, Passarotti AM, Zhou XJ, et al. Development of superficial white matter and its structural interplay with cortical gray matter in children and adolescents. Hum Brain Mapp. 2014;35:2806–2816. doi: 10.1002/hbm.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 39.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 40.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders (SCID, version 2.0) New York: Biometrics Research Department: New York State Psychiatric Institute; 1995. [Google Scholar]
- 43.Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H. Family psychiatric screening instruments for epidemiologic studies: pilot testing and validation. Psychiatry Res. 1995;57:169–180. doi: 10.1016/0165-1781(95)02632-7. [DOI] [PubMed] [Google Scholar]
- 44.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 45.Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Integrated Intensity and Point-Feature Nonrigid Registration. Med Image Comput Comput Assist Interv. 2001;3216:763–770. doi: 10.1901/jaba.2001.3216-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 47.Staib LH, Jackowski M, Papademetris X. Brain shape characterization from deformation. Proc IEEE Int Symp Biomed Imaging. 2006;3:1140–1143. [PMC free article] [PubMed] [Google Scholar]
- 48.Adleman NE, Fromm SJ, Razdan V, Kayser R, Dickstein DP, Brotman MA, et al. Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. J Child Psychol Psychiatry. 2012;53:1149–1156. doi: 10.1111/j.1469-7610.2012.02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arango C, Rapado-Castro M, Reig S, Castro-Fornieles J, Gonzalez-Pinto A, Otero S, et al. Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry. 2012;69:16–26. doi: 10.1001/archgenpsychiatry.2011.150. [DOI] [PubMed] [Google Scholar]
- 50.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 51.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12:1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 52.McIntosh AM, Munoz Maniega S, Lymer GK, McKirdy J, Hall J, Sussmann JE, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 53.Versace A, Almeida JR, Hassel S, Walsh ND, Novelli M, Klein CR, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Munoz Maniega S, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Versace A, Almeida JR, Quevedo K, Thompson WK, Terwilliger RA, Hassel S, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. 2010;68:560–567. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sui J, Pearlson G, Caprihan A, Adali T, Kiehl KA, Liu J, et al. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage. 2011;57:839–855. doi: 10.1016/j.neuroimage.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emsell L, Chaddock C, Forde N, Van Hecke W, Barker GJ, Leemans A, et al. White matter microstructural abnormalities in families multiply affected with bipolar I disorder: a diffusion tensor tractography study. Psychol Med. 2013:1–12. doi: 10.1017/S0033291713002845. [DOI] [PubMed] [Google Scholar]
- 59.Versace A, Andreazza AC, Young LT, Fournier JC, Almeida JR, Stiffler RS, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry. 2014;19:200–208. doi: 10.1038/mp.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linke J, King AV, Poupon C, Hennerici MG, Gass A, Wessa M. Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biol Psychiatry. 2013;74:908–916. doi: 10.1016/j.biopsych.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Chen CH, Fiecas M, Gutierrez ED, Panizzon MS, Eyler LT, Vuoksimaa E, et al. Genetic topography of brain morphology. Proc Natl Acad Sci U S A. 2013;110:17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bitter SM, Mills NP, Adler CM, Strakowski SM, DelBello MP. Progression of amygdala volumetric abnormalities in adolescents after their first manic episode. J Am Acad Child Adolesc Psychiatry. 2011;50:1017–1026. doi: 10.1016/j.jaac.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 65.Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, et al. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1275–1289. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 70.Starkstein SE, Fedoroff P, Berthier ML, Robinson RG. Manic-depressive and pure manic states after brain lesions. Biol Psychiatry. 1991;29:149–158. doi: 10.1016/0006-3223(91)90043-l. [DOI] [PubMed] [Google Scholar]
- 71.Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Arch Neurol. 1982;39:210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- 72.Stanfield AC, Moorhead TW, Job DE, McKirdy J, Sussmann JE, Hall J, et al. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord. 2009;11:135–144. doi: 10.1111/j.1399-5618.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 73.Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, Blumberg HP. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord. 2012;14:432–441. doi: 10.1111/j.1399-5618.2012.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: state-and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 78.Perlis RH, Dennehy EB, Miklowitz DJ, Delbello MP, Ostacher M, Calabrese JR, et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tohen M, Strakowski SM, Zarate C, Jr, Hennen J, Stoll AL, Suppes T, et al. The McLean-Harvard first-episode project: 6-month symptomatic and functional outcome in affective and nonaffective psychosis. Biol Psychiatry. 2000;48:467–476. doi: 10.1016/s0006-3223(00)00915-x. [DOI] [PubMed] [Google Scholar]
- 80.Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore GJ, Cortese BM, Glitz DA, Zajac-Benitez C, Quiroz JA, Uhde TW, et al. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 83.Mitsunaga MM, Garrett A, Howe M, Karchemskiy A, Reiss A, Chang K. Increased subgenual cingulate cortex volume in pediatric bipolar disorder associated with mood stabilizer exposure. J Child Adolesc Psychopharmacol. 2011;21:149–155. doi: 10.1089/cap.2010.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]