Abstract
Purpose
The purpose of this study is to describe impaired oocyte fertilization from phospholipase C-zeta (PLC-ζ) deficiency in normal-appearing sperm that was successfully treated using calcium (Ca2+) ionophore with intracytoplasmic sperm injection (ICSI) of oocytes matured in vitro.
Methods
An infertile couple undergoing in vitro fertilization (IVF) experienced failed oocyte fertilization following ICSI with normal-appearing sperm. A semen sample collected from the patient was used to assess the expression of sperm PLC- ζ protein by Western blot analysis and immunofluorescence and PLC-ζ bioactivity by an in vitro model of Ca2+ release. A second IVF cycle was performed using Ca2+ ionophore with ICSI to enhance Ca2+-induced oocyte activation of oocytes matured in vitro.
Results
Sperm PLC-ζ protein deficiency was demonstrated by Western blot analysis and immunofluorescence and confirmed by reduced PLC-ζ bioactivity using an in vitro model of Ca2+ release. Nevertheless, with this sperm and supplementation of Ca2+ ionophore following ICSI, fertilization of four of six oocytes matured in vitro was obtained. In addition, four embryos underwent cleavage and two of them reached the blastocyst stage. Transfer of these blastocysts into the uterus led to a single pregnancy and live birth.
Conclusions
Deficiency of PLC-ζ in normal-appearing human sperm is associated with impaired Ca2+-dependent oocyte activation during ICSI. Under this condition, use of Ca2+ ionophore following ICSI of oocytes matured in vitro improves embryo developmental competence, possibly through the activation of Ca2+-dependent mechanisms governing fertilization and preimplantation embryogenesis.
Keywords: Sperm, Phospholipase C-zeta, Oocyte activation, Assisted reproductive technology (ART), Calcium ionophore, In vitro maturation
Introduction
Oocyte fertilization failure during in vitro fertilization (IVF) occurs in approximately 4 % of couples [1]. When it occurs, intracytoplasmic sperm injection (ICSI) is usually performed to bypass sperm-oocyte membrane fusion by injecting sperm directly into the mature oocyte, thereby inducing calcium (Ca2+)-dependent oocyte activation. Central to this process is phospholipase C-zeta (PLC- ζ) [2], a sperm-specific protein, which when released into the oocyte upon sperm entry induces Ca2+ release in the ooplasm [2]. In vitro studies confirm that injection of human PLC-ζ cRNA as well as recombinant human PLC-ζ protein into human oocytes induces release of Ca2+ from the endoplasmic reticulum (ER) leading to oocyte activation and zygote formation [2–6]. Furthermore, absence of PLC-ζ from sperm extracts, or inhibition of PLC-ζ expression in sperm by RNA interference, impairs cytosolic Ca2+ release and reduces the amplitude and duration of Ca2+ oscillations [2, 3, 7].
Clinical observations further suggest that PLC-ζ deficiency in sperm with normal morphology can underlie oocyte fertilization failure after ICSI [8]. Furthermore, use of Ca2+ ionophore with ICSI using PLC-ζ-deficient sperm has been shown to improve oocyte fertilization [9–12]. The present report describes the clinical outcome of an infertile couple with normal-appearing sperm but with PLC-ζ deficiency that was discovered after oocyte fertilization failure following ICSI [8]. It introduces the use of Ca2+ ionophore (A23187) following ICSI of oocytes matured in vitro in combination with embryo vitrification to successfully induce oocyte activation, fertilization, and preimplantation embryogenesis, leading to the birth of a healthy term female offspring.
Case
A 32-year-old nulliparous woman presented with a 5-year history of infertility. Her past medical history was significant for hypothyroidism corrected with Synthroid (50 μg orally daily) and hyperandrogenic anovulation due to polycystic ovarian syndrome (PCOS). Her hysterosalpingogram showed a normal intrauterine cavity and bilaterally patent fallopian tubes. Her husband was a 31-year-old male with type 1 diabetes mellitus adequately controlled with Humalog 60 units and Lantus 30 units subcutaneously (sc) daily. His semen analysis was normal by previously established criteria [5] (volume, 4.2 mL; pH, 7.2; sperm concentration, 41.3 million per mL; motility, 49 %; normal forms by strict morphology, 6.0 % according to strict Kruger criteria). The couple did not conceive despite ten cycles of successful ovulation induction with clomiphene citrate administered to the female and adequately timed sexual relations.
In vitro fertilization was subsequently performed using a standard GnRH-antagonist ovarian stimulation protocol (Ganirelix; Merck & Co.) [13]. Recombinant human follicle-stimulating hormone (rhFSH) and urinary gonadotropins were started at a dose of 300 IU sc daily for 2 days and then changed thereafter as clinically indicated. Serial estradiol (E2) levels and transvaginal sonographic measurements of ovarian follicles were performed until at least three follicles reached ≥17 mm in diameter, at which time serum E2 levels reached 1167 pg/mL. Choriogonadotropin alfa (500 μg sc, Ovidrel, EMD Serono, Inc., Rockland, MA) was then administered, and transvaginal oocyte retrieval was performed 35.5 h later.
Nine metaphase II (MII) oocytes were retrieved and were subjected to ICSI (N = 4) or inseminated with motile sperm (N = 5). All four MII oocytes subjected to ICSI failed to fertilize, consistent with a fertilization problem. Only one of five oocytes inseminated with motile sperm fertilized, as previously reported [8], resulting in the embryo transfer of one cleavage-stage embryo that did not implant.
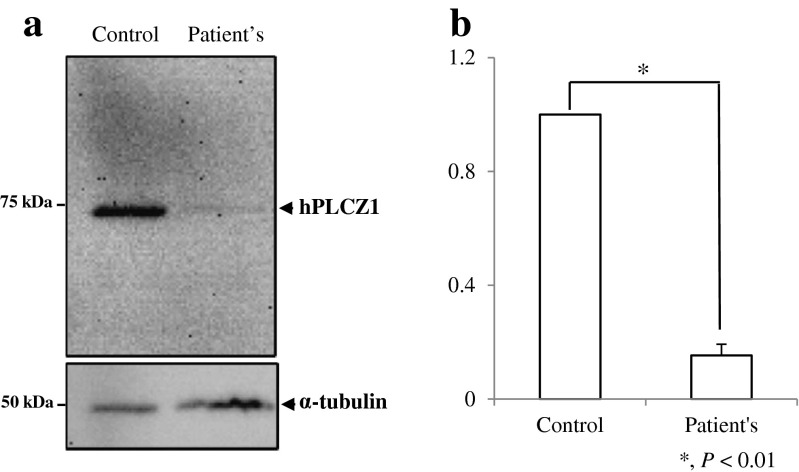
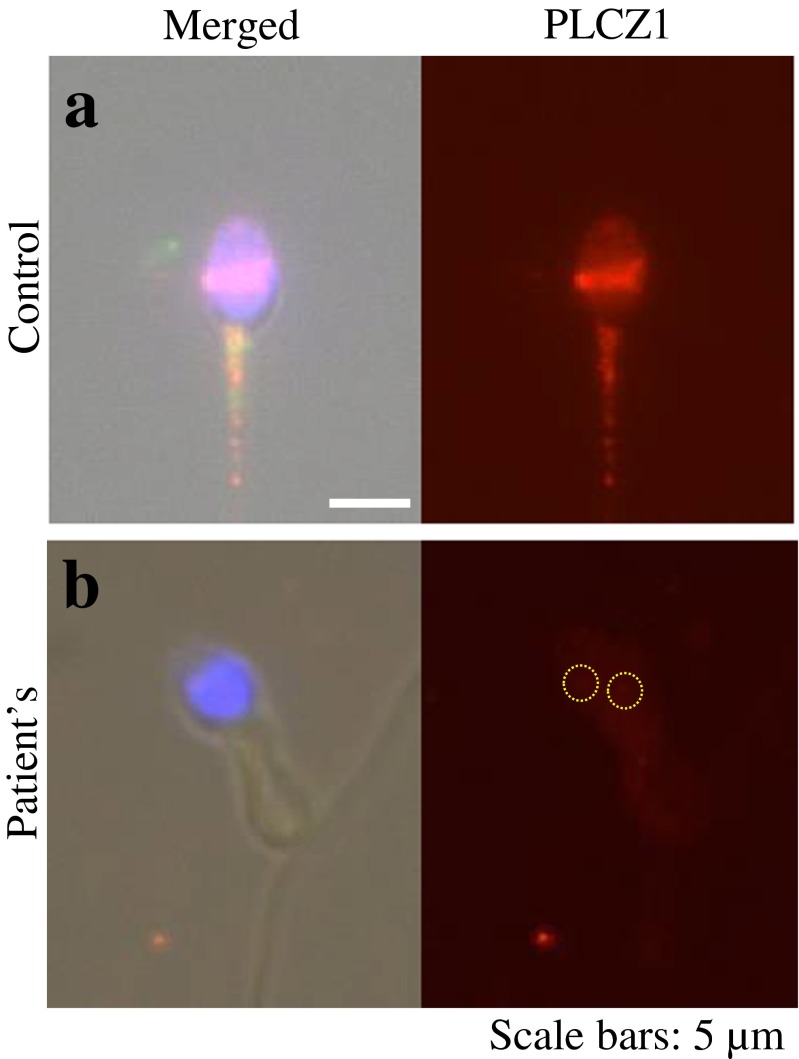
Sperm deficiency of PLC-ζ was considered as a possible cause for oocyte fertilization failure so that additional semen samples were collected from the male for further analysis. Compared to fertile men, sperm from the patient were deficient in PLC-ζ protein, as demonstrated by Western blot analysis and quantification (Fig. 1a, b), and contained PLC-ζ in aberrant locations or none at all [8], as shown by immunofluorescence (Fig. 2). Furthermore, sperm from the patient, but not from fertile men, failed to induce robust Ca2+ release following sperm injection into mouse oocytes and in the majority of cases failed to induce oscillations (Fig. 3) [8].
Fig. 1.
a Western blot analysis of PLC- ζ protein in sperm of reported male (i.e., patient) and fertile male (i.e., control). b Bar graph displaying the relative intensity of the PLC-ζ. Only 15 % of PLC-ζ protein was present in the patient’s sperm as compared to the fertile male
Fig. 2.
Immunofluorescence analysis of PLC- ζ located in the male sperm. As compared to the proven fertile control male (a), the patient’s sperm demonstrates decreased quantity and abnormal localization of PLC- ζ in the post-acrosomal region (b). Note that normal location of PLC- ζ is in the equatorial region of the sperm, although some variation can be observed
Fig. 3.

Calcium release assay demonstrating the functional capacity of PLC- ζ. Patient’s sperm does not initiate as robust a Ca2+ response as compared to the proven fertile control male. Nine out of 24 sperms initiated a lower than normal Ca2+ response. The remaining 15 sperms failed to induce a Ca2+ response entirely
A second GnRH-antagonist ovarian stimulation with IVF/ICSI was performed using Ca2+ ionophore with ICSI to enhance Ca2+-induced oocyte activation, as previously reported [9, 14, 15]. This time, rhFSH and urinary gonadotropins were started at a higher dose of 375 IU sc daily for 2 days and then changed as clinically needed. Serial E2 levels and transvaginal sonographic ovarian measurements again were performed until at least three follicles reached ≥17 mm in diameter when serum E2 levels reached 1991 pg/mL. Choriogonadotropin alfa was then administered, and transvaginal oocyte retrieval was performed as before.
Eleven MII oocytes were subjected to ICSI followed by exposure to Ca2+ ionophore (A23187: 5 μmol/L, Sigma Scientific) in G-1 media (Vitrolife) for 30 min [15]. Oocytes were then washed and placed in the incubator. All 11 MII oocytes failed to fertilize. In the same IVF cycle, Ca2+ ionophore and ICSI were used in six oocytes matured in vitro (four progressed from MI to M2 and two from GV to M2). These oocytes, matured in vitro, were subjected to delayed ICSI 27 h after oocyte retrieval and exposed to Ca2+ ionophore for 30 min after ICSI. Delayed ICSI resulted in clearly defined, normal two-pronuclear fertilization of two of the six oocytes. Twenty-four hours later, four cleaving embryos were observed despite only observing normal fertilization in two of them, and two of the cleaving embryos progressed to the blastocyst stage. The blastocysts were vitrified 6 days after egg retrieval [16]. Approximately 6 weeks later, the two embryos were thawed and transferred as good-quality expanded blastocysts into the patient’s uterus, resulting in a singleton pregnancy.
A female infant weighing 7 lb 10 oz was subsequently delivered vaginally at 37.5 weeks gestation following labor induction for preeclampsia. The infant was born healthy, except for mild prematurity, and she has reached all of the normal milestones of growth and development at her current age of 10 months.
Discussion
Calcium-dependent oocyte activation is a critical step in oocyte fertilization and preimplantation embryo development [8, 14, 17]. As a crucial sperm protein governing Ca2+-dependent oocyte activation, PLC-ζ released into the oocyte during sperm-oocyte membrane fusion [2] acts on phosphatidylinositol 4,5-biphosphate (PIP2) [18] to induce its hydrolysis forming the second messengers inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG) [18]. Subsequent IP3 binding to its own receptor on the endoplasmic reticulum (ER) [19] opens Ca2+ channels, which release Ca2+ from the ER into the cytoplasm of the oocyte [19]. The resulting Ca2+ oscillations within the oocyte [18, 19] disseminate from the cortical region throughout the ooplasm [20, 21] at 10- to 20-min intervals [22], triggering oocyte activation [23] to initiate oocyte fertilization and preimplantation embryogenesis [8, 14, 17].
The present report provides the clinical outcome of one of three previously reported men who had normal-appearing sperm despite reduced PLC-ζ protein and bioactivity as well as poor oocyte fertilization capacity [8]. Specifically, the sperm from these men showed PLC- ζ protein levels that were 40–80 % lower than those of normal-appearing control sperm that were capable of oocyte fertilization [8]. Interestingly, PLC-ζ in the sperm of our patient was either absent or located in the post-acrosomal region and showed a punctate pattern rather than a uniform band in the equatorial region, which is the most consistent location in fertile males [5, 24, 25]. Nevertheless, it is worth noting that several distribution patterns of PLC-ζ have been reported within sperm without a history of failed oocyte fertilization [25].
From a clinical perspective, the present case report emphasizes the difficult clinical scenario that develops when oocyte fertilization failure follows ICSI with the use of normal-appearing gametes. Recurrent ICSI-related oocyte fertilization failure occurs in 13 % of successive IVF cycles [26], raising a dilemma regarding the use of homologous versus non-homologous gametes in subsequent IVF cycles. Under this circumstance, detecting sperm PLC-ζ deficiency implicates the sperm, rather than the oocyte, as the cause of failed oocyte fertilization, thereby avoiding unnecessary oocyte donation. A critical amount of PLC-ζ in sperm may be required for optimal Ca2+-dependent oocyte activation, below which low rates of oocyte fertilization after ICSI are observed (i.e., <30 %) [14], although variable sperm PLC-ζ expression between samples and patients may limit quantitative analysis of PLC-ζ as a diagnostic indicator of oocyte activation [25].
As a divalent cation, calcium ionophore A23187 enhances Ca2+ transport across the oolemma (from the culture media to the oocyte) to increase intracellular Ca2+, thereby mimicking the first Ca2+ release caused by PLC-ζ [27]. This A23187-enhanced Ca2+ transport within the oocyte is mediated through a single, large Ca2+ rise rather than the smaller, naturally repeating Ca2+ oscillations [10, 18]. Although previous studies have shown the benefit of using Ca2+ ionophore with ICSI to enhance Ca2+-induced activation of mature oocytes fertilized with PLC-ζ-deficient sperm [11, 14, 15, 28–32], our use of Ca2+ ionophore with in vivo matured PCOS oocytes following ICSI did not overcome the failed fertilization, raising caution with the use of Ca2+ ionophore and ICSI in this way. Instead, Ca2+ ionophore was successful in overcoming PLC-ζ-deficient sperm fertilization failure when used after ICSI of PCOS oocytes matured in vitro, supporting a report of successful pregnancy occurring after ICSI with artificial activation of in vitro matured oocytes [33]. It is possible that androgen-induced impairment of Ca2+ oscillations in PCOS oocytes matured in vivo reduced the benefit of artificial oocyte activation, without diminishing the value of using Ca2+ ionophore after ICSI of PCOS oocytes matured in vitro [34–36].
The finding that one blastocyst arose from an oocyte stimulated with Ca2+-induced activation from a PCOS patient, which led to a term infant female, raises the additional possible benefit of Ca2+ ionophore on preimplantation embryogenesis. Although beyond the scope of this report, Ca2+ ionophore has been shown to improve embryo development and pregnancy outcome in patients with a history of previous impaired embryo development [17]. Therefore, it is also possible that successful pregnancies after ICSI supplemented with artificial activation of oocytes matured in vitro [33] might be the result of enhanced embryo competence from both improved fertilization and preimplantation embryogenesis [10, 15, 17].
Although the term female offspring of our couple was normal at birth and has grown appropriately at 10 months of age, the safety of using of Ca2+ ionophore with ICSI remains unclear. Data regarding Ca2+ ionophore use with ICSI and its relationship to human oocyte competence, embryo development, and neurodevelopmental outcomes in children appear reassuring [17, 37, 38], but immature oocytes or mature oocytes failing to fertilize may harbor inherent defects that potentially harm the embryo [39]. Also relevant is the potential impact of Ca2+ ionophore use with ICSI on the rates of miscarriage, genetic abnormalities, and minor as well as major congenital malformations [15, 40]. Theoretically, non-physiological patterns of calcium oscillation in artificially activated oocytes might perturb calcium-mediated signal transduction, mitochondrial respiration, developmental programming, or epigenetic events in ways that alter post-implantation embryo development [41, 42].
Thus, normal-appearing human sperm can be deficient in PLC-ζ and unable to induce Ca2+-dependent oocyte activation during ICSI. If sperm PLC-ζ deficiency is detected, successful oocyte fertilization and preimplantation embryogenesis is possible through ICSI and artificial activation with Ca2+ ionophore of oocytes matured in vivo and in vitro. Future challenges with the management of this scenario are the usefulness of quantifying PLC-ζ in sperm as a diagnostic indicator of oocyte activation, the efficacy of Ca2+ ionophore use with ICSI in enhancing IVF-related pregnancy outcome following failed oocyte fertilization and/or impaired embryogenesis, and the implications of Ca2+ ionophore exposure on pregnancy complications and the long-term health of offspring [40].
Footnotes
Capsule
In an IVF couple with impaired oocyte fertilization during ICSI from deficient PLC-ζ in normal-appearing sperm, Ca2+ ionophore use following ICSI of oocytes matured in vitro improved oocyte competence, leading to the birth of a healthy term female offspring.
References
- 1.Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9(3):511–518. doi: 10.1093/oxfordjournals.humrep.a138537. [DOI] [PubMed] [Google Scholar]
- 2.Saunders CM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 3.Ramadan WM, et al. Oocyte activation and phospholipase C zeta (PLCzeta): diagnostic and therapeutic implications for assisted reproductive technology. Cell Commun Signal. 2012;10(1):12. doi: 10.1186/1478-811X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers NT, et al. Phospholipase Czeta causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction. 2004;128(6):697–702. doi: 10.1530/rep.1.00484. [DOI] [PubMed] [Google Scholar]
- 5.Yoon SY, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118(11):3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon SY, et al. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum Reprod. 2012;27(6):1768–1780. doi: 10.1093/humrep/des092. [DOI] [PubMed] [Google Scholar]
- 7.Knott JG, et al. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol Reprod. 2005;72(4):992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- 8.Lee HC, et al. Protein phospholipase C Zeta1 expression in patients with failed ICSI but with normal sperm parameters. J Assist Reprod Genet. 2014;31(6):749–756. doi: 10.1007/s10815-014-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldar-Geva T, et al. Successful pregnancy and delivery after calcium ionophore oocyte activation in a normozoospermic patient with previous repeated failed fertilization after intracytoplasmic sperm injection. Fertil Steril. 2003;79:1656–1658. doi: 10.1016/S0015-0282(03)00369-8. [DOI] [PubMed] [Google Scholar]
- 10.Ebner T, et al. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril. 2012;98(6):1432–1437. doi: 10.1016/j.fertnstert.2012.07.1134. [DOI] [PubMed] [Google Scholar]
- 11.Rybouchkin AV, et al. Fertilization and pregnancy after assisted oocyte activation and intracytoplasmic sperm injection in a case of round-headed sperm associated with deficient oocyte activation capacity. Fertil Steril. 1997;68(6):1144–1147. doi: 10.1016/S0015-0282(97)00378-6. [DOI] [PubMed] [Google Scholar]
- 12.Taylor SL, et al. Complete globozoospermia associated with PLCzeta deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod Biomed Online. 2010;20(4):559–564. doi: 10.1016/j.rbmo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarlatzis BC, et al. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. 2006;12(4):333–340. doi: 10.1093/humupd/dml001. [DOI] [PubMed] [Google Scholar]
- 14.Montag M, et al. The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reprod Biomed Online. 2012;24(5):521–526. doi: 10.1016/j.rbmo.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Borges E, Jr, et al. Artificial oocyte activation using calcium ionophore in ICSI cycles with spermatozoa from different sources. Reprod Biomed Online. 2009;18(1):45–52. doi: 10.1016/S1472-6483(10)60423-3. [DOI] [PubMed] [Google Scholar]
- 16.Kuwayama M, et al. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11(3):300–308. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 17.Ebner T, et al. Treatment with Ca2+ ionophore improves embryo development and outcome in cases with previous developmental problems: a prospective multicenter study. Hum Reprod. 2015;30(1):97–102. doi: 10.1093/humrep/deu285. [DOI] [PubMed] [Google Scholar]
- 18.Horner VL, Wolfner MF. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev Dyn. 2008;237(3):527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- 19.Fissore RA, et al. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124(6):745–754. doi: 10.1530/rep.0.1240745. [DOI] [PubMed] [Google Scholar]
- 20.Parrington J, et al. Expression of inositol 1,4,5-trisphosphate receptors in mouse oocytes and early embryos: the type I isoform is upregulated in oocytes and downregulated after fertilization. Dev Biol. 1998;203(2):451–461. doi: 10.1006/dbio.1998.9071. [DOI] [PubMed] [Google Scholar]
- 21.Fissore RA, et al. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod. 1999;60(1):49–57. doi: 10.1095/biolreprod60.1.49. [DOI] [PubMed] [Google Scholar]
- 22.Swann K, et al. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction. 2004;127(4):431–439. doi: 10.1530/rep.1.00169. [DOI] [PubMed] [Google Scholar]
- 23.Ducibella T, et al. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250(2):280–291. doi: 10.1006/dbio.2002.0788. [DOI] [PubMed] [Google Scholar]
- 24.Grasa P, et al. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod. 2008;23(11):2513–2522. doi: 10.1093/humrep/den280. [DOI] [PubMed] [Google Scholar]
- 25.Kashir J, et al. Variance in total levels of phospholipase C zeta (PLC-zeta) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril. 2013;99(1):107–117. doi: 10.1016/j.fertnstert.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Yanagida K. Complete fertilization failure in ICSI. Hum Cell. 2004;17(4):187–193. doi: 10.1111/j.1749-0774.2004.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 27.Steinhardt RA, et al. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- 28.Murase Y, et al. Pregnancy following chemical activation of oocytes in a couple with repeated failure of fertilization using ICSI: case report. Hum Reprod. 2004;19(7):1604–1607. doi: 10.1093/humrep/deh294. [DOI] [PubMed] [Google Scholar]
- 29.Chi HJ, et al. Successful fertilization and pregnancy after intracytoplasmic sperm injection and oocyte activation with calcium ionophore in a normozoospermic patient with extremely low fertilization rates in intracytoplasmic sperm injection cycles. Fertil Steril. 2004;82(2):475–477. doi: 10.1016/j.fertnstert.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Pinto J, Check JH. Correction of failed fertilization despite intracytoplasmic sperm injection with oligoasthenoteratozoospermia but with acrosomes present by oocyte activation with calcium ionophore—case report. Clin Exp Obstet Gynecol. 2008;35(4):252–254. [PubMed] [Google Scholar]
- 31.Stecher A, et al. Case report: live birth following ICSI with non-vital frozen-thawed testicular sperm and oocyte activation with calcium ionophore. J Assist Reprod Genet. 2011;28(5):411–414. doi: 10.1007/s10815-011-9546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmady A, Michael E. Successful pregnancy and delivery following intracytoplasmic injection of frozen-thawed nonviable testicular sperm and oocyte activation with calcium ionophore. J Androl. 2007;28(1):13–14. doi: 10.2164/jandrol.106.000174. [DOI] [PubMed] [Google Scholar]
- 33.Kim JW, et al. Successful pregnancy and delivery after ICSI with artificial oocyte activation by calcium ionophore in in-vitro matured oocytes: a case report. Reprod Biomed Online, 2014. [DOI] [PubMed]
- 34.Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38. doi: 10.1016/j.fertnstert.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80(4):1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 36.Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum Reprod Update. 1997;3(2):95–100. doi: 10.1093/humupd/3.2.95. [DOI] [PubMed] [Google Scholar]
- 37.Ebner T, et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod Biomed Online. 2015;30(4):359–365. doi: 10.1016/j.rbmo.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Vanden Meerschaut F, et al. Neonatal and neurodevelopmental outcome of children aged 3-10 years born following assisted oocyte activation. Reprod Biomed Online. 2014;28(1):54–63. doi: 10.1016/j.rbmo.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Heindryckx B, et al. Aberrant spindle structures responsible for recurrent human metaphase I oocyte arrest with attempts to induce meiosis artificially. Hum Reprod. 2011;26(4):791–800. doi: 10.1093/humrep/deq400. [DOI] [PubMed] [Google Scholar]
- 40.van Blerkom J, Cohen J, Johnson M. A plea for caution and more research in the ‘experimental’ use of ionophores in ICSI. Reprod Biomed Online. 2015;30(4):323–324. doi: 10.1016/j.rbmo.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128(6):917–928. doi: 10.1242/dev.128.6.917. [DOI] [PubMed] [Google Scholar]
- 42.Vincent C, Cheek TR, Johnson MH. Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. J Cell Sci. 1992;103(Pt 2):389–396. doi: 10.1242/jcs.103.2.389. [DOI] [PubMed] [Google Scholar]