Abstract
Background
Progesterone (P4) is essential for support of the endometrium and implantation of an embryo in the normal menstrual cycle. In programed frozen embryo transfer cycles using exogenous P4 is necessary, as the endogenous production of P4 requires a functioning corpus luteum that is not present in programed cycles. To date, there is continuing debate about ideal serum estradiol and P4 values in frozen embryo transfer cycles.
Methods
Patients underwent single euploid embryo frozen transfer cycles from 2010 to 2013 at a single large academic center. Patients using donor oocytes and patients with changes in progesterone dose during the cycles in question were excluded. All cycles were programed and intramuscular P4 was used exclusively. Only patients administering the same daily dose of P4 throughout the cycle were included (N = 213 patients). Main outcomes were ongoing pregnancy/live birth rates (OPR/LBR), clinical pregnancy rates (CPR), and spontaneous abortions/biochemical pregnancies. CPR was defined by the presence of a sac on 1st trimester ultrasound. Missed abortions were calculated per pregnancy with a sac. Receiver operator characteristic curves (ROC curves) and chi-squared tests were performed for statistical analysis.
Results
Two groups based on day 19 P4 levels were compared (group A, P4 < 20 ng/ml; group B, P4 > 20 ng/ml). OPR/LBRs were 65 vs. 49 %, group A vs. B, p value = 0.02, RR = 1.33 (1.1–1.7). Missed abortion and biochemical rates were higher in group B as opposed to group A, 27 vs. 12 %, p = 0.01, RR = 0.45(0.24–0.86). When P4 was stratified into five groups based on nanogram per milliliter of progesterone on day 19 (10–15, 15–20, 20–30, 30–40, and >40), there was a trend downward in OPR/LBR (70, 62, 52, 50, and 33 %, respectively). There was also an increase in missed abortion/biochemical rates (7, 15, 27, 32, and 20 %, respectively). Multiple logistic regression showed an increase in OPR/LBR when accounting for age, day 2 FSH, weight, number of embryos biopsied, and number of euploid embryos.
Conclusion
P4 levels >20 ng/ml on the day of transfer (during frozen single euploid embryo transfer cycles) were associated with decreased OPR/LBR.
Keywords: Luteal support, In vitro fertilization, Pregestational screening, Progesterone, Frozen embryo transfer
Introduction
Progesterone (P4) is required for successful embryonic implantation into the endometrium and maintenance of the pregnancy in natural cycles, fresh in vitro fertilization cycles, and frozen embryo transfer (FET) cycles [1]. Women undergoing frozen transfer cycles, specifically, are unable to provide adequate endogenous P4 and require progesterone supplementation to initiate and maintain the secretory endometrium and pregnancy. There has been previous research into luteal phase support in frozen cycles, which has demonstrated that supplementation of progesterone does impact outcome in FET [2]. Several studies have shown that there is an increase in live birth rate with P4 luteal support but there is mixed data on whether P4 support lowers the miscarriage or biochemical pregnancy rate [3, 4].
Despite this evidence for P4’s role, there is surprisingly little data on the optimal values for serum P4 during the luteal phase and specifically on the day of embryo transfer in frozen cycles. Following years of a prevalent belief that the higher values of P4 are better [5], it now seems that there may be an optimal window for P4 values during the luteal phase in bovine IVF [10]. Though progesterone levels on the day of transfer have not yet been studied extensively in humans, timing the transfer of a frozen embryo based on serial P4 values rather than cycle day number alone results in higher pregnancy rates [11].
This is the first study to specifically look at autologous euploid single embryo transfers and associated P4 levels during FET. The objective of this study was to determine if P4 levels on the day of frozen embryo transfer predict clinical outcome.
Materials and methods
A retrospective chart review of frozen embryo transfers was performed at our Fertility Center from 2010 to 2013. Inclusion criteria were patients that underwent ovarian stimulation and egg retrieval followed by blastocyst trophectoderm (TE) biopsy and cryopreservation of all embryos. Only those FETs with a single embryo transfer of a euploid embryo were analyzed. Preimplantation genetic screening (PGS) was offered to all patients. Specifically, patients >40 years of age and those with recurrent pregnancy loss and documented cytogenic abnormalities on the products of conception were counseled about the benefits of pregestational screening. About ~50 % of the patients included in this study were first time IVF patients and ~50 % had failed IVF in other institutions and or had recurrent pregnancy loss. The majority of the patients in our center and in this study underwent standard insemination technique as opposed to ICSI. Exclusion criteria included patients using oocytes retrieved from donors as well as embryos that resulted from previous egg freezing and thaw cycles. Patients were also excluded if intramuscular P4 dosage was changed for any reason during their frozen embryo transfer cycle. Patients on luteal support methods other than intramuscular injectable P4 were also excluded from analysis. Finally, two ectopic pregnancies were also excluded from analysis. A total of 213 patients were thus included.
Stimulation protocols were chosen based on patient characteristics and baseline ovarian reserve lab values. Maximum day 2 FSH level was 10.3 mIU/ml. The majority of ovarian stimulation cycles involved gonadotropins/GnRH antagonists with the exception of 11 cycles which were microdose leuprolide acetate cycles and 11 leprolide acetate long protocol cycles. In the gonadotropin/GnRH antagonist cycles when a lead follicle reached 13 mm or if estradiol >1000 pg/mL, the GnRH antagonist was initiated. When at least two lead follicles reached ≥17–18 mm, an ovulation trigger was administered using 10,000 IU of HCG or 40 units of leuprolide acetate or both 40 units of leuprolide acetate and 1000 IUs of HCG, depending on the estrogen level at trigger and number of follicles at trigger. Approximately 35 h later, oocytes were collected via ultrasound-guided transvaginal aspiration.
Incubator conditions were set at 37 °C, 6 % CO2, 5 % O2, and 89 % N2. Oocytes were placed in 25 ul drops of single-step medium (LifeGlobal) overlaid with 1.2 ml mineral oil (LifeGlobal; Ontario, Canada). Insemination was performed in the afternoon after the oocyte retrieval. If sperm appeared to be severely compromised, ICSI was performed rather than standard insemination. On day 1, fertilization was assessed by visualization of two nuclei. Embryos were checked for progress again on day 3 of culture. Embryos were removed from culture, briefly, on day 3, and an opening was made in the zona pellucida using a Cronus laser (Research Instruments; Falmouth, United Kingdom) before being returned to the incubator.
Embryos were graded on days 5/6 according to standard morphologic criteria as described by Gardner and Lane [12]. When embryos achieved the expanding blastocyst stage, they underwent TE biopsy (days 5, 6, or 7). Immediately following biopsy, embryos were vitrified on cryolocks (Biotech, Inc.) using Vitrification Freeze Solutions for Embryo cryopectants (Irvine Scientific, Santa Ana, CA). Biopsy material was analyzed using aCGH as per our standard protocol [13, 14]. Our culture and freezing protocol have been previously described [15].
Single embryo transfer was performed in subsequent frozen embryo transfer cycles per our center’s standard protocol. Patients were given oral estradiol in a stepwise manner to a max of 6 mg by day 14 of their menstrual cycle. On day 14, estradiol and progesterone values were checked to ensure adequate levels of estradiol and to ensure the patient had not ovulated. Endometrial thickness was also evaluated with transvaginal sonography on day 14. When endometrial thickness reached 7 mm or greater and was trilaminar in appearance, patients were initiated on intramuscular (IM) P4 for 6 days at a constant 50 or 75 mg per day. P4 was given as supplementation because normal ovarian steroid production and the ovarian follicular-to-luteal transition were suppressed with estradiol. Given the lack of ovulation, corpus luteum formation, and endogenous P4 production, exogenous P4 administration was continued until 9-week gestation. P4 values were checked 2 days after initiation to ensure adequate levels. Patients are instructed at our center to administer progesterone in the evening between 6 and 9 p.m. and to return two calendar days later between 7 and 9 a.m. to ensure adequate response to the P4 values. Embryo transfer occurred on the 6th day of IM P4 administration. On day 19, estradiol and P4 values were checked. Specifically, blood was drawn for every patient ~30 min to 1 h prior to the embryo transfer. It is important to note that the P4 value was not yet known prior to the transfer and did not influence decisions to continue with the transfer. There is published literature to support the notion that a steady state of P4 in endometrial tissues is reached within 24–48 h as well as peak values of P4 reached 7.3 h after P4 in oil injections. This steady state data provides credibility to the notion that timing of injection is less important once values are analyzed >48 h after initiation of P4 in oil [16].
Vitrified euploid blastocysts were warmed on the day of embryo transfer (defined as Day 19), using Thawing, Equilibration and Washing Media and the protocol from the package insert (Irvine Scientific, Santa Ana, CA). Warmed blastocysts were maintained in culture medium within an incubator until the time of embryo transfer (between 30 and 300 min after warming). Single embryos were loaded into a transfer catheter (Sureview, Wallace, Smith’s Medical, XXX UK) and transferred into the patient’s uterus. During the transfer procedure, placement of the catheter within the uterus was confirmed by abdominal ultrasound.
The patients were divided into two groups (group A, day 19 P4 values of <20 ng/dl and group B, day 19 P4 values of ≥20 ng/dl). Twenty nanogram per deciliter was chosen based on the published literature and the accepted general practices. Pregnancy tests were completed on day 28 of the menstrual cycle. First ultrasound was typically performed on cycle day 40–42. If no gestational sac was seen within the uterus, positive pregnancy tests (HCG levels > 5.3 mIU/ml) were considered biochemical pregnancies. If a gestational sac was seen in the uterus, and fetal cardiac activity was observed, the pregnancy was considered a clinical pregnancy. If a gestational sac was seen in the uterus, and fetal cardiac activity was never seen or did not continue once observed, the pregnancy was considered a spontaneous abortion.
Statistical analysis was performed using a free online epidemiologic calculator (http://www.openepi.com/v37/Menu/OE_Menu.htm). T tests of means were completed where appropriate baseline patient characteristics. Receiver operator characteristic (ROC) curves were also constructed where appropriate. Chi-square tests were used to evaluate pregnancy outcome between the two groups stratified by their P4 levels. Multiple regression was used to confirm the findings for OPR, LBR, and missed abortions/biochemical pregnancies.
Results
For each FET cycle, information from the original gonadotropin stimulation/egg retrieval cycle was collected. All embryos transferred were euploid. Cycle characteristics between group A (P4 < 20 ng/ml) and group B (P4 ≥ 20 ng/ml) were not found to be significant for age, day 2 estradiol and FSH values, total gonadotropins used, estradiol at trigger, number of eggs retrieved, number of fertilized embryos (2PN), and number of biopsied embryos (see Table 1). Progesterone levels were significantly different between the two groups.
Table 1.
Baseline cycle characteristics
| P4 < 20 ng/ml day 19 | P4 > 20 ng/ml day 19 | p value | |
|---|---|---|---|
| Age | 37 ± 4.2 | 37 ± 4.3 | NS |
| Total gonadotropins (IU) | 3400 ± 1375 | 3571 ± 1497 | NS |
| Day 2 FSH (mIU/ml) | 5.99 ± 2.74 | 5.87 ± 2.94 | NS |
| Day 2 Estradiol (pg/ml) | 38.3 ± 14.2 | 46.1 ± 34 | NS |
| Estradiol at trigger (pg/ml) | 2904 ± 1173 | 2656 ± 1131 | NS |
| # Eggs | 17.3 ± 8.1 | 16.2 ± 8.9 | NS |
| # 2PN embryos | 10.9 ± 5.4 | 10.9 ± 6.7 | NS |
| # Biopsy embryos | 5.9 ± 4.2 | 5.5 ± 4.6 | NS |
| P4 at day 19 (ng/ml) | 15.9 ± 2.5 | 30.5 ± 11 | <0.001 |
T test of means
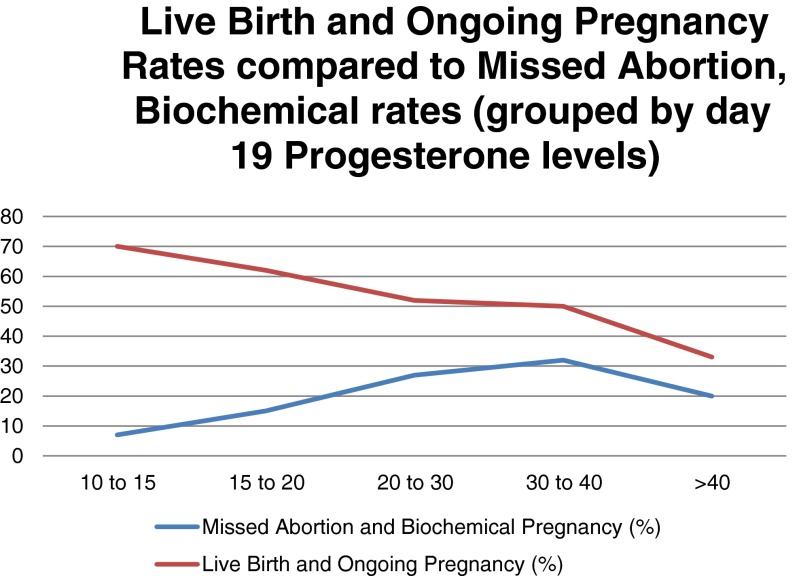
Following transfer of thawed euploid embryos, OPR/LBR were 65 vs. 49 %, group A vs. B, p value = 0.02, RR = 1.33 (1.1–1.7). Spontaneous abortion and biochemical rates were higher in group B as opposed to group A, 27 vs. 12 %, p = 0.01, RR = 0.45(0.24–0.86). When P4 was stratified into groups based on nanogram per milliliter of P4 on day 19 (10–15, 15–20, 20–30, 30–40, and >40), there was a trend downward in OPR/LBR (70, 62, 52, 50, and 33 %, respectively). There was also an increase in spontaneous abortion/biochemical rates (7, 15, 27, 32, and 20 %, respectively) (Fig. 1).
Fig. 1.
Spontaneous abortion/biochemical pregnancy rates
There was a significant decrease in OPR/LBR when progesterone crossed the 40 ng/ml mark at cycle day 19 (33 % for those cycles). The largest spontaneous abortion/biochemical pregnancy rates were found in the 30–40 ng/ml P4 range and averaged 32 % (Fig. 1).
ROC curves were constructed and the area under the curves can be seen in Table 2. The area under the curve examining P4 level on day 19 as a predictor of ongoing pregnancy or live birth was found to be 0.60. E2/P4 ratio on day 19 was then evaluated for its ability to predict ongoing pregnancy or livebirth and was found to have an AUC of 0.53. The low value for the AUC of the ROC curve for E2/P4 ratio on day 19 indicated that this ratio was not a good predictor or ongoing pregnancy or live birth so we performed no further analysis for this ratio.
Table 2.
Area under the curve for day 19 progesterone and estradiol/progesterone ratio
| OPR/LBR | Clinical pregnancy | Missed abortion | |
|---|---|---|---|
| P4 day 19 | AUC 0.60 | AUC 0.56 | AUC 0.60 |
| E2/P4 ratio day 19 | AUC 0.53 | n/a | n/a |
Multiple logistic regression was also performed. The patients’ ages, day 2 FSH, weight, number of embryos biopsied, and number of euploid embryos were examined in the analysis. OPR/LBR was significantly associated with the P4 value on the day of transfer and with none of the other variables. Spontaneous abortion/biochemical pregnancies were not associated with the P4 value on the day of transfer, however, were associated with age.
Discussion
The numbers of women undergoing frozen embryo transfer has increased for many reasons including both elective and medically indicated oocyte and embryo preservation. Currently, there are three different routes of administration of exogenous P4; vaginal, intramuscular, and oral [5, 6]. When compared to human chorionic gonadotropin (HCG), the use of P4 luteal support is preferable to the use of HCG because it performs better in FET cycles [7] and has a lower chance of ovarian hyperstimulation syndrome [5]. Therefore, it is important to understand the impact of P4 support and optimal P4 values during FET.
This study is the first to associate day 19 P4 values during frozen embryo transfer cycles to outcomes following single thawed euploid embryo transfers (STEETs). An error rate of 1.9 % in ploidy status of embryos is established and accepted in the literature [14]. Therefore, we believe that the embryos that we transferred are assumed to have truly been euploid in 98.1 % of the transfer cycles based on previously published literature on the error rate using the same technique employed in this study, array comparative genomic hybridization [14]. Our use of euploid embryos, in turn, allows for the more direct assessment of role of P4 levels on implantation without the fear of genetically abnormal embryos confounding the overall analysis.
Several studies have shown that P4 values within a narrow range on the day of HCG trigger correlate with higher pregnancy rates [8, 9]. Many studies have reported that a premature rise in progesterone is unfavorable on live birth outcome [17–22] following transfer of embryos in fresh IVF cycles. This phenomenon (premature luteinization) is unique to fresh cycles when the ovaries are driven by exogenously administered gonadotropins. Our study examines the levels of P4 in cycles controlled exogenously but by using only estrogens and progesterones without uncontrolled ovarian responses driven by exogenous gonadotropins.
Recently, P4 values lower than 20 ng/ml on the day of embryo transfer were found to be associated with lower rates of live birth and clinical pregnancy rates in donor recipient cycles [23]. Their study is notably different because embryos were not genetically screened (although they were generated from donor oocytes for which we expect a high incidence of euploidy), and they were not cryopreserved. Their conclusion depends on the assumption that the embryos had a high euploidy rate. In contrast, our manuscript ensures the exclusion of confounding effects of aneuploid embryos by limiting the embryos used to known euploid embryos. We believe that the use of only demonstrably euploid embryos is important in eliminating embryo effects when attempting to determine the effect of P4 on outcomes.
It is very likely that the elevated P4 values on day 19 indicate that higher P4 values are attained sooner as well as being maintained at higher levels. This may lead to a direct effect on the “implantation window” by shifting it earlier in time. Accelerated endometrial development could de-synchronize uterine and embryo development. When the uterus becomes receptive to implantation earlier than the embryo attains a state competent to implant this could lead to decreased implantation or “poor quality” implantation. The data presented here supports this notion. Based on this notion, our data indicates that P4 values should be kept between 10 and 20 ng/ml on day 19. Perhaps we should attempt to maintain the levels between 10 and 20 ng/ml during the entire pre-implantation luteal period, at least during frozen embryo transfer cycles. We acknowledge that the OPR/LBR was not drastically changed if P4 was between 20 and 30 ng/ml. P4 values >30 ng/ml on the other hand showed a clear detriment to cycle outcome and should be avoided. There were no P4 values that were documented below 10 ng/ml. It is our protocol to increase IM P4 if the serum levels are less than 10 ng/ml 48 h after initial administration. Since these patients with levels less than 10 ng/ml required elevation in their progesterone values, they were excluded from this analysis and we are not aware of the effects of lower P4 levels.
In conclusion, controversy continues to exist in the literature regarding optimal P4 values and this is likely due to variation in patient characteristics, including uterine dynamics, progesterone absorption, and utilization/metabolism. We propose that maintenance of levels between 10 and 20 ng/ml prior to implantation will maximize implantation and ongoing pregnancy rates for FETs using single euploid embryos (ESTEET). However, this is a small study at one facility. Further studies will be necessary to investigate the universality of this proposed range for optimizing outcomes following euploid FET.
Footnotes
Capsule Our findings indicate that excessive progesterone during FET cycles may lead to worse outcomes, presumably secondary to dyssynchronous endometrium and embryonic developmental stage.
References
- 1.Daya S. Luteal support: progestogens for pregnancy protection. Maturitas. 2009;65(s1):S29–34. doi: 10.1016/j.maturitas.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Veleva Z, Orava M, Nuojua-Huttunen S, Tapanainen JS, Martikainen H. Factors affecting the outcome of frozen-thawed embryo transfer. Hum Reprod. 2013;28(9):2425–31. doi: 10.1093/humrep/det251. [DOI] [PubMed] [Google Scholar]
- 3.Bjuresten K, Landgren B, Hovatta O, Stavreus-Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertil Steril. 2011;95(2):534–7. doi: 10.1016/j.fertnstert.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Kim C, Lee Y, Lee K, Kwon S, Kim S, Chae H, et al. The effect of luteal phase progesterone supplementation on natural frozen-thawed embryo transfer cycles. Obstet Gynecol Sci. 2014;57(4):291–6. doi: 10.5468/ogs.2014.57.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penzias AS. Luteal phase support. Fertil Steril. 2002;77(2):318–23. doi: 10.1016/S0015-0282(01)02961-2. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP. Progesterone replacement with vaginal gel versus IM injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod. 2014;29(8):1706–11. doi: 10.1093/humrep/deu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Datab Syst Rev 2011;(10):CD009154. doi:10.1002/14651858.CD009154.pub2. [DOI] [PubMed]
- 8.Silverberg KM, Burns WN, Olive DL, Riehl RM, Schenken RS. Serum progesterone levels predict success of in vitro fertilization/embryo transfer in patients stimulated with leuprolide acetate and human menopausal gonadotropins. J Clin Endocrinol Metabol. 1991;73(4):797–803. doi: 10.1210/jcem-73-4-797. [DOI] [PubMed] [Google Scholar]
- 9.Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97(6):1321–7. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Niemann H, Sacher B, Elsaesser F. Pregnancy rates relative to recipient plasma progesterone levels on the day of nonsurgical transfer of frozen/thawed bovine embryos. Theriognology. 1985;23(4):631–9. doi: 10.1016/0093-691X(85)90197-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhe D, Sun L, Zhang H, Chen Z, Jian Y. The frozen-thawed embryo transfer timing determined by serum progesterone level: a retrospective follow-up study. Eur J Obstet Gynecol Reprod Biol. 2014;181:210–3. doi: 10.1016/j.ejogrb.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–82. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Mateo C, Colls P, Sanchez-Garcia J, Escudero T, Prates R, Ketterson K, et al. Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril. 2011;95:953–8. doi: 10.1016/j.fertnstert.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Colls P, Escudero T, Fischer J, Cekleniak NA, Ben-Ozer S, Meyer B, et al. Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod BioMed Online. 2012;24(6):621–9. doi: 10.1016/j.rbmo.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Kramer YG, Kofinas JD, Melzer K, Noyes N, McCaffrey C, Buldo-Licciardi J, et al. Assessing Morphokinetic Parameters via Time Lapse Microscopy (TLM) to predict euploidy: are aneuploidy risk classification models universal? J Assist Reprod Genet. 2014;31(9):1231–42. doi: 10.1007/s10815-014-0285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulson RJ, Collins MG, Yankov VI. Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert. J Clin Endocrinol Metab. 2014;99(11):4241–9. doi: 10.1210/jc.2013-3937. [DOI] [PubMed] [Google Scholar]
- 17.Santos-Ribeiro S, Polyzos NP, Haentjens P, Smitz J, Camus M, Tournaye H, et al. Live birth rates after IVF are reduced by both low and high progesterone levels on the day of human chorionic gonadotrophin administration. Hum Reprod. 2014;29(8):1698–705. doi: 10.1093/humrep/deu151. [DOI] [PubMed] [Google Scholar]
- 18.Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60,000 cycles. Reprod Biomed Online. 2012;97:1321–7. doi: 10.1093/humupd/dmt014. [DOI] [PubMed] [Google Scholar]
- 19.Huang R, Fang C, Xu S, Yi Y, Liang X. Premature progesterone rise negatively correlated with live birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertil Steril. 2012;98:644–70. doi: 10.1016/j.fertnstert.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Burns WN, Witz CA, Klein NA, Silverberg KM, Schenken RS. Serum progesterone concentrations on the day after human chorionic gonadotrophin administration and progesterone/oocyte ratios predict In vitro fertilization/embryo transfer outcome. J Assist Reprod Genet. 1994;11:17–23. doi: 10.1007/BF02213692. [DOI] [PubMed] [Google Scholar]
- 21.Huang CC, Lien YR, Chen HF, Chen MJ, SHieh CJ, et al. The duration of pre-ovulatory serum progesterone elevation before hcg administration affects the outcome of IVF/ICSI cycles. Hum Reprod. 2012;27:2036–45. doi: 10.1093/humrep/des141. [DOI] [PubMed] [Google Scholar]
- 22.Check JH, AMui J, Choe JK, Brasile D. Relationship of serum progesterone level the day after human chorionic gonadotropin injection on outcome following in vitro fertilization embryo transfer. Clin Exp Obstet Gynecol. 2009;36:214–5. [PubMed] [Google Scholar]
- 23.Brady PC, Kaser DJ, Ginsburg ES, Ashby RK, Missmer SA, Correia KF. Racowsky. Serum progesterone concentration on day of embryo transfer in donor oocyte cycles. J Assist Reprod Genet. 2014;31(5):569–75. doi: 10.1007/s10815-014-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]