Abstract
Purpose
The in vitro fertilization (IVF) pregnancy rate of women with advanced stage endometriosis is nearly half that of the general population, suggesting incomplete targeting of the pathophysiology underlying endometriosis-associated infertility. Compelling evidence highlights inflammation as the etiologic link between endometriosis and infertility and a potential target for adjunctive treatment. The objective of this study was to examine the effect of dexamethasone on murine embryos exposed to human endometriotic peritoneal fluid (PF) using the established murine embryo assay model.
Methods
PF was obtained from women with and without severe endometriosis. Murine embryos were harvested and randomly allocated to five groups of culture media conditions: (1) human tubal fluid (HTF), (2) HTF and 10 % PF from women without endometriosis, (3) HTF and 10 % PF from women with endometriosis (PF-E), (4) HTF with PF-E and 0.01 mcg/mL dexamethasone, and (5) HTF with PF-E and 0.1 mcg/mL dexamethasone. Embryos were cultured in standard conditions and evaluated for blastocyst development.
Results
A total of 266 mouse embryos were cultured. Baseline blastulation rates were 63.6 %. The addition of peritoneal fluid from women with endometriosis decreased the blastocyst development rate to 38.9 % (P = 0.008). The addition of 0.1 mcg/mL of dexamethasone to the culture media restored the blastulation rate to near baseline levels (61.2 %; P = 0.019).
Conclusions
The results of our in vitro study demonstrate the capacity of dexamethasone to mitigate the deleterious impact of endometriotic PF on embryo development. If confirmed in vivo, dexamethasone may prove a useful adjunct for the treatment of endometriosis-associated infertility.
Keywords: Endometriosis, Infertility, Dexamethasone, Embryo, Blastocyst
Introduction
Endometriosis is a chronic inflammatory condition characterized by the implantation and growth of endometrial glands and stroma outside of the uterus, typically involving the peritoneum and other pelvic structures. Approximately, 6–10 % of reproductive age females and up to 50 % of women with unexplained infertility are affected with this disease [1]. Compelling evidence exists to support the detrimental impact of endometriosis on female fertility, to include a meta-analysis demonstrating a nearly 50 % decrease in the in vitro fertilization (IVF) pregnancy rate [2]. This finding suggests that IVF as currently practiced incompletely addresses the underlying pathophysiology of endometriosis-associated infertility, particularly in patients with advanced stage disease, and signals the need for adjunct(s) to IVF for the treatment of these women.
Endometriosis is considered a chronic inflammatory condition as evidenced by alterations in the cytokine and prostaglandin profiles within the peritoneal microenvironment [3]. Women with endometriosis demonstrate elevated levels of interleukin-6 (IL-6) and tumor necrosis factor (TNF) [4]. Oocytes and early embryos may be adversely affected by exposure to inflammatory mediators [5]. Other groups previously reported a detrimental effect of peritoneal fluid from women with endometriosis on embryo development using a murine embryo assay model [6–11].
Inflammation represents a biologically plausible target in the treatment of endometriosis-associated infertility. Glucocorticoids, such as dexamethasone, reduce the production of mediators of inflammation such as prostaglandins by binding to and inhibiting the enzyme, phospholipase A2 (PLA2) [12]. In a comparison of gene expression profiles of endometriotic implants with normal eutopic endometrium, PLA2 was found to be the most highly upregulated gene [13]. In addition to the attenuation of prostaglandin production via inhibition of PLA2, dexamethasone is known to attenuate IL-6 action [14]. Dexamethasone is easy to use, well tolerated, and has been used to treat infertility associated with other conditions such as polycystic ovary syndrome, diminished ovarian reserve, and tubal factor infertility [15]. We used the in vitro murine embryo assay to study the effect of dexamethasone on embryo development in embryos exposed to endometriotic peritoneal fluid.
Materials and methods
Peritoneal fluid specimens
Peritoneal fluid from women with (n = 3) and without endometriosis (n = 3) was obtained in a study approved by the Institutional Review Board of the Madigan Healthcare System. At laparoscopy for infertility, peritoneal fluid was aspirated from the posterior cul-de-sac and collected for study purposes. Inspection of the abdomen and pelvis was conducted and those areas of suspected endometriosis were biopsied for histologic confirmation. In cases of endometriosis, surgical staging was performed in accordance with the revised American Fertility Society (rAFS) system [16].
Peritoneal fluid was centrifuged for 10 min at 600×g and 4 °C to remove the cellular fraction and the supernatant stored at −70 °C in 1 mL aliquots [17]. Aliquots used in embryo culture experiments were filtered through a 0.22-μm filter (Millipore, Bedford, MA). All specimens were collected during the secretory phase of the menstrual cycle as delineated by a combination of reported cycle day, serum hormone (estradiol and progesterone) profile, and endometrial histology using Noyes criteria [18].
The levels of prostaglandins F2a (PGF2a) and E2 (PGE2), vascular endothelial growth factor (VEGF), monocyte chemotactic protein 1 (MCP1), interleukin 6 (IL6), tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ) were determined for each peritoneal fluid specimen using multiplex electrochemiluminescence (Sector Imager, Meso Scale, Gaithersburg, MD). The normal detection range for VEGF in the assay is 1.8 to 3864 pg/mL and for PGF2a is 0.002 to 1.0 ng/mL. Each specimen was run in triplicate and calibrated with standards according to manufacturers’ instructions. After assay of prostaglandin and cytokine levels, the three normal specimens (PF-NL) and three endometriosis specimens (PF-E) were each pooled for use in the murine embryo assay experiments.
Murine embryo assay
Approval for this study was provided by the Institutional Animal Care and Use Committee of the Madigan Healthcare System. The C57BL/6 mouse strain (Jackson Laboratory, Sacramento, CA) was selected due to widespread use in developmental biology studies and superior reproductive performance in IVF compared to other strains [19]. The care and use procedures for the mice were in accordance with the Helsinki Declaration and Institutional Guide for Laboratory Animals. Mice were fed ad libitum with a standard diet and water and housed under a 12/12-h light/dark cycle at 25 °C and 50 % humidity.
Six-week-old female C57BL/6 mice were superovulated using 5 IU intraperitoneal injection (IP) of gonadotropin (equine chorionic gonadotropin (PMSG); Sigma, St. Louis, MO), followed 46 h later by 5 IU injection of human chorionic gonadotropin (HCG; Sigma, St. Louis, MO) [20]. Immediately after receiving the HCG injection, the female mice were mated with a male wild-type C57BL/6 mouse in a 1:1 ratio. Females were then evaluated 20–24 h later for evidence of mating via examination for a vaginal copulation plug. Fertilized females were euthanized, oviducts surgically harvested, and single cell, pronuclear (2PN) embryos isolated from the oviducts in M2 media (Sigma, St. Louis, MO). Embryos were released from their surrounding cumulus complex by rinsing in 0.5 % hyaluronidase followed by serial rinses in microdrops consisting of commercially available human tubal fluid (HTF) with 10 % serum substitute supplement (SSS) (Irvine Scientific, Irvine, CA).
Embryos (over three replicates) were randomly and equally allocated to five groups of culture media compositions:
Control: HTF only
PF-NL: HTF with 10 % PF from patients without endometriosis at surgery (PF-NL)
PF-E: HTF with 10 % PF from patients with endometriosis at surgery (PF-E)
PF-E + Dex, 0.01: HTF with 10 % PF-E and 0.01 mcg/mL dexamethasone
PF-E + Dex, 0.1: HTF with 10 % PF-E and 0.1 mcg/mL dexamethasone
Embryos were cultured in groups of 8–10 embryos per 30 μL microdrop of culture medium (HTF + 10 % SSS) overlaid with mineral oil and incubated in a designated embryo culture incubator at 37 °C with 5 % CO2 [21]. Culture plates were prepared the evening prior to use and equilibrated overnight prior to embryo allocation [22]. The day of embryo harvest and allocation was designated as day 1.
Embryos were evaluated on day 5 using bright field inverted optics. Digital microscopy was used to archive embryo images. Embryos were evaluated on day 5 for developmental stage in categories of blastocyst, morula, or arrest. The number of embryos differentiating to the blastocyst stage (early, expanding, hatching, or hatched) was recorded for each group. Embryo grading was performed by a single examiner who was blinded to study group. The blastocyst development rate (BDR) was calculated by dividing the number of blastocysts by the total number of embryos cultured.
Statistical analysis
The multiplex electrochemiluminescence results from the pooled peritoneal fluid (PF) samples with and without endometriosis were reported as mean values and compared using Student’s t test. The blastocyst development rates among groups were compared using a one-tailed Chi-square analysis. A P value of less than 0.05 was considered statistically significant.
Results
Three PF specimens from infertile women with surgically staged and histologically confirmed stage III endometriosis (PF-E) were compared with three PF specimens collected from fertile women undergoing laparoscopic evaluation for tubal anastomosis procedure and found to have no endometriosis at surgery (PF-NL). The levels of selected prostaglandins and cytokines in the pooled peritoneal fluid specimens used in the murine embryo assay are provided in Table 1. Other than PGE2, each analyte was higher in the pooled endometriotic PF relative to the normal PF, with levels of VEGF, IFN, and TNF reaching statistical significance (P < 0.05).
Table 1.
Peritoneal fluid comparison
| Assay | Pooled peritoneal fluid No endometriosis (n = 3) |
Pooled peritoneal fluid Endometriosis (n = 3) |
P |
|---|---|---|---|
| PGF2a (ng/mL) | 0.007 ± 0.001 | 0.017 ± 0.007 | 0.11 |
| PGE2 (ng/mL) | 3.070 ± 0.062 | 3.057 ± 0.155 | 0.47 |
| VEGF (pg/mL) | 0.005 ± 0.003 | 0.014 ± 0.008 | <0.01 |
| MCP1 (pg/mL) | 99.60 ± 12.58 | 211.2 ± 60.0 | 0.07 |
| IL-6 (pg/mL) | 36.60 ± 5.15 | 189.2 ± 90.9 | 0.08 |
| IFN-γ (pg/mL) | 140.0 ± 5.5 | 167.7 ± 11.5 | <0.05 |
| TNF-α (pg/mL) | 0.541 ± 0.10 | 1.061 ± 0.21 | <0.05 |
Prostaglandin and cytokine levels in pooled peritoneal fluid from infertile patients with histologically confirmed rAFS stage III endometriosis compared with that of fertile patients with no endometriosis at surgery. The pooled peritoneal fluid was added to embryo culture media to model exposure to the in vivo peritoneal fluid microenvironment
Values are given as mean ± standard error of the mean
Prior to experimental replicates, embryos were evaluated on days 2, 3, and 5 for optimization of the embryo culture conditions. Representative embryos at each of these developmental stages are depicted in Fig. 1. A preliminary experiment involving 40 embryos in each arm compared growth in HTF culture media alone to growth in HTF media containing dexamethasone at two different concentrations, 0.01 and 0.1 mcg/mL, and demonstrated no statistically significant differences in the blastocyst development rate (data not shown). Consequently, this arm was not continued in experimental replicates.
Fig. 1.
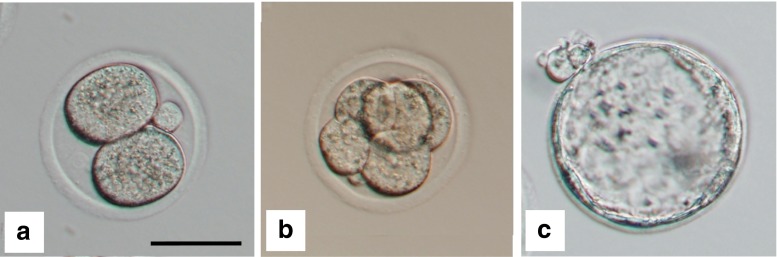
Murine embryo development. Representative images of murine embryos at sequential time points in the murine embryo assay. a Day 2 (HCG + 48 h) embryo at 2 cell stage. b Cleavage stage (HCG + 72 h) embryo at the 6–8 cell stage C. Hatching blastocyst (HCG + 120 h). Images taken at ×20 with scale bar, 50 μm
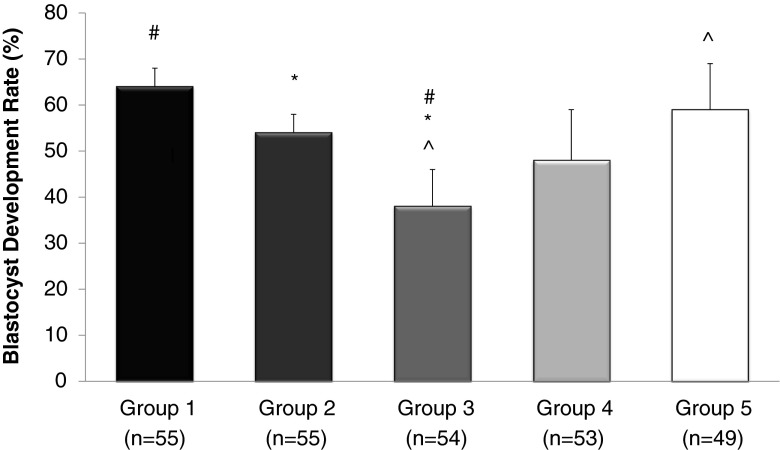
A total of 266 murine embryos were randomly allocated among culture groups and evaluated by an investigator blinded as to group on day 5 following fertilization in three separate replicates of the experiment. In HTF culture medium alone (group 1), 63.6 % (35/55) of embryos reached the blastocyst stage on day 5 (Fig. 2). The BDR decreased to 54.5 % (30/55) in embryos cultured in HTF with addition of 10 % peritoneal fluid from women without endometriosis (PF-NL; group 2), and this difference was not statistically significant relative to embryos cultured in HTF alone (P = 0.22). Embryos cultured in HTF with a 10 % titration of peritoneal fluid from women with endometriosis (PF-E; group 3) evidenced a significant decline in the rate of blastulation on day 5 (38.9 % (21/54), when compared to HTF (P < 0.01) and also with PF-NL (P = 0.03). A dose-dependent improvement in the BDR of embryos cultured in media with peritoneal fluid from women with endometriosis was observed with the addition of dexamethasone (groups 4 and 5). Blastulation rates in group 4 improved to 49.0 % (26/55). The rate of blastulation improved to 61.2 % (30/49) at the 0.10 mcg/mL dose (group 5), and represented a significant improvement relative to group 3 (P < 0.05), and approached the baseline BDR observed in group 1 (P = 0.48). Consistent results were observed in each of the three replicates of the experiment.
Fig. 2.
Blastocyst development in culture media conditions. The blastocyst development rates are calculated for each experimental group. All results expressed as percentages. Error bars represent standard deviation, P < 0.05 considered significant. #Comparison between groups 1 and 3, P = 0.008. *Comparison between groups 2 and 3, P = 0.03. ^Comparison between groups 3 and 5, P = 0.019
Discussion
A large meta-analysis revealed a nearly 50 % reduction in the clinical pregnancy rate among women with advanced stages of endometriosis undergoing IVF [2], suggesting that IVF as traditionally practiced incompletely addresses the underlying pathophysiology of endometriosis-associated infertility. The mechanisms by which endometriosis reduces fertility are incompletely understood, yet critical to the development of targeted therapies [23]. Proposed mechanisms include adverse oocyte and embryo development, impaired fertilization, altered tubal transport, and impaired embryo implantation. A shared oocyte study provided compelling evidence that endometriosis exerts a deleterious effect at the level of oocyte and/or embryo development [24].
The present study was designed to investigate whether dexamethasone improved embryo development in the setting of endometriosis using a well-established murine embryo assay as the in vitro preclinical model. Other investigators have established the fidelity of this model, demonstrating a detrimental effect of peritoneal fluid from women with endometriosis on the growth and development of mouse embryos in culture at dilutions between 10 and 30 % [9, 25, 26]. Ding et al. reported embryo arrest rates of 36, 55, and 93 % at 10, 30, and 50 % dilutions of endometriotic peritoneal fluid, respectively [25]. At higher concentrations of PF, the deleterious impact on embryos may be more consequent to lack of media than nature of PF, and we observed a low BDR at 30 % PF-NL in a pilot experiment (data not shown). Consequently, we chose to use a 10 % dilution of PF for this study. We observed a significant decrease in blastocyst development from 63.6 to 38.9 % with the addition of 10 % PF-E, confirming the embryotoxic impact of endometriotic peritoneal fluid [27]. The addition of 10 % PF-NL to the embryo culture media did not result in a significant decrease in the BDR. The finding of a significant decrease in BDR with addition of endometriotic peritoneal fluid (PF-E) when compared to both HTF and PF-NL suggests that the deleterious effect is a function of PF composition rather than concentration, and may result from inflammatory mediators in PF-E. We measured significantly higher levels of TNF-α, IFN-γ, and VEGF in peritoneal fluid from women with endometriosis relative to that from women surgically confirmed to be free of disease. The levels of IL-6, MCP-1, and PGF2α were increased in PF-E though not statistically significant perhaps owing to reduced sample size. Targeted protein knockdown experiments are warranted to further delineate whether these or other inflammatory mediators contribute to impaired embryo development.
Given the inflammatory nature of PF-E and the known anti-inflammatory action of dexamethasone, we hypothesized that dexamethasone may ameliorate the deleterious effect of PF-E on embryo development. Our results revealed a dose-dependent improvement in the blastulation rate of embryos exposed to endometriotic peritoneal fluid with the addition of dexamethasone. This finding is consistent with a study of in vitro murine embryo development in which the embryotoxicity of serum from women with endometriosis was significantly reduced in patients who received a 3 day course of glucocorticoids prior to phlebotomy [28]. The use of dexamethasone in conjunction with IVF is not without precedent in other patient populations. This agent has been investigated in women with polycystic ovary syndrome (PCOS) and in a general population of women undergoing IVF [29, 30]. Though the latter study showed a reduced cycle cancellation with dexamethasone use, the data was not stratified by infertility diagnosis. No significant differences in IVF outcomes and no harmful short- or long-term consequences were observed in women with PCOS undergoing glucocorticoid treatment.
The concentration of dexamethasone used in our embryo assay experiments was set at 0.01 and 0.1 mcg/mL. The maximum serum concentration after 1 mg oral dexamethasone dose is 0.0084 mcg/mL [31], and the therapeutic dose of orally administered dexamethasone results in a basal serum concentration of 0.012 mcg/mL [32]. Of note, at a dose of 10 mcg/mL (nearly 1000x the therapeutic level), dexamethasone impaired murine pre-implantation embryo hatching [33]. We observed no detrimental effect of HTF + dexamethasone at the study doses on blastocyst development when compared to HTF alone. In the setting of endometriotic PF, the addition of dexamethasone to murine embryo culture improved rates of blastocyst development, and nearly returned blastocyst development to the basal rate at the 0.10 mcg/mL dose.
Glucocorticoids exert their anti-inflammatory effect via several mechanisms to include induction of annexin-1, induction of mitogen-activated protein kinase (MAPK) phosphatase, and repression of cyclooxygenase 2 (COX 2) transcription. Annexin-1 interacts with cytosolic phospholipase A2 (PLA2) to inhibit the release of arachidonic acid and its subsequent conversion to eicosanoids [12]. The addition of dexamethasone is not likely to alter the concentrations of inflammatory mediators, but may mitigate their apoptotic consequences. Ding et al. reported that the level of phosphorylated extracellular regulated protein kinase (p-ERK), required to activate MAPKs in several growth factor receptor-mediated signal transduction pathways, was significantly reduced in embryos exposed to PF-E [25]. Interestingly, dexamethasone has been shown in both primary ovarian follicular cells [34] and HK-2 cells [35] to induce glucocorticoid receptor-mediated rapid phosphorylation of ERK, and this has been suggested as a potential mechanism for dexamethasone’s anti-apoptotic effects. Glucocorticoid receptors are present in pre-implantation embryos [36, 37]. Finally, rodent studies demonstrate that TNF-alpha receptors are present in blastocysts [38], trophectoderm cells [39], and embryonic stem cells [40, 41], and TNF-alpha has been shown to decrease the rate of cell proliferation in the inner cell mass [42]. These findings provide plausible targets for further studies to determine the mechanism by which dexamethasone improves the embryo development rate in the setting of endometriosis.
Limitations of the murine embryo assay are recognized. Pooled peritoneal fluid was added to embryo culture media to recapitulate exposure to the inflammatory peritoneal fluid microenvironment that exists in vivo. In natural conception cycles, oocyte development, fertilization, and embryo development all occur in an inflammatory milieu when endometriosis is present, whereas the murine embryo model only exposes embryos to endometriotic PF. By isolating the embryo from the complex milieu of inflammatory and immunological cascades found in vivo, the model represents an incomplete approximation. Nonetheless, the well-described murine embryo assay is an excellent model for testing adjuncts prior to engaging in preclinical study.
Although the detrimental impact of endometriosis on fertility and IVF outcomes is well described, too few clinical approaches have been validated to improve pregnancy rates in affected women. Herein, we provide in vitro evidence for the benefit of dexamethasone in abrogating the embryotoxicity of endometriotic peritoneal fluid. If confirmed by further in vivo preclinical models and prospective clinical study, this agent may prove a useful adjunct toward optimizing the relatively compromised IVF treatment outcomes for women with endometriosis-associated infertility.
Footnotes
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense or the United States Government.
Capsule The addition of dexamethasone mitigates the embryotoxic effects of endometriotic peritoneal fluid in a murine embryo model. Dexamethasone may represent an adjunctive therapy for endometriosis-associated infertility.
References
- 1.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin N Am. 1997;24(2):235–58. doi: 10.1016/S0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 2.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77(6):1148–55. doi: 10.1016/S0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 3.Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004;1034:300–15. doi: 10.1196/annals.1335.032. [DOI] [PubMed] [Google Scholar]
- 4.Ho HN, Wu MY, Chao KH, Chen CD, Chen SU, Chen HF, et al. Decrease in interferon gamma production and impairment of T-lymphocyte proliferation in peritoneal fluid of women with endometriosis. Am J Obstet Gynecol. 1996;175(5):1236–41. doi: 10.1016/S0002-9378(96)70034-0. [DOI] [PubMed] [Google Scholar]
- 5.Oral E, Olive DL, Arici A. The peritoneal environment in endometriosis. Hum Reprod Update. 1996;2(5):385–98. doi: 10.1093/humupd/2.5.385. [DOI] [PubMed] [Google Scholar]
- 6.Prough SG, Aksel S, Gilmore SM, Yeoman RR. Peritoneal fluid fractions from patients with endometriosis do not promote two-cell mouse embryo growth. Fertil Steril. 1990;54(5):927–30. doi: 10.1016/s0015-0282(16)53958-2. [DOI] [PubMed] [Google Scholar]
- 7.Morcos RN, Gibbons WE, Findley WE. Effect of peritoneal fluid on in vitro cleavage of 2-cell mouse embryos: possible role in infertility associated with endometriosis. Fertil Steril. 1985;44(5):678–83. doi: 10.1016/s0015-0282(16)48987-9. [DOI] [PubMed] [Google Scholar]
- 8.Wu MY, Chen SU, Chao KH, Chen CD, Yang YS, Ho HN. Mouse embryo toxicity of IL-6 in peritoneal fluids from women with or without endometriosis. Acta Obstet Gynecol Scand. 2001;80(1):7–11. doi: 10.1034/j.1600-0412.2001.800102.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Torres MJ, Acien P, Campos A, Velasco I. Embryotoxicity of peritoneal fluid in women with endometriosis. Its relation with cytokines and lymphocyte populations. Hum Reprod. 2002;17(3):777–81. doi: 10.1093/humrep/17.3.777. [DOI] [PubMed] [Google Scholar]
- 10.Esfandiari N, Falcone T, Goldberg JM, Agarwal A, Sharma RK. Effects of peritoneal fluid on preimplantation mouse embryo development and apoptosis in vitro. Reprod BioMed Online. 2005;11(5):615–9. doi: 10.1016/S1472-6483(10)61170-4. [DOI] [PubMed] [Google Scholar]
- 11.Noordin L, San GT, Singh HJ, Othman MS, Hafizah W. Pyruvate reduces in vitro the embryotoxic effect of peritoneal fluid from infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;136(1):67–73. doi: 10.1016/j.ejogrb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 13.Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88(6):1505–33. doi: 10.1016/j.fertnstert.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 14.Verhoog NJ, Du Toit A, Avenant C, Hapgood JP. Glucocorticoid-independent repression of tumor necrosis factor (TNF) alpha-stimulated interleukin (IL)-6 expression by the glucocorticoid receptor: a potential mechanism for protection against an excessive inflammatory response. J Biol Chem. 2011;286(22):19297–310. doi: 10.1074/jbc.M110.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bider D, Amoday I, Tur-Kaspa I, Livshits A, Dor J. The addition of a glucocorticoid to the protocol of programmed oocyte retrieval for in-vitro fertilization–a randomized study. Hum Reprod. 1996;11(8):1606–8. doi: 10.1093/oxfordjournals.humrep.a019454. [DOI] [PubMed] [Google Scholar]
- 16.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817-21. [DOI] [PubMed]
- 17.Fassbender A, Vodeolaskaia A, Saunders P, Lebovic D, Waelkens E, De Moor B, et al. Biomarkers of endometriosis. Fertil Steril. 2013;99(4):1135–45. doi: 10.1016/j.fertnstert.2013.01.097. [DOI] [PubMed] [Google Scholar]
- 18.Noyes R, Hertig A, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–3. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 19.Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs) Theriogenology. 2006;65(9):1716–26. doi: 10.1016/j.theriogenology.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 21.Lane M, Gardner DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod. 1992;7(4):558–62. doi: 10.1093/oxfordjournals.humrep.a137690. [DOI] [PubMed] [Google Scholar]
- 22.Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol. 1993;225:153–64. doi: 10.1016/0076-6879(93)25012-Q. [DOI] [PubMed] [Google Scholar]
- 23.Navarro J, Garrido N, Remohi J, Pellicer A. How does endometriosis affect infertility? Obstet Gynecol Clin N Am. 2003;30(1):181–92. doi: 10.1016/S0889-8545(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 24.Simon C, Gutierrez A, Vidal A, Santos MJ d l, Tarin JJ, Remohi J, et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9(4):725–9. doi: 10.1093/oxfordjournals.humrep.a138578. [DOI] [PubMed] [Google Scholar]
- 25.Ding GL, Chen XJ, Luo Q, Dong MY, Wang N, Huang HF. Attenuated oocyte fertilization and embryo development associated with altered growth factor/signal transduction induced by endometriotic peritoneal fluid. Fertil Steril. 2010;93(8):2538–44. doi: 10.1016/j.fertnstert.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Mansour G, Abdelrazik H, Sharma RK, Radwan E, Falcone T, Agarwal A. L-carnitine supplementation reduces oocyte cytoskeleton damage and embryo apoptosis induced by incubation in peritoneal fluid from patients with endometriosis. Fertil Steril. 2009;91(5 Suppl):2079–86. doi: 10.1016/j.fertnstert.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 27.Hauzman EE, Garcia-Velasco JA, Pellicer A. Oocyte donation and endometriosis: what are the lessons? Semin Reprod Med. 2013;31(2):173–7. doi: 10.1055/s-0032-1333483. [DOI] [PubMed] [Google Scholar]
- 28.Simon C, Gomez E, Mir A, Santos MJ D l, Pellicer A. Glucocorticoid treatment decreases sera embryotoxicity in endometriosis patients. Fertil Steril. 1992;58(2):284–9. doi: 10.1016/s0015-0282(16)55198-x. [DOI] [PubMed] [Google Scholar]
- 29.Bider D, Hourvitz A, Tur Kaspa I, Dirnfeld M, Dor J. Dexamethasone supplementation to gonadotropin stimulation for in vitro fertilization in polycystic ovarian disease. J Assist Reprod Genet. 1999;16(5):233–5. doi: 10.1023/A:1020307227273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keay SD, Lenton EA, Cooke ID, Hull MG, Jenkins JM. Low-dose dexamethasone augments the ovarian response to exogenous gonadotrophins leading to a reduction in cycle cancellation rate in a standard IVF programme. Hum Reprod. 2001;16(9):1861–5. doi: 10.1093/humrep/16.9.1861. [DOI] [PubMed] [Google Scholar]
- 31.Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44(1):61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Lamiable D, Vistelle R, Sulmont V, Millart H, Caron J, Choisy H. Pharmacokinetics of dexamethasone administered orally in obese patients. Therapie. 1990;45(4):311–4. [PubMed] [Google Scholar]
- 33.Van Merris V, Van Wemmel K, Cortvrindt R. In vitro effects of dexamethasone on mouse ovarian function and pre-implantation embryo development. Reprod Toxicol. 2007;23(1):32–41. doi: 10.1016/j.reprotox.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Sasson R, Amsterdam A. Pleiotropic anti-apoptotic activity of glucocorticoids in ovarian follicular cells. Biochem Pharmacol. 2003;66(8):1393–401. doi: 10.1016/S0006-2952(03)00489-1. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Allen DA, Kieswich JE, Patel NS, Harwood S, Mazzon E, et al. Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol : JASN. 2009;20(11):2412–25. doi: 10.1681/ASN.2008080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korgun ET, Dohr G, Desoye G, Demir R, Kayisli UA, Hahn T. Expression of insulin, insulin-like growth factor I and glucocorticoid receptor in rat uterus and embryo during decidualization, implantation and organogenesis. Reproduction (Cambridge, England) 2003;125(1):75–84. doi: 10.1530/rep.0.1250075. [DOI] [PubMed] [Google Scholar]
- 37.Siemieniuch MJ, Majewska M, Takahashi M, Sakatani M, Lukasik K, Okuda K, et al. Are glucocorticoids auto- and/or paracrine factors in early bovine embryo development and implantation? Reprod Biol. 2010;10(3):249–56. doi: 10.1016/S1642-431X(12)60045-X. [DOI] [PubMed] [Google Scholar]
- 38.Pampfer S, Wuu YD, Vanderheyden I, De Hertogh R. Expression of tumor necrosis factor-alpha (TNF alpha) receptors and selective effect of TNF alpha on the inner cell mass in mouse blastocysts. Endocrinology. 1994;134(1):206–12. doi: 10.1210/endo.134.1.8275935. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Yair E, Less A, Lev S, Ben-Yehoshua L, Tartakovsky B. Tumour necrosis factor alpha binding to human and mouse trophoblast. Cytokine. 1997;9(11):830–6. doi: 10.1006/cyto.1997.0236. [DOI] [PubMed] [Google Scholar]
- 40.Kohchi C, Tanabe Y, Noguchi K, Mizuno D, Soma G. Induction of differentiation in embryonic stem cells by 26-kD membrane-bound tumor necrosis factor (TNF) and 17-kD free TNF. In vivo (Athens, Greece). 1996;10(1):19-27. [PubMed]
- 41.Wuu YD, Pampfer S, Vanderheyden I, Lee KH, De Hertogh R. Impact of tumor necrosis factor alpha on mouse embryonic stem cells. Biol Reprod. 1998;58(6):1416–24. doi: 10.1095/biolreprod58.6.1416. [DOI] [PubMed] [Google Scholar]
- 42.Pampfer S, Vanderheyden I, McCracken JE, Vesela J, De Hertogh R. Increased cell death in rat blastocysts exposed to maternal diabetes in utero and to high glucose or tumor necrosis factor-alpha in vitro. Development (Cambridge, England) 1997;124(23):4827–36. doi: 10.1242/dev.124.23.4827. [DOI] [PubMed] [Google Scholar]