Abstract
Background
Fluralaner is a novel systemic ectoparasiticide for dogs providing immediate and persistent flea, tick and mite control after a single oral dose. Ivermectin has been used in dogs for heartworm prevention and at off label doses for mite and worm infestations. Ivermectin pharmacokinetics can be influenced by substances affecting the p-glycoprotein transporter, potentially increasing the risk of ivermectin neurotoxicity. This study investigated ivermectin blood plasma pharmacokinetics following concurrent administration with fluralaner.
Findings
Ten Beagle dogs each received a single oral administration of either 56 mg fluralaner (Bravecto™), 0.3 mg ivermectin or 56 mg fluralaner plus 0.3 mg ivermectin/kg body weight. Blood plasma samples were collected at multiple post-treatment time points over a 12-week period for fluralaner and ivermectin plasma concentration analysis.
Ivermectin blood plasma concentration profile and pharmacokinetic parameters Cmax, tmax, AUC∞ and t½ were similar in dogs administered ivermectin only and in dogs administered ivermectin concurrently with fluralaner, and the same was true for fluralaner pharmacokinetic parameters.
Conclusions
Concurrent administration of fluralaner and ivermectin does not alter the pharmacokinetics of either compound. Based on the plasma pharmacokinetic profile and the clinical observations, there is no evident interaction between fluralaner and ivermectin, and co-administration does not increase the risk of ivermectin associated neurotoxicity.
Keywords: Fluralaner, Bravecto™, Ivermectin, Dog, Pharmacokinetic, P-glycoprotein, MDR1
Findings
Background
Fluralaner is a novel systemically administered isoxazoline class compound that provides immediate and persistent insecticidal and acaricidal efficacy after oral administration to dogs. A field study has shown that a single fluralaner dose administered orally to dogs provides at least 12 weeks of flea and tick control [1] and another study demonstrated efficacy against mites (Demodex spp.) [2]. Fluralaner was shown to be safe when administered orally at overdoses of up to 5 times the maximum clinical dose at 8-week intervals in healthy Beagle dogs [3] and at overdoses of 3 times the maximum clinical dose in Collies bearing a homozygous defect of the multi-drug-resistance 1 gene (MDR1 −/−) [4]. There are no known interactions of fluralaner with other veterinary medicinal drugs [5] and fluralaner was shown to be safe when administered concurrently with macrocyclic lactones like milbemycin oxime [6] and moxidectin [7].
Ivermectin is registered for the use in dogs at monthly oral doses of 6 mcg/kg BW for heartworm protection [8]; some veterinarians may choose to administer ivermectin at higher off label doses to treat dogs for different worm or mite infestations (for example 0.05 mg/kg for hookworm, 0.1 mg/kg BW for whipworms, 0.2 mg/kg for Toxocara canis, 0.2-0.4 mg/kg for sarcoptic mange, 0.2 mg/kg for nasal mites Pneumonyssus caninum, 0.3 mg/kg for cheyletiellosis, 0.3–0.6 mg/kg for demodicosis; orally or subcutaneous as single or repeated treatments) [9–20]; however, such high doses of ivermectin cannot safely be administered to “ivermectin-sensitive” dogs carrying a MDR1 mutation [21, 22]. Ivermectin is a substrate for the p-glycoprotein (p-gp) transporter encoded by the MDR1 gene [22, 23]. This transporter limits the entry of its substrates into the body by an efflux-based mechanism, particularly at the blood–brain barrier [24]. Dogs with a homozygous defect of the MDR1 gene do not carry a functional p-glycoprotein transporter and are therefore more susceptible to neurotoxicity caused by ivermectin [21]. Furthermore, drug-drug interactions at the p-glycoprotein transporter may occur following the concurrent use of ivermectin and drugs, leading to an increased risk of neurotoxicity of ivermectin in MDR1 intact dogs. One example is spinosad that inhibits the p-glycoprotein transporter-mediated elimination of ivermectin in MDR1 intact dogs, thereby increasing ivermectin blood concentrations, which leads to a higher risk of neurotoxicity when administering high off-label doses of ivermectin concurrently with spinosad [25–30].
Veterinarians may choose to administer fluralaner and ivermectin concurrently. To ensure that the concurrent use does not increase the risk of ivermectin-associated neurotoxicity, the pharmacokinetic profile of ivermectin was investigated when administered concurrently with fluralaner. For pharmacokinetic characterization over time, fluralaner and ivermectin were administered at high dose rates (i.e. 56 mg fluralaner/kg BW, the highest expected dose in clinical use, and 0.3 mg ivermectin/kg BW) and on a single occasion.
Methods
Thirty healthy Beagle dogs (15 males and 15 females) were included in the study. Dogs were kept indoors in pens with sealed floors and were housed in groups of two or three, with the exception of the 3 days after ivermectin/fluralaner administration, when dogs were housed individually. Dogs had access to water ad libitum throughout the study period and were fed a standard dog diet.
This study was conducted in Ireland in compliance with Directive 2010/63/EU S.I. No. 543 of 2012 and the Irish national animal protection legislation framework (experimental license no. B100\4500), and the study plan was approved by the research organization institutional (Charles River Laboratories Preclinical Services Ireland Ltd.) ethics committee.
The 30 dogs were allocated to three study groups by sorting within gender according to descending body weight and random allocation to a group (Table 1). Ivermectin (Ivomec Classic Injection for Cattle and Sheep; Merial Animal Health) was administered orally at a dose of 0.3 mg/kg BW and fluralaner (Bravecto™; Merck/MSD Animal Health) was administered orally at the maximum clinical dose of 56 mg/kg BW on study day 0. Blood samples for plasma concentration determination were collected prior to administration and at 1, 2, 4, 6, 8, 10, 24, 48, 72, 120, 168, 240, 336, 504, 672, 1008, 1344, 1656 and 2016 h (84 days) after administration. Ivermectin and fluralaner blood plasma concentrations were determined using validated methods (lower limit of quantification 1 ng ivermectin/mL and 10 ng fluralaner/mL).
Table 1.
Study groups for evaluation of the pharmacokinetic profile of ivermectin and fluralaner when administered concurrently to dogs
| Ivermectin | Fluralaner | Ivermectin plus Fluralaner | ||
|---|---|---|---|---|
| Ivermectin dose (mg/kg BW) | 0.3 | - | 0.3 | |
| Fluralaner dose (mg/kg BW) | - | 56 | 56 | |
| Gender | Male | 5 | 5 | 5 |
| Female | 5 | 5 | 5 | |
| Body weight (kg) | Mean ± SD | 13.1 ± 1.2 | 13.3 ± 1.5 | 13.1 ± 1.0 |
SD standard deviation
Standard pharmacokinetic parameters including maximum plasma concentration (Cmax), time to Cmax (tmax), the extrapolated area under the curve (AUC∞) and the elimination half-life (t½) were calculated using non-compartmental and linear trapezoidal methods. Statistical analysis of pharmacokinetic parameters was performed after natural logarithmic transformation, with the exception of tmax, using ANOVA models and 90 % confidence intervals, and the individual animal being the experimental unit. Pharmacokinetic and statistical analyses were performed using SAS/STAT® (Language: Reference, Version 9.3, SAS Institute Inc., Cary, NC, USA).
Results and discussion
The plasma concentration versus time profile of ivermectin was comparable in dogs administered ivermectin only and in dogs administered ivermectin concurrently with fluralaner (Fig. 1). Similarly, the plasma concentration versus time profile of fluralaner was comparable in dogs administered fluralaner only and in dogs administered fluralaner concurrently with ivermectin (Fig. 2). The pharmacokinetic parameters of both, ivermectin and fluralaner, were also comparable across groups (Tables 2 and 3), with no statistical significant differences between groups.
Fig. 1.
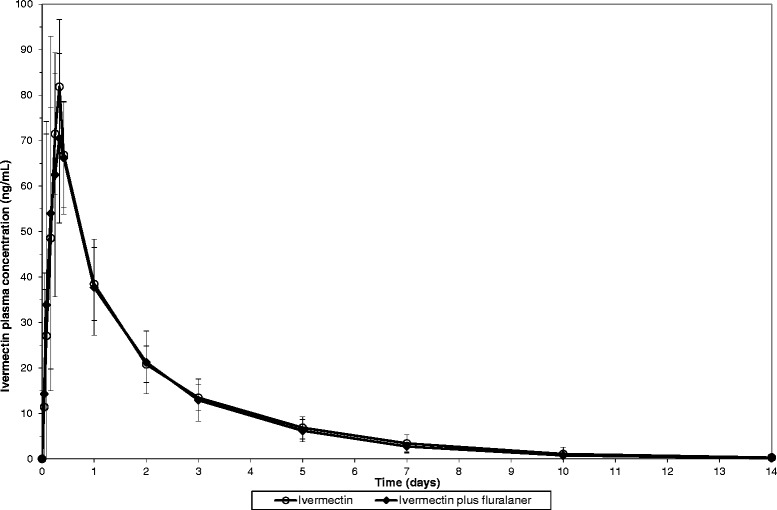
Mean ivermectin plasma concentration (± standard deviation) in dogs following oral administration (0.3 mg/kg BW) alone or concurrently with fluralaner (56 mg/kg BW)
Fig. 2.

Mean fluralaner plasma concentration (± standard deviation) in dogs following oral administration (56 mg/kg BW) alone or concurrently with ivermectin (0.3 mg/kg BW)
Table 2.
Ivermectin pharmacokinetic parameters in dogs following oral administration (0.3 mg/kg BW) alone or concurrently with fluralaner (56 mg/kg BW)
| Parameter | Unit | Ivermectin | Ivermectin plus Fluralaner | P-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Cmax | (ng/mL) | 92.70 ± 26.77 | 80.52 ± 21.41 | 0.2465 |
| tmax | (day) | 0.29 ± 0.10 | 0.31 ± 0.11 | 0.7269 |
| AUC∞ | (day*ng/mL) | 141.96 ± 27.23 | 134.26 ± 37.99 | 0.5073 |
| t½ | (days) | 2.07 ± 0.71 | 1.84 ± 0.42 | 0.4888 |
SD standard deviation
Table 3.
Fluralaner pharmacokinetic parameters in dogs following oral administration (56 mg/kg BW) alone or concurrently with ivermectin (0.3 mg/kg BW)
| Parameter | Unit | Fluralaner | Fluralaner plus Ivermectin | P-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Cmax | (ng/mL) | 7976 ± 4239 | 9312 ± 1767 | 0.1529 |
| tmax | (day) | 3.00 ± 1.49 | 3.20 ± 2.66 | 0.8379 |
| AUC∞ | (day*ng/mL) | 175778 ± 75122 | 184030 ± 49524 | 0.5373 |
| t½ | (days) | 14.27 ± 2.53 | 13.45 ± 1.68 | 0.5107 |
SD standard deviation
Conclusions
Concurrent administration of fluralaner and ivermectin does not alter the pharmacokinetics of either compound. There is no evident interaction of fluralaner and ivermectin indicating an increased risk of ivermectin-associated neurotoxicity in fluralaner-treated dogs.
Acknowledgements
The authors thank Charles River Laboratories Preclinical Services, Ballina, Ireland, for assistance with the study.
Footnotes
Competing interests
FMW, MJA and RKAR are employees of Merck/MSD Animal Health.
Authors’ contributions
FMW, MJA and RKAR authored the study design, monitored the study and interpreted the results. All authors revised and approved the final version of the manuscript.
Contributor Information
Feli M. Walther, Email: feli.walther@merck.com
Mark J. Allan, Email: mark.allan@msd.de
Rainer KA Roepke, Email: rainer.roepke@msd.de.
References
- 1.Rohdich N, Roepke RKA, Zschiesche E. A randomized, blinded, controlled and multi-centered field study comparing the efficacy and safety of Bravecto™(fluralaner) against Frontline™(fipronil) in flea- and tick-infested dogs. Parasit Vectors. 2014;7:83. doi: 10.1186/1756-3305-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fourie JJ, Liebenberg JE, Horak IG, Taenzler J, Heckeroth AR, Frénais R. Efficacy of orally administered fluralaner (Bravecto™) or topically applied imidacloprid/moxidectin (Advocate®) against generalized demodicosis in dogs. Parasit Vectors. 2015;8:187. doi: 10.1186/s13071-015-0775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walther FM, Allan MJ, Roepke RKA, Nuernberger MC. Safety of fluralaner chewable tablets (Bravecto™), a novel systemic antiparasitic drug, in dogs after oral administration. Parasit Vectors. 2014;7:87. doi: 10.1186/1756-3305-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walther FM, Paul AJ, Allan MJ, Roepke RKA, Nuernberger MC. Safety of fluralaner, a novel systemic antiparasitic drug, in MDR1(−/−) Collies after oral administration. Parasit Vectors. 2014;7:86. doi: 10.1186/1756-3305-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Commission: Community register of veterinary medicinal products, Product information Bravecto, Annex 1 Summary of product characteristics.2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/veterinary/002526/WC500163859.pdf
- 6.Walther FM, Fisara P, Allan MJ, Roepke RKA, Nuernberger MC. Safety of concurrent treatment of dogs with fluralaner (Bravecto™) and milbemycin oxime – praziquantel. Parasit Vectors. 2014;7:481. doi: 10.1186/s13071-014-0481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walther FM, Fisara P, Allan MJ, Roepke RKA, Nuernberger MC. Safety of the concurrent treatment of dogs with Bravecto™ (fluralaner) and Scalibor™ protectorband (deltamethrin) Parasit Vectors. 2014;7:105. doi: 10.1186/1756-3305-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul AJ, Todd KS, Jr, Acre KE, Sr, Plue RE, Wallace DH, French RA, et al. Efficacy of ivermectin chewable tablets and two new ivermectin tablet formulations against Dirofilaria immitis larvae in dogs. Am J Vet Res. 1991;52:1922–3. [PubMed] [Google Scholar]
- 9.Anderson DL, Roberson EL. Activity of ivermectin against canine intestinal helminths. Am J Vet Res. 1982;43:1681–3. [PubMed] [Google Scholar]
- 10.Egerton JR, Eary CH, Suhayda D. Dose-titration studies of ivermectin against experimental Ancylostoma caninum and Uncinaria stenocephala infections. Am J Vet Res. 1985;46:1057–9. [PubMed] [Google Scholar]
- 11.Campbell WC, Benz GW. Ivermectin: a review of efficacy and safety. Vet Pharmacol Ther. 1984;7:1–16. doi: 10.1111/j.1365-2885.1984.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghubash R. Parasitic Miticidal Therapy. Clin Tech Small Anim Pract. 2006;21:135–44. doi: 10.1053/j.ctsap.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Pin D, Bensignor E, Carlotti D-N, Cadiergues MC. Localised sarcoptic mange in dogs: a retrospective study of 10 cases. J Small Anim Pract. 2006;47:611–4. doi: 10.1111/j.1748-5827.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 14.Brandt RW. Pneumonyssus caninum (Nasal mite) in Four Golden Retrievers. Can Vet J. 1988;29:741. [PMC free article] [PubMed] [Google Scholar]
- 15.Mundell AC, Ihrke PJ. Ivermectin in the treatment of Pneumonyssoides caninum: a case report. J Am Anim Hosp Assoc. 1990;26:393–6. [Google Scholar]
- 16.Saari S. The nasal mites (Pneumonyssus caninum) in dogs. The first report from Finland. Suomen Eläinlääkärilehti. 1992;98:647–52. [Google Scholar]
- 17.Paradis M, Villeneuve A. Efficacy of Ivermectin against Cheyletiella yasguri Infestation in Dogs. Can Vet J. 1988;29:633–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller R. Treatment protocols for demodicosis: an evidence-based Review. Vet Dermatol. 2004;15:75–89. doi: 10.1111/j.1365-3164.2004.00344.x. [DOI] [PubMed] [Google Scholar]
- 19.Paterson TE, Halliwell RE, Fields PJ, Louw ML, Louw JP, Ball GS, et al. McKibben JS:Treatment of canine-generalized demodicosis: a blind, randomized clinical trial comparing the efficacy ofAdvocate® (Bayer Animal Health) with ivermectin. Vet Dermatol. 2009;20:447–55. doi: 10.1111/j.1365-3164.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 20.Nolan TJ, Lok JB. Macrocyclic lactones in the treatment and control of parasitism in small companion animals. Curr Pharm Biotechnol. 2012;13:1078–94. doi: 10.2174/138920112800399167. [DOI] [PubMed] [Google Scholar]
- 21.Paul AJ, Tranquilli WJ, Seward RL, Todd KS, Jr, DiPietro JA. Clinical observations in collies given ivermectin orally. Am J Vet Res. 1987;48:684–5. [PubMed] [Google Scholar]
- 22.Mealey KL, Bentjen SA, Gay JM, Cantor GH. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;8:727–33. doi: 10.1097/00008571-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Didier A, Loor F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anticancer Drugs. 1996;7:745–51. doi: 10.1097/00001813-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol. 1997;8:161–70. doi: 10.1006/scbi.1997.0068. [DOI] [PubMed] [Google Scholar]
- 25.Hugnet C, Lespine A, Alvinerie M. Multiple oral dosing of ketoconazole increases dog exposure to ivermectin. J Pharm Pharm Sci. 2007;10:311–8. [PubMed] [Google Scholar]
- 26.Schrickx JA, Fink-Gremmels J. Implications of ABC transporters on the disposition of typical veterinary medicinal products. Eur J Pharmacol. 2008;585:510–9. doi: 10.1016/j.ejphar.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Fenner KS, Troutman MD, Kempshall S, Cook JA, Ware JA, Smith DA, et al. Drug–Drug Interactions Mediated Through P-Glycoprotein: Clinical Relevance and In Vitro–In Vivo Correlation Using Digoxin as a Probe Drug. Clin Pharmacol Ther. 2009;85:173–81. doi: 10.1038/clpt.2008.195. [DOI] [PubMed] [Google Scholar]
- 28.Dunn ST, Hedges L, Sampson KE, Lai Y, Mahabir S, Balogh L, et al. Pharmacokinetic Interaction of the Antiparasitic Agents Ivermectin and Spinosad in Dogs. Drug Metab Dispos. 2011;39:789–95. doi: 10.1124/dmd.110.034827. [DOI] [PubMed] [Google Scholar]
- 29.Schrickx JA, Fink-Gremmels J. A porcine lymphocyte model for P-gp inhibition studies. J Vet Pharmacol Ther. 2011;34:499–501. doi: 10.1111/j.1365-2885.2011.01270.x. [DOI] [PubMed] [Google Scholar]
- 30.Schrickx AJ. Spinosad is a potent inhibitor of canine P-glycoprotein. Vet J. 2014;200:195–6. doi: 10.1016/j.tvjl.2014.01.012. [DOI] [PubMed] [Google Scholar]


