Abstract
Purpose
The purpose of this paper is to determine whether antimullerian hormone (AMH) levels were associated with BMI in patients with diagnosed infertility, and more specifically, in patients with polycystic ovarian syndrome (PCOS).
Methods
A retrospective cohort study reviewed all females who presented to the clinical investigators’ practice between November 2011 and March 2013. The following data was retrieved from the medical record: (1) AMH level, (2) age, (3) BMI, (4) ethnicity, and (5) if infertile, etiology of infertility.
Results
AMH levels were available for 489 women. Of these, 104 were diagnosed with PCOS. Overall, there was no association between BMI and AMH (r −0.04, p > 0.05). On the other hand, in the women with PCOS, there was a significant association between BMI and AMH (r −0.31, p < 0.01).
Conclusions
BMI was not associated with AMH levels in the general population of infertile women or in patients without PCOS. However, BMI appeared to be significantly and inversely correlated with AMH in women with PCOS.
Keywords: Antimullerian hormone, Body mass index, Polycystic ovarian syndrome
Introduction
Antimullerian hormone (AMH) is a glycoprotein which is involved in tissue growth and differentiation [1]. In women, it is produced by granulosa cells, pre-antral, and small antral follicles and is secreted into circulation. Serum AMH levels decline throughout reproductive life until they become undetectable in menopause [2]. The serum levels of AMH reflect the size of the ovarian follicular pool. The main function of AMH seems to be the inhibition of the early stages of follicular development and of the FSH-dependent selection process [3]. By antagonizing FSH, AMH serves as one of the gate‐keepers for the cohort of follicles and prevents selection of multiple dominant follicles [3, 4]. The level of AMH diminishes in larger follicles, and this results in improved FSH sensitivity in the face of falling FSH levels as estradiol levels rise. Diminished ovarian reserve seems to be associated with low serum AMH levels. Recently, AMH has emerged as a promising marker of ovarian reserve and even as a predictor of in vitro fertilization (IVF) success [5]. In a systematic review and meta-analysis by Tal [6], AMH has been shown to be associated with implantation and, albeit weakly, clinical pregnancy. As an effective marker of ovarian reserve AMH has the advantage that, unlike FSH and other hormones produced by the ovary, it does not significantly change during the menstrual cycle. A study by Kissell [7] did show a variation in AMH levels throughout the menstrual cycle; however, clinically, the variability was not great enough to warrant a change in clinical practice.
While AMH can be used as a measure of ovarian reserve, it is also useful in pointing to other factors affecting fertility. Elevated AMH levels have been noted in patients with polycystic ovarian syndrome (PCOS), reflecting the higher number of small follicles that give PCOS its name. PCOS affects 4 to 12 % of women of reproductive age and is the leading cause of infertility [8, 9]. The cardinal clinical features of PCOS are hirsutism and menstrual irregularity related to anovulation. In addition, obesity occurs in approximately 50 % of hyperandrogenic anovulatory women. However, there is a subtype of women with anovulatio and ultrasound-confirmed polycystic ovaries who are not obese and in fact appear to have a normal or even a below normal BMI. These women are often said to be “thin polycystics.”
AMH levels have been shown to be higher in patients with PCOS than in controls because of the increased number of antral follicles [10, 11]. Additionally, these high AMH levels are probably related to the follicular arrest, during the selection process of the dominant follicle, through a negative interaction between AMH and FSH [12]. Clinical observation at our institution suggested that people with thin PCOS may have higher AMH levels than those with classic PCOS. Our goal was to determine whether AMH levels were associated with BMI in patients presenting to an academic, infertility clinic, and more specifically, in patients with PCOS.
Materials and methods
Our study was conducted with approval from the Institutional Review Board at Baylor College of Medicine in Houston, TX. We performed a cross-sectional study in a cohort of female patients who presented to our practice at the Texas Children’s Hospital Pavilion for Women, Houston, TX, between November 2011 and March 2013. The following data was retrieved from the medical records of all patients with a documented antimullerian hormone level: (1) AMH level, (2) age, (3) BMI, (4) ethnicity, (5) gestation/parity, (6) past surgical history, (7) past medical history, (8) metabolic factors including hemoglobin A1c and physical findings, (9) small antral follicle count, and (10) etiology of infertility (PCOS, endometriosis, tubal disease, male factor, advanced maternal age). All information was obtained strictly from patients’ charts. No areas of bias were noted. Patients were excluded only if the AMH level, and/or, BMI were not available. Patients were identified as having polycystic ovary syndrome by the Rotterdam criteria as defined by as meeting two of the following three criteria: oligo/anovulation, hyperandrogenism, or polycystic ovaries on ultrasound. A GE 8 ultrasound machine was used to visualize ovaries. Polycystic ovaries were defined as having ≥12 small antral follicles per ovary. Patients were considered “lean” PCOS if their BMI was less than 25. Antimullerian hormone levels were measured on the initial visit irrespective of day of cycle and established prior to hormone therapy. AMH levels were measured in ng/ml using the dual monoclonal antibodies in a chemiluminescent immunoassay (CLIA). The serum for the assay was frozen within 3 hours of collection. The same assay was used for all AMH samples collected. Our primary outcome was to determine if AMH was correlated with BMI in patients with PCOS as well as evaluating this relationship in the overall infertility population in our clinic.
The correlation between age, BMI, and AMH was examined using the Spearman Correlation Coefficient test. The correlation coefficient assesses the measure of the strength and direction of the linear relationship between two variables using a monotonic function with values ranging from−1 to +1. The comparison of the average AMH within each BMI subcategory was examined using the t test. The data is presented as mean ± standard deviation. Our secondary goal was to evaluate demographic information with regard to AMH levels. Demographic information was analyzed using the chi square test.
Results
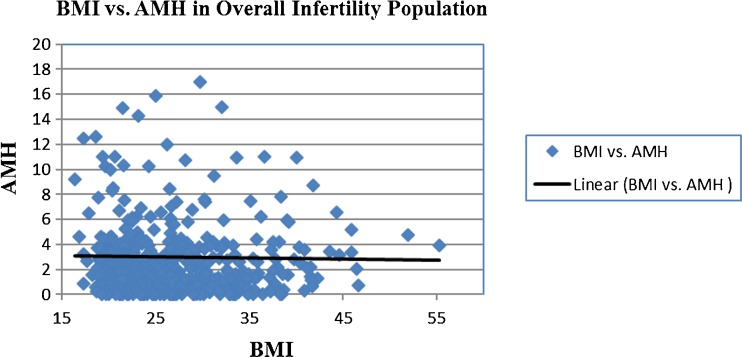
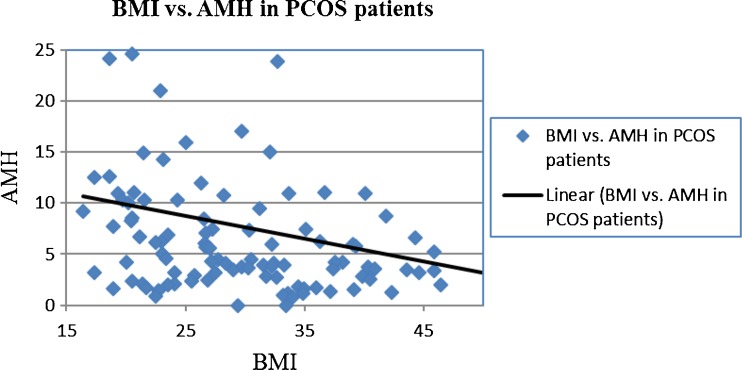
Information was obtained for a total of 512 patients. Twenty-three patients were excluded from the study because AMH levels were not available. The total number of women for whom an AMH level was available was 489. The average age, BMI, and AMH were 34.2 ± 5.4, 26.7 ± 6.4, and 3.0 ± 5.4, respectively. Of these 489 women, 104 were diagnosed with PCOS. In the 385 women without PCOS, the average age, BMI, and AMH were 35.2 ± 5.2, 25.9 ± 5.5, and 1.75 ± 2.3, respectively. The average age, BMI, and AMH of women with PCOS were 31.0 ± 5.1, 29.9 ± 8.4, and 7.6 ± 9.5, respectively. There was no association between BMI and AMH in the overall population of patients (r −0.04, p > 0.05) (Fig. 1). On the other hand, in women with PCOS, there was a significant inverse correlation between BMI and AMH (r = −0.29, p = 0.002) and (r = −0.31, p = 0.002) when adjusted for age (Fig. 2).
Fig. 1.
BMI vs. AMH in all infertility patients. Seven outliers were removed from graph for scaling purposes
Fig. 2.
BMI vs. AMH in PCOS patients. Four outliers were removed from graph for scaling purposes
In the PCOS population, 36 patients had a BMI ≤25, 20 patients had a BMI 25–29.9, 21 patients had a BMI of 30–34.9, and 27 patients had a BMI ≥35. The average AMH levels within each category were 10.9 ± 13.4, 6.4 ± 3.8, 5.2 ± 5.7, and 5.9 ± 7.5, respectively. With a 90 % confidence interval, the ≤25 BMI category had significantly higher AMH levels than each of the other BMI categories. The difference was most notable between the average AMH in the ≤25 group and that in the BMI group of 30–34.9 (p = 0.02, CI 95 %).
Using the criteria of ≥12 follicles on one ovary as a diagnosis of a polycystic ovary, we found 79 patients in the PCOS population to have PCO ovaries. Of the remaining 25 patients with PCOS, information was not available for 11 patients, and ovaries were not well visualized for 2 patients. Of the remaining 12 PCOS patients without polycystic ovaries on imaging, the average age, BMI, and AMH were 36.5 ± 5.5, 33.2 ± 5.4, and 1.1 ± 0.6, respectively. All of the PCOS patients had both ovaries, and only two patients had ever undergone any ovarian surgery. Both patients had a cystectomy; one of which was for struma ovarii. Medical history among the PCOS patients was most notable for hypothyroidism (12 %). Excluding patients with known diabetes, 28 patients (27 %) in the PCOS population had evidence of insulin resistance apparent in physical findings or lab abnormalities. Fifteen patients had elevated HA1c (≥5.7), and fifteen patients had acanthosis nigricans; two of the patients had both an elevated HA1c and acanthosis. Clinical findings of androgen excess (acne, hirsutism) were noted in 42 % (n = 44) of PCOS patients. Of the total 489 women in the study, 402 reported their ethnicity. The largest number of the women were Caucasians comprising 50 % of the patient population. African Americans comprised 15 %; Asians comprised 10 %; Hispanics comprised 4 %, and “Other” comprised 3 %. The remaining 18 % did not report their ethnicity. Among the patients studied, the ethnic distribution of patients with a diagnosis of PCOS and those without PCOS was approximately equal with no statistically significant difference between groups.
Conclusions
In our study of 489 women, BMI was not correlated with AMH levels in our general clinic population. However, in the subject population with PCOS, there was a strong correlation, with AMH levels being inversely related to BMI. Likewise in the PCOS population, the average AMH in the lean PCOS group (BMI ≤25 category) was significantly higher than in the higher BMI categories. This is the first large study of its kind. In a small study, Piouka [13] showed an inverse relationship between BMI and AMH levels in 25 PCOS patients. Our findings are consistent with the therapeutic challenges that are encountered in treating “thin” PCOS patients, given that the higher the AMH level, the more difficulties there are in attaining response to fertility medications [14, 15]. A possible explanation of higher AMH levels in lean PCOS patients could be related to LH levels. Higher LH levels have been noted in normal weight PCOS patients than in overweight or obese PCOS patients. AMH and LH levels have been shown to be positively correlated [16]. It has been suggested that lower LH concentrations in obese women are a result of increased aromatization of androgens to estrogens in the peripheral fat tissue, resulting in LH suppression [17]. Other working theories suggest that obesity may affect the catabolism of AMH, that obesity could reduce the ovarian potential, or that obesity may be related to ovarian dysfunction [12]. However, there is no clear explanation for this interesting subgroup of PCOS women.
Several studies have noted increased AMH levels in PCOS patients. In our PCOS population, the majority of patients had an increased small antral follicle count. Of those patients with non-PCO ovaries, the average AMH was 1.13. Of note, the patients with non-PCO ovaries were on average 5 years older. These findings are consistent with findings that AMH levels decrease with age. This also lends further evidence that increased small antral follicle count is one of the factors in higher AMH levels in PCOS patients.
Limitations of our study include the need for larger numbers in both groups, i.e., lean and non-lean patients. However, the study represents the largest study of its kind. We would have liked to follow these patients over time to determine subsequent treatment outcomes in those seeking pregnancy. Also, FSH and LH levels were not routinely measured in our study population, so we could not determine if LH and AMH were related. In addition, the data was obtained from a single infertility center where our population was limited predominantly to Caucasians.
Our future direction is to examine the effect of AMH levels on outcomes in patients with thin PCOS. Is there a certain upper AMH level at which fertility response to oral ovulation induction medications diminishes significantly, and these more conservative treatments should be bypassed for more advanced assisted reproductive therapies like in vitro fertilization (IVF)? Given the potential cumulative effect of Clomid and the increased resistance of PCOS patients with high AMH levels to Clomid, should we move to IVF more quickly to avoid the risk of higher order multiples that have been observed [18]. Likewise, recent data [19] has shown an increased incidence of ovarian hyperstimulation syndrome in lean PCOS patients in comparison to classic PCOS patients. With our results confirming that lean PCOS patients have higher AMH levels, can AMH levels be used as a predictor of risk for ovarian hyperstimulation syndrome in the thin women?
Our initial reason for looking at AMH and BMI levels was the impression that thin women with PCOS had higher AMH levels than patients with “classic” PCOS, and that is suggested by our results. This is a good preliminary study to help understand differences between the two PCOS subtypes.
Acknowledgments
Conflict of interest
The authors declare that they have no competing interests.
Financial support
None.
Footnotes
Capsule
There is a significant inverse correlation between body mass index and antimullerian hormone level in women with polycystic ovary syndrome.
References
- 1.Durlinger AL, et al. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124(5):601–9. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 2.Siefer DB, et al. Age-specific serum anti-Müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–50. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Köninger A, et al. Predictive markers for the FSH sensitivity of women with polycystic ovarian syndrome. Hum Reprod. 2014;29(3):518–24. doi: 10.1093/humrep/det468. [DOI] [PubMed] [Google Scholar]
- 4.Jonard S, et al. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107–17. doi: 10.1093/humupd/dmh010. [DOI] [PubMed] [Google Scholar]
- 5.van Rooij A, et al. Serum anti-mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 6.Tal R, et al. Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception: a systematic review and meta-analysis. Fertil Steril. 2015;103(1):119–30.e3. doi: 10.1016/j.fertnstert.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Kissell KA. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764–72. doi: 10.1093/humrep/deu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheehan M. Polycystic ovarian syndrome: diagnosis and management. Clin Med Res. 2004;2(1):13–27. doi: 10.3121/cmr.2.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.March WA, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 10.Homburg, et al. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod. 2013;28(4):1077–83. doi: 10.1093/humrep/det015. [DOI] [PubMed] [Google Scholar]
- 11.Caglar, et al. Anti-Mullerian hormone and insulin resistance in classic phenotype lean PCOS. Arch Gynecol Obstet. 2013 Apr 4. [DOI] [PubMed]
- 12.Karkanaki A, et al. The clinical significance of anti-Mullerian hormone evaluation in gynecological endocrinology. Horm. 2011;10(2):95–103. doi: 10.14310/horm.2002.1299. [DOI] [PubMed] [Google Scholar]
- 13.Piouka A, et al. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296(2):E238–43. doi: 10.1152/ajpendo.90684.2008. [DOI] [PubMed] [Google Scholar]
- 14.Amer SA, et al. The influence of circulating anti-Müllerian hormone on ovarian responsiveness to ovulation induction with gonadotrophins in women with polycystic ovarian syndrome: a pilot study. Reprod Biol Endocrinol. 2013;11:115. doi: 10.1186/1477-7827-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahran A, et al. The predictive value of circulating anti-Müllerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate: a prospective observational study. J Clin Endocrinol Metab. 2013;98(10):4170–5. doi: 10.1210/jc.2013-2193. [DOI] [PubMed] [Google Scholar]
- 16.Panidis D. Serum LH levels are markedly increased and significantly correlated with Delta 4-androstendione levels in lean women with polycystic ovary syndrome.2005. Fertil Steril 84: 538–540. [DOI] [PubMed]
- 17.Katsikis I, et al. Phenotypic expression, body mass index and insulin resistance in relation to LH levels in women with polycystic ovary syndrome. 2011. Eur J Obstet Gynecol Reprod Biol [DOI] [PubMed]
- 18.McDowell S, et al. Clomiphene ovulation induction and higher-order multiple pregnancy. Aust N Z J Obstet Gynaecol. 2013;53(4):395–8. doi: 10.1111/ajo.12106. [DOI] [PubMed] [Google Scholar]
- 19.Bailey AP, et al. Effect of body mass index on in vitro fertilization outcomes in women with polycystic ovary syndrome. Am J Obstet Gynecol. 2014;211(2):163–e1-6. doi: 10.1016/j.ajog.2014.03.035. [DOI] [PubMed] [Google Scholar]