Abstract
Purpose
This study aimed to determine whether the presence of an uncleaved embryo on day 3 is predictive of cycle outcome after day 5 transfer (D5 ET).
Methods
In vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles from January 2013 to November 2014 with D5 ET were analyzed for the presence of at least one uncleaved embryo on day 3 (D3). Each index cycle (n = 70) was compared with two matched control cycles without uncleaved embryos. The main outcome measures included embryo quality, implantation rate, and clinical pregnancy rate.
Results
Fifty-nine of 3896 total embryos in this study were uncleaved on D3 (1.5 %). Cycles with uncleaved embryos had more oocytes retrieved (20.6 vs. 17.5), lower proportions of good quality embryos on D3 (52.4 vs. 66.1 %), and fewer usable embryos (transferred or frozen) on D5 (42.4 vs. 50.8 %). However, there were no significant differences in the incidence of cycles with a positive hCG, or in the rates of implantation, clinical pregnancy, or live birth.
Conclusions
Although an uncleaved embryo on D3 is associated with reduced conversion of sibling embryos to the blastocyst stage on D5, overall quality of those embryos forming blastocysts is not markedly decreased and clinical outcomes are not compromised.
Keywords: IVF, Uncleaved embryo, Day 5 transfer
Introduction
Clinical in vitro fertilization (IVF) was established following transfer of cleavage stage embryos [1], a practice largely due to limitations of the prevailing culture system to support development of human embryos to the blastocyst stage in vitro. However, with advancements in embryo culture media, use of extended culture and transfer of blastocysts on day 5 became possible [2, 3]. These additional days in culture aid in the deselection of poorly developing embryos as aneuploid, and other defective embryos are less likely to reach the blastocyst stage [4, 5]. As a consequence, fewer embryos of higher quality may be transferred. Also, refinements of morphological systems for day 5 embryo selection [6–12] as well as the significant advances in genomic approaches for ploidy assessment using a few biopsied trophectoderm cells [13–15] all support the use of day 5 transfer in clinical IVF. Furthermore, improved implantation rates with blastocyst transfer may result from improved temporal synchronization between the embryo and endometrium at the time of transfer [16], as well as a possible reduced risk of embryo expulsion [17].
Despite the advantages associated with blastocyst transfer, potential downsides include a small increased risk of monozygotic [18, 19] and monochorionic [20] twinning, as well as the possibility of transfer cancellation in the event that no embryos have progressed beyond cleavage by day 5. Of note, the incidence of transfer cancellation is significantly higher in patients who are not specifically selected for extended culture, but is not different in “good” prognosis patients [21].
Several randomized controlled trials have investigated when, and with which parameters, during treatment, patients should be selected for extended culture (reviewed by Glujovsky et al 2012). Several cycle and embryo variables have been identified as predictors of blastocyst formation, and models for predicting pregnancy rates [22] and cycle cancellation [23] have been proposed. However, there is currently no standard protocol for prospectively selecting which patients will benefit from blastocyst transfer despite the numerous recent time-lapse imaging studies reporting that various morphokinetic parameters improve prediction of blastocyst formation compared with conventional morphology grading [reviewed by 24, 25]. The need for such a protocol is highlighted by recent studies showing that outcomes for patients with poor cleavage stage morphology are likely optimized by transferring embryos on day 3 [26, 27].
Current practice for deciding whether a patient should have a day 3 or a day 5 transfer has primarily focused on the number of embryos on day 1 (at the fertilization check) and/or the number and assessment of the lead, highest quality embryos in the cohort on day 3 [19]. Of note, no specific attention has previously been given to the possibility that embryos which have failed to cleave by day 3 may also have value in predicting success following transfer on day 5. The present study, designed to do just this, evaluated whether developmentally incompetent embryos are an independent marker for the quality of the total cohort. In particular, our objective was to determine if the presence of an uncleaved (i.e., an arrested one-cell) embryo on day 3 is predictive of blastocyst yield, quality, and clinical outcomes following a day 5 transfer and, thus, can be used when selecting patients to proceed to a day 5 transfer.
Materials and methods
This study was approved by the Partners’ Healthcare System Institutional Review Board.
Experimental design
Medical records of all patients undergoing IVF with or without intracytoplasmic sperm injection (ICSI) at Brigham and Women’s Hospital from January 2013 to November 2014 were reviewed. Only cycles with a day 5 embryo transfer (ET) using either autologous or donor oocytes and without preimplantation genetic diagnosis were included in this analysis. Index cases were defined as cycles with at least one uncleaved embryo identified on day 3 evaluation. Controls were those without uncleaved embryos on day 3. Each index cycle was matched to two control cycles for the following four variables: patient age, cycle type (IVF, ICSI, etc.), egg donor status, and type of stimulation protocol used. Of note, during the study period, all patients having a day 5 ET were required to have at least three day 3 embryos having >8 cells and <10 % of their volume fragmented. The patients in this data set were therefore considered to be of good prognosis.
Clinical protocols
Standard protocols were used for ovarian stimulation, as described elsewhere [28]. Based on patient age, ovarian reserve, and/or response in previous cycles, patients underwent one of the following ovarian stimulation protocols: GnRH antagonist, microflare, or luteal phase leuprolide acetate downregulation at full (20 to 10 units), very low (4 to 2 units), or ultra-low (2 to 1 unit) doses. Alternatives, including pseudoluteal, estradiol patch, ovulation induction (OI), and no suppression, were less common but also utilized.
Laboratory protocols
Gametes and embryos were incubated as described elsewhere [29] in humidified air with 5 % CO2, 5 % O2, and 90 % N2. Fertilization checks were done 15–18 h after insemination or ICSI. Oocytes which exhibited two pronuclei (2PN) were then identified and individually placed in 25 μL drops of medium (global® total® w/human serum albumin; LifeGlobal® Group, Toronto, Ontario, Canada) under 8 mL of Ov OilTM (Vitrolife, Englewood, CO) in Falcon 1007 dishes (Becton Dickinson Labware, Franklin Lakes, NJ). In addition, those oocytes exhibiting either 1PN or 3PN were recorded, and those lacking any PN were graded for meiotic maturity or an abnormality (defined as “giant”, i.e., having a diameter ≥50 % greater than normal; multi-cell, i.e., having two or more cells; or presenting as a ball of fragments).
Day 3 embryo morphology was assessed by a team of trained embryologists, who employ standard criteria to assign scores for cell number, fragmentation, and symmetry. Fragmentation was assessed using grades of 0 through 4, where scores correlated to 0 %, 1–9 %, 10–25 %, 26–50 %, or >50 % fragmentation, respectively. Blastomere asymmetry was graded using a numerical score of 1 through 3 according to uniformity in size and shape, where a score of 1 represented perfect symmetry, 2 moderate asymmetry, and 3 severe asymmetry. A good quality embryo was defined as ≥8 cells with <10 % fragmentation on day 3 (symmetry was not considered in this definition).
On day 5, morphology of each embryo was scored regarding stage of blastocyst development, inner cell mass (ICM) quality, and trophectoderm (TE) quality [7, 8, 30]. A day 5 stage of 1 signified a degenerate embryo, 2 a morula with incomplete compaction, 3 a morula with more than 50 % compaction, 4 an early blastocyst, 5 an expanding blastocoele occupying more than half the volume of the embryo, 6 a full blastocyst, 7 a fully expanded blastocyst with a thin zona pellucida, 8 a hatching blastocyst, and 9 a fully hatched blastocyst. For ICM grade, grade A represented a prominent ICM with many cells tightly adhered together, B a loosely adherent ICM with fewer cells, and C a few cells visible and D no cells discernible. For TE grade, grade A represented a continuous layer of cells, B fewer and larger cells without a continuous layer, C sparse cells, and D degenerate cells.
Outcome variables
Demographic characteristics, standard laboratory outcomes, and standard clinical outcomes between all index cycles and controls were compared in this analysis. Laboratory cycle outcomes included total number of oocytes retrieved, proportion of mature (MII) oocytes, fertilization rate (% 2PN/MII oocytes), proportion of good quality embryos on day 3, incidence of oocytes with 1PN and 3PN at the fertilization check, number of embryos transferred, and number of embryos cryopreserved. Further analysis was conducted comparing embryo morphology of index cohorts and controls on day 5, including stage of embryo development, ICM score, and TE score. Standard clinical outcomes examined in this study included chemical pregnancy, implantation, and clinical pregnancy rates. Chemical pregnancy was defined as an initial rise in β-hCG level with no detectable sac and spontaneous subsequent decline in β-hCG. Implantation was defined as the number of gestational sacs visualized by a 5–6-week ultrasound of the total number of embryos transferred. Clinical pregnancy rate was defined as the number of transfers resulting in the presence of at least one gestational sac by 5–6 weeks gestation.
Statistical analyses
Statistical analysis was performed using R’s statistics base-package (R Foundation for Statistical Computing, Vienna, Austria). Differences in continuous variables between the index and control groups were calculated using the Wilcoxon rank-sum test. Categorical variables were analyzed using Fisher’s exact test or Fisher-Freeman-Halton test. In all cases, P < 0.05 was considered statistically significant.
Results
Cycle demographics and incidence of uncleaved embryos on day 3
Seventy index cycles, defined as having at least one embryo that failed to cleave (i.e., that exhibited only one cell on day 3), and 140 control cycles were included in the final dataset. Patient demographics of the index and control groups are presented in Table 1. There were no significant differences between the two groups for any of the variables evaluated.
Table 1.
Demographic comparison of cycles in the control and index (uncleaved embryo) groups
| Control group (n = 140) | Index group (n = 70) | P value | |
|---|---|---|---|
| Age (year) | 33.7 ± 4.5a | 33.8 ± 4.8 | 0.815b |
| Attempt number | 1.8 ± 1.3 | 1.7 ± 1.4 | 0.174b |
| Day 3 FSH (mIU/mL) | 7.1 ± 2.9 | 6.8 ± 2.1 | 0.244b |
| Primary infertility diagnosis, n (% of total) | 0.998c | ||
| Male factor | 24 (17.1) | 12 (17.1) | |
| Decreased ovarian reserve | 17 (12.1) | 7 (10.0) | |
| An/oligoovulation | 26 (18.6) | 13 (18.6) | |
| Tubal | 11 (7.9) | 6 (8.6) | |
| Endometriosis | 2 (1.4) | 1 (1.4) | |
| Unexplained | 52 (37.1) | 28 (40.0) | |
| Other | 8 (5.7) | 3 (4.3) | |
| % Cycles with donor oocyte | 17 (12.1) | 9 (12.9) | >.999d |
| % Cycles IVF | 96 (68.8) | 47 (67.1) | 0.876d |
| % Cycles ICSI | 42 (30.0) | 22 (31.4) | 0.874d |
| % Cycles IVF/ICSI | 2 (1.4) | 1 (1.4) | >.999d |
| Stimulation protocol, n (% of total) | 0.450c | ||
| LDL | 57 (40.7) | 30 (42.9) | |
| Antagonist | 63 (45.0) | 30 (42.9) | |
| Pseudoluteal | 3 (2.1) | 1 (1.4) | |
| Microflare | 7 (5.0) | 1 (1.4) | |
| VLDL | 2 (1.4) | 4 (5.7) | |
| Patch | 5 (3.6) | 2 (2.9) | |
| OI | 2 (1.4) | 0 (0) | |
| ULDL | 0 (0) | 1 (1.4) | |
| No suppression | 1 (0.7) | 1 (1.4) | |
| Units of HMG + FSH taken | 23.6 ± 17.7 | 23.0 ± 15.8 | 0.917b |
| Peak E2 (d hCG) pg/mL | 2405.1 ± 880.4 | 2260.7 ± 759.8 | 0.416b |
aValues represent mean ± SD, except where otherwise indicated; those in parentheses are percentages
bWilcoxon rank-sum test
cFisher-Freeman-Halton test
dFisher’s exact test
The incidence of uncleaved embryos was 1.5 % (59/3896) for all embryos in the dataset and 4.1 % (59/1440) among embryos in the index cycles alone. In eleven of the 70 index cycles, there were multiple uncleaved embryos: one cycle had five uncleaved embryos, one cycle had four, two cycles had three, and seven cycles had two.
Oocyte maturity and fertilization
As seen in Table 2, significantly more oocytes were retrieved in the index cycles compared to the controls (20.6 vs. 17.5; P = 0.016). There was no significant difference in the percentage of metaphase II (MII) oocytes, immature oocytes, abnormal oocytes in total or by phenotype, or those that were degenerate. The percentages of 1PN, 2PN, or 3PN zygotes also did not differ between the two groups.
Table 2.
Cycle outcome variables in the control and index (uncleaved embryo) groups
| Control group (n = 140) | Index group (n = 70) | P valuec | |
|---|---|---|---|
| No. oocytes | 17.5 ± 6.4 | 20.6 ± 8.1 | 0.016 |
| % MII oocytes/total | 83.8 ± 14.3 | 84.2 ± 13.0 | 0.995 |
| % Immature/totala | 15.0 ± 13.0 | 13.4 ± 12.5 | 0.254 |
| % Abnormal oocytes/total | 1.06 | 1.67 | 0.107d |
| % Giant oocytes | 0.33 | 0.76 | 0.092d |
| % Multi-cell oocytes | 0.24 | 0.42 | 0.378d |
| % Fragmented oocytes | 0.04 | 0.14 | 0.559d |
| % Degenerate/total | 1.8 ± 3.8 | 1.4 ± 2.8 | 0.892 |
| % 1PN/MII | 3.8 ± 5.6 | 3.4 ± 5.0 | 0.703 |
| % 2PN/MII | 78.2 ± 14.0 | 80.1 ± 14.6 | 0.310 |
| % 3PN/MII | 4.3 ± 6.6 | 3.4 ± 5.7 | 0.484 |
| No. good quality D3 embryosb | 7.2 ± 3.2 | 6.8 ± 3.4 | 0.232 |
| % Good quality D3 embryos/2PN | 66.1 ± 18.2 | 52.4 ± 14.9 | <0.001 |
| No. D5 embryos frozen | 4.1 ± 3.2 | 4.1 ± 3.7 | 0.491 |
| % D5 embryos frozen (of 2PN) | 35.2 ± 20.9 | 28.3 ± 19.8 | 0.030 |
| No. embryos transferred | 1.5 ± 0.6 | 1.6 ± 0.7 | 0.576 |
| Total no. usable embryos (transferred and frozen) | 5.6 ± 3.0 | 5.6 ± 3.6 | 0.599 |
| % Transferred and frozen/2PN | 50.8 ± 19.1 | 42.4 ± 18.0 | 0.005 |
aImmature = no. of oocytes at germinal vesicle (GV) and metaphase I (MI) stages
b ≥8 blastomeres with <10 % fragmentation on day 3
cWilcoxon rank-sum test
dFisher’s exact test
Day 3 morphology
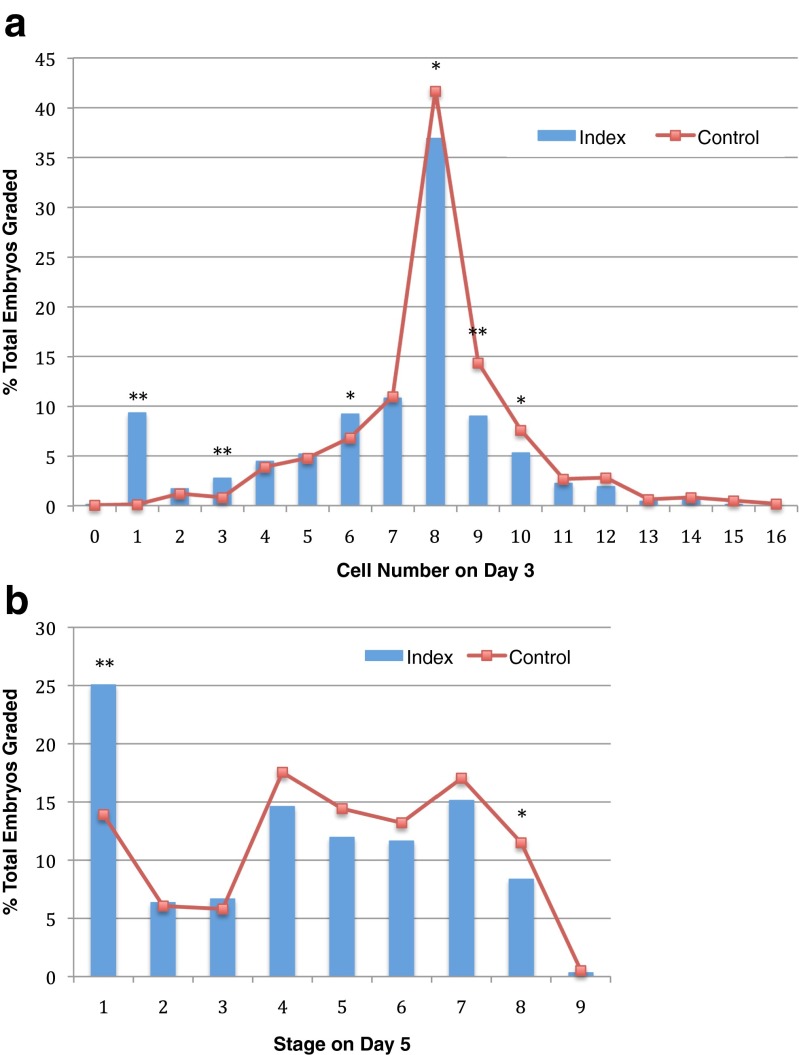
The index cases had significantly lower proportions of good quality day 3 embryos (52.4 vs. 66.1 %; P < 0.001; Table 2). Furthermore, there were significant differences between the two groups regarding the distribution of day 3 embryos for cell counts, with the index group having less 8-cell, 9-cell, and 10-cell embryos on average and more 3-cell, 6-cell, and, as expected, 1-cell embryos (Fig. 1a). The index group also had a lower proportion of embryos having seven or more cells of the total number of 2PN examined (67.3 vs. 82.3 %; P < 0.001).
Fig 1.
a Distribution of cell counts in day 3 embryos in the index group and control group. b Distribution of stages on day 5 in the index group and control group. *Fisher’s exact P value <0.05; **Fisher’s exact P value <0.001
Day 5 morphology
The index group had significantly more stage 1 embryos (P < 0.001) and significantly fewer stage 8 embryos (P = 0.012, respectively) when compared to controls (Fig. 1b). In addition, the index group had fewer embryos with TE grade B (45.5 vs. 51.6 %; P = 0.002) and more embryos with grade C (37.1 vs. 32.3 %; P < 0.001). There was no difference in the distribution of embryos according to the ICM grades. The index group had a significantly lower percentage of embryos cryopreserved (28.3 vs. 35.2 %; P = 0.030) and a lower percentage of usable embryos (transferred and frozen combined; 42.4 vs. 50.8 %; P = 0.005) (Table 2). However, the two groups were similar with regard to number of embryos transferred (1.6 ± 0.7 vs. 1.5 ± 0.6; P = 0.576).
Clinical outcomes
Of the seventy patients in the index group, a total of 111 embryos were transferred. Among the controls, there were 209 embryos transferred during the 139 cycles (Table 3). Of note, one control cycle had all embryos cryopreserved for future transfer and thus was not included in the clinical outcomes analysis.
Table 3.
Clinical outcomes in the control and index (uncleaved embryo) groups
| Control group (n = 139)c | Index group (n = 70) | P valued | |
|---|---|---|---|
| Positive hCG | 97 (69.8)e | 54 (77.1) | 0.327 |
| Biochemical pregnancy | 16 (11.5) | 11 (15.7) | 0.391 |
| Ectopic pregnancy | 2 (1.4) | 1 (1.4) | >0.999 |
| Spontaneous abortion | 16 (11.5) | 6 (8.6) | 0.636 |
| Therapeutic abortion | 0 (0) | 0 (0) | >0.999 |
| Implantation ratea | 100/209 (47.8) | 46/111 (41.4) | 0.290 |
| Clinical pregnancyb | 63 (45.3) | 36 (51.4) | 0.464 |
aNumber of sacs/embryos transferred
bNumber of transfers resulting in the presence of at least one gestational sac by 5–6 weeks gestation
cOne of the control cycles had all embryos frozen and is not included in this table
dFisher’s exact test
eNumbers in parentheses are percentages of the number of transfers
No significant differences were observed in the positive hCG, implantation, and clinical pregnancy rates between index cycles and controls. In addition, there were no significant differences between the two groups regarding the rates of biochemical pregnancy, ectopic pregnancy, spontaneous abortion, or therapeutic abortion.
As of January 2015, 18 singletons and five sets of twins have been born to women in the index group. One hundred and twelve singletons and 26 sets of twins have been born to women in the control group. Unfortunately, one index patient spontaneously aborted both of her twins, one at gestational age of 8 weeks and the other at 17 weeks. This is the only patient to have a spontaneous abortion after the first trimester. The number of cycles with live births thus far suggests that the overall live birth rates will not be significantly different between index and control groups. All babies are healthy to the knowledge of the authors.
Discussion
In this study, we evaluated cohorts of embryos with at least one uncleaved embryo present on day 3 and characterized the development of day 5 sibling embryos and reproductive outcomes from these cycles. We found that while more oocytes were retrieved in the index cycles, their yield of good quality embryos was significantly reduced on day 5. In particular, index cycles demonstrated an increased incidence of embryos that had arrested without even progressing to the morula stage by day 5, as well as a decreased proportion of stage 8 (i.e., hatching blastocysts). Nevertheless, the clinical outcomes, including implantation rate, clinical pregnancy rate, and live birth rate, did not differ between the two groups.
Despite the index cycles producing an increased number of oocytes, our analyses suggest that these extra oocytes were of poorer quality. The absolute numbers of MII oocytes, 2PN zygotes, and good quality embryos on day 3 were similar between index cycles and controls. However, the proportion of good quality day 3 embryos of the total number of oocytes retrieved was significantly lower in the index group (35.6 vs. 43.1 %; P = 0.001). Furthermore, of the 2PN zygotes in an index cohort, the proportion of good quality day 3 embryos was also significantly reduced (Table 2). Thus, while index and control cohorts had similar proportions of MII oocytes retrieved, which subsequently fertilized and formed 2PN zygotes at similar rates, their incidence of developing into usable embryos by day 3 was reduced in the index group. While the etiology for this poorer development is unknown, it may relate to incomplete cytoplasmic maturation in these oocytes which, although capable of supporting nuclear maturation, fertilization and syngamy, were compromised in their ability to support entry into the first mitotic cycle and subsequent development.
Although the index cycles had a decreased incidence of good quality day 3 embryos, there was no significant difference in the absolute numbers of day 5 embryos cryopreserved. Nevertheless, the lower proportion of embryos with grade B and the higher proportion with grade C TE scores, as well as the lower percentage of usable embryos (transferred and frozen), suggest that the quality of day 5 embryos within each cycle was lower in the index group when compared to controls. Our observations of similar implantation, clinical pregnancy, and live birth rates between index cases and controls are reassuring in that the presence of an uncleaved embryo at least does not impact the quality of the leading embryo(s) selected for day 5 transfer. In addition, there were no significant differences in the absolute number of embryos frozen and transferred, further endorsing that similar numbers of leading, highest quality embryos were produced in index and control groups.
To our knowledge, the present study is the first to investigate the association between the presence of an uncleaved embryo on day 3 and the quality of remaining sibling embryos within its cohort either on day 3 or day 5. While the index cohorts demonstrated a higher proportion of poor quality embryos on day 3 and lower overall blastocyst conversion rates on day 5, there were no significant differences between index and control cycles regarding the number of embryos frozen on day 5 or clinical outcomes following day 5 transfer. Thus, at least in good prognosis patients, our findings do not support use of an uncleaved embryo on day 3 as a good predictor of day 5 transfer success.
Footnotes
Capsule Our findings show that the presence of an uncleaved embryo on D3 is not a valuable predictor of clinical outcome after D5 ET in good prognosis patients and is, therefore, unlikely to be a useful criterion when deciding to take a patient to D5 transfer.
References
- 1.Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87(9):737–56. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 2.Bolton VN, Wren ME, Parsons JH. Pregnancies after in vitro fertilization and transfer of human blastocysts. Fertil Steril. 1991;55:830–2. doi: 10.1016/s0015-0282(16)54257-5. [DOI] [PubMed] [Google Scholar]
- 3.Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–8. doi: 10.1016/S0015-0282(97)00438-X. [DOI] [PubMed] [Google Scholar]
- 4.Adler A, Lee HL, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod Biomed Online. 2014;28(4):485–91. doi: 10.1016/j.rbmo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Vega M, Breborowicz A, Moshier EL, McGovern PG, Keltz MD. Blastulation rates decline in a linear fashion from euploid to aneuploid embryos with single versus multiple chromosomal errors. Fertil Steril. 2014;102(2):394–8. doi: 10.1016/j.fertnstert.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: an indicator of developmental potential? Hum Reprod. 1993;8:2119–27. doi: 10.1093/oxfordjournals.humrep.a137993. [DOI] [PubMed] [Google Scholar]
- 7.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond. London: Parthenon Publishing; 1999. pp. 378–88. [Google Scholar]
- 8.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Gardner D, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–58. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 10.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertil Steril. 2000;74:282–7. doi: 10.1016/S0015-0282(00)00645-2. [DOI] [PubMed] [Google Scholar]
- 11.Balaban B, Yakin K, Urman B. Randomized comparison of two different blastocyst grading systems. Fertil Steril. 2006;85:559–563. doi: 10.1016/j.fertnstert.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Richter K, Harris D, Daneshmand S, Shapiro B. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril. 2001;76:1157–67. doi: 10.1016/S0015-0282(01)02870-9. [DOI] [PubMed] [Google Scholar]
- 13.Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT., Jr Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril. 2012;97(4):819–24. doi: 10.1016/j.fertnstert.2012.01.115. [DOI] [PubMed] [Google Scholar]
- 14.Scott RT, Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Wells D, Kaur K, Grifo J, Glassner M, Taylor JC, Fragouli E, et al. Clinical utilization of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J Med Genet. 2014;51(8):553–62. doi: 10.1136/jmedgenet-2014-102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valbuena D, Martin J, de Pablo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962–8. doi: 10.1016/S0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 17.Fanchin R, Ayoubi JM. Uterine dynamics: impact on the human reproduction process. Reprod Biomed Online. 2009;18(Suppl 2):57–62. doi: 10.1016/S1472-6483(10)60450-6. [DOI] [PubMed] [Google Scholar]
- 18.Kawachiya S, Bodri D, Shimada N, Kato K, Takehara Y, Kato O. Blastocyst culture is associated with an elevated incidence of monozygotic twinning after single embryo transfer. Fertil Steril. 2011;95(6):2140–2. doi: 10.1016/j.fertnstert.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Glujovsky D, Blake D, Bardach A, Farquhar C. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7:CD002118. doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- 20.Skiadas CC, Missmer SA, Benson CB, Gee RE, Racowsky C. Risk factors associated with pregnancies containing a monochorionic pair following assisted reproductive technologies. Hum Reprod. 2008;23(6):1366–71. doi: 10.1093/humrep/den045. [DOI] [PubMed] [Google Scholar]
- 21.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis C, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts and cleavage stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MR, Sparks AE, Ryan GL, van Voorhis BJ. Clinical predictors of human blastocyst formation and pregnancy after extended culture and transfer. Fertil Steril. 2010;94:543–8. doi: 10.1016/j.fertnstert.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 23.Dessolle L, Freour T, Barriere P, Darai E, Ravel C, Jean M, et al. A cycle-based model to predict blastocyst transfer cancellation. Hum Reprod. 2010;25:598–604. doi: 10.1093/humrep/dep439. [DOI] [PubMed] [Google Scholar]
- 24.Braga DP, Setti AS, de Cássia S, Figueira R, Machado RB, Iaconelli A, Jr, et al. Patient selection criteria for blastocyst transfers in extended embryo culture programs. J Assist Reprod Genet. 2012;29(12):1357–62. doi: 10.1007/s10815-012-9875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;20(5):617–31. doi: 10.1093/humupd/dmu023. [DOI] [PubMed] [Google Scholar]
- 26.Kirkegaard K, Ahlstrom A, Ingerslev HJ, Hardarson T. Choosing the best embryo by time lapse versus standard morphology. Fertil Steril. 2015;103(2):323–32. doi: 10.1016/j.fertnstert.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Braga DP, Setti AS, Figueira RC, Iaconelli A, Jr, Borges E., Jr The importance of the cleavage stage morphology evaluation for blastocyst transfer in patients with good prognosis. J Assist Reprod Genet. 2014;31(8):1105–10. doi: 10.1007/s10815-014-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichman DE, Jackson KV, Racowsky C. Incidence and development of zygotes exhibiting abnormal pronuclear disposition after identification of two pronuclei at the fertilization check. Fertil Steril. 2010;94:965–70. doi: 10.1016/j.fertnstert.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsburg ES. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73(3):558–564. doi: 10.1016/S0015-0282(99)00565-8. [DOI] [PubMed] [Google Scholar]
- 30.Ceyhan ST, Jackson KV, Racowsky C. Selecting the most competent embryo. In: Carrell DT, Racowsky C, Schlegel PN, Van Voorhis BJ, editors. Biennial Review of Infertility. New York: Humana Press; 2009. pp. 143–69. [Google Scholar]