Abstract
Background
IL–10 is an important immunosuppressive cytokine which is frequently elevated in tumor microenvironment. Some studies have reported that overexpression of serous IL–10 is correlated with worse outcome in patients with malignant tumor. Here, we conducted a meta-analysis to assess the prognostic impact of serous IL–10 expression in cancer patients.
Methods
We searched PubMed and EBSCO for studies in evaluating the association of IL–10 expression—in serum and clinical outcome in cancer patients. Overall survival (OS) was the primary prognostic indicator and disease-free survival (DFS) was the secondary indicator. Extracted data were computed into odds ratios (ORs) and 95% confidence interval (CI) or a P value for survival at 1, 3 and 5 years. Pooled data were weighted using the Mantel–Haenszel Fixed-effect model. All statistical tests were two-sided.
Results
A total of 1788 patients with cancer from 21 published studies were incorporated into this meta-analysis. High level of serum IL–10 was significantly associated with worse OS at 1-year (OR = 3.70, 95% CI = 2.81 to 4.87, P < 0.00001), 3-year (OR = 3.33, 95% CI = 2.53 to 4.39, P < 0.0001) and 5-year (OR = 2.80, 95% CI = 1.90 to 4.10, P < 0.0001) of cancer. Subgroup analysis showed that the correlation between serous IL–10 expression and outcome of patients with solid tumors and hematological malignancies are consistent. The association of IL–10 with worse DFS at 1-year (OR = 3.34, 95% CI = 1.40 to 7.94, P = 0.006) and 2-year (OR = 3.91, 95% CI = 1.79 to 8.53, P = 0.0006) was also identified.
Conclusions
High expression of serous IL–10 leads to an adverse survival in most types of cancer. IL–10 is a valuable biomarker for prognostic prediction and targeting IL–10 treatment options for both solid tumors and hematological malignancies.
Introduction
Chronic inflammation is closely linked to cancer [1–4]. Cancer-related inflammation promotes the development and progression of tumor by different mechanisms, specifically by subverting immune response, inducing gene mutations, stimulating angiogenesis and cell proliferation and inhibiting apoptosis [5, 6]. Multifunctional cytokines are a significant mediator in the development of malignant tumor by participating in bidirectional regulation of inflammatory responses [7, 8]. A large number of studies demonstrated that cytokines could facilitate carcinogenesis by both provoking inflammation [9–11] and eliciting immunosuppression [12–14].
Interleukin 10 (IL–10) is an immunoregulatory cytokine mainly produced by regulatory T cells and helper T cells [15, 16]. The primarily function of IL–10 is initially considered as an effective anti-inflammatory cytokine, which functions through suppressing macrophage/T cell cytokine expression and inhibiting their antigen-presenting capacity by activating STAT3 signal pathway [17–19]. Accumulating evidence showed that IL–10 played a pleiotropic role in both immune stimulation and suppression in tumor inflammatory microenvironment [20–23]. As an inflammatory modulatory cytokine, IL–10 was reported to exert both anti-tumor and pro-tumor function [19, 24, 25]. In previous studies, high level of IL–10 was reported to correlate with poor survival of some cancer patients [26–29], while some other studies provided opposite results [30–32]. Both human recombined IL–10 and IL–10 antagonist have been launched for cancer therapy [33–36]. Nowadays, deep insight into the controversial functions of IL–10 in cancer is urgently needed. Moreover, the potential of IL–10 as an effective biomarker in prognostic prediction and targeted therapy is necessary to be explored.
Here, we performed this meta-analysis to test OS and DFS as outcomes in cancer patients with known serum IL–10 levels. The purpose of this study was to quantitatively summarize the association between serum IL–10 overexpression and clinical outcomes in cancer patients, and thereby shed more light on the clinical value of IL–10 as a prognostic biomarker and therapeutic target for both solid and hematological malignances.
Methods
Search and Selection of Studies
We searched PubMed and EBSCO for studies evaluating the expression of IL–10 in serum and survival in cancer patients from 1993 to December 2012. The research term was ("Neoplasms"[Mesh]) AND ("Interleukin–10"[Mesh]). Results were restricted to serum IL–10 detection in human cancer. A total of 2023 and 3091 entries were identified in PubMed and EBSCO respectively. Inclusion criteria were the measurement of IL–10 by Enzyme-Linked Immunosorbent Assay (ELISA), available data of overall survival (OS) or disease free survival (DFS) for at least 1 year, and publication in English. Exclusion criteria included studies evaluating gene expression of IL–10, detecting IL–10 in tissues, and studies on animals or in lab. The association between IL–10 and survival was the primary consideration for study selection. The most complete study was chosen when a clinical trial had more than one publication. All the studies selected were assessed by the Newcastle-Ottawa Scale (NOS).
Endpoints of Interest
OS at 1, 3 and 5 years was recorded as the primary outcome of interest, and DFS at 1 and 2 years was recorded as the secondary clinical outcomes. Cut-offs of IL–10 expression level defined by individual studies classified cancer patients into high- and low- groups
Data Collection
Data were independently extracted by two authors (Shuai Zhao and Dang Wu) using standardized data abstraction forms. The following details were collected from individual studies: types of tumor, patient numbers, time of follow up, technique for IL–10 detection, and cut-off to determine IL–10 positivity. In all cases, survival data were extracted from tables or Kaplan–Meier curves for both IL–10 negative (control group) and IL–10 positive (experimental group) patients.
Data Synthesis
All inclusion study data were pooled in this meta-analysis initially. Odds Ratio (OR) and its 95% confidence interval (CI) were derived to express the relative frequency of survival at 1, 3 and 5 years between the negative and positive groups. Two subgroups were generated for patients of hematological malignancies and solid tumors.
Statistical Analysis
Extracted data were combined into a meta-analysis using RevMan 5.3 analysis software (Cochrane Collaboration, Copenhagen, Denmark). Pooled estimates of ORs were computed using the Mantel–Haenszel fixed-effect model. Cochran’s Q and I2 statistics were used to assess statistical heterogeneity. Differences between the subgroups were assessed using methods as described previously by Deeks et al [37]. Batch Effects was assessed between overall survival and batch using ComBat. Sensitivity analyses were performed for batch correction and different cut-offs in defining IL–10 expression to assess the robustness. All statistical tests were two-sided, and P value less than 0.05 are considered statistically significant.
Results
Search results
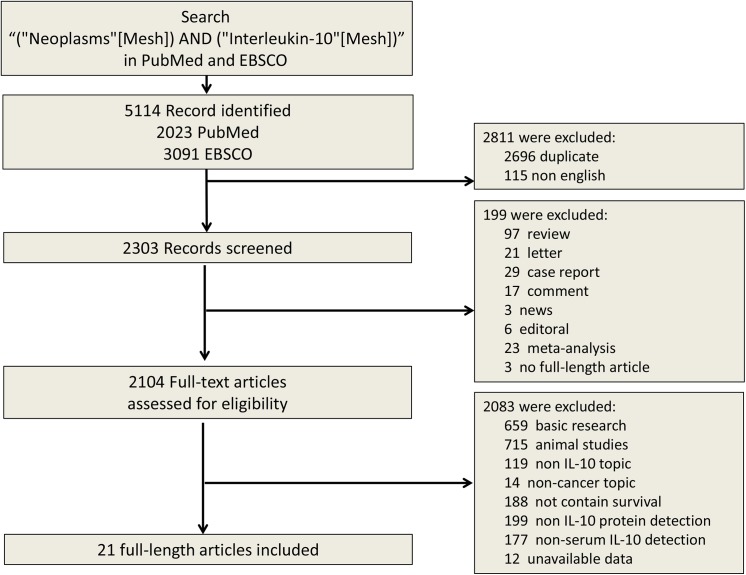
The search results have been shown in Fig 1. The primary literature research retrieved 5114 records. After screening the title of citations, 3010 records were excluded because of the non-relevance with the theme and duplicated literatures. Next, 2083 citations were excluded after screening abstracts of the records. Then we read carefully the full text of the left citations and at last 21 studies were included.
Fig 1. Selection of studies included in the analysis.
IL–10 = interleukin–10.
Description of studies
21 studies including 1788 patients were identified for the assessment of IL–10 expression [38–58]. All the studies were evaluated by the Newcastle-Ottawa Scale (NOS), and were in accordance with the inclusion criteria and suitable for data consolidation. Table 1 and Table 2 show characteristics of included studies for OS and DFS respectively. Seven studies evaluated lymphoma, two evaluated lymphocytic leukemia, five evaluated gastrointestinal cancer, three evaluated hepatocellular carcinoma, and one each evaluated head and neck cancer, pancreatic cancer, melanoma, and lung cancer. Eighteen studies reported data for 1-year OS, 3-year OS and 5-year OS and three studies reported data for 1-year DFS and 2-year DFS.
Table 1. Characteristics of the included studies for OS analysis.
| References | Type of cancer | Patient NO. | Age(range) | Male/female | stage | cut-off | Follow up months (range) | IL–10(-/+) NO. | 1-y OS(-/+)% | 3-y OS(-/+)% | 5-y OS(-/+)% | Quality Score (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alhamarneh, O., et al.(2011)[38] | head and neck squamous cell carcinoma | 107 | 64(26–90) | 85/22 | I-IV | 0.2 pg/ml | 15(1–36) | 66/41 | 90/78 | 74.4/52.8 | NR | 8 |
| Blay, J. Y., et al.(1993) [39] | Non-Hodgkin’s Lymphoma | 70 | 46 (3–89) | NR | I-IV | 100 pg/mL | NR | 32/38 | 81.4/67.9 | 78/53.6 | 78/49.3 | 8 |
| Chan, S. L., et al. (2012) [40] | hepatocellular carcinoma | 222 | 59.9±12.3 | 198/24 | I-IV | 1.00 pg/mL | 31(0–48) | 146/76 | 57.7/25.6 | 25.6/7 | NR | 8 |
| Cortes, J. E., et al. (1995) [41] | Diffuse Large Cell Lymphoma | 52 | 56(24–78) | 32/20 | I-IV | 8 pg/ml | 26(12–44) | 26/26 | 75.5/73.6 | 43.7/61.4 | NR | 7 |
| De Vita, F., et al. (1999) [42] | Gastrointestinal Malignancies | 58 | <60:50%. >60:50% | 39/19 | III-IV | 18 pg/mL | 12.9±6.4 | 29/29 | 92.4/32.1 | NR | NR | 7 |
| De Vita, F., et al. (2000) [43] | Non-small Cell Lung Cancer | 60 | <60:46.7%. >60:53.3% | 49/11 | III-IV | 19.6 pg/mL | 12.8±7.8 | 21/39 | 74.3/9.6 | 5.1/0 | NR | 8 |
| Ebrahimi, B., et al. (2004) [44] | Pancreatic Carcinoma | 50 | 65(43–79) | 30/20 | NR | 9.8 pg/ml | 10(0–22) | 9/41 | 44/0 | NR | NR | 7 |
| Evans, C., et al. (2006) [45] | colorectal cancer | 33 | 76.5 | 22/11 | NR | NR | 89 | 17/16 | 59/53.1 | 41.2/23.6 | 37.5/17.6 | 7 |
| Fayad, L., et al. (2001) [46] | chronic lymphocytic leukemia | 159 | 60(21–82) | NR | I-IV | 10 pg/ml | 30(1–40) | 54/105 | 96.4/78.3 | 85/45 | NR | 8 |
| Green, V. L., et al. (2012) [47] | head and neck squamous cell carcinoma | 106 | 64(26–90) | 84/22 | I-IV | 0.2 pg/ml | 33(1–67) | 64/42 | 85.3/73.3 | 74.4/54.6 | 59.1/54.6 | 8 |
| Hattori, E., et al. (2003) [48] | hepatocellular carcinoma | 74 | 65(41–88) | 54/20 | I-IV | 10 pg/ml | NR | 35/39 | 45/16.8 | 15.5/0 | NR | 8 |
| Lech-Maranda, E., et al. (2010) [49] | Diffuse Large B-Cell Lymphoma | 106 | <60:46.2%. >60:53.8% | 50/56 | I-IV | 5 pg/ml | NR | 24/82 | NR | 70.7/36 | NR | 8 |
| Lech-Maranda, E., et al. (2012) [50] | chronic lymphocytic leukemia | 160 | <60:32.5%. >60:67.5% | 86/74 | I-IV | 17.8 pg/ml | 48(1.2–200) | 82/78 | NR | NR | 92.1/80.52 | 8 |
| Nacinovic, A., et al. (2008) [51] | diffuse large B-cell lymphoma | 46 | 58 (17–82) | 25/21 | I-IV | 20.2 pg/ml | 50(1–69) | 16/30 | 85.8/74.9 | 75/31 | 71/25 | 7 |
| Nemunaitis, J., et al. (2001) [52] | Melanoma | 41 | NR | NR | advance | 10 pg/ml | NR | 18/23 | 60.5/28.1 | 27.3/11.4 | NR | 7 |
| Szaflarska, A., et al. (2009) [53] | Gastric Cancer | 136 | 61.5±11.9 | 84/52 | I-IV | 10 pg/ml | 82.6 | 49/87 | 71.6/52.9 | 55.4/26.2 | NR | 7 |
| Vassilakopoulos, T. P., et al. (2001) [54] | Hodgkin’s lymphoma | 122 | 31.5(15–76) | 74/48 | I-IV | 10 pg/mL | 27(6–136) | 55/67 | 93.4/79.7 | 88.3/75 | 85.4/66.5 | 8 |
| Viviani, S., et al. (2000) [55] | Hodgkin's disease | 73 | 27(17–61) | 44/29 | I-IV | 6 pg/ml | 7.3y(1-8y) | 33/40 | 100/87.8 | 95.4/82 | 95.4/79 | 8 |
Table 2. Characteristics of the included studies for DFS analysis.
| References | Type of cancer | Patient NO. | Age(range) | Male/female | stage | cut-off | Follow up months (range) | IL–10(-/+) NO. | 1-y OS(-/+)% | 2-y OS(-/+)% | Quality Score (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chau, G. Y., et al. (2000) [56] | Hepatocellular Carcinoma | 67 | 63.4±1.5 | 60/7 | NR | IL–10 >12 pg/mL | NR | 21/46 | 66.3/46.6 | 62/31 | 8 |
| Galizia, G., Orditura,M., et al. (2002) [57] | Colon Cancer | 30 | 65.4±10.5(30–83) | NR | NR | IL–10 >15 pg/mL | 22.2±6.6(5.2–26.1) | 15/15 | 93.3/86.2 | 93.2/60 | 7 |
| Galizia, G., Lieto,E., et al. (2002) [58] | Colon Cancer | 50 | 65.4±10.5 (37–83) | 34/16 | NR | IL–10 >14 pg/mL | 15.5±6.7 (0.3–26.1) | 25/25 | 100/75.5 | 83.4/67.3 | 7 |
Evaluation and Expression of IL–10
Serum level of IL–10 in samples was tested by ELISA in companies according to the manufacturer’s instructions. The cut-off for high level depended on the serum concentration of IL–10. No significant difference of IL–10 level was observed among different types of cancer.
Association of IL–10 with Survival
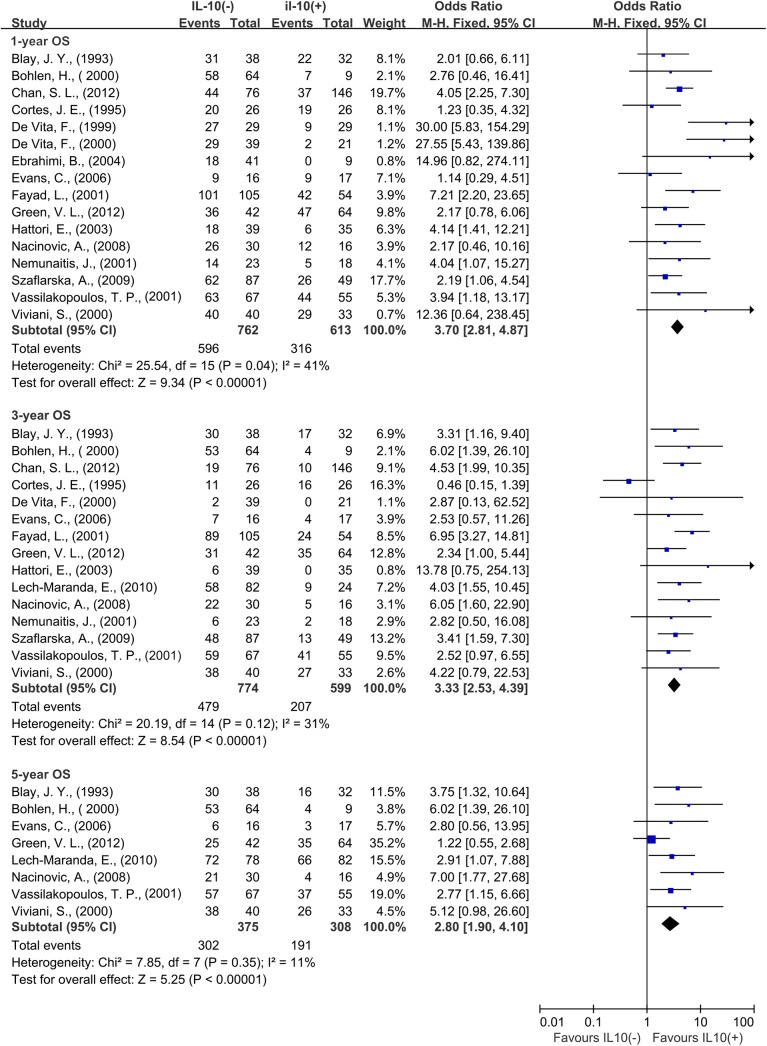
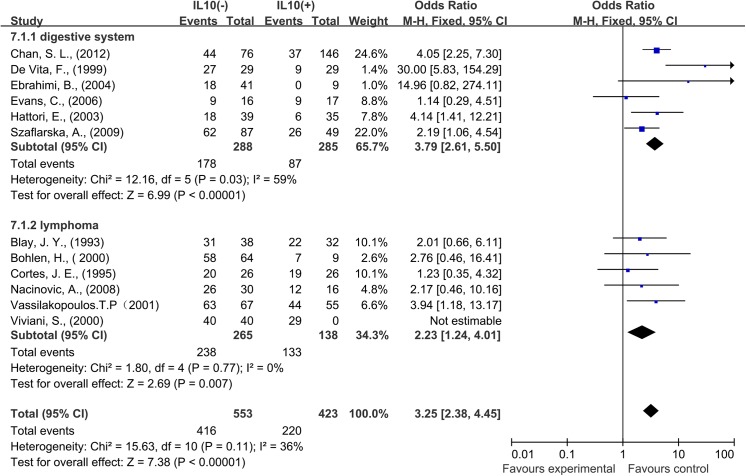
A total of 16 studies reported data for OS at 1-year. Results showed that IL–10 overexpression was associated with worse 1-year OS in cancer patients (OR = 3.70, 95% CI = 2.81 to 4.87, P < 0.00001) (Fig 2). There was no significant heterogeneity among studies (OS: Cochran’s Q P = 0.04, I2 = 41%). In the stratified analysis by cancer types, IL–10 overexpression was associated with worse 3-year OS of digestive system cancer (OR = 3.79, 95% CI = 2.61 to 5.50, P < 0.0001) and lymphoma (OR = 2.23, 95% CI = 1.24 to 4.01, P < 0.0001) (Fig 3). As solid and hematological malignances were both included in our study, so we conducted subgroup meta-analysis to explore whether the results were consistent in different types of cancer.
Fig 2. Forest plots describing odds ratios of the association between serous interleukin–10 (IL–10) expression and overall survival (OS) at 1, 3 and 5 years.
Fig 3. Forest plots describing analysis of the association between serous interleukin–10 (IL–10) expression and overall survival (OS) in digestive system cancer patients and lymphoma patients at 1 year.
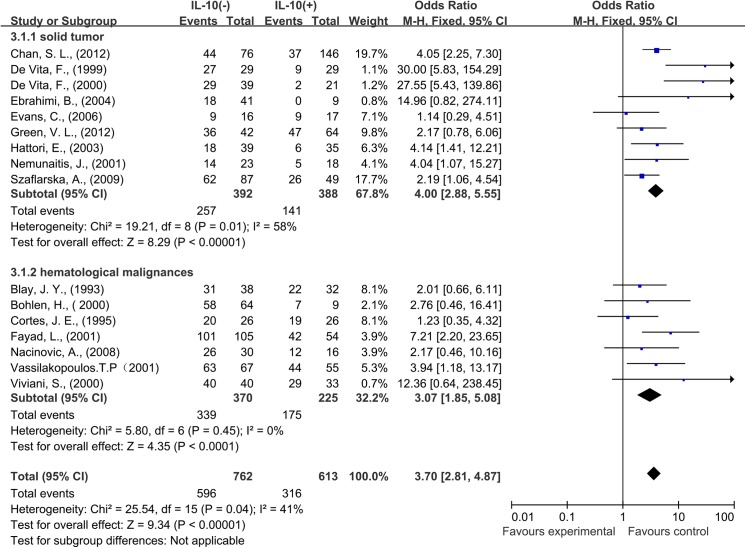
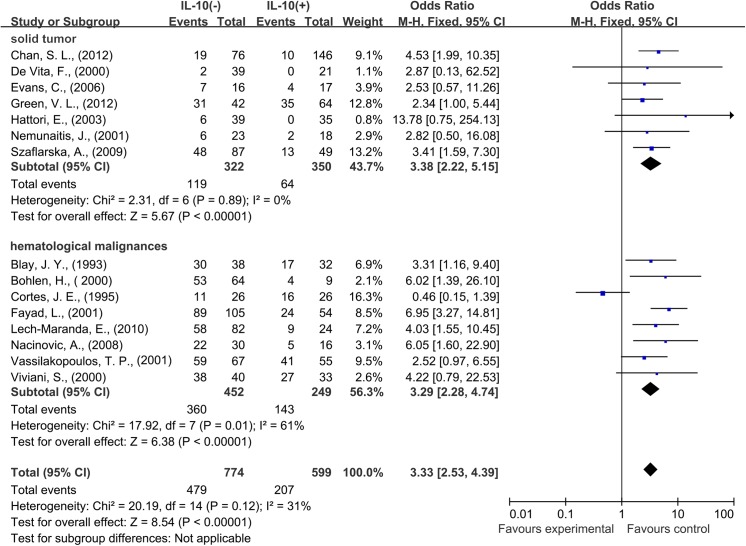
Nine studies provided 1-year OS for solid tumors, and seven studies for hematological malignances. In the stratified analysis by cancer types, IL–10 overexpression was associated with worse 1-year OS in solid tumors (OR = 4.00, 95% CI = 2.88 to 5.55, P < 0.0001). The similar result was also observed in hematological malignancies, high IL–10 level correlated with worse OS at 1 year (OR = 3.07, 95% CI = 1.85 to 5.08, P < 0.0001) (Fig 4).
Fig 4. Forest plots describing subgroup analysis of the association between serous interleukin–10 (IL–10) expression and overall survival (OS) in solid tumor patients and hematological malignances patients at 1 year.
A total of 15 studies reported data for OS at 3 years. IL–10 overexpression was also associated with worse 3-year OS in cancer patients (OR = 3.33, 95% CI = 2.53 to 4.39, P < 0.0001) (Fig 2). No significant heterogeneity was observed among studies (Cochran’s Q P = 0.12, I2 = 31%). Subgroup meta-analysis was also conducted to explore whether the results were consistent in different types of cancer.
Seven studies provided 3-year OS for solid tumors, eight studies for hematological malignances. IL–10 overexpression was found to be associated with worse 3-year OS in both solid tumors (OR = 3.38, 95% CI = 2.22 to 5.15, P < 0.0001) and hematological malignances (OR = 3.29, 95% CI = 2.28 to 4.74, P < 0.0001) (Fig 5).
Fig 5. Forest plots describing subgroup analysis of the association between serous interleukin–10 (IL–10) expression and overall survival (OS) in solid tumor patients and hematological malignances patients at 3 years.
A total of 8 studies reported data for OS at 5-years. The result was similar to that of 1-year and 3-year, IL–10 overexpression was significantly associated with worse 5-year OS of cancer (OR = 2.80, 95% CI = 1.90 to 4.10, P < 0.0001) (Fig 2). There was no significant heterogeneity among studies (Cochran’s Q P = 0.35, I2 = 11%). Subgroup analysis showed that IL–10 overexpression was associated with worse 5-year OS in hematological malignancies (OR = 3.59, 95% CI = 2.26 to 5.72, P < 0.0001), however the amount of 5-year OS data in solid tumors was not enough for meta-analysis.
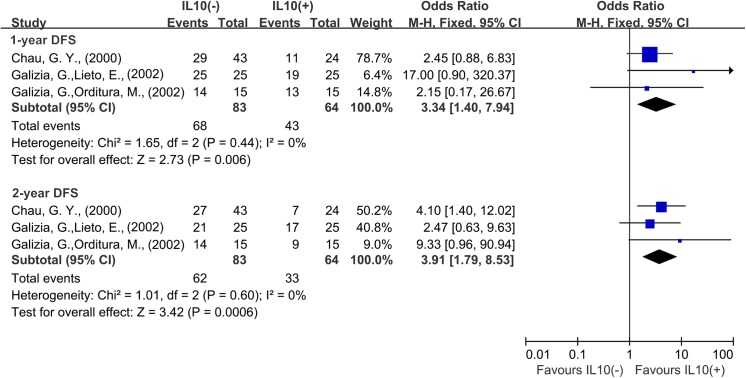
A total of 3 studies reported data for DFS at 1 year and 2 years. Results showed that IL–10 overexpression was associated with worse 1-year DFS (OR = 3.34, 95% CI = 1.40 to 7.94, P = 0.0006) and worse 2-year DFS (OR = 3.91, 95% CI = 1.79 to 8.53, P = 0.0006) of cancer. There was no significant heterogeneity among studies (1 year: Cochran’s Q P = 0.44, I2 = 0%; 2 years: Cochran’s Q P = 0.60, I2 = 0%) (Fig 6).
Fig 6. Forest plots describing odds ratios of the association between serous interleukin–10 (IL–10) expression and disease-free survival (DFS) at 1 year and 3 years.
Sensitivity Analyses
Batch correction using the ComBat method had no important impact on the results for 1-year, 3-year or 5-year OS. The summary results were not significantly influenced when 3 studies which reported unsocial cut-off value were removed (OR = 4.05, 95% CI = 2.88 to 5.70; OR = 3.36, 95% CI = 2.42 to 4.66; OR = 3.48, 95% CI = 2.12 to 5.69, respectively). No obvious heterogeneity was observed after exclusion of these studies (Cochran’s Q P = 0.02, I2 = 49%, Cochran’s Q P = 0.06, I2 = 42%; Cochran’s Q P = 0.89, I2 = 0%, respectively).
Discussion
IL–10 shows its bidirectional functions both in pro-tumor and anti-tumor effect. In previous cancer studies, high level of IL–10 was reported to correlate with poor clinical outcome [29], while some others suggested IL–10 as a beneficial factor in cancer prognosis [32]. Here we meta-analyzed the published data about the expression of IL–10 in serum of 1788 cancer patients from 21 published studies and their association with survival for studies that evaluated IL–10 by ELISA.
IL–10 was generally known as an immunosuppressive cytokine which mainly promoted the proliferation and metastasis of tumor cells [20]. However, IL–10 was newly found to active anti-tumor immunity in tumor microenvironment [36]. Our results showed that high level of IL–10 in serum was associated with worse 1, 3, and 5-year OS and worse 1-year DFS for 21 studies analyzed totally, suggesting IL–10 is a potential biomarker for the prognosis evaluation and IL-10-targeted therapy. We believe that our study provides significative statistical evidence to declare the important prognostic value of IL–10 as a tumor promoter in cancer patients for the first time.
Actually, IL–10 has been explored as a novel therapeutic target for a long time. Significant restrain of colon and breast tumors in mice was induced by delivery of anti-IL-10-receptor antibody in combination with CpG and CCL16, which supported the effectiveness of anti-IL–10 therapy [35]. In contrast, a recent study reported that recombined IL–10 induced tumor rejections by specifically targeting the tumor-infiltrating memory CD8+ T cells to activate the anti-tumor immune response [36]. Therefore, applying IL–10 antagonist or recombinant IL–10 is still a matter of some debate. Our findings provide statistical evidence for this argument. High level of serum IL–10 showed poor prognosis of cancer patients, suggesting IL–10 antagonist may be a novel therapeutic technique for clinical treatment of cancer patients.
Subgroup analysis of solid tumors and hematological malignancies was conducted to identify the effect of IL–10 on different types of cancer. IL–10 showed a close correlation with poor prognosis of both solid tumors and hematological malignancies. Interestingly, patients with solid tumors showed mild higher ORs at 1-year and 3-year than patients with hematological malignancies. Among specific cancer types, both digestive system cancer and lymphoma linked with a poor prognosis for patients who expressed high serum IL–10. This finding suggests that IL–10 plays a crucial role in tumor progression both in solid tumors and hematological malignancies, probably by inducing systemic immunosuppression.
Several important implications were put forward in these analyses. First, we show that high level of serum IL–10 has a tight correlation with poor prognosis in cancer patients, which suggests that IL–10 is a promising biomarker for the evaluation of disease progression and survival time. Second, it provides statistical evidence to support that IL–10 antagonist in anticancer target therapy could have a better response than recombinant human IL–10. Finally, this analysis emphasizes the discrepant application of IL-10-targeted drugs toward solid tumors and hematological malignancies. Following these discoveries, further clinic trials on IL–10 antagonist is imperatively needed to detect the clinical response in cancer patients.
Several limitations should be noted from this analysis. It is hard to eliminate the publication bias in a literature-based analysis. The studies reporting negative results may have few chances to be published. Besides, separate analysis for every types of cancer was not allowed in this study because some types of cancer did not have enough data. The solid/hematological-tumor differentiation may increase the heterogeneity in each subgroup.
In conclusion, our analyses show that high level of serum IL–10 is associated with worse clinical outcome of cancer patients, which indicates that IL–10 might be a potential biomarker for prognostic prediction and targeted treatment in human cancer. Additionally, our findings present statistical evidence for the clinical application of IL–10 antagonists in cancer therapy.
Supporting Information
(DOCX)
Acknowledgments
We thank all the members of the laboratory who helped in this study.
Abbreviations
- IL–10
interleukin–10
- OS
overall survival
- DFS
disease-free survival
- OR
odds ratios
- Cl
confidence interval
- ELISA
Enzyme-Linked Immunosorbent Assay
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81520108024, JH; 81572800, PW) and Natural Science Foundation of Zhejiang Province (LY15H160041, PW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. 2005;7(3):211–7. 10.1016/j.ccr.2005.02.013 . [DOI] [PubMed] [Google Scholar]
- 2. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. 10.1016/S0140-6736(00)04046-0 . [DOI] [PubMed] [Google Scholar]
- 3. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6. 10.1038/nature04870 . [DOI] [PubMed] [Google Scholar]
- 5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. 10.1038/nature07205 . [DOI] [PubMed] [Google Scholar]
- 6. Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Seminars in cancer biology. 2006;16(1):80–8. 10.1016/j.semcancer.2005.12.002 . [DOI] [PubMed] [Google Scholar]
- 7. Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunological reviews. 2004;202:275–93. 10.1111/j.0105-2896.2004.00199.x . [DOI] [PubMed] [Google Scholar]
- 8. Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine & growth factor reviews. 2002;13(4–5):413–21. . [DOI] [PubMed] [Google Scholar]
- 9. Chambers SK. Role of CSF–1 in progression of epithelial ovarian cancer. Future oncology. 2009;5(9):1429–40. 10.2217/fon.09.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. . [DOI] [PubMed] [Google Scholar]
- 11. Apte RN, Voronov E. Interleukin-1—a major pleiotropic cytokine in tumor-host interactions. Seminars in cancer biology. 2002;12(4):277–90. . [DOI] [PubMed] [Google Scholar]
- 12. Villa-Morales M, Fernandez-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert opinion on therapeutic targets. 2012;16(1):85–101. 10.1517/14728222.2011.628937 . [DOI] [PubMed] [Google Scholar]
- 13. Waldner MJ, Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert opinion on therapeutic targets. 2012;16(1):5–13. 10.1517/14728222.2011.641951 . [DOI] [PubMed] [Google Scholar]
- 14. Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, et al. TGF-beta: duality of function between tumor prevention and carcinogenesis. Journal of the National Cancer Institute. 2014;106(2):djt369 10.1093/jnci/djt369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nature immunology. 2007;8(9):931–41. 10.1038/ni1504 . [DOI] [PubMed] [Google Scholar]
- 16. Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL–10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. Journal of immunology (Baltimore, Md: 1950). 1993;150(2):353–60. . [PubMed] [Google Scholar]
- 17. Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. The Journal of experimental medicine. 1989;170(6):2081–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin–10. Annual review of immunology. 1993;11:165–90. 10.1146/annurev.iy.11.040193.001121 . [DOI] [PubMed] [Google Scholar]
- 19. Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL–10. Trends in immunology. 2003;24(1):36–43. Epub 2002/12/24. . [DOI] [PubMed] [Google Scholar]
- 20. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin–10 and the interleukin–10 receptor. Annual review of immunology. 2001;19:683–765. 10.1146/annurev.immunol.19.1.683 . [DOI] [PubMed] [Google Scholar]
- 21. Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin–10 and regulatory T-cells in cancer. Current opinion in oncology. 2013;25(6):637–45. Epub 2013/10/01. 10.1097/cco.0000000000000006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamidullah, Changkija B, Konwar R. Role of interleukin–10 in breast cancer. Breast cancer research and treatment. 2012;133(1):11–21. Epub 2011/11/08. 10.1007/s10549-011-1855-x . [DOI] [PubMed] [Google Scholar]
- 23. Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunologic research. 2011;51(2–3):170–82. Epub 2011/12/06. 10.1007/s12026-011-8262-6 . [DOI] [PubMed] [Google Scholar]
- 24. Vicari AP, Trinchieri G. Interleukin–10 in viral diseases and cancer: exiting the labyrinth? Immunological reviews. 2004;202:223–36. Epub 2004/11/18. 10.1111/j.0105-2896.2004.00216.x . [DOI] [PubMed] [Google Scholar]
- 25. Kundu N, Beaty TL, Jackson MJ, Fulton AM. Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. Journal of the National Cancer Institute. 1996;88(8):536–41. . [DOI] [PubMed] [Google Scholar]
- 26. Sarris AH, Kliche KO, Pethambaram P, Preti A, Tucker S, Jackow C, et al. Interleukin–10 levels are often elevated in serum of adults with Hodgkin's disease and are associated with inferior failure-free survival. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 1999;10(4):433–40. Epub 1999/06/17. . [DOI] [PubMed] [Google Scholar]
- 27. Axdorph U, Sjoberg J, Grimfors G, Landgren O, Porwit-MacDonald A, Bjorkholm M. Biological markers may add to prediction of outcome achieved by the International Prognostic Score in Hodgkin's disease. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2000;11(11):1405–11. Epub 2001/01/06. . [DOI] [PubMed] [Google Scholar]
- 28. Salgami EV, Efstathiou SP, Vlachakis V, Sekara EV, Syrigos KN, Roussou PP. High pretreatment interleukin–10 is an independent predictor of poor failure-free survival in patients with Hodgkin's lymphoma. Haematologia. 2002;32(4):377–87. Epub 2003/06/14. . [PubMed] [Google Scholar]
- 29. Visco C, Vassilakopoulos TP, Kliche KO, Nadali G, Viviani S, Bonfante V, et al. Elevated serum levels of IL–10 are associated with inferior progression-free survival in patients with Hodgkin's disease treated with radiotherapy. Leukemia & lymphoma. 2004;45(10):2085–92. Epub 2004/09/17. 10.1080/10428190410001712234 . [DOI] [PubMed] [Google Scholar]
- 30. Kaio E, Tanaka S, Oka S, Hiyama T, Kitadai Y, Haruma K, et al. Clinical significance of thrombospondin–1 expression in relation to vascular endothelial growth factor and interleukin–10 expression at the deepest invasive tumor site of advanced colorectal carcinoma. International journal of oncology. 2003;23(4):901–11. . [PubMed] [Google Scholar]
- 31. Soria J-C, Moon C, Kemp BL, Liu DD, Feng L, Tang X, et al. Lack of interleukin–10 expression could predict poor outcome in patients with stage I non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9(5):1785–91. . [PubMed] [Google Scholar]
- 32. Toiyama Y, Miki C, Inoue Y, Minobe S, Urano H, Kusunoki M. Loss of tissue expression of interleukin–10 promotes the disease progression of colorectal carcinoma. Surgery today. 2010;40(1):46–53. 10.1007/s00595-009-4016-7 . [DOI] [PubMed] [Google Scholar]
- 33. Terai M, Tamura Y, Alexeev V, Ohtsuka E, Berd D, Mastrangelo MJ, et al. Human interleukin 10 receptor 1/IgG1-Fc fusion proteins: immunoadhesins for human IL–10 with therapeutic potential. Cancer immunology, immunotherapy: CII. 2009;58(8):1307–17. 10.1007/s00262-008-0644-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chernoff AE, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, et al. A randomized, controlled trial of IL–10 in humans. Inhibition of inflammatory cytokine production and immune responses. Journal of immunology (Baltimore, Md: 1950). 1995;154(10):5492–9. . [PubMed] [Google Scholar]
- 35. Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. The Journal of experimental medicine. 2002;196(4):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mumm JB, Oft M. Pegylated IL–10 induces cancer immunity: the surprising role of IL–10 as a potent inducer of IFN-gamma-mediated CD8(+) T cell cytotoxicity. BioEssays: news and reviews in molecular, cellular and developmental biology. 2013;35(7):623–31. Epub 2013/05/15. 10.1002/bies.201300004 . [DOI] [PubMed] [Google Scholar]
- 37. Deeks JJ H. AD J.. Analysing and presenting results In: Higgins JPT, Green S, ed Cochrane Handbook for Systematic Reviews of Interventions 425 Chichester, UK: John Wiley & Sons ncer Res; 2006;15: p. 6341–7. [Google Scholar]
- 38. Blay JY, Burdin N, Rousset F, Lenoir G, Biron P, Philip T, et al. Serum interleukin–10 in non-Hodgkin's lymphoma: a prognostic factor. Blood. 1993;82(7):2169–74. Epub 1993/10/01. . [PubMed] [Google Scholar]
- 39. Bohlen H, Kessler M, Sextro M, Diehl V, Tesch H. Poor clinical outcome of patients with Hodgkin's disease and elevated interleukin–10 serum levels. Clinical significance of interleukin–10 serum levels for Hodgkin's disease. Annals of hematology. 2000;79(3):110–3. Epub 2000/05/10. . [DOI] [PubMed] [Google Scholar]
- 40. Chan SL, Mo FKF, Wong CSC, Chan CML, Leung LKS, Hui EP, et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer. 2012;118(16):3984–92. 10.1002/cncr.26726 . [DOI] [PubMed] [Google Scholar]
- 41. Cortes JE, Talpaz M, Cabanillas F, Seymour JF, Kurzrock R. Serum levels of interleukin–10 in patients with diffuse large cell lymphoma: lack of correlation with prognosis. Blood. 1995;85(9):2516–20. . [PubMed] [Google Scholar]
- 42. De Vita F, Orditura M, Galizia G, Romano C, Infusino S, Auriemma A, et al. Serum interleukin–10 levels in patients with advanced gastrointestinal malignancies. Cancer. 1999;86(10):1936–43. . [DOI] [PubMed] [Google Scholar]
- 43. De Vita F, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E, et al. Serum interleukin–10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest. 2000;117(2):365–73. 10.1378/chest.117.2.365 . [DOI] [PubMed] [Google Scholar]
- 44. Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101(12):2727–36. 10.1002/cncr.20672 . [DOI] [PubMed] [Google Scholar]
- 45. Evans C, Morrison I, Heriot AG, Bartlett JB, Finlayson C, Dalgleish AG, et al. The correlation between colorectal cancer rates of proliferation and apoptosis and systemic cytokine levels; plus their influence upon survival. British journal of cancer. 2006;94(10):1412–9. 10.1038/sj.bjc.6603104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fayad L, Keating MJ, Reuben JM, O'Brien S, Lee BN, Lerner S, et al. Interleukin–6 and interleukin–10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97(1):256–63. 10.1182/blood.V97.1.256 . [DOI] [PubMed] [Google Scholar]
- 47. Green VL, Irune E, Prasai A, Alhamarneh O, Greenman J, Stafford ND. Serum IL10, IL12 and circulating CD4+CD25high T regulatory cells in relation to long-term clinical outcome in head and neck squamous cell carcinoma patients. International journal of oncology. 2012;40(3):833–9. 10.3892/ijo.2011.1259 . [DOI] [PubMed] [Google Scholar]
- 48. Hattori E, Okumoto K, Adachi T, Takeda T, Ito J-i, Sugahara K, et al. Possible contribution of circulating interleukin–10 (IL–10) to anti-tumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatology Research. 2003;27(4):309. [DOI] [PubMed] [Google Scholar]
- 49. Lech-Maranda E, Bienvenu J, Broussais-Guillaumot F, Warzocha K, Michallet A-S, Robak T, et al. Plasma TNF-alpha and IL–10 level-based prognostic model predicts outcome of patients with diffuse large B-Cell lymphoma in different risk groups defined by the International Prognostic Index. Archivum immunologiae et therapiae experimentalis. 2010;58(2):131–41. 10.1007/s00005-010-0066-1 . [DOI] [PubMed] [Google Scholar]
- 50. Lech-Maranda E, Grzybowska-Izydorczyk O, Wyka K, Mlynarski W, Borowiec M, Antosik K, et al. Serum tumor necrosis factor-alpha and interleukin–10 levels as markers to predict outcome of patients with chronic lymphocytic leukemia in different risk groups defined by the IGHV mutation status. Archivum immunologiae et therapiae experimentalis. 2012;60(6):477–86. 10.1007/s00005-012-0197-7 . [DOI] [PubMed] [Google Scholar]
- 51. Nacinovic-Duletic A, Stifter S, Dvornik S, Skunca Z, Jonjic N. Correlation of serum IL–6, IL–8 and IL–10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. International journal of laboratory hematology. 2008;30(3):230–9. 10.1111/j.1751-553X.2007.00951.x . [DOI] [PubMed] [Google Scholar]
- 52. Nemunaitis J, Fong T, Shabe P, Martineau D, Ando D. Comparison of serum interleukin–10 (IL–10) levels between normal volunteers and patients with advanced melanoma. Cancer investigation. 2001;19(3):239–47. 10.1081/cnv-100102550 . [DOI] [PubMed] [Google Scholar]
- 53. Szaflarska A, Szczepanik A, Siedlar M, Czupryna A, Sierzega M, Popiela T, et al. Preoperative plasma level of IL–10 but not of proinflammatory cytokines is an independent prognostic factor in patients with gastric cancer. Anticancer research. 2009;29(12):5005–12. . [PubMed] [Google Scholar]
- 54. Vassilakopoulos TP, Nadali G, Angelopoulou MK, Siakantaris MP, Dimopoulou MN, Kontopidou FN, et al. Serum interleukin–10 levels are an independent prognostic factor for patients with Hodgkin's lymphoma. Haematologica. 2001;86(3):274–81. . [PubMed] [Google Scholar]
- 55. Viviani S, Notti P, Bonfante V, Verderio P, Valagussa P, Bonadonna G. Elevated pretreatment serum levels of Il–10 are associated with a poor prognosis in Hodgkin's disease, the milan cancer institute experience. Medical oncology (Northwood, London, England). 2000;17(1):59–63. 10.1007/bf02826218 . [DOI] [PubMed] [Google Scholar]
- 56. Chau GY, Wu CW, Lui WY, Chang TJ, Kao HL, Wu LH, et al. Serum interleukin–10 but not interleukin–6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Annals of surgery. 2000;231(4):552–8. Epub 2000/04/05. ; PubMed Central PMCID: PMCPmc1421032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, et al. Prognostic significance of circulating IL–10 and IL–6 serum levels in colon cancer patients undergoing surgery. Clinical immunology (Orlando, Fla). 2002;102(2):169–78. 10.1006/clim.2001.5163 . [DOI] [PubMed] [Google Scholar]
- 58. Galizia G, Lieto E, De Vita F, Romano C, Orditura M, Castellano P, et al. Circulating levels of interleukin–10 and interleukin–6 in gastric and colon cancer patients before and after surgery: relationship with radicality and outcome. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2002;22(4):473–82. Epub 2002/05/30. 10.1089/10799900252952262 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.