Abstract
Background
Eosinophils recognize various stimuli, such as cytokines, chemokines, immunoglobulins, complement, and external pathogens, resulting in their accumulation in mucosal tissues and the progression of inflammation. Eosinophils are also involved in innate Th2-type immune responses mediated through endogenous danger signals, including IL-33, uric acid (UA), or ATP, in non-sensitized mice exposed to environmental allergens. However, the mechanism involved in eosinophil responses to these danger signals remains insufficiently understood.
Methods
We examined migration, adhesion, superoxide production and degranulation of human eosinophils. Isolated eosinophils were incubated with monosodium urate (MSU) crystals and ATPγS, a nonhydrolysable ATP analogue. To determine the involvement of P2 or P2Y2 receptors in eosinophil responses to UA and ATP, eosinophils were preincubated with a pan-P2 receptor inhibitor, oxidized ATP (oATP), or anti-P2Y2 antibody before incubation with MSU crystals or ATPγS.
Results
MSU crystals induced adhesion of eosinophils to recombinant human (rh)-ICAM-1 and induced production of superoxide. oATP abolished eosinophil responses to MSU crystals, suggesting involvement of endogenous ATP and its receptors. Furthermore, exogenous ATP, as ATPγS, induced migration of eosinophils through a model basement membrane, adhesion to rh-ICAM-1, superoxide generation, and degranulation of eosinophil-derived neurotoxin (EDN). oATP and anti-P2Y2 significantly reduced these eosinophil responses.
Conclusions
ATP serves as an essential mediator of functional responses in human eosinophils. Eosinophil responses to ATP may be implicated in airway inflammation in patients with asthma.
Keywords: ATP, Danger signal, Eosinophils, P2Y2 receptor, Uric acids
Introduction
Eosinophils are known as effector cells involved in host protection against parasite infections and in pathological processes involved in allergic diseases.1,2 In response to various stimuli, eosinophils release toxic granule proteins and produce proinflammatory mediators (cytokines and chemokines), which may cause tissue damage, dysfunction,3 and remodeling.4,5 Eosinophils accumulate in inflamed tissue in response to Th2 cytokine derived group 2 innate lymphoid cells and conventional Th2-type CD4+ T cells, and may serve as a pivotal player in asthma.
Recently, eosinophils have been shown to recognize a wide variety of pathogens and environmental allergens through engagement of receptors for pathogen-associated molecular patterns. Likewise, damage-associated molecular patterns (DAMPs), or danger signals, are also involved in eosinophil activation. DAMPs are endogenous factors that are usually stored within cells and can be released into extracellular spaces under conditions of cellular stress or injury.6 For example, IL-33, an IL-1β family cytokine, is a DAMP that can be released quickly from human bronchial epithelial cells in response to several environmental allergens and initiate allergic airway inflammation.7 Other prototypical DAMPs include ATP, K+ ions, uric acid (UA), HMGB-1, and S100 calcium-binding protein family members.8–10 Recent studies show that DAMPs might either directly or indirectly mediate pathophysiological processes in asthma and allergic disorders.7,11–14 UA serves as a potent Th2 cell adjuvant, when administered into airways with an innocuous OVA antigen, by recruiting DCs.11 Upon exposure to inhaled allergens, UA can be released into the airways and lungs of mice.13,14 In addition, administration of UA into the airways of naive animals induces extracellular release of IL-33, leading to both innate and adaptive type 2 immune responses.13 Although these findings suggest that UA might be implicated in the induction of allergic airway inflammation, our knowledge of the immunologic mechanism underlying the effects of UA on type 2 immune responses is limited. Indeed, UA induced cytokine production in human eosinophils,15 suggesting that UA is involved in the initiation of eosinophilic inflammation.
Similar to UA, ATP is also recognized as a DAMP that is implicated in Th2-type innate and adaptive immune responses. Inhaled aeroallergens elicit secretion of ATP in the lungs of naive or OVA-sensitized mice.7,14 ATP also mediates release of IL-33, IL-5, and IL-13 in the lungs of naive mice exposed to aeroallergens and induces pathophysiologic features of asthma.14 Likewise, ATP is involved in the release of IL-33 from airway epithelial cells in response to environmental fungus and the recruitment of DCs.7,14 In addition, ATP mediates recruitment of eosinophils to lung in OVA-sensitized mice, suggesting that ATP promotes a wide variety of eosinophilic responses. Several studies showed that P2-type purinergic receptors are involved in triggering diverse eosinophil functions.16 Nonetheless, the effect of ATP on eosinophils remains poorly understood.
In this study, to clarify the mechanism of cellular accumulation and effector functions of human eosinophils in response to danger signals, we examined the response of eosinophils to monosodium urate (MSU) crystals and ATP. We found that MSU crystals induced chemotaxis and adhesion of eosinophils. Pharmacological agents, a broad P2 receptor antagonist, inhibited eosinophil chemotaxis in response to MSU crystals, suggesting the involvement of ATP endogenous to eosinophils.We also found exogenous ATP promoted diverse eosinophil function, including adhesion to rh-ICAM-1, transbasement membrane migration (TBM), production of superoxide, and degranulation. Inhibition of P2 receptors by a pharmacological agent or anti-P2Y2 reduced eosinophil responses to ATP. These findings suggest that eosinophils recognize extracellular danger signals and produce effector functions. Endogenous and exogenous ATP may play a pivotal role in mediating such eosinophil responses.
Methods
Reagents
Percoll was obtained from Pharmacia (Uppsala, Sweden). Anti-CD16 antibody-coated magnetic beads were purchased from Miltenyl Biotec (Auburn, CA, USA). Hanks' balanced salt solution (HBSS), PBS and fetal calf serum (FCS) were obtained from Life Technologies (Grand Island, NY, USA). Recombinant human IL-5 was obtained from GeneTex (Irvine, CA, USA). RPMI 1640 medium was from Life Technologies. Monosodium urate (MSU) crystals, ATP periodate oxidized sodium salt (oATP), ATP-5′ γ-[thio]tetralithium salt (ATPγS) were from Sigma–Aldrich (St. Louis, MO, USA). The MSU crystals were suspended in the 5% GEL/HBSS at 10 mg/ml and diluted serially. Rabbit polyclonal anti-P2Y2 Ab was from Thermo Fisher Scientific (Santa Cruz, CA, USA). Control rabbit IgG was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Eosinophil isolation
Human eosinophils were isolated from the peripheral blood of 60 healthy volunteers. They did not have history of allergic diseases or have mild hay fever with a peripheral blood differential eosinophil count of <5%. The numbers of males and females were comparable. They ranged in age from 20 to 50 years. Human eosinophils were isolated by negative selection with anti-CD16 microbeads as previously described, with minor modifications.17 This protocol consistently yielded >99% eosinophil purity (mean, 99.2%) as determined according to morphologic criteria using May-Grünwald-Giemsa staining. Eosinophil viability was >99%, as determined by Trypan blue dye exclusion. Eosinophils were resuspended in HBSS supplemented with gelatin to a final concentration of 0.1% (HBSS/gel). The responses of eosinophils from normal individuals and from patients with mild hay fever were quantitatively equivalent; therefore, the data were pooled. The Saitama Medical University Institutional Review Board approved the protocol to obtain blood from volunteers, all of who provided informed consent.
Eosinophil adhesion assay
Eosinophil adhesion to rh-ICAM-1-coated plates was examined by measuring the residual eosinophil peroxidase (EPO) activity of adherent eosinophils, as previously described.18–22 Eosinophils (100 µl of 1 × 105 cells/ml in HBSS/gel) were incubated at 37 °C for 20 min in rh-ICAM-1-coated plates in the presence or absence of the MSU crystal suspensions (0.01–1 mg/ml) or ATPγS (1–100 µM). Eosinophils were pre-incubated with oATP (30 mM) at 4 °C for 15 min before to the assay in selected experiments. After washing the plate with HBSS 5 times, 100 µl of HBSS/gel was then put into the wells. Standards comprised of 100 µl of serially diluted cell suspensions (1 × 103, 3 × 103, 1 × 104, 3 × 104, and 1 × 105 cells/ml) were put into empty wells. Subsequently, the EPO substrate (1 mM o-phenylenediamine, 1 mM H2O2, and 0.1% Triton X-100 in Tris buffer, pH 8.0) was added to all wells and the plates were incubated for 30 min at room temperature. The reaction was stopped by adding 50 µl of 4 M H2SO4 and absorbance was measured at 490 nm. Each experiment was performed in quadruplicate through use of eosinophils from a single volunteer. The percentage of eosinophil adhesion was calculated from log dose response curves and mean values were used. Eosinophil viability after incubation was greater than 98%, as determined by Trypan blue dye exclusion.
Eosinophil trans-basement membrane migration
The TBM of eosinophils was examined using a modified Boyden's chamber method.15,23 A Matrigel®-coated Transwell® insert (pore size 3 µm, Becton Dickinson Labware) was used as the upper chamber, and an ordinary tissue-culture plate well was used as the lower chamber. Eosinophils were suspended in HBSS/gel at 1 × 106 cells/ml. One hundred microliters of the eosinophil suspension were put into the upper chamber and serial dilutions of ATPγS suspension were placed onto the lower chamber. Eotaxin (100 ng/ ml) was used as a positive control. Alternatively, eosinophils suspended with 100 µM ATPγS were put into the upper chamber, and 100 µM ATPγS were placed onto the lower chamber in a checker-board fashion. Eosinophils were pre-incubated with oATP (30 mM) at 4 °C for 15 min before assay in selected experiments. After 2 h of incubation at 37 °C and 5% CO2, the cells that migrated to the lower chamber were collected and counted using light microscopy. The study was conducted in duplicate and results were presented as the percentage of the initial total number of cells that migrated.
Eosinophil superoxide anion generation
Eosinophil superoxide generation was measured in 96-well plates (Becton Dickinson Labware, NY, USA) using a method based on the superoxide dismutase (SOD)-inhibitable reduction of cytochrome C, as previously described.18–20,22 We initially added SOD (0.2 mg/ml in HBSS/gel; 20 µl) to SOD control wells and then HBSS/gel to all wells to obtain the final volume (100 µl). The eosinophil density was adjusted to 1.25 × 106 cells/ml of HBSS/gel mixed 4:1 with cytochrome C (12 mg/ml of HBSS/gel), and 100 µl of eosinophil suspension was then placed onto all wells. Immediately after adding MSU crystal suspensions (0.01–1 mg/ml) or ATPγS (1–100 µM), the absorbance of the cell suspensions in the wells was measured at 550 nm in an Immuno-Mini (NJ-2300; Japan Intermed, Tokyo, Japan). Subsequently, the measurements were repeated over the next 240 min. To determine inhibition of generation induced by ATP, eosinophils were pre-incubated with oATP (30 mM) at 4 °C for 15 min in selected experiments. The plates were incubated in a 5% CO2 incubator at 37 °C between measurements. Each reaction was performed in duplicate against the control reaction in wells containing 20 µg/ml of SOD. The results were adjusted for a 1 ml reaction volume, and generation was calculated at an extinction coefficient of 21.1 mM−1 cm−1, as nanomoles of cytochrome C attenuated per 1.0 × 106 cells/ml minus the SOD control.18,24,25 Each value during the incubation period was examined to evaluate the effects of MSU crystal or ATPγS on eosinophil generation. Cell viability, examined by Trypan blue exclusion at the end of each experiment, remained at 95% after the 240-min incubation period with the activator.
Eosinophil degranulation assay
Eosinophil degranulation was analyzed by quantification of eosinophil-derived neurotoxin (EDN) release into cell-free supernatants.15,26 Briefly, freshly isolated eosinophils were suspended in HBSS with 25 mM HEPES and 0.01% gelatin (240 min culture) at 1 × 106 cells/ml. Cells were placed onto the wells of 96-well tissue culture plates. To examine the role of the P2Y2 nucleotide receptor in eosinophil EDN degranulation in response to ATPγS, eosinophils were pre-incubated with 30 mM rabbit polyclonal anti-P2Y2 Ab or control rabbit IgG for 15 min before the addition of ATPγS. After incubation, cell-free supernatants were collected and stored at −20 °C until EDN was measured by ELISA. The EDN ELISA was performed using ELISA kits (Medical and Biological Laboratory, Nagoya, Japan) as previously described.27 The lowest detection limit of the standard curve was 0.6 ng/ml for this assay. All assays were conducted in duplicate.
Statistical analysis
Results were expressed as mean ± SEM. Statistical analyses were performed using one-way ANOVA (Tukey–Kramer multiple comparisons test), and differences between pairs of groups were analyzed with the paired Student t test. Values of P < 0.05 were considered statistically significant.
Results
Endogenous ATP is involved in eosinophil adhesion and superoxide generation stimulated by MSU crystals
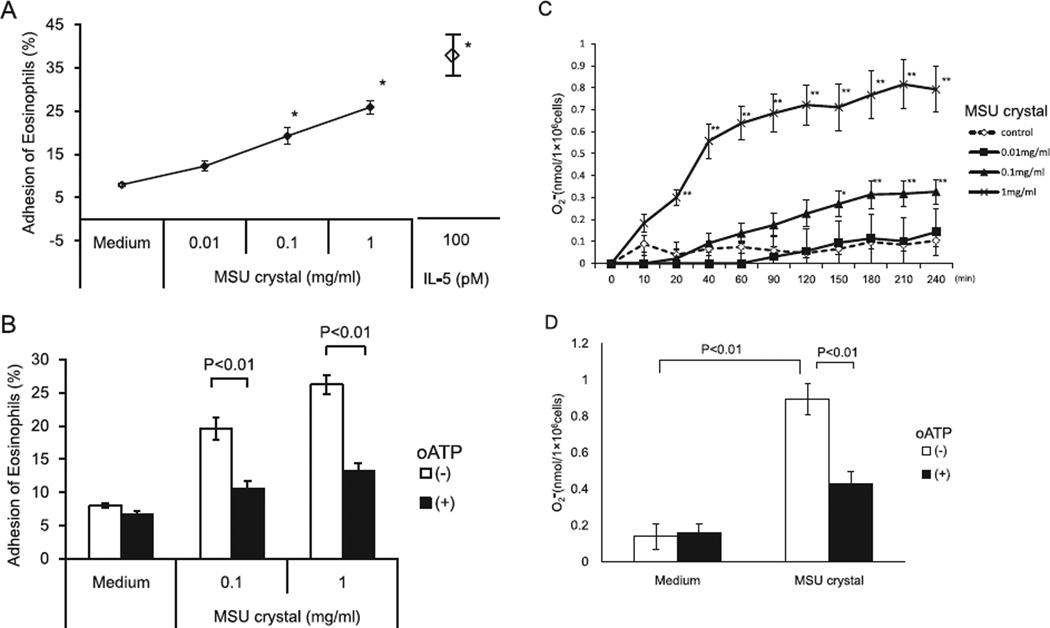
Because UA may be abundant in inflamed tissues,6,13,28 we examined whether MSU crystals induce eosinophil adhesion to rh-ICAM-1 in a model endothelium, and whether MSU crystals promote superoxide generation by eosinophils. IL-5 is a potent activator of human eosinophils29 and was used as a control. MSU crystals induced adhesion of eosinophils to rh-ICAM-1 in a concentration-dependent manner (Fig. 1A). When eosinophils were stimulated with 0.1 and 1 mg/ml MSU crystals, the increase in eosinophil adhesion was significant, as compared with the medium control (4.8 ± 0.4% by medium versus 14.8 ± 1.9% by 0.1 mg/ml MSU crystals, and 20.8 ± 1.6% by 1 mg/ml MSU crystals, P < 0.01, N = 5). Maximum eosinophil adhesion reached about 70% of the IL-5-induced eosinophil adhesion, suggesting that MSU crystals are a potent initiator of eosinophil adhesion. In addition, MSU crystals at >0.1 mg/ml significantly induced superoxide production by eosinophils as compared with the medium control (0.1 ± 0.03 nmol by medium versus 0.3 ± 0.1 nmol by 0.1 mg/ml MSU crystals, P < 0.01; N = 6, at 240 min) (Fig. 1C). Additionally, at higher concentrations (e.g., 1 mg/ml), MSU crystals induced rapid induction of superoxide production (0.3 ± 0.04 nmol at 20 min, 0.6 ± 0.08 nmol at 40 min, 0.7 ± 0.08 nmol at 60 min, 0.07 ± 0.09 nmol at 90 min, and 0.7 ± 0.09 nmol at 120 min, P < 0.01, N = 6), which plateaued at 120 min.
Fig. 1.
Effects of MSU crystals on eosinophil adhesion to rh-ICAM-1 (A, B), production of superoxide anion (C, D) and modification of these function by P2 receptor pharmacological antagonist (B, D). (A) Eosinophils were incubated with or without MSU crystals suspensions (0.01–1 mg/ml) at 37 °C for 20 min in rh-ICAM-1 coated plates. After washing the plates, the EPO substrate and 100 µl of HBSS/FCS were added to all wells, followed by incubation for 30 min at room temperature. The reaction was stopped by 4 M H2SO4 (50 µl) and absorbance at 490 nm was determined. The percentage of eosinophil adhesion was determined from mean values calculated from log dose response curves. IL-5 was used as a positive control. (B) Eosinophils were pre-incubated with or without 30 mM oATP at 4 °C for 15 min and subsequently incubated with or without serial dilution of an MSU crystal suspension under the same condition. The residual EPO activity of adherent eosinophils was measured as in A. (C) A 4:1 mixture of eosinophil suspension and cytochrome C was incubated with or without various concentrations of MSU crystal suspension at 37 °C for up to 240 min. generation was evaluated by measuring absorbance of the cell suspensions at 550 nm every 30 min for up to 240 min, and was calculated at an extinction coefficient of 21.1 mM−1 cm−1 as nanomoles of cytochrome C reduced per 1.0 × 106 cells/ml minus the SOD control. (D) Eosinophils were pre-incubated with oATP (30 mM) or medium at 4 °C for 15 min before assay and then were incubated with or without 1 mg/ml. MSU crystal suspension under the same condition. generation at 240 min was measured as in (C). Results show the mean ± SEM from 5 different eosinophil preparations in (A) and (B), and 6 in (C) and (D). *p < 0.01, **p < 0.05, compared with medium alone. MSU, monosodium urate; EPO, eosinophil peroxidase; oATP, ATP periodate oxidized sodium salt.
We previously reported that autocrine ATP is involved in MSU crystals-induced cytokine production by human eosinophils.15 Hence, we hypothesized that ATP may be involved in MSU crystals-induced eosinophil adhesion and superoxide generation. To test this hypothesis, we examined the effects of oATP, a broad pharmacological inhibitor of P2 receptors. oATP significantly reduced eosinophil adhesion to rh-ICAM-1 in response to MSU crystals (19.6 ± 1.6% by 0.1 mg/ml MSU crystals with medium versus 10.6 ± 1.1% with oATP, P < 0.01; 26.2 ± 1.4% by 1 mg/ml MSU crystals with medium versus 13.3 ± 1.1% with oATP, P < 0.01, N = 5) (Fig. 1B). oATP also significantly attenuated superoxide production by eosinophils stimulated with 1 mg/ml MSU crystals (0.9 ± 0.09 nmol with medium versus 0.4 ± 0.07 nmol with oATP, P < 0.01, N = 6, at 240 min) (Fig. 1D). Up to an approx. 50–60% reduction in eosinophil adhesion and superoxide production was observed with optimal concentrations of oATP.
Exogenous ATP induces adhesion of eosinophils to rh-ICAM-1 and trans-basement membrane migration by engaging P2 purinergic receptors
Involvement of endogenous ATP in eosinophil adhesion and effector function induced by MSU crystals led us to examine the effect of exogenous ATP on eosinophil function. We previously found that exogenous ATP exerts chemoattractant activity for human eosinophils.15 In addition, autocrine ATP promoted neutrophil chemotaxis through P2Y2 and A3 receptors.29 Therefore, we hypothesized that exogenous ATP can induce eosinophil infiltration to tissues and that P2 receptors are involved in this process.
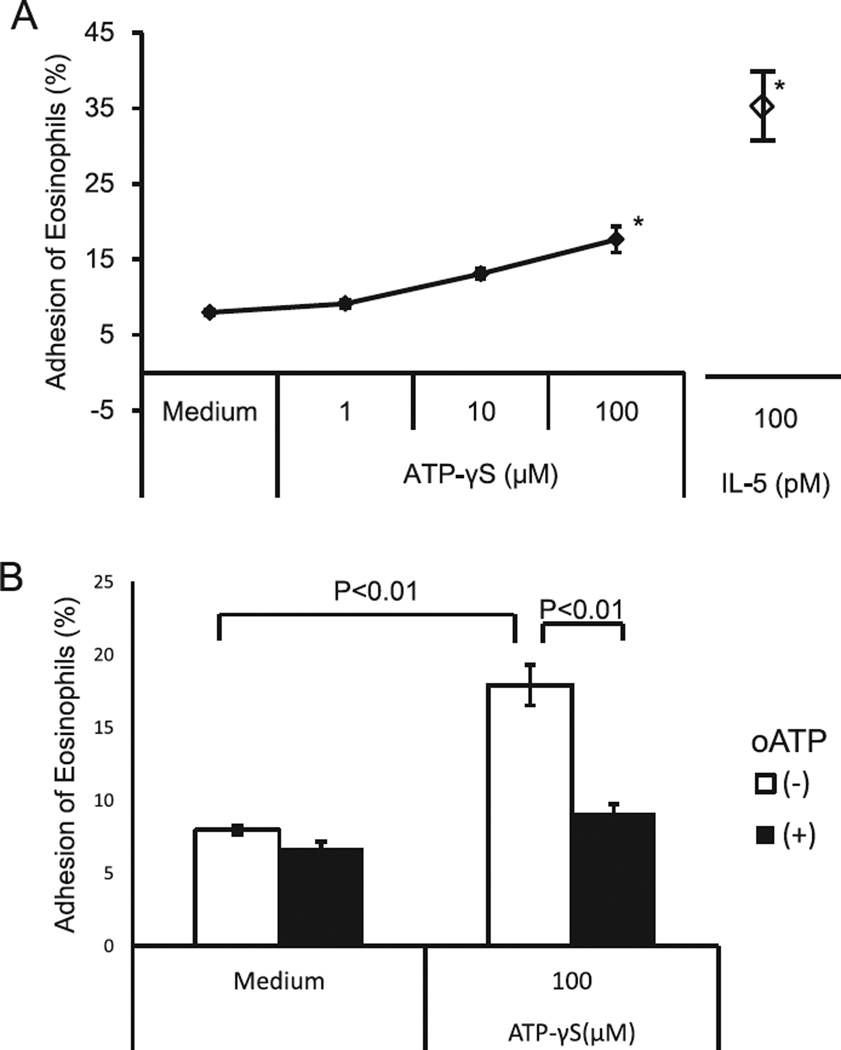
Thus, we incubated eosinophils with non-hydrolysable ATP, ATPγS, and examined eosinophil adhesion to rh-ICAM-1-coated plates. ATPγS modestly, but significantly, induced eosinophil adhesion to rh-ICAM-1 (adhesion of eosinophils: 7.9 ± 0.4% by medium versus 17.6 ± 1.8% by ATPγS (P < 0.01) and 36.3 ± 4.1% by IL-5 (P < 0.01), N = 5) (Fig. 2A). The magnitude of eosinophil adhesion induced by ATPγS was approximately 50% that induced by IL-5. To examine the involvement of P2 receptors, we pre-incubated eosinophils with oATP. oATP reduced eosinophil adhesion to the near baseline level (eosinophil adhesion: 17.9 ± 1.4% by ATPγS versus 9.2 ± 0.6% by ATPγS with oATP (P < 0.01), N = 5). Thus, exogenous ATP can induce eosinophil adhesion through P2 receptors (Fig. 2B).
Fig. 2.
Effects of ATPγS on eosinophil adhesion to rh-ICAM-1 by engagement of P2 receptors. (A) Eosinophils were incubated with or without various concentrations of an ATPγS at 37 °C for 20 min in rh-ICAM-1 coated plates. The adhesion rate of eosinophils was measured as described in Fig. 1. IL-5 was used as a positive control. (B) Eosinophils were pre-incubated with or without 30 mM oATP at 4 °C for 15 min and then were incubated with or without 100 µM ATPγS under the same condition. Results show the mean ± SEM from 5 different eosinophil preparations. *p < 0.01, compared with medium alone.
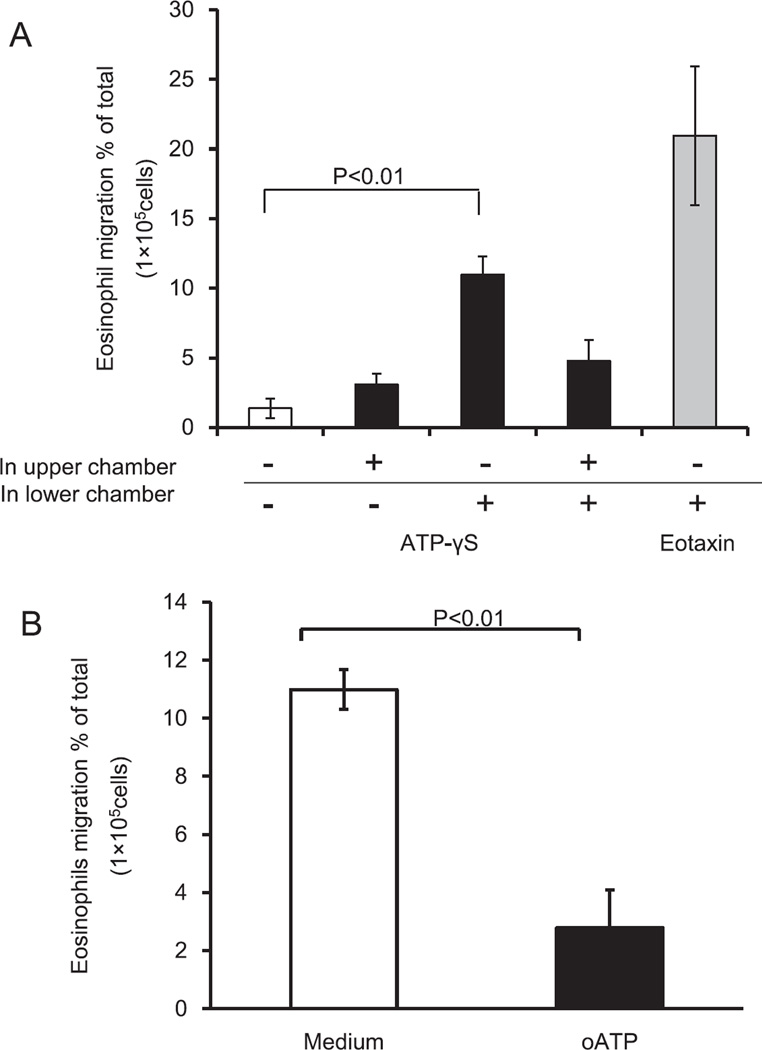
To examine whether ATP induces migration of eosinophils, we employed a basement membrane model. The effects of ATP were analyzed in a checkerboard system by placing eosinophils, with or without ATPγS (100 µM), in the upper chamber and placing medium, with or without ATPγS (100 µM), in the lower chamber. ATPγS in the lower chamber alone promoted significant transbasement membrane migration of eosinophils whereas ATPγS in the upper chamber alone did not (1.4 ± 0.7% by medium versus 11.8 ± 1.3% by ATPγS in lower chamber, P < 0.05) (Fig. 3A). The magnitude of ATPγS-induced migration was approximately 60% of eotaxin (20.1 ± 5.0% by eotaxin in lower chamber, P < 0.01, N = 5) (Fig. 3A). Furthermore, when ATPγS was added to the upper chamber (i.e., ATPγS in both upper and lower chambers), the migration of eosinophils was suppressed, suggesting that ATPγS serves as a chemotactic factor for eosinophils.
Fig. 3.
Effects of ATPγS on eosinophil trans-basement membrane migration by engagement of P2 receptors. (A) Eosinophil suspensions with or without 100 µM ATPγS were added to the upper chamber, and 100 µM ATPγS was added to the lower chamber in a checkerboard fashion. Eotaxin (100 ng/ml) in the lower chamber was used as a positive control. After 2 h at 37 °C, the cells that migrated to the lower chambers were collected and counted using light microscopy. The data show the percentage of migrated cells to the total number of initial cells. Results show the mean ± SEM from five different eosinophil preparations. p < 0.01, compared with medium alone in the lower chamber. (B) Eosinophils were pre-incubated with or without 30 mM oATP at 4 °C for 15 min. Subsequently, eosinophil suspensions were added to the upper chamber and 100 µM ATPγS was added to the lower chamber. The migration ratio was determined as described in (A). Results show the mean ± SEM from five different eosinophil preparations. p < 0.01, compared with medium alone in the lower chamber.
To investigate the role of P2 purinergic receptors, eosinophils were preincubated with or without oATP before being placed in the upper chamber. oATP significantly inhibited ATPγS-mediated eosinophil migration (11.8 ± 1.3% by medium versus 2.8 ± 0.7% by oATP, P < 0.05, N = 5) (Fig. 3B). Thus, ATP likely induces transbasement membrane migration of human eosinophils through P2 receptors.
ATP induces superoxide production and EDN degranulation of eosinophils by engaging P2 receptors
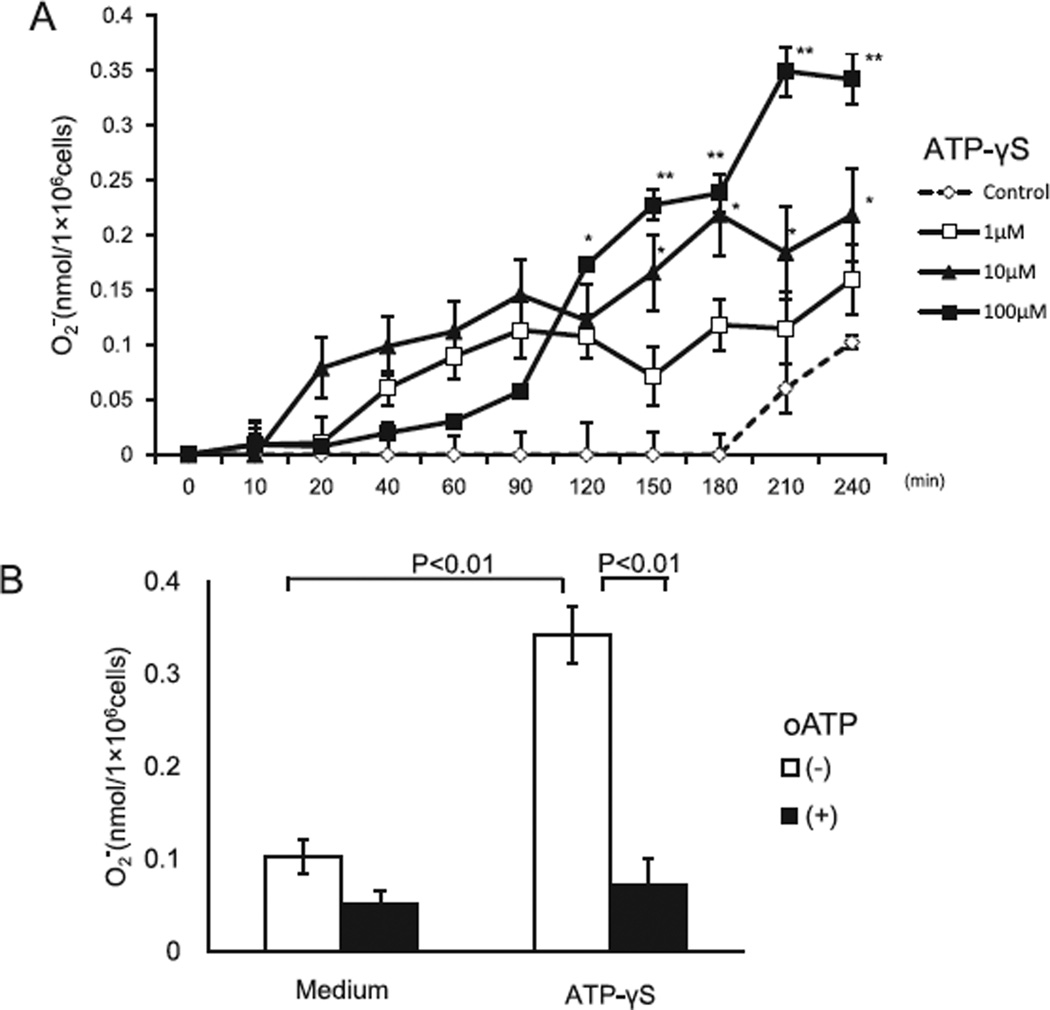
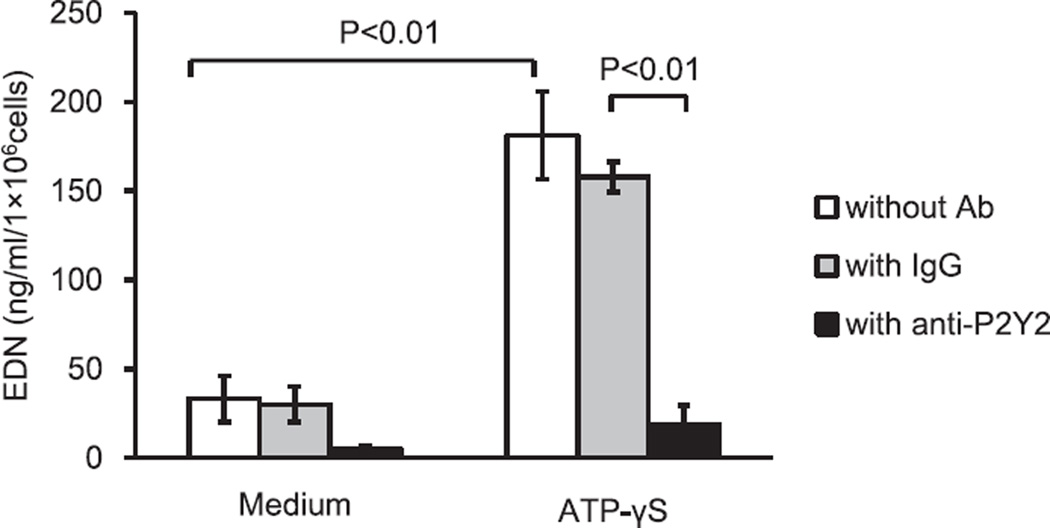
Previous study showed that ATP induces eosinophil cytokine production through P2Y2 receptors.15,30 To examine whether exogenous ATP promotes eosinophil effector function, we incubated eosinophils with ATPγS and analyzed superoxide production. ATPγS significantly induced superoxide generation (0.1 ± 0.04% by medium at 240 min versus 0.3 ± 0.03 nmol by 100 µM ATPγS at 240 min, P < 0.01, N = 6) (Fig. 4A). In addition, the eosinophil granule protein, EDN, was detectable in cell-free supernatants (33 ± 12.8 ng/ml by medium versus 181.1 ± 24.7 ng/ml by ATPγS, P < 0.01, N = 5), suggesting that ATPγS also induces eosinophil degranulation. oATP completely inhibited eosinophil superoxide generation in response to ATPγS at 240 min (0.34 ± 0.03 nmol with ATPγS versus 0.07 ± 0.03 nmol with oATP, P < 0.05, N = 6) (Fig. 4B).
Fig. 4.
Effects of ATPγS on the eosinophil production of superoxide anion by activation of P2 receptors. (A) Eosinophil and cytochrome C were incubated with or without various concentrations of ATPγS at 37 °C for up to 240 min. generation was measured as described in Fig. 1 (B) Eosinophils were pre-incubated with oATP (30 mM) or medium at 4 °C for 15 min before assay, and were then incubated with or without 100 µM ATPγS suspension under the same condition. generation at 240 min was measured as in (A). Results show the mean ± SEM from 6 different eosinophil preparations. *p < 0.05, **p < 0.01, compared with medium alone.
Although inhibition of eosinophil responses by a broad-range P2 receptor inhibitor suggested the involvement of P2 receptors, the role of specific P2 receptor(s) remained unclear. The P2Y2 receptor appears to be a possible candidate, since it is consistently expressed in eosinophils, and eosinophil cytokine production is dependent on receptor expression.15,31 To examine the role of P2Y2 receptors in eosinophil degranulation, an anti-P2Y2 receptor antibody was employed. The anti-P2Y2 receptor antibody completely inhibited EDN release by eosinophils (157 ± 8.4 ng/ml by murine IgG1 antibody versus 18.9 ± 10.6 ng/ml by anti-P2Y2 antibody, P < 0.01, N = 5) (Fig. 5). Thus, the P2Y2 receptor is likely involved in eosinophil degranulation in response to exogenous ATP.
Fig. 5.
Effects of ATPγS on eosinophil degranulation by activation of the P2Y2 receptor. Eosinophils were preincubated with or without rabbit polyclonal anti-P2Y2 Ab or control IgG (30 mM) at 4 °C for 15 min, and were then incubated with 100 µM ATPγS for 4 h at 37 °C and 5% CO2. The EDN ELISA was performed as described in the Materials and Methods using ELISA kits. Results show the mean ± SEM from 5 different eosinophil preparations. p < 0.01, compared with medium alone or control IgG.
Discussion
In this study, we found that two major DAMPs are involved in the induction of eosinophil function. MSU crystal induced eosinophil adhesion and promoted the release of inflammatory mediators. Exogenous ATP also promoted TBM migration of eosinophils and promoted the production of inflammatory mediators. These processes are likely driven through P2 receptors, in particular P2Y2 receptors. Importantly, eosinophil responses to UA were abolished following P2 receptors blockade, suggesting that endogenous eosinophil ATP is involved in eosinophil responses to UA. These findings suggest that exogenous and endogenous danger signals coordinately drive the inflammatory process attributed to activated eosinophils. These observations may partially explain why eosinophilic inflammation is prolonged in certain patients with asthma once the process is initiated.
Earlier, UA or MSU crystals were shown to initiate Th1- or Th17-type immune responses, including activation of neutrophil functions,32 inflammation by neutrophil extracellular traps in gout,33 IL-1β release from macrophages, and mediating adjuvant activities associated with infection and cell death.27 We previously found that eosinophils respond to MSU crystal, resulting in chemotaxis, pro-inflammatory cytokine production and degranulation.15 In this study, we add to this knowledge by proposing that other important eosinophil functions were induced by MSU crystal, such as adhesion and superoxide production. UA may therefore induce eosinophil accumulation at the site of inflammation and accelerate tissue damage by promoting the production of proinflammatory factors by eosinophils. In fact, UA was shown to drive Th2-type immune responses in the lungs.12,13 UA was involved in allergic bronchial inflammation induced by airway exposure to house dust mite (HDM) allergens. In addition, UA was also involved in protease-induced Th2-type airway immune responses by triggering IL-33 secretion by bronchial epithelial cells in naive mice. Consequently, UA may contribute not only to the progression of Th2-type immune responses, but also to the connection between innate and Th2-type adaptive responses.
ATP is another DAMP that is known to drive Th1-type immune responses, similar to UA. ATP was shown to mediate IL-1β secretion from macrophages by engaging P2X7 receptors11 and to promote neutrophil migration thorough P2Y2 receptors.28 Furthermore, ATP promoted both innate and adaptive Th2-type immune responses.7,14 For example, ATP regulated the function of DCs and induced Th2-type cytokine release in bronchial epithelial cells exposed to aeroallergens. In this study, we found that eosinophils also responded to ATP and exhibited various functions, including adhesion, migration, production of pro-inflammatory mediators and degranulation. For example, eosinophils migrated through the basement membrane matrix towards ATP in a similar manner to the potent chemotactic factor eotaxin.
We also observed that the inhibition of P2 receptors by a pharmacologic agent or antibody reduced eosinophil adhesion, superoxide production and degranulation in response to UA and ATP. Therefore, ATP itself appears to regulate immune cells involved in allergic responses. Furthermore, endogenous ATP may be involved in cellular responses to other danger signaling molecules such as UA. ATP is increased at inflammation sites as a result of various triggers.6,7 Thus, as judged by its potent chemotactic and stimulatory activities for eosinophils, ATP may play a role in persistent eosinophil infiltration in patients with severe asthma. Taken together, ATP may serve as pivotal player in allergic inflammation in lung.
In this study, ATP induced various functions in human eosinophils. These results are consistent with several other studies, which demonstrate the activities of extracellular nucleotides and ATP analogues to induce eosinophil functions, including expression of integrin CD11b, chemotaxis and production of reactive oxygen metabolites and cytokines.30,34–37 However, little information has been available on the nature of P2 purinergic receptor(s) involved because of the broad P2 receptor antagonists used. Eosinophils express mRNA for P2X1, P2X4, P2X7, P2Y1, P2Y2, P2Y4, P2Y11 and P2Y14.31 We previously verified the expression of P2Y2 receptors using flow cytometry.15 We also found that anti-P2Y2 antibody abrogated cytokine production by eosinophils15 and degranulation (this study). These observations are consistent with those in in vivo mouse studies. For example, P2Y2-deficient mice showed reduced accumulation of eosinophils in the lungs when they were sensitized and challenged with OVA antigen.38 Also, ATP failed to induce the migration of eosinophils generated from bone marrow of P2Y2-deficient mice.39 Thus, P2Y2 receptors likely play key roles in the interaction between eosinophils and ATP.
In summary, human eosinophils exerted diverse functions by recognizing two danger signals, UA and ATP. ATP was also involved in UA-induced eosinophil functions, suggesting that ATP potentially provides a self-sustaining inflammation signal to eosinophils, even when environmental triggers such as viruses and allergens have been eliminated. Such a mechanism explains the immunologic mechanism involved in prolonged and sustained airway inflammation in patients with difficult-to-treat asthma. Thus, ATP and the P2Y2 receptor pathway may provide a novel therapeutic target in future.
Acknowledgements
We greatly thank Ms. Akemi Yokote for her excellent technical assistance. This work was supported in part by Grant 24791004 from the National Institutes of Health in Japan, by Saitama Medical University Foundation (24-C-1-11), by Grant AI34486 from the National Institutes of Health in USA and by the Mayo Foundation for Medical Education and Research (AI34486), United States.
Abbreviations
- DAMPs
damage-associated molecular patterns
- UA
uric acid
- HMGB-1
high mobility group box 1
- OVA
ovalbumin
- DCs
dendritic cells
- MSU
monosodium urate
- rh-ICAM-1
recombinant human intercellular adhesion molecule-1
- TBM
trans-basement membrane migration
- oATP
oxidized ATP
- EPO
eosinophil peroxidase
- SOD
superoxide dismutase
- EDN
eosinophil-derived neurotoxin
- HEPES
4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid
- HDM
house dust mite
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
Authors' contributions
TK designed the study, performed the examination and wrote the manuscript. TS designed the study, interpreted the results and wrote the main text. TN contributed to the preparation of the examination. KN and HN contributed to interpretation of the results. HK contributed to design of the study, interpretation of the results and writing the manuscript. MN designed the study and interpreted the results. All authors discussed the results and implications, read and approved the final manuscript.
References
- 1.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 6.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 9.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 11.Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy. 2009;39:12–19. doi: 10.1111/j.1365-2222.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- 12.Kool M, Willart MAM, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Hara K, Iijima K, Elias MK, Seno S, Tojima I, Kobayashi T, et al. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J Immunol. 2014;192:4032–4042. doi: 10.4049/jimmunol.1400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 2010;184:6350–6358. doi: 10.4049/jimmunol.0902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari D, la Sala A, Panther E, Norgauer J, Di Virgilio F, Idzko M. Ctivation of human eosinophils via P2 receptors: novel findings and future perspectives. Leukoc Biol. 2006;79:7–15. doi: 10.1189/jlb.0505286. [DOI] [PubMed] [Google Scholar]
- 17.Ide M, Weiler D, Kita H, Gleich GJ. Ammonium chloride exposure inhibits cytokine-mediated eosinophil survival. J Immunol Methods. 1994;168:187–196. doi: 10.1016/0022-1759(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 18.Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194–2202. [PubMed] [Google Scholar]
- 19.Nagata M, Sedgwick JB, Kita H, Busse WW. Granulocyte macrophage colony-stimulating factor augments ICAM-1 and VCAM-1 activation of eosinophil function. Am J Respir Cell Mol Biol. 1998;19:158–166. doi: 10.1165/ajrcmb.19.1.3001. [DOI] [PubMed] [Google Scholar]
- 20.Mori M, Takaku Y, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. Eosinophil superoxide anion generation induced by adhesion molecules and leukotriene D4. Int Arch Allergy Immunol. 2009;149:31–38. doi: 10.1159/000210651. [DOI] [PubMed] [Google Scholar]
- 21.Nagata M, Saito K, Tsuchiya K, Sakamoto Y. Leukotriene D4 upregulates eosinophil adhesion via the cysteinyl leukotriene 1 receptor. J Allergy Clin Immunol. 2002;109:676–680. doi: 10.1067/mai.2002.122841. [DOI] [PubMed] [Google Scholar]
- 22.Takaku Y, Nakagome K, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. IFNγ-inducible protein of 10 kDa upregulates the effector functions of eosinophils through β2 integrin and CXCR3. Respir Res. 2011;12:138. doi: 10.1186/1465-9921-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kikuchi I, Kikuchi S, Kobayashi T, Hagiwara K, Sakamoto Y, Kanazawa M, et al. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am J Respir Cell Mol Biol. 2006;34:760–765. doi: 10.1165/rcmb.2005-0303OC. [DOI] [PubMed] [Google Scholar]
- 24.Grünberg K, Smits HH, Timmers MC, de Klerk EP, Dolhain RJ, Dick EC, et al. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 25.Bochner BS, Schleimer RP. The role of adhesion molecules in human eosinophil and basophil recruitment. J Allergy Clin Immunol. 1994;94:427–438. doi: 10.1016/0091-6749(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 26.Horie S, Gleich GJ, Kita H. Cytokines directly induce degranulation and superoxide production from human eosinophils. J Allergy Clin Immunol. 1996;98:371–381. doi: 10.1016/s0091-6749(96)70161-6. [DOI] [PubMed] [Google Scholar]
- 27.Saito K, Nagata M, Kikuchi I, Sakamoto Y. Leukotriene D4 and eosinophil transendothelial migration, superoxide generation, and degranulation via β2 integrin. Ann Allergy Asthma Immunol. 2004;93:594–600. doi: 10.1016/S1081-1206(10)61269-0. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 29.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Idzko M. Stimulation of P2 purinergic receptors induces the release of eosinophil cationic protein and interleukin-8 from human eosinophils. Br J Pharmacol. 2003;138:1244–1250. doi: 10.1038/sj.bjp.0705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;292:R423–R431. doi: 10.1152/ajpregu.00495.2006. [DOI] [PubMed] [Google Scholar]
- 32.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauer C, Janko C, Munoz LE, Zhao Y, Hoffmann M, Herrmann M, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari D. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000;486:217–224. doi: 10.1016/s0014-5793(00)02306-1. [DOI] [PubMed] [Google Scholar]
- 35.Idzko M. Functional characterization of P2Y and P2X receptors in human eosinophils. J Cell Physiol. 2001;188:329–336. doi: 10.1002/jcp.1129. [DOI] [PubMed] [Google Scholar]
- 36.Dichmann S. Adenosine triphosphate induced oxygen radical production and CD11b up-regulation: Ca++ mobilization and actin reorganization in human eosinophils. Blood. 2000;95:973–978. [PubMed] [Google Scholar]
- 37.Mohanty JG. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: activation of purinergic P2Y receptors. J Allergy Clin Immunol. 2001;107:849–855. doi: 10.1067/mai.2001.114658. [DOI] [PubMed] [Google Scholar]
- 38.Vanderstocken G, Bondue B, Horckmans M, Di Pietrantonio L, Robaye B, Boeynaems JM, et al. P2Y2 receptor regulates VCAM-1 membrane and soluble forms and eosinophil accumulation during lung inflammation. J Immunol. 2010;185:3702–3707. doi: 10.4049/jimmunol.0903908. [DOI] [PubMed] [Google Scholar]
- 39.Müller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]