Abstract
Background
Hypertensive disorders complicating pregnancy (HDCP) continues to be a leading cause of maternal and neonatal mortality and morbidity. The clinical value of placental three-dimensional power Doppler (3DPD) in assessing HDCP requires further confirmation. The research was developed to assess changes of placental vascularity in HDCP using 3DPD and to investigate the placental vascularity in small for gestational age (SGA) compared with not-SGA in patients with HDCP.
Methods
There were 126 normotensive and 128 hypertensive pregnant women included in this prospective case–control study from March 2011 to March 2013. Pregnant women underwent 3DPD. Vascularization index (VI), flow index (FI) and vascularization flow index (VFI) were obtained. The placental 3DPD indices, umbilical artery systolic and diastolic ratio (S/D) and pregnancy outcomes were compared between the groups.
Results
The placental VI and VFI were significantly lower in hypertensive women compared with normotensive women (P < 0.001 and P = 0.014, respectively), and these parameters were significantly reduced in severe preeclampsia (P < 0.001 and P = 0.003, respectively). A weak correlation was found between VI and umbilical artery S/D in HDCP group (r = -0.277, P = 0.001). In HDCP population, neonates who were postnatally diagnosed with SGA had lower VI (P = 0.041) and higher S/D (P < 0.001).
Discussion
The placental vascularity indices decreased in hypertensive women and the reduction inplacental perfusion was consistent with the severity of the hypertensive disorder. The associations betweenplacental vascularization and umbilical artery impedance may be valuable for further researches and arerequired confirmation. The significant differences in the 3DPD placental vascularization between SGA andnot-SGA in hypertensive pregnancy population may show some clinical importance that we could use tobetter assess or predict the progression and adverse outcomes in the future. Although 3DPD quantificationhas been widely used in multiple publications, we have to acknowledge its limitations.
Conclusions
The intraplacental vascularization was poor in HDCP, and especially in severe preeclampsia. Neonates with SGA had poor placental vascularization and higher umbilical artery S/D. Further studies should focus on the clinical assessment of placental 3DPD as well as a combination of placental 3DPD and other fetal Doppler indices to better predict the development and outcomes of preeclampsia.
Keywords: Hypertensive disorders complicating pregnancy (HDCP), Placenta, Small for gestational age (SGA), Three-dimensional power Doppler (3DPD)
Background
Hypertensive disorders complicating pregnancy (HDCP) continues to be a leading cause of maternal and neonatal mortality and morbidity [1]. The prevalence of HDCP is approximately 5.22 % of all pregnancies in China and 8–10 % worldwide [2, 3]. In recent years, important advances have been made in our understanding of the pathogenesis and pathophysiology of preeclampsia, but most of the factors that contribute to the disease remain unclear. Multiple tests and combination tests have been developed as screening methods for preeclampsia, including maternal serum biomarkers and Doppler parameters. To date, there is no effective screening test for HDCP [4–6].
HDCP is currently considered as a chronic placental disease. Placental dysfunction and hypo-perfusion play an important role in HDCP pathophysiology and are considered to be responsible for pathologic pregnancy outcomes, such as fetal growth restriction and perinatal loss [7–9]. Three-dimensional power Doppler (3DPD) has been a focal point of recent placental research; it is superior to 2D Doppler in several ways, including its ability to detect the secondary and tertiary stem vessels in the placenta and intraplacental vessel characteristics, such as the vessel density, branching, caliber changes and tortuosity, which can be shown using 3DPD [8, 10, 11]. Quantitative 3DPD analysis has been used to assess placental perfusion and vascularization indices, which potentially reflect both utero-placental and feto-placental blood perfusion [10].
Assessment of placental perfusion using 3DPD seems to be more helpful in understanding HDCP pathophysiology. However, the clinical value of placental 3DPD in assessing HDCP requires confirmation. The aim of our study was to compare changes in placental vascularity between HDCP and normotensive subjects using 3DPD, and to assess whether placental vascularity changes presenting in small for gestational age (SGA) compared with not-SGA in HDCP.
Patients and methods
Enrolment
This was a prospective case–control study that was conducted at the First Affiliated Hospital of Xi’an Jiaotong University, ShaanXi, China, from March 2011 to March 2013. Women with HDCP were enrolled, and normotensive pregnant women were used as controls. HDCP and normotensive pregnant subjects were individually matched by maternal age ± 2 years and gestational week at examination ±2 weeks.
The hypertensive group was further stratified into the following subgroups: (1) gestational hypertension (hypertension for the first time during pregnancy without proteinuria); (2) severe preeclampsia (diastolic BP ≥110 mmHg, systolic BP ≥160 mmHg; proteinuria from none to positive; elevated serum creatinine and transaminase levels; obvious fetal growth restriction (FGR); multi-organ disturbances, such as headache, visual disturbance, upper abdominal pain, oliguria, convulsion, thrombocytopenia and pulmonary edema); (3) nonsevere preeclampsia (diastolic BP <110 mmHg, systolic BP <160 mmHg; proteinuria from none to positive; no multi-organ disturbances or only minimal serum transaminase elevation). Nonsevere preeclampsia includes “mild” and “moderate” hypertension, which is not specifically defined. Overt proteinuria may not be a necessary element to characterize preeclampsia syndrome [12]. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg on at least two occasions at a 6-hour interval.
All women with fetal chromosomal abnormalities, fetal malformations, placental anomalies (bipartite placenta or velamentous insertions), umbilical anomalies, complications associated with pregnancy (including diabetes mellitus, intrahepatic cholestasis, thrombophilia and thrombocytopenia), systemic vascular or autoimmune disorders, multiple pregnancies, or rupture of amniotic membrane as well as women receiving vasoactive drugs or those in active labor were excluded from the study. Control participants were healthy and normotensive pregnant women.
The study was approved by the ethics and research committee of the First Affiliated Hospital of Xi’an Jiaotong University. The purpose and procedures of the study were explained to all enrolled participants and written informed consent was obtained from each participant.
3DPD assessment
A single operator (Ting Yuan) performed all ultrasound scans using a Voluson E8 (GE Medical Systems, Milwaukee, WI, USA) ultrasound system. In addition to routine ultrasound, the placental 3DPD was performed in all women.
Placental vascularization was assessed using 3DPD. Because placental blood perfusion varied from region to region, we standardized the process by choosing five different sampling sites, according to Guiot [13]. The five spherical sampling sites in each placenta were as follows: one central, two peripheral at opposite sides and one between the central site and each peripheral site.
The peripheral sampling spheres were always positioned to maintain a minimum distance of approximately 4 mm between the insonated region and both the chorionic and basal plates. Maximal sensitivity was ensured using the following settings: pulse repetition frequency (PRF), 0.6 kHz; wall motion filter (WMF), low 1; frequency, mid; dynamic, 3; balance, 4150; smooth, 4/5; ensemble, 11; line density, 8; power Doppler map, 4; artefact suppression, off; power Doppler line filter, off; and quality, high. The placenta was investigated using a constant volume and the same 25-degree sector angle throughout. The spheres were kept away from the spiral arteries and they were placed near the basal and/or chorionic plate (Fig. 1). The 3D Glass Body render mode was used, in which the colour and the grey value information are processed and combined to give a 3D image. The vascularization indices were obtained using the Virtual Organ Computer-aided Analysis (VOCAL) program. The manual mode was set, and the five spherical regions of interest in each placenta were manually encircled by rotating 30 degrees six times, respectively. The sphere volume did not include vessels from the basal and chorionic plates, and it was kept constant. A histogram automatically showed the vascularization indices. The three-dimensional volume was formed using basic units called voxels. The voxel contains all information about the grey scale and colour, according to an intensity scale that ranges from 0 to 100. The vascularization indices are as follows: vascularization index (VI) refers to the colour voxel-to-total voxel ratio, indicating the number of vessels that could be detected within the placental volume; flow index (FI) refers to the weighted colour voxel divided by the total colour voxel ratio and provides an amplitude value for the colour signal, which indirectly estimates the placental blood flow during a 3D sweep; vascularization flow index (VFI) refers to the weighted colour voxel-to-total voxel ratio, which indicates a combination of vascularity and blood flow. The three index values were obtained from each sampling measurement, and the average value of the three indices from five measurements was provided for the final statistical analysis.
Fig. 1.
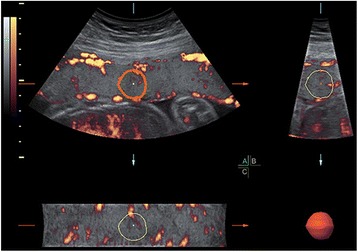
Assessment of the placental 3DPD at 25 weeks gestation in normal pregnancy
The systolic and diastolic ratio (S/D) of the umbilical artery Doppler measurement was used for the final analysis. Other records were reviewed to evaluate the pregnancy outcomes, including the delivery mode, delivery week, neonatal birth weight, Apgar scores and the diagnosis of small for gestational age (SGA). SGA was confirmed at birth by a newborn weight <2 standard deviations (SDs) below the mean for gestational age [14]. The gestational age was calculated using the last menstruation period (LMP) or confirmed based on the results of the first trimester ultrasound.
Statistical analysis
Data gathered from the study subjects were verified and double-entered into a data management system. Variables are presented as the mean ± SD or median (range) and percentages, as appropriate. Statistical analysis was performed using the Pearson, χ2, Fisher exact, Student’s t, Mann–Whitney U, analysis of variance, Kruskall-Wallis and Steel Dwass tests, as appropriate. The following variables were compared between the groups: maternal age, gestational week at examination, parity, SBP, DBP, placental vascularity (VI, FI and VFI), umbilical artery S/D, delivery mode, delivery week, Apgar scores and SGA diagnosis. Correlation analysis was performed using Spearman rank analysis between placental vascularity and umbilical artery S/D. All reported P values were 2-tailed, and values <0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 16.0 (IBM, Armonk, NY, USA) and GraphPad Prism version 5.0 (GraphPad, La Jolla, CA).
Results
A total of 264 pregnant women were initially enrolled, but 2 were diagnosed with intrahepatic cholestasis, 2 were diagnosed with gestational diabetes and 6 were lost to follow-up. As a result, 126 normotensive women and 128 hypertensive pregnant women were included in the final analysis. In the hypertensive group, there were 38 patients with gestational hypertension, 40 patients with mild preeclampsia and 50 patients with severe preeclampsia.
Table 1 shows the characteristics of the normotensive and hypertensive pregnant women. There were similar maternal ages, gestational weeks at examination and parity in both groups. The SBP and DBP were significantly higher in the hypertensive group than in normotensive group. The placental VI and VFI were significantly lower in the hypertensive group than in the normotensive women (P < 0.001 and P = 0.014, respectively). The umbilical artery S/D increased significantly in the hypertensive group compared with the normotensive group (P = 0.001). Overall, hypertensive pregnant women (median delivery gestational age, 36.43 weeks) delivered earlier than pregnant women in the control group (median delivery gestational age, 39.57 weeks; P < 0.001). Additionally, the delivery mode was significantly different, with a higher frequency of caesarean section and induced labor with amniotic cavity injection in the hypertensive group compared with the normotensive group (P < 0.001). There were more neonates with 1-minute Apgar scores <7 and more neonates diagnosed with SGA in the hypertensive group compared with the controls (P < 0.001 and P = 0.004, respectively).
Table 1.
Characteristics, placental vascularity and neonatal outcomes in the normotensive and hypertensive groups
| Parametera | Normotensive(n = 126) | Hypertensive(n = 128) | P |
|---|---|---|---|
| Age, yrs | 29.14 ± 4.22 | 29.76 ± 4.82 | 0.341 |
| Gestational age at examination,wks | 35.00(22.57–42.00) | 35.86(21.14–41.57) | 0.320 |
| Parity, n | 0.381 | ||
| Nulliparous | 100 | 95 | |
| Mutiparous | 26 | 33 | |
| SBP, mmHg | 110(85–125) | 150(130–230) | <0.001 |
| DBP, mmHg | 70(56–87) | 100(90–158) | <0.001 |
| VI | 24.47(10.56–48.22) | 18.49(10.49–49.55) | <0.001 |
| FI | 43.89(7.26–92.80) | 41.50(1.02–97.70) | 0.620 |
| VFI | 10.47(0.91–31.54) | 7.55(0.11–44.20) | 0.014 |
| Um-S/D | 2.23(1.44–3.55) | 2.48(1.54–5.92) | 0.001 |
| Delivery mode, n | <0.001 | ||
| vaginal | 59 | 12 | |
| cesarean | 67 | 105 | |
| Induced labor | 0 | 11 | |
| Gestational age at delivery,wks | 39.57(34.14–42.00) | 36.43(22.29–41.71) | <0.001 |
| Postnatal SGA, n | <0.001 | ||
| Yes | 3 | 26 | |
| No | 123 | 91 | |
| 1-m-Apgar score, n | 0.004 | ||
| < 7 | 3 | 12 | |
| ≥ 7 | 123 | 105 |
a SBP systolic blood pressure, DBP diastolic blood pressure, VI vascularization index, FI flow index, VFI vascularization flow index, Um-S/D umbilical artery systolic and diastolic ratio, SGA small for gestational age
Table 2 shows the characteristics and results among normotensive patients and those with different types of hypertension. The demographic information was similar between the groups. Compared with normotensive subjects, patients with severe preeclampsia had a significant reduction in the placental VI (P < 0.001) and VFI (P = 0.001), as well as a significant increase in umbilical artery S/D (P < 0.001), but there was no difference with the other types of hypertension (Figs. 2, 3, 4 and 5). The delivery week, SGA diagnosis and 1-minute Apgar scores <7 were compared between groups, and patients with severe preeclampsia delivered the earliest and had the highest frequency of neonates with SGA as well as a 1-minute Apgar score <7.
Table 2.
Characteristics, placental vascularity and neonatal outcomes in different subgroups
| Parametera | Normotensive (n = 126) | Gestational hypertension (n = 38) | Nonsevere PEb (n = 40) | Severe PEb (n = 50) | P |
|---|---|---|---|---|---|
| Age, yrs | 29.14 ± 4.22 | 29.56 ± 5.21 | 28.96 ± 6.23 | 30.81 ± 5.56 | 0.371 |
| Gestational age at examination, wks | 35.00(22.57–42.00) | 37.23(34.57–41.57) | 36.50(34.00–39.25) | 35.53(21.14–38.56) | 0.070 |
| Parity, n | 0.131 | ||||
| Nulliparous | 100 | 33 | 27 | 35 | |
| Mutiparous | 26 | 5 | 13 | 15 | |
| SBP, mmHg | 110(85–125) | 133(130–145) | 148(140–155) | 180(160–230) | <0.001 |
| DBP, mmHg | 70(56–87) | 95(90–98) | 95(90–105) | 130(115–158) | <0.001 |
| VI | 24.47(10.56–48.22) | 23.38(12.17–40.94) | 23.13(12.88–49.55) | 16.22(10.49–43.06) | <0.001 |
| FI | 43.89(7.26–92.80) | 55.21(2.06–97.70) | 32.70(2.30–95.96) | 38.16(1.02–89.54) | 0.282 |
| VFI | 10.47(0.91–31.54) | 10.56(0.28–32.48) | 11.81(0.30–44.20) | 7.09(0.11–36.62) | 0.003 |
| Um-S/D | 2.23(1.44–3.55) | 2.25(1.63–3.51) | 2.42(1.60–3.27) | 2.74(1.54–5.92) | <0.001 |
| Delivery mode, n | <0.001 | ||||
| vaginal | 59 | 9 | 3 | 0 | |
| cesarean | 67 | 29 | 37 | 39 | |
| Induced labor | 0 | 0 | 0 | 11 | |
| Gestational age at delivery, wks | 39.57(34.14–42.00) | 38.86(33.86–41.71) | 37.71(30.14–40.28) | 34.79(22.29–41.00) | <0.001 |
| Postnatal SGA, n | <0.001 | ||||
| Yes | 3 | 6 | 7 | 13 | |
| No | 123 | 32 | 33 | 26 | |
| 1-m-Apgar score, n | <0.001 | ||||
| < 7 | 3 | 0 | 2 | 10 | |
| ≥ 7 | 123 | 38 | 38 | 29 |
a SBP systolic blood pressure, DBP diastolic blood pressure, VI vascularization index, FI flow index, VFI vascularization flow index, Um-S/D umbilical artery systolic and diastolic ratio, SGA small for gestational age
b PE preeclampsia
Fig. 2.
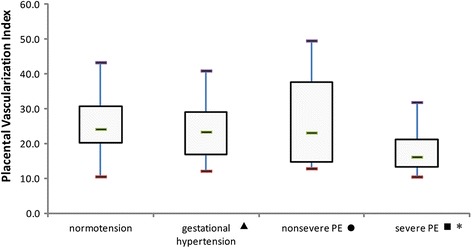
The placental VI in different population subgroups. The horizontal bar within each box corresponds to the median. The upper and lower bars of each box correspond to the first and third quartiles, respectively. The vertical whiskers outside each box extend to the smallest and largest observations within a 1.5 interquartile range (third-first quartiles). The symbols of ▲, ● and ■ represented the comparisons of parameters between normal group and gestational hypertension, normal group and nonsevere PE, normal group and severe PE. * represented a significant difference. PE = preeclampsia. For placental VI, P values of ▲, ● and ■ were 0.32, 0.61 and <0.001, respectively
Fig. 3.
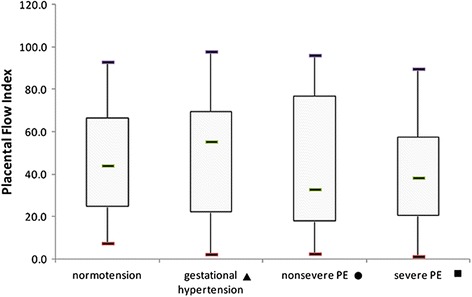
The placental FI in different population subgroups. The horizontal bar within each box corresponds to the median. The upper and lower bars of each box correspond to the first and third quartiles, respectively. The vertical whiskers outside each box extend to the smallest and largest observations within a 1.5 interquartile range (third-first quartiles). The symbols of ▲, ● and ■ represented the comparisons of parameters between normal group and gestational hypertension, normal group and nonsevere PE, normal group and severe PE. * represented a significant difference. PE = preeclampsia. For placental FI, P values of ▲, ● and ■ were 0.24, 0.81 and 0.24, respectively
Fig. 4.
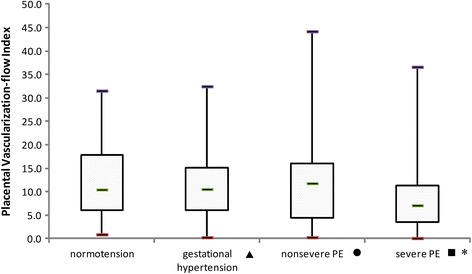
The placental VFI in different population subgroups. The horizontal bar within each box corresponds to the median. The upper and lower bars of each box correspond to the first and third quartiles, respectively. The vertical whiskers outside each box extend to the smallest and largest observations within a 1.5 interquartile range (third-first quartiles). The symbols of ▲, ● and ■ represented the comparisons of parameters between normal group and gestational hypertension, normal group and nonsevere PE, normal group and severe PE. * represented a significant difference. PE = preeclampsia. For placental VFI, P values of ▲, ● and ■ were 0.97, 0.70 and 0.001, respectively
Fig. 5.
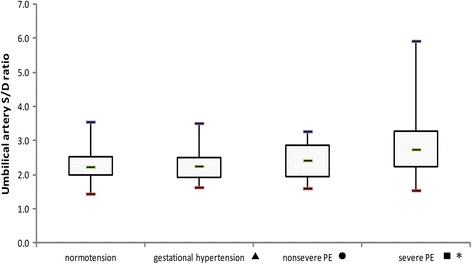
The umbilical artery S/D in different population subgroups. The horizontal bar within each box corresponds to the median. The upper and lower bars of each box correspond to the first and third quartiles, respectively. The vertical whiskers outside each box extend to the smallest and largest observations within a 1.5 interquartile range (third-first quartiles). The symbols of ▲, ● and ■ represented the comparisons of parameters between normal group and gestational hypertension, normal group and nonsevere PE, normal group and severe PE. * represented a significant difference. PE = preeclampsia. For umbilical S/D ratio, P values of ▲, ● and ■ were 0.69, 0.62 and <0.001, respectively
Table 3 shows the correlation between placental vascularity and umbilical artery S/D. There were no correlations in the normotensive population. However, the correlation between VI and S/D was significant (P = 0.001) in the hypertensive population, but the correlation was relatively weak (r = –0.277).
Table 3.
Correlation between placental vascularity and Um-S/D in normotensive and hypertensive population
| Indexa | Normotensive | Hypertensive | ||
|---|---|---|---|---|
| r | P | r | P | |
| VI | 0.132 | 0.248 | −0.277 | 0.001 |
| FI | 0.014 | 0.904 | −0.122 | 0.159 |
| VFI | 0.147 | 0.196 | −0.148 | 0.088 |
a VI vascularization index, FI flow index, VFI vascularization flow index
Table 4 shows the comparison of patient characteristics and placental vascularity based on a postnatal SGA diagnosis only in the hypertensive population (for 128 hypertensive pregnant women, 117 neonates were alive). Neonates that were diagnosed with SGA were born significantly earlier than those with normal birth weights. The placental VI and umbilical artery S/D were significantly different in hypertensive pregnancies, resulting in SGA neonates (P = 0.041 and P < 0.001, respectively).
Table 4.
Characteristics and placental vascularity according to postnatal diagnosis of SGA in the hypertensive population
| Parametera | SGAb | P | |
|---|---|---|---|
| No(n = 91) | Yes(n = 26) | ||
| Diagnosis, n | 0.122 | ||
| Gestational H | 32 | 6 | |
| Mild PE | 33 | 7 | |
| Severe PE | 26 | 13 | |
| Age, yrs | 29.90 ± 4.88 | 30.20 ± 4.94 | 0.800 |
| Gestational age at delivery, wks | 37.57(31.71–41.71) | 33.85(31.14–38.57) | <0.001 |
| VI | 19.15(10.49–47.47) | 15.49(10.62–49.55) | 0.041 |
| FI | 45.06(1.02–96.52) | 38.97(3.94–97.70) | 0.621 |
| VFI | 8.00(0.11–44.20) | 8.93(0.48–32.39) | 0.917 |
| Um-S/D | 2.36(1.54–5.92) | 3.41(1.94–5.77) | <0.001 |
agestational h = gestational hypertension, PE preeclampsia, VI vascularization index, FI flow index, VFI vascularization flow index, Um-S/D umbilical artery systolic and diastolic ratio
b SGA small for gestational age
Discussion
Quantitative 3DPD histogram assessments of placental vascularization and blood flow have become feasible because of recent advances [10, 15]. Merce LT et al. [15] and some other authors reported that there were positive correlations between gestational age and the placental vascularization indices, and more placental trees and placental blood perfusion are available as gestation progresses in normal pregnancies [16, 17]. However, de Paula CF et al. [18] demonstrated that all placental vascular indices estimated by 3DPD presented a constant distribution throughout gestation in spite of the increase in placental volume according to gestational age.
According to previous studies, the placental vascularity indices decreased in hypertensive women compared with normal pregnancies, which has been demonstrated by many authors [19–22]. In the current study, we also found that, compared with normotensive pregnancies, there was less intraplacental vascularization in hypertensive pregnant women, and intraplacental vascularization was the worst in women with severe preeclampsia. Intraplacental vascularization in gestational hypertension and nonsevere preeclampsia did not differ from that in the normotensive group. However, superimposed preeclampsia was also found to have the most significant reduction in placental perfusion when compared amongst hypertensive disorder subgroups [19]. These results reflected the reduction in placental perfusion, which was consistent with the severity of the hypertensive disorder. In preeclampsia, a disease that reduces the number of vessels in the placental territory, a lower VI value could be interpreted in the context of deficient trophoblast invasion of spiral arteries [20]. The reduction in VFI indicates that the number of placental trees and blood flow perfusion are decreased. The FI value could be lower, reflecting placental blood insufficiency, although this was not confirmed in the study. Damages to placental vessels may be relatively mild in gestational hypertension and nonsevere preeclampsia, or in compensatory stage, which presented with no significant alterations in the placental vascularity indices.
Both low impedance of placental blood flow and intraplacental hyperperfusion, which conveys oxygen and nutrients, are necessary for normal fetal development and survival during pregnancy. Placental dysfunction and hypoperfusion are part of the preeclampsia pathophysiology and they correlate with adverse fetal outcomes. Doppler measurement of the umbilical artery has been used for decades to assess the fetal condition. Elevated impedance of the umbilical artery and absent end diastolic velocity (AEDV) or reverse end diastolic velocity (REDV) denotes a higher risk of survival and adverse outcomes for the fetus [23, 24]. Therefore, we assessed the correlation between the placental vascularity and umbilical artery impedance. There was no correlation in normal pregnancies, but a correlation was present in the hypertensive population. A lower placental vascularization was associated with a higher umbilical artery impedance. Therefore, some fetal outcomes may be an indirect result of reduced placental blood flow. Nevertheless, this relatively weak correlation (r = −0.277) should be taken into account. Doppler measurement of the umbilical artery is usually influenced by various factors, such as fetal movement and the patient’s position; thus, further research is required to confirm the actual correlation.
Pomorski M et al. [25] evaluated the placental vascularity indices between normal pregnancies and FGR pregnancies, and the indices were all lower in FGR pregnancies. They further suggested that VI and VFI were the best parameters with the most favorable potential to identify FGR pregnancies. de Almeida PE et al. [19] studied 62 normotensive and 66 hypertensive pregnant women, and no placental vascularity differences were found in neonates who were SGA compared to normal-sized neonates in the entire population. We compared the placental vascularity and umbilical artery S/D based on the postnatal SGA diagnosis in only the hypertensive population. The neonates who were diagnosed with SGA had a significantly lower prenatal VI and a higher umbilical artery S/D. Other studies have shown that FGR and low birth weight were associated with higher umbilical artery resistance, low placental weight and placental hypermaturiy, higher frequency of placentas in the III-level and infarction in the group with a higher umbilical artery [26, 27]. These results were in accordance with our findings. There are dysplastic placental villi and fibrous sediments in the villi, increased areas of atherosclerosis, ischemia and infarctions, resulting in a decreased total cross sectional area of the placental vessels, placental hypo-infusion and hypoxia, increased resistance of fetal circulation, elevated umbilical artery S/D, FGR and low birth weight.
Hata T et al. [28] assessed placental perfusion at 18–22 weeks of gestation in a low-risk population to predict adverse obstetrical complications or outcomes, including FGR and HDCP. It is the first study to assess 3DPD placental vascularization at approximately 20 weeks of gestation in a low-risk population to evaluate whether reduced endovascular trophoblast invasion and uteroplacental artery remodeling occur in pregnant women who develop FGR or HDCP. However, there were no significant differences in the VI, FI, or VFI values between FGR and normal pregnancies or between HDCP and non-HDCP pregnancies. Possible explanations for this lack of difference were the low number of women who developed adverse outcomes and that most HDCP cases were classified as nonsevere type. In spite of this, some authors have reported different results [29, 30]. The VI and VFI between 11–14 weeks of gestation were significantly lower in the pregnant women who developed PE and VI, which may have potential usefulness for the detection of HDCP. Therefore, it remains unclear whether 3DPD can depict early impaired proliferation, migration, and invasion of trophoblasts into the maternal decidua and myometrium [28]. It is necessary to confirm the true predictive value of 3DPD for placental vascularization in a low-risk population. By contrast, the study design we performed was evaluate whether there were significant differences in the 3DPD placental vascularization between SGA and not-SGA in the hypertensive pregnancy population instead of the low-risk population. The findings in Tables 3 and 4 showed the clinical importance of variables that could be used in combination to better assess or predict the progression of high-risk pregnancies and their adverse outcomes in the future. As a result, early precautions and effective interventions could be made to improve the prognosis.
With respect to the reproducibility of 3DPD ultrasound placental index measurements, there is significant variability for placental 3D power Doppler vascular index values among previous studies [31]. Merce LT et al. [32] were the first to reveal the validity of 3DPD, confirming the favourable reproducibility of 3DPD vascular indices in normal pregnancies. Similarly, Hata T et al. [33] and other researchers also provided intra- and inter-observer reproducibility applying 3DPD parameters in the placenta [34–37]. However, though multiple publications and journal submissions have used 3DPD, the reliability and reproducibility of 3DPD parameters are still questioned. Lai PK et al. [38] showed a poor reproducibility at even the most fundamental level in normal pregnancies. Guimarães Filho HA et al. [37] demonstrated that the FI was the highest reproducibility index in normal pregnancies between 26 and 35 weeks. Guiot C et al. [13] investigated normal and FGR pregnancies between 23-37 weeks and reported that the FI showed the least variability between the samples and was lower than controls only in the group with severe impairment of feto-placental blood perfusion. The results by Costa J et al. [22] were consistent with this. However, the study on 11–14 weeks of normal pregnancy by Tuuli MG et al. [39] revealed that VI and VFI were the most reliable indices, while FI showed significant bias towards underestimation in placental sonobiopsy research. It is concluded that further investigations on the reproducibility of placental perfusion and quantification using VOCAL are still required before 3DPD is applied as a clinical tool to quantify placental blood perfusion in normal and complicated pregnancies.
This study had some limitations. While the method of estimating placental vascularization was standardized and we considered the vascularization variability throughout the placenta, the long duration required for the measurement seemed to be a disadvantage.
Although 3DPD quantification has been widely used in recent studies and publications in the field of obstetrics and gynecology, we acknowledge that PD quantification also has several methodological limitations for assessing vascularization [40, 41]. These limitations include the high dependence on gain settings, lack of standardized machine settings [42–45], differences in attenuation [46], and issues with reproducibility [47–49], which are capable of altering the VI, FI and VFI quantification. A meaningful interpatient comparison of vascularity cannot be made without standardization. These issues limit the clinical applicability of 3DPD imaging. One alternative that could compensate for the effects of machine settings, attenuation, organ position and other confounding factors is fractional moving blood volume (FMBV), which can provide a more robust estimation of vascularity [50–54]. However, this method was not performed in the current study, and more obstetrics investigations that use FMBV should be performed in the future. Additionally, the volumetric pulsatility index (vPI) assessing the volumetric vascular impedance with spatiotemporal image correlation (STIC) and VOCAL could overcome these deficiencies, representing an important step in the process of standardization [43]. These alternative methods will be performed in the future.
Additionally, Zalud I et al. [55] demonstrated that there was a significant difference in the uterine spiral vasculature volume between younger and older women and that obstetrical complications could be partially related to the decreased uterine spiral vasculature volume; moreover, parity influenced all placental 3DPD vascularity indices. In the current study, there were no significant differences in maternal age and parity between the groups. However, in studies on hypertensive pregnancies, it seems necessary to adjust or stratify these influencing factors to gain more accurate results in the future.
Conclusion
The intraplacental vascularization was poor in HDCP, especially in patients with severe preeclampsia. Neonates who were diagnosed with SGA had poor placental vascularization and higher umbilical artery S/D. Further studies should focus on the clinical assessment of placental 3DPD as well as a combination of placental 3DPD and other Doppler indices that represent the fetus could better predict the development and outcomes in patients with preeclampsia.
Acknowledgements
We would like to thank Chao Li (doctoral student in the Department of Public Health in medical school of Xi’an Jiaotong university) for the assistance with the data analysis.
Funding
There is no funding for the study.
Abbreviations
- 3DPD
Three-dimensional power Doppler
- FI
Flow index
- HDCP
Hypertensive disorders complicating pregnancy
- SGA
Small for gestational age
- S/D
Systolic and diastolic ratio
- UmA
Umbilical artery
- VI
Vascularization index
- VFI
Vascularization flow index
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TY participated in the study design, collected the data and prepared the manuscript. TZ performed the quality control process and provided the statistical analysis. ZH conceived of the research and was responsible for guidance regarding image scanning before the research was initiated. All authors have read and approved the final manuscript.
Authors’ information
TY and TZ are all PhD candidates, ZH is PhD, professor and doctoral supervisor.
Availability of data and materials
Not applicable.
Author details
All the authors are from Department of Obstetrics & Gynecology, First Affiliated Hospital of Xi’an Jiaotong University College of Medicine, Xi’an, Shaanxi 710061, P.R. China.
Contributor Information
Ting Yuan, Email: y.t.1109@qq.com.
Ting Zhang, Email: tingting711@stu.xjtu.edu.cn.
Zhen Han, Email: hanamy02@163.com.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One. 2014;9:e100180. doi: 10.1371/journal.pone.0100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–9. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Yigiter AB, Kavak ZN, Durukan B, Isci H, Uzuner A, Uyar E, et al. Placental volume and vascularization flow indices by 3D power Doppler US using VOCAL technique and correlation with IGF-1, free beta-hCG, PAPP-A, and uterine artery Doppler at 11–14 weeks of pregnancy. J Perinat Med. 2011;39:137–41. doi: 10.1515/jpm.2010.136. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz N, Mandel D, Shlakhter O, Coletta J, Pessel C, Timor-Tritsch IE, et al. Placental morphologic features and chorionic surface vasculature at term are highly correlated with 3-dimensional sonographic measurements at 11 to 14 weeks. J Ultrasound Med. 2011;30:1171–8. doi: 10.7863/jum.2011.30.9.1171. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo G, Silvestri E, Capponi A, Servadei F, Pietrolucci ME, Capece A, et al. Histomorphometric characteristics of first trimester chorionic villi in pregnancies with low serum pregnancy-associated plasma protein-A levels: relationship with placental three-dimensional power Doppler ultrasonographic vascularization. J Matern Fetal Neonatal Med. 2011;24:253–7. doi: 10.3109/14767058.2010.482627. [DOI] [PubMed] [Google Scholar]
- 7.Dikshit S. Fresh look at the Doppler changes in pregnancies with placental-based complications. J Postgrad Med. 2011;57:138–40. doi: 10.4103/0022-3859.81880. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S. Placental vasculature as visualized by 3D power Doppler angiography and 3D colour Doppler imaging. Ultrasound Obstet Gynecol. 2007;30:917–20. doi: 10.1002/uog.5195. [DOI] [PubMed] [Google Scholar]
- 9.Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D’Souza SW, et al. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58:827–32. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- 10.Guimaraes FH, da Costa LL, Araujo JE, Nardozza LM, Nowak PM, Moron AF, et al. Placenta: angiogenesis and vascular assessment through three-dimensional power Doppler ultrasonography. Arch Gynecol Obstet. 2008;277:195–200. doi: 10.1007/s00404-007-0453-y. [DOI] [PubMed] [Google Scholar]
- 11.Konje JC, Huppertz B, Bell SC, Taylor DJ, Kaufmann P. 3-dimensional colour power angiography for staging human placental development. Lancet. 2003;362:1199–201. doi: 10.1016/S0140-6736(03)14514-X. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL. Hypertensive disorders. In: Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, editors. Williams Obstetrics. Chapter 40. 24. New York: McGraw-Hill; 2014. pp. 728–30. [Google Scholar]
- 13.Guiot C, Gaglioti P, Oberto M, Piccoli E, Rosato R, Todros T. Is three-dimensional power Doppler ultrasound useful in the assessment of placental perfusion in normal and growth-restricted pregnancies? Ultrasound Obstet Gynecol. 2008;31:171–6. doi: 10.1002/uog.5212. [DOI] [PubMed] [Google Scholar]
- 14.Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated fetal weights. Acta Paediatr. 1996;85:843–8. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- 15.Merce LT, Barco MJ, Bau S, Kupesic S, Kurjak A. Assessment of placental vascularization by three-dimensional power Doppler “vascular biopsy” in normal pregnancies. Croat Med J. 2005;46:765–71. [PubMed] [Google Scholar]
- 16.Guimaraes FH, Araujo JE, Mattar R, Da Costa LL, de Mello Junior CF, Nardozza LM, et al. Placental blood flow measured by three-dimensional power Doppler ultrasound at 26 to 35 weeks gestation in normal pregnancies. J Matern Fetal Neonatal Med. 2010;23:69–73. doi: 10.3109/14767050903121431. [DOI] [PubMed] [Google Scholar]
- 17.Yu CH, Chang CH, Ko HC, Chen WC, Chang FM. Assessment of placental fractional moving blood volume using quantitative three-dimensional power Doppler ultrasound. Ultrasound Med Biol. 2003;29:19–23. doi: 10.1016/S0301-5629(02)00695-6. [DOI] [PubMed] [Google Scholar]
- 18.de Paula CF, Ruano R, Campos JA, Zugaib M. Quantitative analysis of placental vasculature by three-dimensional power Doppler ultrasonography in normal pregnancies from 12 to 40 weeks of gestation. Placenta. 2009;30:142–8. doi: 10.1016/j.placenta.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 19.de Almeida PE, de Paula CF S, Duarte Bonini Campos JA, Fox KA, Francisco R, Ruano R, et al. Three-dimensional sonographic assessment of placental volume and vascularization in pregnancies complicated by hypertensive disorders. J Ultrasound Med. 2014;33:483–91. doi: 10.7863/ultra.33.3.483. [DOI] [PubMed] [Google Scholar]
- 20.Mihu CM, Drugan T, Mihu D. Contribution of 3D power Doppler ultrasound to the evaluation of placental circulation in normal pregnancies and pregnancies complicated by preeclampsia. J Perinat Med. 2012;40:359–64. doi: 10.1515/jpm-2011-0105. [DOI] [PubMed] [Google Scholar]
- 21.Chen CY, Wang KG, Chen CP. Alteration of vascularization in preeclamptic placentas measured by three-dimensional power Doppler ultrasound. J Matern Fetal Neonatal Med. 2013;26:1616–22. doi: 10.3109/14767058.2013.793661. [DOI] [PubMed] [Google Scholar]
- 22.Costa J, Rice H, Cardwell C, Hunter A, Ong S. An assessment of vascularity and flow intensity of the placenta in normal pregnancy and pre-eclampsia using three-dimensional ultrasound. J Matern Fetal Neonatal Med. 2010;23:894–9. doi: 10.3109/14767051003649862. [DOI] [PubMed] [Google Scholar]
- 23.Baschat AA, Weiner CP. Umbilical artery Doppler screening for the small for gestational age fetus in need of antenatal surveillance. Am J Obstet Gynecol. 2000;182:154–8. doi: 10.1016/S0002-9378(00)70505-9. [DOI] [PubMed] [Google Scholar]
- 24.Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol. 2000;16:407–13. doi: 10.1046/j.1469-0705.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 25.Pomorski M, Zimmer M, Florjanski J, Michniewicz J, Wiatrowski A, Fuchs T, et al. Comparative analysis of placental vasculature and placental volume innormal and IUGR pregnancies with the use of three-dimensional Power Doppler. Arch Gynecol Obstet. 2012;285:331–7. doi: 10.1007/s00404-011-1968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borges KJ, Hassan N, Hussain R, Akhtar MT. Effects of variation in umbilical artery resistive index on placental morphology and birth weight in pregnancy induced hypertension. J Ayub Med Coll Abbottabad. 2013;25:23–6. [PubMed] [Google Scholar]
- 27.Ruiz-Quiñonez G, Reza-López SA, Chávez-Corral DV, Sánchez-Ramírez B, Leal-Berumen I, Levario-Carrillo M. Placental maturity, hypertensive disorders of pregnancyand birth weight. Hypertens Pregnancy. 2014;33:132–44. doi: 10.3109/10641955.2013.842583. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi J, Tanaka H, Koyanagi A, Miyake K, Hata T. Three-dimensional power Doppler indices at 18–22 weeks’ gestation for prediction of fetal growth restriction or pregnancy-induced hypertension. Arch Gynecol Obstet. 2015;292:75–9. doi: 10.1007/s00404-014-3603-z. [DOI] [PubMed] [Google Scholar]
- 29.Odibo AO, Goetzinger KR, Huster KM, Christiansen JK, Odibo L, Tuuli MG. Placental volume and vascular flow assessed by 3D power Doppler and adverse pregnancy outcomes. Placenta. 2011;32:230–4. doi: 10.1016/j.placenta.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odeh M, Ophir E, Maximovsky O, Grinin V, Bornstein J. Placental volume and three-dimensional power Doppler analysis in prediction of pre-eclampsia and small for gestational age between week 11 and 13 weeks and 6 days of gestation. Prenat Diagn. 2011;31:367–71. doi: 10.1002/pd.2697. [DOI] [PubMed] [Google Scholar]
- 31.Hata T, Tanaka H, Noguchi J, Hata K. Three-dimensional ultrasound evaluation of the placenta. Placenta. 2011;32:105–15. doi: 10.1016/j.placenta.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Mercé LT, Barco MJ, Bau S. Reproducibility of the study of placental vascularization by three-dimensional power Doppler. J Perinat Med. 2004;32:228–33. doi: 10.1515/JPM.2004.043. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi J, Hata K, Tanaka H, Hata T. Placental vascular sonobiopsy using three-dimensional power Doppler ultrasound in normal and growth restricted fetuses. Placenta. 2009;30:391–7. doi: 10.1016/j.placenta.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Bujold E, Effendi M, Girard M, Gouin K, Forest JC, Couturier B, et al. Reproducibility of first trimester three-dimensional placental measurements in the evaluation of early placental insufficiency. J Obstet Gynaecol Can. 2009;31:1144–8. doi: 10.1016/s1701-2163(16)34375-4. [DOI] [PubMed] [Google Scholar]
- 35.Huster KM, Haas K, Schoenborn J, McVean D, Odibo AO. Reproducibility of placental volume and vasculature indices obtained by 3-dimensional power Doppler sonography. J Ultrasound Med. 2010;29:911–6. doi: 10.7863/jum.2010.29.6.911. [DOI] [PubMed] [Google Scholar]
- 36.Morel O, Grangé G, Fresson J, Schaaps JP, Foidart JM, Cabrol D, et al. Vascularization of the placenta and the sub-placental myometrium: feasibility and reproducibility of a three dimensional power Doppler ultrasound quantification technique. A pilot study. J Matern Fetal Neonatal Med. 2011;24:284–90. doi: 10.3109/14767058.2010.486845. [DOI] [PubMed] [Google Scholar]
- 37.Guimarães Filho HA, Mattar R, Araujo Júnior E, da Costa LL, de Mello Junior CF, Nardozza LM, et al. Reproducibility of three-dimensional power Doppler placental vascular indices in pregnancies between 26 and 35 weeks. Arch Gynecol Obstet. 2011;283:213–7. doi: 10.1007/s00404-009-1341-4. [DOI] [PubMed] [Google Scholar]
- 38.Lai PK, Wang YA, Welsh AW. Reproducibility of regional placental vascularity/perfusion measurement using 3D power Doppler. Ultrasound Obstet Gynecol. 2010;36:202–9. doi: 10.1002/uog.7608. [DOI] [PubMed] [Google Scholar]
- 39.Tuuli MG, Houser M, Odibo L, Huster K, Macones GA, Odibo AO. Validation of placental vascular sonobiopsy for obtaining representative placental vascular indices by three dimensional power Doppler. Placenta. 2010;31:192–6. doi: 10.1016/j.placenta.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Nastri CO, Ferriani RA, Raine-Fenning N, Martins WP. Endometrial scratching performed in the non-transfer cycle and outcome of assisted reproduction: a randomized controlled trial. Ultrasound Obstet Gynecol. 2013;42:375–82. doi: 10.1002/uog.12539. [DOI] [PubMed] [Google Scholar]
- 41.Nandi A, Martins WP, Jayaprakasan K, Clewes JS, Campbell BK, Raine-Fenning NJ. Assessment of endometrial and subendometrial blood flow in women undergoing frozen embryo transfer cycles. Reprod Biomed Online. 2014;28:343–51. doi: 10.1016/j.rbmo.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Miyague AH, Raine-Fenning NJ, Pavan TZ, Polanski LT, Baumgarten MN, Nastri CO, et al. Influence of gain adjustment on 3-dimensional power Doppler indices and on spatiotemporal image correlation volumetric pulsatility indices using a flow phantom. J Ultrasound Med. 2013;32:1831–6. doi: 10.7863/ultra.32.10.1831. [DOI] [PubMed] [Google Scholar]
- 43.Martins WP, Welsh AW, Lima JC, Nastri CO, Raine-Fenning NJ. The “volumetric” pulsatility index as evaluated by spatiotemporal imaging correlation (STIC): a preliminary description of a novel technique, its application to the endometrium and an evaluation of its reproducibility. Ultrasound Med Biol. 2011;37:2160–8. doi: 10.1016/j.ultrasmedbio.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Welsh AW, Hou M, Meriki N, Martins WP. Spatiotemporal image correlation-derived volumetric Doppler impedance indices from spherical samples of the placenta: intraobserver reliability and correlation with conventional umbilical artery Doppler indices. Ultrasound Obstet Gynecol. 2012;40:431–6. doi: 10.1002/uog.11113. [DOI] [PubMed] [Google Scholar]
- 45.Martins WP, Raine-Fenning NJ, Ferriani RA, Nastri CO. Quantitative three-dimensional power Doppler angiography: a flow-free phantom experiment to evaluate the relationship between colour gain, depth and signal artifact. Ultrasound Obstet Gynecol. 2010;35:361–8. doi: 10.1002/uog.7562. [DOI] [PubMed] [Google Scholar]
- 46.Miyague AH, Pavan TZ, Grillo FW, Teixeira DM, Nastri CO, Martins WP. Influence of attenuation on three-dimensional power Doppler indices and STIC volumetric pulsatility index: a flow phantom experiment. Ultrasound Obstet Gynecol. 2014;43:103–5. doi: 10.1002/uog.12558. [DOI] [PubMed] [Google Scholar]
- 47.Martins WP, Nastri CO. Reproducibility of 3D power Doppler placental vascular indices. Arch Gynecol Obstet. 2011;283:403–4. doi: 10.1007/s00404-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 48.Martins WP, Lima JC, Welsh AW, Araujo Junior E, Miyague AH, Filho FM, et al. Three-dimensional Doppler evaluation of single spherical samples from the placenta: intra- and interobserver reliability. Ultrasound Obstet Gynecol. 2012;40:200–6. doi: 10.1002/uog.11076. [DOI] [PubMed] [Google Scholar]
- 49.Miyague AH, Raine-Fenning NJ, Polanski L, Martinez LH, Araujo Junior E, Pavan TZ, et al. Assessing repeatability of 3D Doppler indices obtained by static 3D and STIC power Doppler: a combined in-vivo/in-vitro flow phantom study. Ultrasound Obstet Gynecol. 2013;42:571–6. [DOI] [PubMed]
- 50.Stevenson GN, Collins SL, Welsh AW, Impey LW, Noble JA. A technique for the estimation of fractional moving blood volume by using three-dimensional power Doppler US. Radiology. 2015;274:230–7. doi: 10.1148/radiol.14132363. [DOI] [PubMed] [Google Scholar]
- 51.Soares CA, Miyague AH, Filho FM, Raine-Fenning N, Martins WP. Fractional moving blood volume (FMBV) concept can be applied to 3D power Doppler quantification. Ultrasound Obstet Gynecol. 2013;41:98–9. doi: 10.1002/uog.12288. [DOI] [PubMed] [Google Scholar]
- 52.Rubin JM, Adler RS, Fowlkes JB, Spratt S, Pallister JE, Chen JF, et al. Fractional moving blood volume: estimation with power Doppler US. Radiology. 1995;197:183–90. doi: 10.1148/radiology.197.1.7568820. [DOI] [PubMed] [Google Scholar]
- 53.Rubin JM, Bude RO, Fowlkes JB, Spratt RS, Carson PL, Adler RS. Normalizing fractional moving blood volume estimates with power Doppler US:defining a stable intravascular point with the cumulative power distribution function. Radiology. 1997;205:757–65. doi: 10.1148/radiology.205.3.9393532. [DOI] [PubMed] [Google Scholar]
- 54.Welsh AW, Fisk NM. Picture of the month. Assessment of fetal renal perfusion by power Doppler digital analysis. Ultrasound Obstet Gynecol. 2001;17:89–91. doi: 10.1046/j.1469-0705.2001.00343.x. [DOI] [PubMed] [Google Scholar]
- 55.Zalud I, Shaha S. Three-dimensional sonography of the placental and uterine spiral vasculature: influence of maternal age and parity. J Clin Ultrasound. 2008;36:391–6. doi: 10.1002/jcu.20485. [DOI] [PubMed] [Google Scholar]


