Abstract
Purpose
Childbearing delay contributes to the increase of subfertile couples that require assisted reproductive technology (ART). Subfertility relates with reproductive aging (RA). In vitro aging (IvA) (due to extended culture) may also impair oocyte competence. Aims of this study were to evaluate and compare the oocyte ultrastructure after RA and IvA.
Methods
Cumulus-oocyte complexes (COCs) (n = 68), with metaphase II oocyte and expanded cumulus, from consenting patients (<35 years old and ≥35 years old, n = 36), were selected by phase contrast microscopy and fixed at pick up, or after 24 h culture. COCs (n = 44) were studied by light and qualitative/morphometric transmission electron microscopy. Two-way ANOVA, with age and culture as grouping factors, was applied for statistical analysis (p < 0.05). Metaphase II cumulus-free oocytes (n = 24) were selected for confocal microscopy observations.
Results
Significant decrease of mitochondria-smooth endoplasmic reticulum aggregates, increase of mitochondria-vesicle complexes size and amount, decrease of cortical granules and microvilli, and alterations of the spindle structure characterized both RA and IvA oocytes. These changes were significantly more evident in the RA oocytes submitted to IvA. RA oocytes also showed changes of the zona pellucida and occurrence of vacuoles after culture. Cumuli appeared re-compacted after culture, irrespective of the age of the patients.
Conclusions
These data demonstrated that aging is related to decay of oocyte ultrastructural quality, and that oocytes from elder women are more sensitive to prolonged culture (IvA) than the oocytes from younger women. These morphological results should be considered when applying ART in aged patients, rescue ICSI, or artificial oocyte activation.
Keywords: Oocyte, Aging, Quality, Assisted reproduction, Human, Ultrastructure
Introduction
The postponement of childbearing, which is a demographic trend in all Western countries, contributes considerably to the increasing proportion of subfertile couples that require assisted reproductive technology (ART) procedures [1, 2]. Indeed, age of the female partner is one of the most significant factors influencing the clinical outcomes of ART cycles [3].
The relation between the decrease in fecundity and the advancing age is stronger in the middle of the third decade. At present, ART cannot fully compensate this natural decline of fertility [4, 2]. Thirty-five years is considered as a watershed in terms of fertility; indeed, women in the late of third decade or early of fourth decade may be reasonably considered in an advanced reproductive stage, but also in a borderline aging [5, 6].
The reproductive aging (RA) is mainly due aging of the ovary because of the physiological and gradual depletion of ovarian follicles, and the increase of age-related meiotic errors, that likely occur during preovulatory maturation [1, 7–9].
The “postovulatory aging” occurs when fertilization does not happen within the appropriate window of time. It can take place in vivo, when ovulated oocytes remain in oviduct, or in vitro, during extended culture (the latter is also called “in vitro aging,” IvA) [3, 10]. The post-ovulatory “fertilizable lifespan” of oocytes is important for reproductive success, in particular during ART procedures. There is a relationship between embryo grading and the elapsed time between oocyte retrieval and fertilization, with consequent effects on pregnancy rates [11]. Generally, in humans, the optimal fertilization window appears to be less than 24 h, more precisely about 12 to 14 h after ovulation [12]. In ART, IvA may occur if the oocyte is maintained in vitro for a longer period. Actually, several oocytes remain unfertilized after infertility treatments and are commonly considered aged cells. They represent an accessible and abundant source of oocytes that could be used for research purpose, but also in clinical settings, e.g., in protocols of artificial oocyte activation (for a complete review, see [13]) or in protocols of rescue intracytoplasmic sperm injection (ICSI) [14]. In this regard, the outcome of rescue ICSI is poor in terms of clinical pregnancy rate and this may be due to the oocyte IvA [15].
Summarizing, we have to face at least two different interpretations of aging: RA, due to the advanced maternal age, and IvA, due to extended culture during ART procedures.
Oocyte quality profoundly affects fertilization, survival of early embryo, maintenance of pregnancy, and fetal development as well [6]. The specific morphological features of oocytes observed by phase contrast microscopy (PCM) are important and irreplaceable predictive markers of ART success, but a low-resolution morphological assessment may be not sufficient to fully evaluate oocyte developmental competence and age-related damages [16, 17]. Undoubtedly, transmission electron microscopy (TEM) and confocal microscopy cannot be used proactively in ART. However, TEM is effective in evaluating ooplasmic quality [17–24] and confocal microscopy is a technique of choice to assess nuclear (spindle) impairment [25]. Up to date, only rare reports described the ultrastructure of human, mature, metaphase II (MII), IvA oocytes [26, 27]. Confocal microscopy was used to evaluate human MII spindle during RA [8, 28] and during IvA [29]. To our knowledge, no studies have used both confocal and electron microscopy, in order to investigate and compare the ooplasm ultrastructure and spindle arrangement in human MII, aged oocytes.
Thus, we studied oocytes obtained from women of different ages undergoing ART: (1) to provide a detailed ultrastructural evaluation and a morphometric comparison of organelle and spindle features; and (2) to evaluate their characteristics during IvA (24 h).
Our study offers an insight into the structural and ultrastructural changes of aging, suggesting a functional correlation, at the light of recent discoveries, and possibly giving useful recommendation for clinical practice.
Material and methods
The study was approved by the Institutional Review Board of the Clinic (Azienda Policlinico Umberto I, “Sapienza” University, Rome, Italy) and informed consent was obtained from the patients.
A total of 68 oocytes were obtained from 36 patients undergoing ART treatments performed at the Infertility and Assisted Reproduction Unit, Department of Gynecology-Obstetrics & Urology, “Sapienza” University Rome, Italy. All patients included in this study had a normal karyotype, normal hormonal assessments, negative vaginal or urethral cultures, and had no malignancy or systemic diseases.
All patients, who underwent a standard infertility evaluation, were nulliparous with previous failed in vitro fertilization (IVF) cycles ranging from 0 to 4, and none of them showed basal FSH >10 mIU/ml or estradiol (E2) >40 pg/ml on cycle day 3. Infertility was due to male factor in 39.1 % and to female extra-ovarian factor in remaining 60.9 % of couples (respectively 21.8 % uterine factor and 39.1 % tubal factor).
Ovarian stimulation and oocyte retrieval
IVF/ICSI cycle management consisted of down regulation with a short protocol starting from day 1 of treatment cycle with a GnRH agonist (Decapeptyl 0.1 ml, daily subcutaneous (s.c.), Ipsen). Once ovarian suppression was assessed by E2 profiles and ovarian ultrasound scan (US), daily s.c. administration of 150 IU urinary FSH (Fostimon 75 UI Ibsa) or FSH + LH (Meropur 75 UI + 75 UI, Ferring) was commenced.
From the seventh day of stimulation, daily monitoring of follicles size by US was performed and plasma levels of E2 and progesterone were measured. From this stage, the dose of gonadotropins was adjusted depending on the individual response of each patient.
Criteria used for triggering ovulation with 10.000 IU hCG (Gonasi HP 5000, ® Ibsa) s.c. were plasma E2 between 1000 and 3000 pg/ml and at least four follicles >18 mm mean diameter (two perpendicular measurements) with plasma progesterone <1.5 ng/ml.
Oocyte retrieval was performed 36 h after hCG administration, by transvaginal US-guided follicular aspiration under intravenous sedation. Selection of samples for our study was performed according to the Italian law regulating ART at the time of the pick up (law 40/2004 and subsequent modifications).
At the time of follicular aspiration, MII oocytes surrounded by expanded cumulus-corona (CC) cells were identified by PCM and rinsed with a buffer. Only MII oocytes that appeared of good quality when observed by PCM were selected for light/electron/confocal microscopy observations. They should have the following: (1) a rounded, regular shape; (2) a clear, moderately granular cytoplasm; (3) a narrow perivitelline space (PVS) with the 1st polar body (PBI); and (4) an intact, colorless zona pellucida (ZP) [30].
Oocytes were sorted by donor age: <35 years (range 31–34, mean 32 ± 1.069) n. 34 oocytes; ≥35 years (range 35–44, mean 38.06 ± 2.84) n. 34 oocytes. In detail, a total of 16 women <35 years old donated two oocytes each (14 women) and three oocytes each (2 women). A total of 20 women ≥35 years old donated one oocyte each (6 women) and two oocytes each (14 women). In both groups, 50 % of oocytes was fixed at pick up, 50 % was cultured in 50 μl microdrops of Quinn’s Advantage Fertilization Medium (Sage, USA) under oil at 37 °C, 5 % CO2 for 24 h and then fixed. When two (or, more rarely, three) oocytes were obtained by the same patient, we subdivided them in the above groups (oocytes fixed at pick up or cultured), in order to avoid a possible “donor effect” in the evaluation of the data. We have chosen the above culture medium because its main characteristics (similarity with the physiological human tubal fluid, low ammonium concentration [31], low production of reactive oxygen species [32], presence of selected nonessential amino acids and taurine, alanyl-glutamine as a stable source of glutamine) render it suitable to sustain prolonged culture. At the end of the above procedures, we obtained four study groups. (1) n. 17 MII oocytes from women <35 years old (control); (2) n. 17 MII oocytes from women <35 years old cultured in vitro (IvA); (3) n. 17 MII oocytes from women ≥35 years old (RA); (4) n. 17 MII oocytes from women ≥35 years old cultured in vitro (RA + IvA).
For each group, 11 oocytes were processed for light/electron microscopy examination, and 6 oocytes were processed for confocal microscopy analysis as described below.
Light/electron microscopy
Fixation was performed in a 1:3 solution of insemination medium (Quinn’s Advantage Fertilization Medium, Sage, USA) and glutaraldehyde 2.5 % in an Eppendorf vial. After 2–5 days at 4 °C, the samples were processed for light microscopy (LM) and TEM examination as described in [18–20, 22].
According to Motta and colleagues [33] recently updated in [17], the parameters listed below were evaluated and taken into consideration for the qualitative morphological assessment of the preservation of oocytes. By LM, on semithin sections, the general features were evaluated: shape, dimensions, density of ooplasm. By TEM, distribution (microtopography), type and quality of the organelles—mitochondria, mitochondria-smooth endoplasmic reticulum (M-SER) aggregates and mitochondria-vesicle (MV) complexes, vacuoles, lysosomes, cortical granules (CG)—integrity of the oolemma, ZP texture and thickness (ZPt), appearance of the PVS (width, presence of fragments, presence, and characteristics of the PBI), arrangement of the MII spindle (in sections laying on appropriate planes) were taken into consideration.
Confocal microscopy
Oocytes were fixed and processed for microtubules and chromatin immunofluorescence as previously described [21].
Confocal microscopy allowed the evaluation of the position and arrangement of spindle, the chromosomes alignment, and the presence of ooplasmic tubulin and/or chromatin.
Statistical analysis
The evaluation of organelles amount and ZPt was performed through collection of TEM microphotographs at ×6300 magnification on three equatorial sections per oocyte (distance between sections, 3–4 microns) [20]. Images were further enlarged on the PC screen, in order to easily recognize and count organelles. Values were expressed as follows: (a) number of M-SER aggregates per 100 μm2 of the oocyte ooplasmic area, (b) number of MV complexes per 100 μm2 of the oocyte ooplasmic area, (c) number of CG per 10 μm of the oocyte linear surface profile, (d) number of microvilli (Mv) per 10 μm of the oocyte linear surface profile [20, 34].
The mean ± SD of five morphological parameters (M-SER aggregates and MV complexes density, CG and Mv number, ZPt) of the four groups was compared using two-way analysis of variance (ANOVA), with age and culture as grouping factors. When F-testing indicated significance in all variables, Tukey’s Honestly Significant Difference test was performed. The statistical software ezANOVA was used (http://www.mccauslandcenter.sc.edu/mricro/ezanova/index.html, last accessed 14 June 2014). Results were considered significant at p < 0.05.
Results
We did not find overt structural and ultrastructural differences between oocytes belonging to patients treated with FSH alone (<35 years old, N = 9; ≥35 years old, N = 9) or with FSH-LH (<35 years old, N = 8; ≥35 years old, N = 10) in all the groups under examination.
Group 1—control (MII oocytes from women under 35 years old)
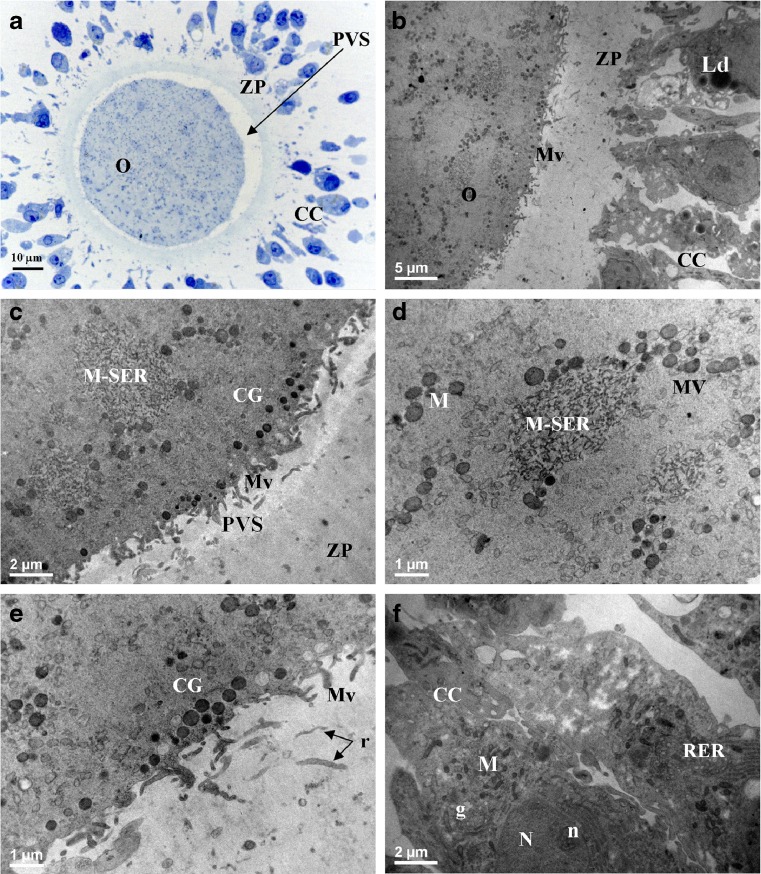
By LM, in semithin sections, the oocytes were round in shape, with a mean diameter of 93 ± 2.8 μm (Fig. 1a). Ooplasm was homogeneous and oolemma appeared intact. PVS was wider, where PBI was visible (in sections laying on appropriate planes). Rare debris were present in PVS, and presumably represented remnants of corona cell processes and/or small fragments of PBI. ZP showed a normal texture (Fig. 1a). Large intercellular spaces separated CC cells, as in expanded cumuli. In addition, the somatic cells closer to the oocyte presented an elongated shape (corona cells), while peripheral ones showed a cuboidal shape (residual cumulus cells). Only a small percentage of CC cells (1–2 %) appeared pyknotic by LM examination. By TEM, the ooplasm showed abundant and uniformly distributed organelles (Fig. 1b, c). Mitochondria were rounded or oval in profile, with a diameter varying from 0.5 to 0.8 μm, and a matrix of a moderate electrondensity. They were frequently associated with tubules of smooth endoplasmic reticulum (SER), anastomosed with each other, and forming numerous (Table 1), typical M-SER aggregates of variable size (Fig. 1b). Large M-SER aggregates were mainly located in the cortical areas of the ooplasm (Fig. 1c, d). Sporadically, mitochondria were also associated with small (0.2–0.3 μm in diameter) rounded SER vesicles filled of a slightly electrondense material and forming the so-called “MV complexes” (Fig. 1d; Table 1). A continuous row of electron-dense CG, varying in diameter from 300 to 400 nm, was present just beneath the oolemma (Fig. 1c, e; Table 1). Mv projecting into PVS were abundant (Table 1) and regularly distributed; their shape and content did not show noteworthy abnormalities (Fig. 1b, c, e). ZP showed some remnants of corona cell projections (Fig. 1e) and ZPt was 18.32 ± 1.93 μm (Table 1). CC cells showed an oval nucleus with finely dispersed chromatin and one or more nucleoli. Numerous elongated mitochondria, electron-dense lipid droplets, and cisternae belonging to rough endoplasmic reticulum and Golgi complex were detectable in the cytoplasm (Fig. 1b, f). Cells with a pyknotic nucleus and/or a vacuolized cytoplasm were present only occasionally. CC cells, whose cytoplasm was rich in lipid droplets, were also very rare.
Fig. 1.
MII oocytes (O) and cumulus-corona (CC) cells from women <35 years old (control) The general morphology and organelle microtopography are shown by light microscopy (a) and transmission electron microscopy (b). Note the presence of voluminous aggregates between mitochondria and elements of smooth endoplasmic reticulum (M-SER) (c, d) and small mitochondria–vesicle (MV) complexes (d). A single, regular row of electrondense cortical granules (CG) is seen just beneath the oolemma (c, e). Remnants of corona cell processes (r plus arrows in e) are seen in the zona pellucida texture. Numerous long microvilli (Mv) are uniformly distributed on the oolemma (b, c, e). Residual CC cells are separated by large intercellular spaces (a, b, f). CC cells are elongated and rich in organelles (b, f). ZP zona pellucida; PVS perivitelline space; M mitochondria; N nucleus; n nucleolus; g Golgi complex; RER rough endoplasmic reticulum; Ld lipid droplets. The apparent reduced dimensions of the oocyte shown in a (and of the oocytes shown in Figs. 3a and 4a) in comparison with the dimensions of the oocyte shown in Fig. 2a is an effect of the section plane (not equatorial in Figs. 1a, 3a, and 4a; equatorial in Fig. 2a)
Table 1.
Morphometric data and statistical analysis of aged human mature oocytes. Values are express as mean ± SD
| M-SER (per 100 μm2 ) |
MV (per 100 μm2 ) |
CG (per 10 μm) |
Mv (per 10 μm) |
ZPt (μm) | |
|---|---|---|---|---|---|
| Control | 2.89 ± 0.68a | 0.35 ± 0.26a | 11.00 ± 1.41a | 11.18 ± 1.47a | 18.32 ± 1.93a |
| IvA | 1.80 ± 0.35b | 1.31 ± 0.36b | 8.73 ± 0.79b | 9.45 ± 1.29b | 18.27 ± 1.74a |
| RA | 0.97 ± 0.28c | 2.05 ± 0.48c | 7.45 ± 1.37c | 7.18 ± 1.08c | 21.73 ± 1.79b |
| RA + IvA | 0.57 ± 0.31d | 3.55 ± 0.61d | 2.55 ± 1.37d | 3.91 ± 1.04d | 21.55 ± 1.57b |
Values with different lower case letters (a, b, c, d) were significantly different as detected by Tukey’s multiple comparison post-hoc analyses (p < 0.01)
Group 2—IvA (MII oocytes from women under 35 years old cultured in vitro)
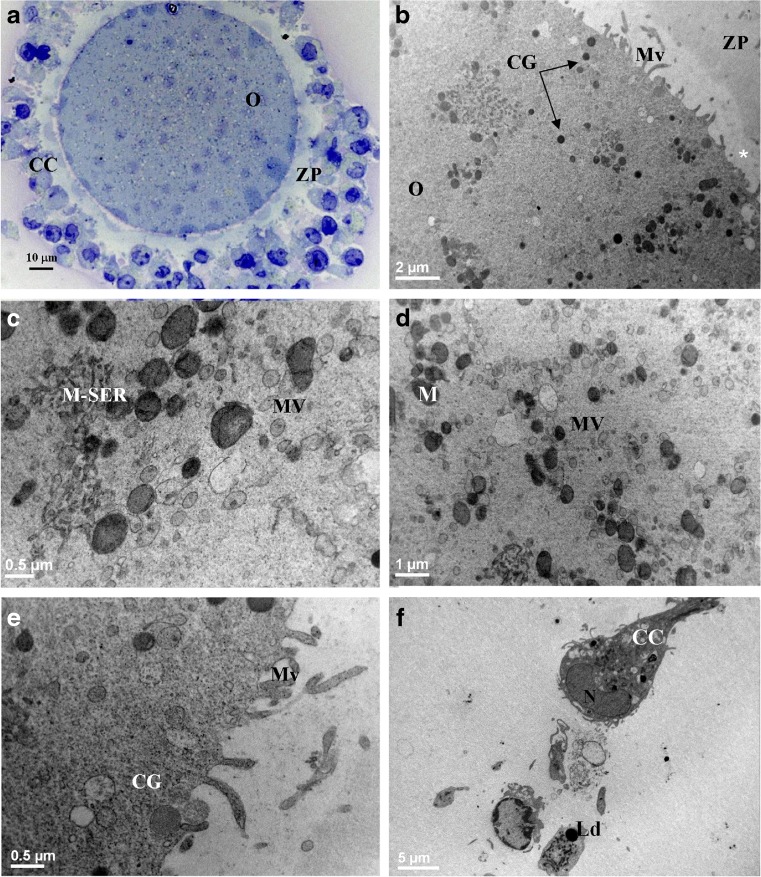
LM features were similar to those of controls except for the presence in most (two/thirds) of the samples of a compact cumulus formed by round or cuboid-shaped cells, very close to each other, with no significant differences between inner and outer elements (Fig. 2a). By TEM, a decreased number (Table 1) and size of M-SER aggregates (Fig. 2b, c) as well as an increased amount and size of MV complexes (Fig. 2b, c, d; Table 1) were present, with statistically significant differences in respect to control group (Table 1). The mean diameter of vesicles was 0.3–1 μm. Vesicles were intact and filled with a slightly electron dense content (Fig. 2c, d). Overall, CG amount appeared reduced in respect to control group (Table 1) and they did not form a continuous suboolemmal layer (Fig. 2b, e). A partial internalization of CG was present (Fig. 2b). The oolemma surface presented areas totally devoid of Mv (Fig. 2b) and regions provided with a normal pattern of Mv (Fig. 2e); consequently, the overall Mv number was significantly decreased in respect to control group (Table 1). ZPt did not differ significantly in respect to control group (Table 1). At ultrastructural level, CC cells did not present significant differences if compared to control ones (Fig. 2f).
Fig. 2.
MII oocytes (O) and cumulus-corona (CC) cells from women <35 years old cultured in vitro (IvA) The general features and organelle microtopography are shown by light microscopy (a) and transmission electron microscopy (b). Note the absence of intercellular spaces between residual CC cells and their polygonal shape (a, f). Small-sized M-SER (c) and MV complexes (c, d) are detected. Cortical granules (CG) are sparse (e), showing occasional internalization (CG plus arrows, b). Note the discontinuous distribution of microvilli (Mv), being either grouped in some areas (b, e) or almost totally absent in other (white asterisk in b). M mitochondria; N nucleus; Ld lipid droplet; ZP zona pellucida
Group 3—RA (MII oocytes from women over or equal 35 years old)
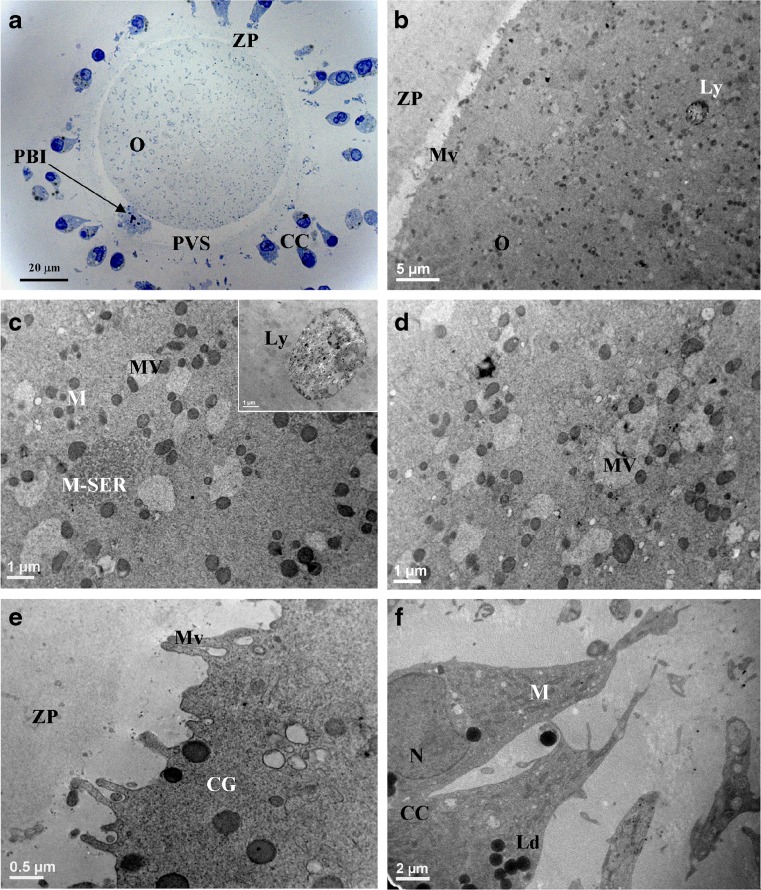
By LM, general features were analogous to those of controls (Fig. 3a). M-SER aggregates (Fig. 3b, c) were few and significantly decreased in respect to control and IvA samples (Table 1). MV complexes were normally organized (Fig. 3b–d). Their amount and size resulted increased if compared with control and IvA samples (Table 1). At higher magnification, vesicles in MV complexes appeared bigger than 1 μm (Fig. 3c, d), reaching 2–3 μm in maximum diameter. The number of CG decreased in an extremely significant manner, if compared to control and IvA samples (Table 1). CG inner electrondensity appeared reduced (Fig. 3e).
Fig. 3.
MII oocytes (O) and cumulus-corona (CC) cells from women ≥35 years old (RA) The general features and organelle microtopography are shown by light microscopy (a) and transmission electron microscopy (b). Ooplasm shows sparse, small-sized M-SER (c) and numerous, large and well-organized MV complexes (c, d). Occasional lysosomes (Ly) are also present (b, inset in c). Microvilli (Mv) are rare and short (b, e). Cortical granules (CG) are few and display variable electrondensity (e). Note the first polar body (PBI) in the perivitelline space (PVS) (a). ZP zona pellucida; M mitochondria; N nucleus; Ld lipid droplets
The oolemma surface was irregular, showing regions with rare Mv nearby to areas with a smooth appearance (Fig. 3b, e). The overall Mv number was statistically lower in respect to control and IvA groups (Table 1). Moreover, they were atypical in shape, being short and thin (Fig. 3e). ZP texture did not show noteworthy damages (Fig. 3b, e) but its thickness was significantly increased in respect to control and IvA groups (Table 1). CC cells did not show noteworthy ultrastructural changes (Fig. 3f).
Group 4—RA + IvA (MII oocytes from women over or equal 35 years old cultured in vitro)
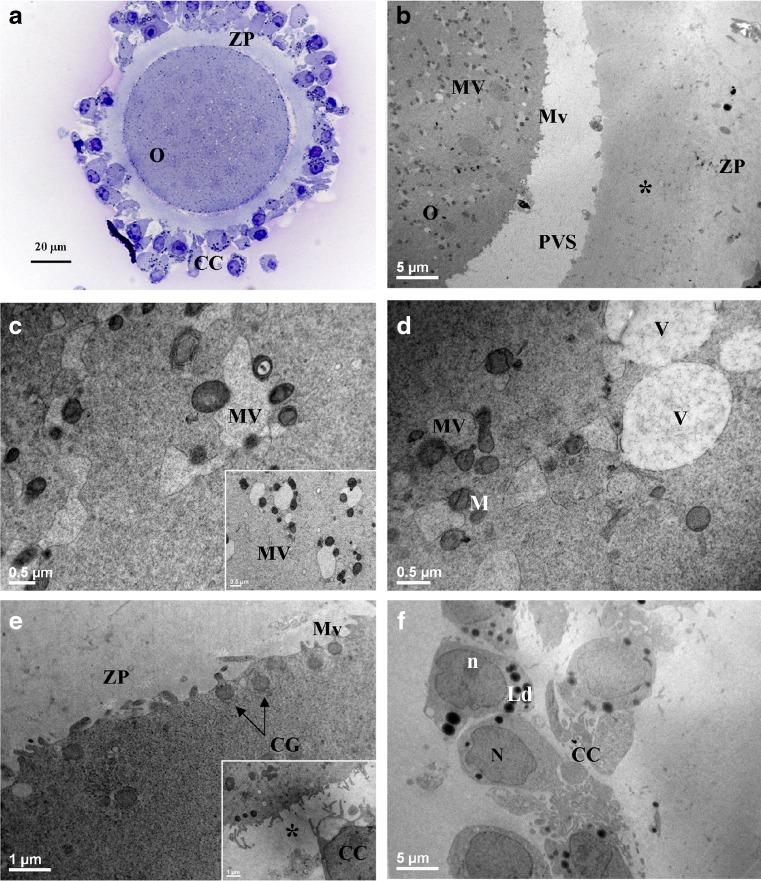
LM observations revealed an oocyte morphology similar to that of IvA samples and the presence of a compact cumulus (Fig. 4a). By TEM, the M-SER aggregates were rare (Table 1) and very small, while MV complexes were numerous and very large (Fig. 4b–d). The latter became the most relevant oocyte structures, and the above differences were significant in respect to control, IvA, and RA samples (Table 1). Vesicles of MV complexes displayed irregular shape, rupture of limiting membranes, and a light matrix, reaching often 4–5 μm in maximum diameter. These features were sometimes associated with a loss of the mitochondrial rim (Fig. 4d). The amount of CG dropped radically (Table 1). Moreover, extensive suboolemmal areas were devoid of CG (Fig. 4b, e), the latter showed a lighter electrondensity. The Mv pattern was altered (Fig. 4b; Table 1). The rare Mv appeared very small (Fig 4e). The ZP width did not differ significantly from RA group (Table 1), but its inner part showed a certain degree of increased electrondensity (Fig. 4b). Moreover, it displayed a consistent number of CC cell projections (inset in fig. 4e). CC cells displayed an overall ultrastructural aspect comparable to previous samples (Fig. 4f).
Fig. 4.
MII oocytes (O) and cumulus-corona (CC) cells from women ≥35 years old cultured (IvA + RA) The general features and organelle microtopography are shown by light microscopy (a) and transmission electron microscopy (b). MV complexes fill the ooplasm (b–d and inset in c), showing large and irregularly shaped vesicles (c, d). Occasional clear vacuoles (V) varying in dimensions are seen in the ooplasm (d). Cortical granules (CG) are hardly detectable (b) and, when detected, they display a reduction of electrondensity (e, CG plus arrows). Microvilli (Mv) are reduced (b, e). The inner zona pellucida (ZP) shows an increased density and corresponds to an area of the cortical ooplasm virtually devoid of CG (asterisk in b and inset in e). Residual CC cells are rounded-shaped and closely associated (a, f). M mitochondria; N nucleus; n nucleolus; Ld lipid droplets; PVS perivitelline space
Statistical analysis
Two-way ANOVA statistical analysis, using culture and age as grouping factors, showed that there were significant differences due to the effects of age and culture on all the parameters evaluated with the exception of ZPt (Table 1). ZPt was influenced only by the age.
Analysis of meiotic spindle
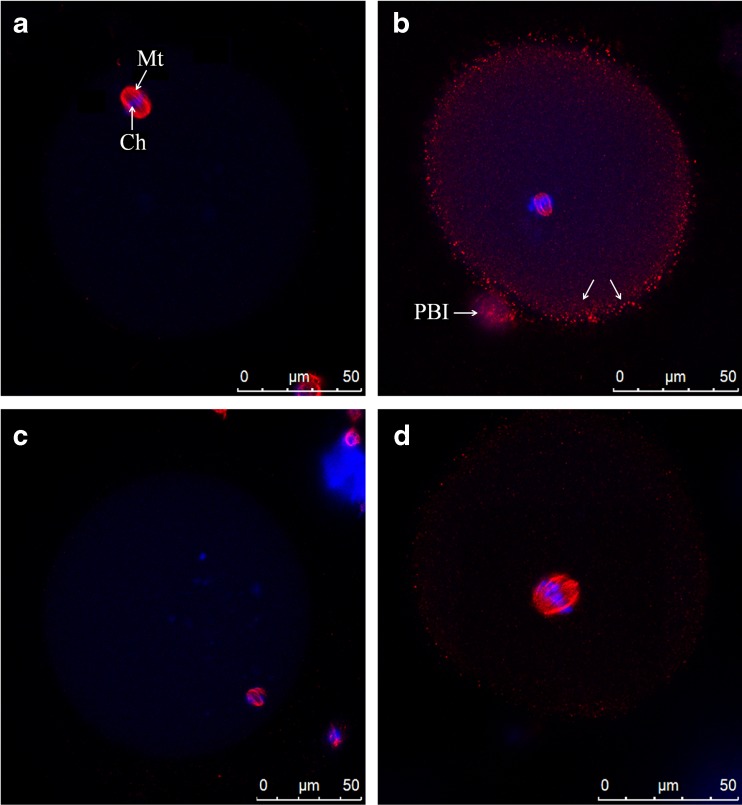
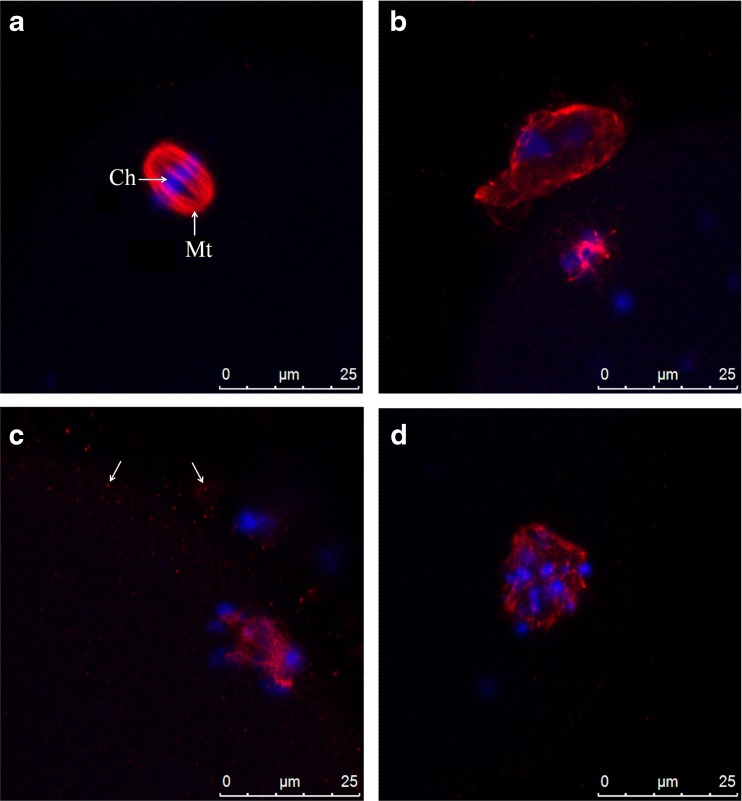
In control oocytes, microtubules and chromatin were concentrated in MII spindle and PBI (Fig. 5a). Spindle was peripheral, located in cortical ooplasm, and orthogonal to the oolemma (Fig. 5a). It was bipolar and barrel-shaped with constriction evident at both poles. Chromosomes were aligned regularly at the equator of the structure (Fig. 6a; Table 2).
Fig. 5.
Representative images of MII oocytes general features at confocal analysis. Control oocyte (a), IvA oocyte (b), RA oocyte (c), RA + IvA oocyte (d). Mt plus arrow MII spindle microtubules; Ch plus arrow chromosomes; arrows spots of tubulin; PBI 1st polar body
Fig. 6.
Representative images of spindle morphology at confocal analysis. Control oocyte (a), IvA oocyte (b), RA oocyte (c), RA + IvA oocyte (d). Mt plus arrow MII spindle microtubules; Ch plus arrow chromosomes; arrows spots of tubulin
Table 2.
Percentage of different phenotypes of meiotic spindle and chromosomes as seen by confocal microscopy
| Number (%) of oocytes | |||||
|---|---|---|---|---|---|
| Treatment | Examined | Position of the spindle | Arrangement of the spindle | Chromosome alignment | Ooplasmic tubulin |
| Control | 6 | Peripheral 6/6 (100 %) |
Regular, bipolar 6/6 (100 %) |
Regularly aligned 6/6 (100 %) |
Absent 0/6 (0 %) |
| IvA | 6 | Central 5/6 (83.3 %) |
Regular, bipolar 5/6 (83.3 %) |
Moderate degree of misalignment 5/6 (83.3 %) |
Absent 1/6 (16.7 %) |
| Peripheral 1/6 (16.7 %) |
Completely disarranged 1/6 (16.7 %) |
Totally misaligned 1/6 (16.7 %) |
Peripheral spots 5/6 (83.3 %) |
||
| RA | 6 | Peripheral 6/6 (100 %) |
Slightly disarranged 1/6 (16.7 %) |
Misaligned 6/6 (100 %) |
Minute spots near the spindle 6/6 (100 %) |
| Completely disarranged 5/6 (83.3 %) | |||||
| RA + IvA | 6 | Central 6/6 (100 %) |
Slightly disarranged 1/6 (16.7 %) |
Misaligned 6/6 (100 %) |
6/6 (100 %) |
| Completely disarranged 5/6 (83.3 %) | |||||
A great percentage of IvA oocytes exhibited numerous, small, punctate spots of tubulin staining in ooplasmic texture, with a higher concentration in the cortical region (Fig. 5b). Frequently, spindle was centrally located and displayed a bipolar shape with a moderate degree of non-aligned chromosomes (Fig. 5b). When, occasionally, the spindle was detected in cortical position, it was completely disarranged with scattered chromosomes (Fig. 6b; Table 2).
All RA oocytes displayed scattered chromosomes in ooplasm (Fig. 5c). Spindle was peripherally located (Fig. 5c). In most of samples, it appeared completely disarranged, with microtubule pole focusing largely missing (Fig. 6c). Minute spots of tubulin were present close to spindle (Fig. 6c; Table 2).
Diffuse spots of tubulin, whose distribution was very similar to IvA oocytes, characterized RA + IvA oocytes (Fig. 5d). A centrally located, disarranged spindle was detected (Fig. 6d; Table 2).
Discussion
The positive outcome of ART’s is strictly dependent on the morpho-functional viability of the oocyte [18]. In recent years, ART protocols have significantly improved, but oocyte aging remains one of the major limiting factors for success [3].
In this study, we evaluated the ooplasmic ultrastructure and the spindle morphology of human mature oocytes during IvA and RA, with the aim to assess the presence, extent, and consequences of subcellular changes related to aging.
We limited our analysis to mature oocytes of good quality (at PCM selection), thus resembling the actual clinical conditions. In fact, only oocytes that appear normal by PCM are routinely selected in ART cycles [18].
It is worth of noting that women of advanced age produce a few mature oocytes per cycle. They rarely donate supernumerary gametes for research purposes. The above considerations explain the limited availability of oocytes in this kind of studies.
Our study especially demonstrated the occurrence in human mature oocytes of changes in ooplasmic aggregates, CG, Mv, ZP, cumulus arrangement, and spindle morphology, clearly related to RA and IvA.
Ooplasmic aggregates
The most characteristic organelles found in fresh oocytes from <35 donors were the numerous, well-preserved mitochondria, associated with tubular SER elements to form M-SER aggregates of varying size. Small MV complexes were less frequent in mature oocytes [26, 33, 35]. These aggregates, typical of mature ooplasm, are likely involved in the energy production and storage at fertilization and during first embryo cleavages.
M-SER aggregates and MV complexes presumably belong to the same system of interconnected membranes [24, 26, 33]. In fact, SER membranes appear capable of shifting into each other, as suggested by the detection of intermediate features between tubules and vesicles by TEM [33]. However, the presence of numerous and large ooplasmic MV complexes in human mature oocytes is an uncommon trait, caused by swelling and coalescence of isolated SER vesicles, probably associated to cytoskeletal defects [35].
The MV complexes should not be confused with the vacuoles, the latter characterized by a discontinuous limiting membrane, empty matrix, and absence of surrounding mitochondria. In humans, vacuoles may occur commonly in response to a cellular injury, as for example in procedures of oocyte cryopreservation at MII stage [17–20, 22].
Our present original observations revealed that during IvA and RA in MII oocytes, the mutual balance of M-SER aggregates/MV complexes was compromised, displaying a general decrease in M-SER aggregates amount and a proportional increase in MV complexes. This suggests that a progressive swelling of SER tubules occurs in both types of aging, but there are considerable differences between the groups that need to be clarified.
When MII oocytes from a younger donor were cultured in vitro for 24 h (IvA), MV complexes increased, replacing M-SER aggregates, but vesicles were small sized (less than 1 μm), moderately electron-dense, and still substantially intact. We instead observed that, in RA, MV complexes represented a large percentage of ooplasmic organelles, and a considerable swollen of vesicles (about 2–3 μm) was also present. This is in line with ultrastructural age-related changes in resting follicle pool [9].
The interaction between RA and IvA caused a dramatic imbalance of M-SER aggregates/MV complexes. Large MV complexes became the most represented organelles in ooplasm. In addition, numerous vesicles showed loss of mitochondrial rim, rupture of membrane, and release of content, becoming electron transparent and eliciting an incipient vacuolization.
Some authors suggested that M-SER aggregates/MV complexes shift, seems to be enhanced by in vitro culture [27], and that it may be interpreted as a sign of postmaturation (also defined “overmaturation” in [26]). In fact, large MV complexes start appearing after 9–10 h of culture [36]. Our morphometric/quantitative evaluation strongly confirms this hypothesis and adds a new information: a similar ultrastructural phenomenon occurs in RA. To better comprehend the clinical implication of this finding is necessary to elucidate briefly the role of M-SER aggregates in fertilization and subsequent embryo development. Structural dysmorphism of SER, detectable by conventional PCM, has been associated with compromised embryo development and implantation in clinical settings [37–39], thus confirming the pivotal role of SER and associated mitochondria during fertilization and during the first embryo cleavages. A panel of experts strongly recommend that oocytes with this feature should not be inseminated [40], although a different opinion has been recently expressed [41].
M-SER aggregates are supposed to be involved in production/secretion of substances (nutrients, growth factors), in neogenesis of plasma and nuclear membranes [33, 42, 43], in regulation of ATP production, and in transmembrane signaling processes [44, 45]; above all, they represent the main calcium (Ca++) stores in mature human oocytes [46]. Ca++ signals are involved in the control of multiple molecular and cellular events during fertilization and early embryonic development, including the triggering of oocyte activation (reviewed in [47]). In particular, it was demonstrated that, after fertilization, a redistribution of SER and mitochondria accompanied by a change in their Ca++ content occurs [46].
Aging seems to prejudice Ca++ metabolism at different levels, as several authors have described (i) alteration of Ca++ oscillations, (ii) disturbances of intracellular Ca++ regulation, and (iii) an increased amount of intracellular Ca++ and reduction of the endoplasmic reticulum Ca++ stores which is likely arisen from dysfunction of the endoplasmic reticulum Ca++-ATPase (reviewed in [3]). Unfortunately, functional data refer mainly to animal model (mouse and pig) since it is difficult to perform extensive studies on human oocytes because of ethical concerns and limitations to obtain sufficient numbers of human oocytes for these kind of studies [3]. Recently, Nikiforaki and colleagues [48] demonstrated modification of Ca++ oscillations and an increase of total Ca++ release in in vitro aged human MII oocytes following ICSI.
Hence, the morphological disorganization of Ca++ storing elements, with a possible alteration of their content, could perturb the dynamic regulation of the ATP supply–demand relationship [49]. Ultimately, these alterations may represent the ultrastructural counterpart of impaired Ca++ pathways and, consequently, of compromised developmental competence.
Dilatation and vesiculation of endoplasmic reticulum in aged oocytes was correlated to oxidative stress [9]. In addition, these changes are also likely due to (i) an ingress of water (with subsequent dilution of contents and electron-transparency of vesicles) or (ii) storage of substances (accompanied by an increased electron density) [50]. Our observations suggest that during extended in vitro culture, an osmotic injury probably occurs, likely due to an alteration of the microenvironment, causing the swelling of SER vesicles that become electron transparent. Younger oocytes are less affected by IvA, maintaining the integrity of the vesicles, while older ones appear to be more sensitive to 24 h culture. The observed alterations in Ca++ storing elements, together with the increase in autophagy and stress-response pathways, have the potential to affecting negatively the oocyte quality by interfering with the normal sequence of biochemical changes that constitute egg activation following fertilization [51]. This observation suggest that suboptimal culture conditions, acting on a SER already compromised, may generate further dilation and rupture of vesicles, with transformation into vacuoles.
The SER-associated mitochondria did not show noteworthy ultrastructural modifications of individual profile in all section planes, likewise in the ooplasm of aged resting follicles [9]. In IvA oocytes, mitochondria begin to show structural alterations after two or more days of culture [12, 27], a period of time not clinically relevant. In fact, mitochondria of oocytes aged in vitro for 48–72 h tend to clump together, increase the electron density, acquire inner dense granules, or show a disorganized matrix structure [27]. Besides, after 3 or 4 days, they display abnormal translucent spaces within the matrix [12]. The culture period (24 h) used in our study that reproduces clinical settings did not allow mitochondrial ultrastructural alterations. The variation in quantity of mitochondria, even if recorded in some studies, is controversial [9, 52], and likely not indicative of a specific damage, being more relevant the metabolic changes. The bioenergetic deficit is one of the most accountable and intriguing factors thought responsible for age-related reproductive failure [6].
From a functional point of view, fresh mature human oocytes are characterized by a mitochondrial activity that showed a negative correlation with maternal age. On the other hand, ATP levels in oocytes from women over 40 years of age are similar to those detected in younger women. Thus, ATP deficiency resulting from mitochondrial dysfunction cannot be judged, a priori, responsible for the impairment of oocyte competence occurring in women of advanced age [12]. Moreover, net cytoplasmic ATP content seems to remain at relatively normal levels even after the fertilization window has passed [53].
Taken together, all the above considerations suggest that the compromised viability of aged oocytes may not primarily or exclusively due to a mitochondrial impairment. SER dysmorphism, other than mitochondrial impairment, can play a pivotal role in compromising oocyte viability in both types of aging.
Cortical granules
In human mature oocytes, CG are linearly arranged under the oolemma [26, 33, 54]. Although some of these granules may occasionally discharge their content independently of fertilization [18], nevertheless, a sudden and massive physiological release of CG content in the PVS occurs only at fertilization (“cortical reaction”). The cortical reaction and the consequent hardening of the internal part of the ZP (“zona reaction”) are necessary to block polyspermy [54, 55].
The evaluation of the fate of CG during aging appears of obvious importance, but despite this, morphological data regarding the amount of CG in human are poor and limited to IvA. To our knowledge, this is the first study that tries to estimate, by carrying out a combined TEM and morphometric analysis, the amount of CG during RA in human MII oocytes, comparing these data with those obtained during IvA.
Numerous morphological and/or functional alterations of CG have been described in mouse oocytes recovered from old female and during postovulatory in vivo and in vitro aging [3, 56–59].
In humans, a conglomeration and centripetal migration of CG was observed in oocytes aged in culture for 48–72 h [27], while a shorter permanence in culture (3–6 h) seems to increase their number [60]. Morphofunctional studies of human MII oocytes aged in culture show contradictory result: Ducibella et al. [61] found an occurrence of CG exocytosis and consequent zona hardening, while Talevi et al. [62] showed an intrinsic alteration in ZP with no alteration of CG distribution. Our results underline an abnormal reduction in amount (and inner density in some cases) of suboolemmal CG in all aged samples, when compared with those of fresh controls. This phenomenon may have a threefold interpretation, reflecting an altered production, an impaired distribution or a massive premature exocitosis of CG. In IvA oocytes from young women, a certain degree of internalization was detected, but the inner matrix density appeared preserved, in line with previous findings [27]. The above observation may exclude a defect in production (since inner density was unaffected), and so it can be likely attributable to a defective distribution accompanied by a slight degree of exocytosis (since ZP did not show noteworthy features, see below). The finding that only a moderate ZP hardening occurs in 24 h cultured human MII oocytes [63] supports this assumption.
All RA oocytes, in addition to the significantly decreased amount, displayed a reduction in CG matrix density. This suggests a primary defect in assembling CG and this may be supported by the occurrence of a dilated Golgi in immature aged oocytes [9]. Moreover, the occurrence of an evident compaction of the inner part of the ZP, with the loss of its typical regular filamentous texture [64], is strongly suggestive of an additional quick granule release in RA + IvA oocytes. Summarizing, the decrease in suboolemmal CG during aging is evident, but this event should be better elucidated and understood.
Microvilli
CG are separated from the oolemma by a distinct band of microfilaments (actin) [27, 54] forming a cortical layer [65–67]. This actin microfilamentous texture controls organelle movements, including CG migration, anchoring, and release [68], and forms a terminal web within the core of plasma membrane Mv [27, 54]. Human oocytes reveal an abnormal microfilament assembly during IvA and in vitro maturation [65, 66].
Our results showed that control oocytes were provided with numerous Mv projecting into a normal-looking PVS in terms of shape, width, and content, as already described in previous studies [33, 54, 64]. Deformation, disruption, as well as different degrees of Mv loss were instead found in all aged oocytes, in line with previous findings regarding humans [27, 54, 65], mouse, and porcine [3]. The proper amount and positioning of Mv on the oocyte surface is determinant for the initial contact and fusion of the fertilizing sperm with the oolemma [69]. In addition, experiments using zona pellucida-free human oocytes suggest that oolemma has its own role in blocking polyspermy [70]. In humans, in vitro aged oocytes are defective in supporting sperm-egg fusion [71] and in blocking polyspermy, as demonstrated in ZP-free model [72]. So structural damage of Mv in aged oocytes could reflect primary alteration of the actin microfilamentous web and, in turn, could be in part responsible for the reduced fertilizability/viability of aged oocytes.
Zona pellucida
The ZP has a role in oocyte development and protection, fertilization, spermatozoa binding, preventing polyspermy, blastocyst development, and preventing premature implantation (ectopic pregnancy) [73]. The ZP of human oocytes consists of granulofibrillar, interconnected reticulum with fenestrations [55, 64].
Our data showed that the ZP was significantly thicker in RA and RA + IvA oocytes if compared with both younger groups, suggesting a direct connection with women’s age. Moreover, a compaction of inner part of ZP was seen in RA + IvA oocytes, probably related to the above-mentioned altered CG release. A thickened ZP may impair IVF procedures influencing sperm penetration, and it was related to lower fertilization rate of older patients [74]. Even if ICSI may overcome this problem, we must highlight that ZP thickening may also impair early embryo development, limiting metabolic exchanges between the embryo and the surrounding microenvironment [73].
There is controversy in the literature regarding this aspect, as some studies suggest a significant increase in ZPt with age [74, 75] whereas others have shown the contrary [76]. All this data were obtained by light microscopy that cannot be efficient in revealing fine variations in thickness.
Cumulus-corona cells
Ultrastructural features of CC cells did not differ between groups, appearing characteristic for maturational stage. They also appeared generally healthy and well preserved, without evident signs of degeneration such as pyknosis, vacuolization, and excess of lipid inclusions [77–79]. On the other hand, altered molecular profiles have been demonstrated in cumulus cell from woman of advanced maternal age [80]. The apparently contradictory results could be explained by the small amount of residual CC cells examined. In fact, residual somatic cells are easily lost during sample preparation for microscopy, so our observations seem to be not consistent with functional findings. The only noteworthy structural variation was the re-compaction of the CC cells combined with the loss of the elongated shape of the corona cells after in vitro culture, irrespective of the age of the patients. A similar cumulus re-compaction has been described in unruptured, luteinized ovarian follicles, in either spontaneous or stimulated cycles, if ovulation does not occur due to insufficient LH surge or, conversely, to the presence of too numerous follicles after overstimulation (in this case, most follicles may ovulate before follicular aspiration but a few may remain unruptured) [81]. However, the cumulus re-compaction observed in our study occurs in an extraovarian milieu, suggesting that the extended culture, and possibly, the consequent prolonged exposure to some components of the culture medium, may exert a negative effect on the CC cell cytoskeleton [82], as also reported above for the oocyte microfilament web. Cumulus re-compaction may be also suggestive of a modification/alteration of CC cell extracellular matrix occurring during culture. All these hypotheses need to be better elucidated. The above concerns could be overcome by performing extensive ultrastructural/functional studies of human aged CC cells.
Spindle morphology
The meiotic spindle is highly dynamic [8], and its structural integrity is fundamental to successful chromosome congression, segregation, and cytoplasmic partitioning [3]. Disturbances in spindle formation and function may create conditions for aneuploidy and/or maturation arrest through depolymerization of microtubules. Incorrect assembly of the spindle or its disruption during meiosis will likely result in anomalies of chromosome disjunction. Impaired developmental competence may be due to chromosomal aberrations, as is commonly seen in human oocytes after suboptimal culture conditions and/or excessive gonadotropin stimulation [83].
The detrimental effect of advanced maternal age and extended culture on the structure of the human meiotic spindle was described in several papers. Our results on spindle position and morphology are in line with earlier reports obtained with confocal [8, 29] and widefield fluorescence microscopy [65, 84, 85], which showed specific defects in spindle location, chromosome alignment, and tubulin distribution in aged human oocytes.
Prolonged culture seems to influence the occurrence of a centrally located spindle and the presence of diffuse tubulin spots in ooplasm [29, 84, 85]. These alterations are more evident in oocytes from older women and are indicative of a general cytoskeletal damage. Displacement of spindle probably depends on a weakening of actin network. The latter regulate various dynamic processes during oocyte maturation. It seems to be responsible for the correct positioning of the meiotic spindle, movement and separation of chromosomes, and extrusion of the PBI [66, 68]. Characteristically, the spindle migrates to the cell periphery for an unequal division after germinal vesicle break down, and the correct positioning of the meiotic spindle was shown to be actin-dependent in mouse [68]. Recently, actin distribution was studied in human MII oocytes and was supposed to play a key role in cell polarization [67].
The presence of diffuse tubulin spots in ooplasm is suggestive of a loss of microtubule integrity and this seems to be mainly due to in vitro culture, since spindle is highly responsive to environmental changes [3].
Our data confirm that advanced maternal age influence scattering and misalignment of chromosomes. This is probably due not only to decreased chromosome cohesion (loss of cohesive proteins at centromeric sites) [86] but also to the presence of abnormal centrosomal domains at the spindle poles, which may induce an abnormal spindle assembly [87].
Conclusions
In conclusion, we describe that ultrastructural markers of quality are impaired in human metaphase II aged oocytes. The extended culture produces ultrastructural damages resembling those of RA. In addition our findings revealed that the younger the oocyte, less sensitive to IvA it is. The use of PCM, TEM, and confocal microscopy, allowing the evaluation of both ooplasmic and nuclear (spindle) quality, also revealed that not always a good spindle morphology at PCM selection means a healthy oocyte because ooplasmic quality may be anyway compromised. At this regard, clinicians should be aware that oocytes of older women should be inseminated as soon as possible after retrieval, in order to avoid further ultrastructural damages.
Acknowledgments
The authors wish to acknowledge Prof. Stefano Necozione of the Department of Life, Health and Environmental Sciences, University of L’Aquila, for providing statistical advice and Mr. Ezio Battaglione of the Laboratory for Electron Microscopy “Pietro M. Motta”, Department of Anatomy, Histology, Forensic Medicine and Orthopaedics, “Sapienza” University, Rome, Italy, for his technical assistance.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
The present study was supported by grants from the Italian Ministry of Education, University and Research (Sapienza and L’Aquila university grants), years 2011–2013.
Conflicts of interest
The authors declare that they have no conflict of interest.
Abbreviations
- ART
Assisted reproductive technology
- Ca++
Calcium
- COCs
Cumulus-oocyte complexes
- CC cells
Cumulus-corona cells
- CG
Cortical granules
- E2
Estradiol
- ICSI
Intracytoplasmic sperm injection
- IVF
In vitro fertilization
- IvA
In vitro aging
- LM
Light microscopy
- MII
Metaphase II
- M-SER
Mitochondria-smooth endoplasmic reticulum
- Mv
Microvilli
- MV
Mitochondria-vesicle
- PBI
1st polar body
- PCM
Phase contrast microscopy
- PVS
Perivitelline space
- RA
Reproductive aging
- s.c.
Subcutaneous
- SER
Smooth endoplasmic reticulum
- TEM
Transmission electron microscopy
- US
Ultrasound scan
- ZP
Zona pellucida
- ZPt
Zona pellucida thickness
Footnotes
Capsule
Aging is related to decay of oocyte ultrastructural quality. In vitro aging negatively affects morphofunctional features of mature oocytes in women of advanced age.
References
- 1.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 2.Kocourkova J, Burcin B, Kucera T. Demographic relevancy of increased use of assisted reproduction in European countries. Reprod Health. 2014;26:11–37. doi: 10.1186/1742-4755-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–85. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- 4.Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548–53. doi: 10.1093/humrep/deh304. [DOI] [PubMed] [Google Scholar]
- 5.Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–83. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, et al. The root of reduced fertility in aged women and possible therapeutic options: current status and future prospects. Mol Aspects Med. 2013;38:54–85. doi: 10.1016/j.mam.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–7. doi: 10.1016/0140-6736(91)93060-M. [DOI] [PubMed] [Google Scholar]
- 8.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–22. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 9.de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, et al. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod. 2004;70:419–24. doi: 10.1095/biolreprod.103.015784. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory aging of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–7. doi: 10.1093/humrep/13.2.394. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod. 2003;18:2118–21. doi: 10.1093/humrep/deg325. [DOI] [PubMed] [Google Scholar]
- 12.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010;94:520–6. doi: 10.1016/j.fertnstert.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 14.Beck-Fruchter R, Lavee M, Weiss A, Geslevich Y, Shalev E. Rescue intracytoplasmic sperm injection: a systematic review. Fertil Steril. 2014;101:690–8. doi: 10.1016/j.fertnstert.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Coticchio G. Polarization microscopy and rescue ICSI. Reprod Biomed Online. 2013;26:222–3. doi: 10.1016/j.rbmo.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Stensen MH, Tanbo T, Storeng R, Byholm T, Fèdorcsak P. Routine morphological scoring systems in assisted reproduction treatment fail to reflect age-related impairment of oocyte and embryo quality. Reprod Biomed Online. 2010;21:118–25. doi: 10.1016/j.rbmo.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Khalili MA, Maione M, Palmerini MG, Bianchi S, Macchiarelli G, Nottola SA. Ultrastructure of human mature oocytes after vitrification. Eur J Histochem. 2012;56:e38. doi: 10.4081/ejh.2012.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nottola SA, Macchiarelli G, Coticchio G, Bianchi S, Cecconi S, De Santis L, et al. Ultrastructure of human mature oocytes after slow cooling cryopreservation using different sucrose concentrations. Hum Reprod. 2007;22:1123–33. doi: 10.1093/humrep/del463. [DOI] [PubMed] [Google Scholar]
- 19.Nottola SA, Coticchio G, De Santis L, Macchiarelli G, Maione M, Bianchi S, et al. Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod Biomed Online. 2008;17:368–77. doi: 10.1016/S1472-6483(10)60220-9. [DOI] [PubMed] [Google Scholar]
- 20.Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, et al. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online. 2009;19:17–27. doi: 10.1016/S1472-6483(10)60280-5. [DOI] [PubMed] [Google Scholar]
- 21.Coticchio G, De Santis L, Rossi G, Borini A, Albertini D, Scaravelli G, et al. Sucrose concentration influences the rate of human oocytes with normal spindle and chromosome configurations after slow-cooling cryopreservation. Hum Reprod. 2006;21:1771–6. doi: 10.1093/humrep/del073. [DOI] [PubMed] [Google Scholar]
- 22.Coticchio G, Borini A, Distratis V, Maione M, Scaravelli G, Bianchi V, et al. Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols. J Assist Reprod Genet. 2010;27:131–40. doi: 10.1007/s10815-010-9394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahedi A, Hosseini A, Khalili MA, Norouzian M, Salehi M, Piriaei A, et al. The effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytes. Eur J Obstet Gynecol Reprod Biol. 2013;167:69–75. doi: 10.1016/j.ejogrb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Palmerini MG, Antinori M, Maione M, Cerusico F, Versaci C, Nottola SA, et al. Ultrastructure of immature and mature human oocytes after cryotop vitrification. J Reprod Dev. 2014;60:411–20. doi: 10.1262/jrd.2014-027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coticchio G, Sciajno R, Hutt K, Bromfield J, Borini A, Albertini DF. Comparative analysis of the metaphase II spindle of human oocytes through polarized light and high-performance confocal microscopy. Fertil Steril. 2010;93:2056–64. doi: 10.1016/j.fertnstert.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Sundstrom P, Nilsson BO, Liedholm P, Larsson E. Ultrastructural characteristics of human oocytes fixed at follicular puncture or after culture. J In Vitro Fert Embryo Transf. 1985;2:195–206. doi: 10.1007/BF01201797. [DOI] [PubMed] [Google Scholar]
- 27.Sathananthan AH. Ultrastructure of the human egg. Hum Cell. 1997;10:21–38. [PubMed] [Google Scholar]
- 28.Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998;13:154–60. doi: 10.1093/humrep/13.1.154. [DOI] [PubMed] [Google Scholar]
- 29.George MA, Pickering SJ, Braude PR, Johnson MH. The distribution of alpha- and gamma-tubulin in fresh and aged human and mouse oocytes exposed to cryoprotectant. Mol Hum Reprod. 1996;2:445–56. doi: 10.1093/molehr/2.6.445. [DOI] [PubMed] [Google Scholar]
- 30.Rienzi L, Balaban B, Ebner T, Mandelbaum J. The oocyte. Hum Reprod. 2012;27:i2–21. doi: 10.1093/humrep/des200. [DOI] [PubMed] [Google Scholar]
- 31.Lin YH, Hwang JL, Huang LW, Seow KM, Hsieh BC, Tzeng CR. Comparison of Quinn’s Advantage fertilization medium and tissue culture medium 199 for in vitro maturation of oocytes. Taiwan J Obstet Gynecol. 2014;53:17–20. doi: 10.1016/j.tjog.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Shih YF, Lee TH, Liu CH, Tsao HM, Huang CC, Lee MS. Effects of reactive oxygen species levels in prepared culture media on embryo development: a comparison of two media. Taiwan J Obstet Gynecol. 2014;53:504–8. doi: 10.1016/j.tjog.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Motta PM, Nottola SA, Micara G, Familiari G. Ultrastructure of human unfertilized oocytes and polyspermic embryos in an IVF-ET program. Ann N Y Acad Sci. 1988;541:367–83. doi: 10.1111/j.1749-6632.1988.tb22274.x. [DOI] [PubMed] [Google Scholar]
- 34.Gualtieri R, Iaccarino M, Mollo V, Prisco M, Iaccarino S, Talevi R. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil Steril. 2009;91:1023–34. doi: 10.1016/j.fertnstert.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 35.El Shafie M, Sousa M, Windt M-L, Kruger TF. An atlas of the ultrastructure of human oocytes. New York, USA: Parthenon; 2000. [Google Scholar]
- 36.Bianchi V, Macchiarelli G, Borini A, Lappi M, Cecconi S, Miglietta S, et al. Fine morphological assessment of quality of human mature oocytes after slow freezing or vitrification with a closed device: a comparative analysis. Reprod Biol Endocrinol. 2014;12:110. doi: 10.1186/1477-7827-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, Barros A, Sousa M. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96:143–49. doi: 10.1016/j.fertnstert.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 38.Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod Biomed Online. 2008;16:113–8. doi: 10.1016/S1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 39.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–7. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 40.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 41.Mateizel I, Van Landuyt L, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28:2111–7. doi: 10.1093/humrep/det241. [DOI] [PubMed] [Google Scholar]
- 42.Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15:129–47. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- 43.Motta PM, Nottola SA, Familiari G, Makabe S, Stallone T, Macchiarelli G. Morphodynamics of the follicular-luteal complex during early ovarian development and reproductive life. Int Rev Cytol. 2003;223:177–288. doi: 10.1016/S0074-7696(05)23004-8. [DOI] [PubMed] [Google Scholar]
- 44.Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406. doi: 10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- 45.Van Blerkom J, Davis P. Mitochondrial signaling and fertilization. Mol Hum Reprod. 2007;13:759–70. doi: 10.1093/molehr/gam068. [DOI] [PubMed] [Google Scholar]
- 46.Sousa M, Barros A, Silva J, Tesarik J. Developmental changes in calcium content of ultrastructurally distinct subcellular compartments of preimplantation human embryos. Mol Hum Reprod. 1997;3:83–90. doi: 10.1093/molehr/3.2.83. [DOI] [PubMed] [Google Scholar]
- 47.Swann K, Ozil JP. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol. 1994;152:183–222. doi: 10.1016/S0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- 48.Nikiforaki D, Vanden Meerschaut F, Qian C, De Croo I, Lu Y, Deroo T, et al. Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization. Hum Reprod. 2014;29:29–40. doi: 10.1093/humrep/det404. [DOI] [PubMed] [Google Scholar]
- 49.Stricker SA. Structural reorganizations of the endoplasmic reticulum during egg maturation and fertilization. Semin Cell Dev Biol. 2006;17:303–13. doi: 10.1016/j.semcdb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Ghadially FN. Ultrastructural pathology of the cell and matrix. 4. Boston, USA: Butterworth-Heinemann; 1997. [Google Scholar]
- 51.McGinnis LK, Pelech S, Kinsey WH. Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis. Mol Reprod Dev. 2014;81:928–45. doi: 10.1002/mrd.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller-Hocker J, Schafer S, Weis S, Munscher C, Strowitzki T. Morphological-cytochemical and molecular genetic analysis of mitochondria in isolated human oocytes in the reproductive age. Mol Hum Reprod. 1996;2:951–8. doi: 10.1093/molehr/2.12.951. [DOI] [PubMed] [Google Scholar]
- 53.Van Blerkom J, Davis P, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 54.Sathananthan AH. Ultrastructure of human gametes, fertilization and embryos in assisted reproduction: a personal survey. Micron. 2013;44:1–20. doi: 10.1016/j.micron.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Familiari G, Heyn R, Relucenti M, Nottola SA, Sathananthan AH. Ultrastructural dynamics of human reproduction, from ovulation to fertilization and early embryo development. Int Rev Cytol. 2006;249:53–141. doi: 10.1016/S0074-7696(06)49002-1. [DOI] [PubMed] [Google Scholar]
- 56.Abbott AL, Xu Z, Kopf GS, Ducibella T, Schultz RM. In vitro culture retards spontaneous activation of cell cycle progression and cortical granule exocytosis that normally occur in in vivo unfertilized mouse eggs. Biol Reprod. 1998;59:1515–21. doi: 10.1095/biolreprod59.6.1515. [DOI] [PubMed] [Google Scholar]
- 57.Tarín JJ, Pérez-Albalá S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–50. doi: 10.1095/biolreprod65.1.141. [DOI] [PubMed] [Google Scholar]
- 58.Díaz H, Esponda P. Aging-induced changes in the cortical granules of mouse eggs. Zygote. 2004;12:95–103. doi: 10.1017/S0967199404002680. [DOI] [PubMed] [Google Scholar]
- 59.Ducibella T, Duffy P, Reindollar R, Su B. Changes in the distribution of mouse oocyte cortical granules and ability to undergo the cortical reaction during gonadotropin-stimulated meiotic maturation and aging in vivo. Biol Reprod. 1990;43:870–6. doi: 10.1095/biolreprod43.5.870. [DOI] [PubMed] [Google Scholar]
- 60.Sathananthan AH, Trounson AO. Ultrastructural observations on cortical granules in human follicular oocytes cultured in vitro. Gamete Res. 1982;5:191–8. doi: 10.1002/mrd.1120050209. [DOI] [Google Scholar]
- 61.Ducibella T, Dubey A, Gross V, Emmi A, Penzias AS, Layman L, et al. A zona biochemical change and spontaneous cortical granule loss in eggs that fail to fertilize in in vitro fertilization. Fertil Steril. 1995;64:1154–61. [PubMed] [Google Scholar]
- 62.Talevi R, Gualtieri R, Tartaglione G, Fortunato A. Heterogeneity of the zona pellucida carbohydrate distribution in human oocytes failing to fertilize in vitro. Hum Reprod. 1997;12:2773–80. doi: 10.1093/humrep/12.12.2773. [DOI] [PubMed] [Google Scholar]
- 63.Manna C, Rienzi L, Greco E, Sbracia M, Rahman A, Poverini R, et al. Zona pellucida solubility and cortical granule complements in human oocytes following assisted reproductive techniques. Zygote. 2001;9:201–10. doi: 10.1017/S0967199401001216. [DOI] [PubMed] [Google Scholar]
- 64.Familiari G, Nottola SA, Macchiarelli G, Micara G, Aragona C, Motta PM. Human zona pellucida during in vitro fertilization: an ultrastructural study using saponin, ruthenium red, and osmium-thiocarbohydrazide. Mol Reprod Dev. 1992;32:51–61. doi: 10.1002/mrd.1080320109. [DOI] [PubMed] [Google Scholar]
- 65.Pickering SJ, Johnson MH, Braude PR, Houliston E. Cytoskeletal organization in fresh, aged and spontaneously activated human oocytes. Hum Reprod. 1988;3:978–89. doi: 10.1093/oxfordjournals.humrep.a136828. [DOI] [PubMed] [Google Scholar]
- 66.Kim NH, Chung HM, Cha KY, Chung KS. Microtubule and microfilament organization in maturing human oocytes. Hum Reprod. 1998;13:2217–22. doi: 10.1093/humrep/13.8.2217. [DOI] [PubMed] [Google Scholar]
- 67.Coticchio G, Guglielmo MC, Albertini DF, Dal Canto M, Mignini Renzini M, De Ponti E, et al. Contributions of the actin cytoskeleton to the emergence of polarity during maturation in human oocytes. Mol Hum Reprod. 2014;20:200–7. doi: 10.1093/molehr/gat085. [DOI] [PubMed] [Google Scholar]
- 68.Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction. 2006;131:193–205. doi: 10.1530/rep.1.00847. [DOI] [PubMed] [Google Scholar]
- 69.Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, et al. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol. 2007;304:317–25. doi: 10.1016/j.ydbio.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 70.Sengoku K, Tamate K, Horikawa M, Takaoka Y, Ishikawa M, Dukelow WR. Plasma membrane block to polyspermy in human oocytes and preimplantation embryos. J Reprod Fertil. 1995;105:85–90. doi: 10.1530/jrf.0.1050085. [DOI] [PubMed] [Google Scholar]
- 71.Park KS, Song HB, Chun SS. Late fertilization of unfertilized human oocytes in in vitro fertilization and intracytoplasmic sperm injection cycles: conventional insemination versus ICSI. J Assist Reprod Genet. 2000;17:419–24. doi: 10.1023/A:1009409100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malter H, Talansky B, Gordon J, Cohen J. Monospermy and polyspermy after partial zona dissection of reinseminated human oocytes. Gamete Res. 1989;23:377–86. doi: 10.1002/mrd.1120230403. [DOI] [PubMed] [Google Scholar]
- 73.Nottola SA, Makabe S, Stallone T, Familiari G, Correr S, Macchiarelli G. Surface morphology of the zona pellucida surrounding human blastocysts obtained after in vitro fertilization. Arch Histol Cytol. 2005;68:133–41. doi: 10.1679/aohc.68.133. [DOI] [PubMed] [Google Scholar]
- 74.Nawroth F, Muller P, Wolf C, Sudik R. Is the zona pellucida thickness of metaphase-II oocytes in an IVF/ICSI program influenced by the patient’s age? Gynecol Obstet Invest. 2001;52:55–8. doi: 10.1159/000052942. [DOI] [PubMed] [Google Scholar]
- 75.Kilani SS, Cooke S, Kan AK, Chapman MG. Do age and extended culture affect the architecture of the zona pellucida of human oocytes and embryos? Zygote. 2006;14:39–44. doi: 10.1017/S0967199406003625. [DOI] [PubMed] [Google Scholar]
- 76.Valeri C, Pappalardo S, De Felici M, Manna C. Correlation of oocyte morphometry parameters with woman’s age. J Assist Reprod Genet. 2011;28:545–52. doi: 10.1007/s10815-011-9555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nottola SA, Familiari G, Micara G, Aragona C, Motta PM. The ultrastructure of human cumulus-corona cells at the time of fertilization and early embryogenesis. A scanning and transmission electron microscopic study in an in vitro fertilization program. Arch Histol Cytol. 1991;54:145–61. doi: 10.1679/aohc.54.145. [DOI] [PubMed] [Google Scholar]
- 78.Motta PM, Nottola SA, Pereda J, Croxatto HB, Familiari G. Ultrastructure of human cumulus oophorus: a transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum Reprod. 1995;10:2361–7. doi: 10.1093/oxfordjournals.humrep.a136299. [DOI] [PubMed] [Google Scholar]
- 79.Motta PM, Nottola SA, Familiari G, Macchiarelli G, Correr S, Makabe S. Structure and function of the human oocyte-cumulus-corona cell complex before and after ovulation. Protoplasma. 1999;206:270–7. doi: 10.1007/BF01288215. [DOI] [Google Scholar]
- 80.McReynolds S, Dzieciatkowska M, McCallie BR, Mitchell SD, Stevens J, Hansen K, et al. Impact of maternal aging on the molecular signature of human cumulus cells. Fertil Steril. 2012;98:1574–80. doi: 10.1016/j.fertnstert.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 81.Bomsel-Helmreich O, Huyen LV, Durand-Gasselin I, Salat-Baroux J, Antoine JM. Mature and immature oocytes in large and medium follicles after clomiphene citrate and human menopausal gonadotropin stimulation without human chorionic gonadotropin. Fertil Steril. 1987;48:596–604. doi: 10.1016/s0015-0282(16)59470-9. [DOI] [PubMed] [Google Scholar]
- 82.Salhab M, Papillier P, Perreau C, Guyader-Joly C, Dupont J, Mermillod P, et al. Thymosins β-4 and β-10 are expressed in bovine ovarian follicles and upregulated in cumulus cells during meiotic maturation. Reprod Fertil Dev. 2010;22:1206–21. doi: 10.1071/RD10015. [DOI] [PubMed] [Google Scholar]
- 83.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 84.Eichenlaub-Ritter U, Stahl A, Luciani JM. The microtubular cytoskeleton and chromosomes of unfertilized human oocytes aged in vitro. Hum Genet. 1988;80:259–64. doi: 10.1007/BF01790094. [DOI] [PubMed] [Google Scholar]
- 85.Miyara F, Aubriot FX, Glissant A, Nathan C, Douard S, Stanovici A, et al. Multiparameter analysis of human oocytes at metaphase II stage after IVF failure in non-male infertility. Hum Reprod. 2003;18:1494–503. doi: 10.1093/humrep/deg272. [DOI] [PubMed] [Google Scholar]
- 86.Tsutsumi M, Fujiwara R, Nishizawa H, Ito M, Kogo H, Inagaki H, et al. Age-related decrease of meiotic cohesins in human oocytes. PLoS ONE. 2014;9:e96710. doi: 10.1371/journal.pone.0096710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schatten H, Sun QY. Centrosome and microtubule functions and dysfunctions in meiosis: implications for age-related infertility and developmental disorders. Reprod Fertil Dev. 2015 doi: 10.1071/RD14493. [DOI] [PubMed] [Google Scholar]