Abstract
Objective
The aim of this study was to determine whether interleukin-6 (IL-6) −174 G/C, IL-6 −634 G/C, and interferon-γ (IFN-γ) +874 A/T polymorphisms are associated with susceptibility to recurrent pregnancy loss (RPL).
Methods
We conducted a literature search using PubMed and EMBASE databases and performed a meta-analysis using fixed- or random-effects models.
Results
A total of 15 articles met the study inclusion criteria. When all study subjects were considered together, meta-analysis showed no association between RPL and the IL-6 −174 GG + GC genotype (odds ratio [OR] = 0.794, 95 % confidence interval [CI] = 0.542–1.163, p = 0.236). However, stratification of the data by ethnicity indicated an association between this genotype and RPL in non-Caucasians (OR = 0.528, 95 % CI = 0.302–0.925, p = 0.028), but not in Caucasian populations. Moreover, meta-analysis revealed an association between RPL and the IL-6 −634 GG + GC genotype in all study subjects (OR = 0.556, 95 % CI = 0.383–0.806, p = 0.002), while stratification by ethnicity revealed a negative association between this genotype and RPL in Asian (OR = 0.545, 95 % CI = 0.371–0.800, p = 0.002) but not Middle Eastern populations. Furthermore, a relationship between the IFN-γ +874 A allele and RPL was identified in non-Caucasians (OR = 1.403, 95 % CI = 1.133–1.734, p = 0.002), but not in Caucasians.
Conclusions
This meta-analysis demonstrates that IL-6 −174 G/C, IL-6 −634 G/C, and IFN-γ +874 A/T polymorphisms are associated with susceptibility to RPL, particularly in non-Caucasians.
Keywords: IL-6, IFN-γ, Polymorphism, RPL, Meta-analysis
Introduction
Recurrent pregnancy loss (RPL) is defined as two or more losses of pregnancies having lasted less than 20 weeks, and involves a multifactorial etiology. Although a number of etiological factors have been identified, the cause of RPL remains unclear in approximately 50 % of cases [1]. These unexplained occurrences may be due to immunological and genetic factors [2].
Pro-inflammatory and anti-inflammatory cytokines are known to be involved in the pathogenesis of RPL [3], and successful pregnancy may be dependent on a balance between T-helper 1 (Th1) and T-helper 2 (Th2) immunity [4]. Specifically, a change of cytokine balance in favor of Th2 cytokines such as interleukin-6 (IL-6) is considered essential for maintaining a normal pregnancy [5]. In addition, abnormal immune reactivity in the context of the Th1/Th2 paradigm has been observed in RPL [5] and there is evidence of a diminished Th2 immune response to placental antigens in women with this condition [6]. Trophoblast antigens activate lymphocytes, resulting in the production of embryotoxic cytokines such as interferon-γ (IFN-γ), which inhibit embryonic and fetal development [7]. IL-6, involved in the inflammatory response and in the modulation of immune responses including B cell and T cell differentiation, functions as both a pro- and anti-inflammatory cytokine [8]. Significantly higher levels of IL-6 are present in healthy pregnant women compared to those with RPL [9], while the opposite is true for IFN-γ [10].
In part, the production of cytokines is under genetic control, and polymorphisms, including those of IL-6 and IFN-γ, are associated with variations in their expression. The IL-6 gene is located on chromosome 7p21, and of the several known polymorphisms in its promoter region, −174 G/C (rs1800795) and −634 G/C (rs1800796) have been the most frequently studied in RPL. IL-6 −174 G/C and −634 G/C polymorphisms are functionally significant and are known to exhibit weak linkage disequilibrium. This cytokine is regulated principally at the transcriptional level by regulatory elements in its 5ʹ flanking region [11], within which, the −174 G/C polymorphism acts as an important regulator of transcription [12]. Likewise, a functional polymorphism (rs2430561) of the IFN-γ gene located on chromosome 12q24, consisting of an A to T substitution at position +874 from the translation start site in the first intron and coinciding with a putative NF-κB binding site, is known to increase IFN-γ levels [13]. This polymorphism is correlated with IFN-γ levels, the T allele being associated with increased production [13].
Based on their functional significance, the roles of IL-6 −174 G/C, IL-6 −634 G/C, and IFN-γ +874 A/T polymorphisms in RPL susceptibility have been examined, with conflicting results [14–28]. This disparity may have been caused by small sample sizes, low statistical power, ethnic variability between studies, and/or clinical heterogeneity. To overcome the limitations of individual studies, resolve inconsistencies, and reduce the likelihood of random errors being responsible for false-positive or false-negative associations, we employed a meta-analysis approach [29–31]. Our aim in this study was to determine whether IL-6 −174 G/C, IL-6 −634 G/C, and IFN-γ +874 A/T polymorphisms are associated with RPL risk in different ethnicities.
Methods
Identification of eligible studies and data extraction
A literature search was conducted to identify studies having examined associations between IL-6 or IFN-γ polymorphisms and RPL susceptibility. PubMed and EMBASE citation indices were used to locate articles published before June 2015, in which the presence of IL-6 or IFN-γ polymorphisms had been determined in RPL patients and controls. Combinations of keywords such as “interleukin-6,” “interferon,” “polymorphism,” “recurrent miscarriage,” and “recurrent pregnancy loss” were entered as both Medical Subject Headings and Text Words. In addition, all references cited in retrieved articles were reviewed to identify additional studies not indexed by PubMed and EMBASE. Studies were included in the analysis if they met all of the following criteria: (i) they were case–control studies; (ii) they included patients with RPL, which was defined as two or more unexplained pregnancy losses in the first two trimesters of pregnancy; and (iii) they included genotype or allele data concerning IL-6 −174 G/C, IL-6 −634 G/C, or IFN-γ +874 A/T polymorphisms. No language restriction was applied. The following were excluded: (i) studies containing overlapping data; (ii) those in which genotype or allele data could not be ascertained; (iii) reviews; and (iv) studies in which family members had been included and the analysis was based on linkage considerations. Information relating to the methods and results of the included investigations was extracted by two independent researchers. Disagreements were resolved by consensus or the adjudication of a third researcher. The following information was extracted from each study: author(s), year of publication, ethnicity of the study population, number of cases and controls, and genotype and allele frequencies of IL-6 and IFN-γ polymorphisms.
Evaluation of statistical associations
We performed meta-analyses using allelic contrast, homozygote contrast, and recessive and dominant models. IL-6 polymorphism allele frequencies in the relevant studies were determined using the allele counting method. The chi-square test was used to establish whether observed genotype frequencies in the control groups conformed to Hardy–Weinberg equilibrium (HWE). Point estimates of risks, odds ratios (ORs), and 95 % confidence intervals (CIs) were determined for each study, and Cochran’s Q statistic was used to assess within- and between-study variation and heterogeneity [32]. The heterogeneity test was used to assess the probability of the null hypothesis that all studies were evaluating the same effect. When a significant Q statistic (p < 0.10) indicated heterogeneity across studies, a random-effects model was used in the meta-analysis, while a fixed-effects model was employed in the absence of such heterogeneity. The fixed-effects model assumes that genetic factors have similar effects on RPL susceptibility across all studies and that observed variations between studies are caused by chance alone [33]. The random-effects model however assumes substantial diversity among different investigations and assesses both within-study sampling errors and between-study variance [34]. We quantified the effect of heterogeneity using I2, which is equal to 100 % × (Q − df)/Q, where “Q” represents Cochran’s Q and “df” the degrees of freedom [35]. This measure ranges between 0 and 100 % and represents the proportion of inter-study variation attributable to heterogeneity rather than chance. I2 values of 25, 50, and 75 % were defined as low, moderate, and high estimates, respectively. Statistical tests were carried out using the Comprehensive Meta-Analysis computer program (Biostat, Englewood, NJ, USA).
Evaluation of publication bias
Funnel plots are often used to detect publication bias. However, because of the limitations of this method, which requires a range of studies of varying sizes and involves subjective judgments, publication bias was evaluated using Egger’s linear regression test [36].
Results
Studies included in the meta-analysis
We identified 100 reports using electronic and manual searches and selected 18 for full-text review based on their title and abstract details. Subsequently, three documents were excluded for being review articles [37–39]. Thus, 15 relevant studies met the inclusion criteria [14–28]), of which, eight were related to the IL-6 −174 G/C polymorphism and involved 859 patients and 1018 controls, four concerned the IL-6 −634 G/C polymorphism and included 343 patients and 340 controls, while six referred to the IFN-γ +874 A/T polymorphism and comprised 622 patients and 712 controls (Table 1).
Table 1.
Characteristics of the studies included in the meta-analysis
| Study | Ethnicity | Polymorphism | Number | RPL | Control | p value for associationa | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||||||
| GG | GC | CC | GG | GC | CC | |||||||
| Camil, 2014 [14] | Caucasian | IL-6 −174 G/C | 69 | 64 | 60 | 9 | 0 | 56 | 8 | 0 | 0.928 | Yes |
| Demirturk, 2014 [15] | Turkish | IL-6 −174 G/C | 113 | 113 | 72 | 36 | 5 | 100 | 11 | 2 | 4.8 × 10−5 | No |
| Bahadori, 2014 [16] | Middle Eastern | IL-6 −174 G/C | 85 | 104 | 36 | 43 | 6 | 44 | 51 | 9 | 0.866 | Yes |
| Parveen, 2013 [17] | Asian | IL-6 −174 G/C | 200 | 300 | 120 | 67 | 13 | 223 | 67 | 10 | 4.8 × 10−5 | Yes |
| von Linsingern, 2005 [18] | Latin American | IL-6 −174 G/C | 57 | 74 | 21 | 26 | 10 | 40 | 31 | 3 | 0.009 | Yes |
| Prigoshin, 2004 [27] | Caucasian | IL-6 −174 G/C | 38 | 54 | 35b | 3 | 49b | 5 | 0.819b | NA | ||
| Saijo, 2004 [19] | Asian | IL-6 −174 G/C | 76 | 93 | 76 | 0 | 0 | 93 | 0 | 0 | 1.000 | NA |
| Daher, 2003 [20] | Caucasian | IL-6 −174 G/C | 44 | 108 | 39b | 5 | 99b | 9 | 0.559b | NA | ||
| Unfried, 2003 [21] | Caucasian | IL-6 −174 G/C | 161 | 124 | 66 | 72 | 23 | 43 | 58 | 23 | 0.199 | Yes |
| GG | GC | CC | GG | GC | CC | |||||||
| Alkjuriji, 2013 [22] | Middle Eastern | IL-6 −634 G/C | 65 | 65 | 53 | 9 | 3 | 54 | 8 | 3 | 0.844 | No |
| Ma, 2011 [23] | Asian | IL-6 −634 G/C | 162 | 156 | 0 | 46 | 116 | 11 | 52 | 93 | 0.002 | Yes |
| El-Hamid, 2011 [24] | Middle Eastern | IL-6 −634 G/C | 40 | 30 | 33 | 5 | 2 | 27 | 3 | 0 | 0.203 | Yes |
| Saijo, 2004 [19] | Asian | IL-6 −634 G/C | 76 | 93 | 18b | 58 | 37b | 56 | 0.028b | NA | ||
| AA | AT | TT | AA | AT | TT | |||||||
| Zastavna, 2014 [25] | Caucasian | IFN-γ +874 A/T | 163 | 60 | 56 | 79 | 28 | 15 | 26 | 19 | 0.025 | Yes |
| Parveen, 2013 [17] | Asian | IFN-γ +874 A/T | 200 | 300 | 85 | 82 | 33 | 160 | 110 | 30 | 0.004 | Yes |
| Kamali, 2005 [26] | Middle Eastern | IFN-γ +874 A/T | 132 | 132 | 37 | 63 | 32 | 44 | 65 | 23 | 0.162 | Yes |
| Prigoshin, 2004 [27] | Caucasian | IFN-γ +874 A/T | 40 | 53 | 8 | 26 | 6 | 22 | 19 | 12 | 0.345 | Yes |
| Daher, 2003 [20] | Caucasian | IFN-γ +874 A/T | 46 | 104 | 16 | 18 | 12 | 39 | 50 | 15 | 0.243 | Yes |
| Babbage, 2001 [28] | Caucasian | IFN-γ +874 A/T | 41 | 63 | 11 | 19 | 11 | 20 | 32 | 11 | 0.313 | Yes |
RPL recurrent pregnancy loss, HWE Hardy–Weinberg equilibrium, NA not available
aAllele contrast
bGG + GC
Meta-analysis of the association between IL-6 −174 G/C and −634 G/C polymorphisms and RPL
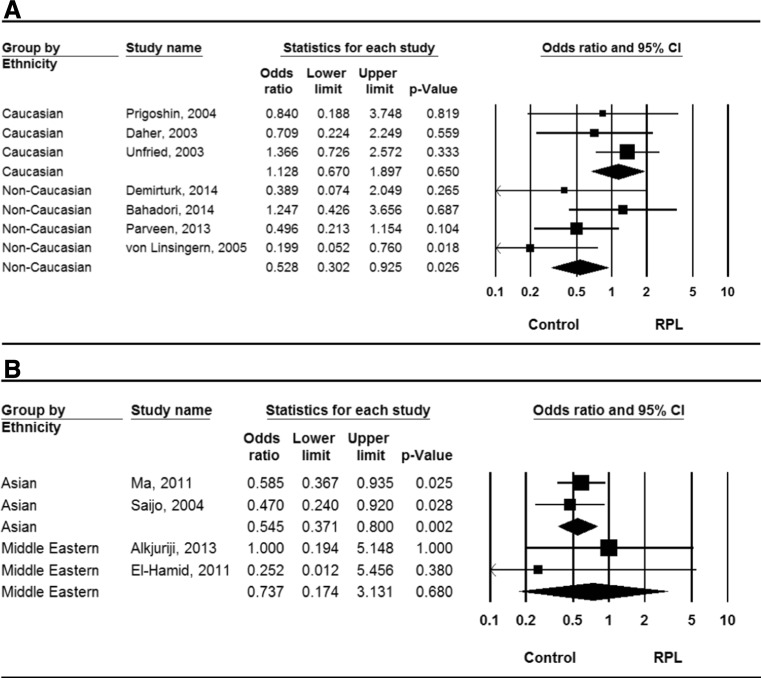
A summary of the findings of our meta-analyses concerning the association between IL-6 −174 G/C and −634 G/C polymorphisms and RPL is provided in Table 2. When all study subjects were considered together, no association was found between RPL and the −174 GG + GC genotype (OR = 0.794, 95 % CI = 0.542–1.163, p = 0.263). However, stratification of the data by ethnicity revealed an association between this genotype and RPL in non-Caucasian (OR = 0.528, 95 % CI = 0.302–0.925, p = 0.026) but not Caucasian study subjects (OR = 1.128, 95 % CI = 0.670–1.897, p = 0.650; Fig. 1). Meta-analyses using the recessive model and homozygote contrast identified the same pattern, suggesting that the IL-6 −174 G allele protects against the development of RPL, at least in non-Caucasian populations. Our meta-analysis also found an association between RPL and the IL-6 −634 GG + GC genotype in all study subjects (OR = 0.556, 95 % CI = 0.383–0.806, p = 0.002), while stratification by ethnicity revealed a negative association between this genotype and RPL in Asians (OR = 0.545, 95 % CI = 0.371–0.800, p = 0.002), but not Middle Eastern subjects (OR = 0.737, 95 % CI = 0.171–3.131, p = 0.680; Fig. 1). Under allelic contrast, the same result was observed (OR = 0.532, 95 % CI = 0.354–0.799, p = 0.002), suggesting that the IL-6 −634 G allele reduces the risk of developing RPL in Asian populations.
Table 2.
Meta-analysis of the association between the IL-6 −174 G/C and −634 G/C polymorphisms and RPL
| Polymorphism | Population | No. of studies | Subject no. | Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | OR | 95 % CI | p value | Model | p value | I 2 | |||
| IL-6 −174 G/C G vs. C allele | All subjects | 6 | 685 | 779 | 0.681 | 0.436–1.062 | 0.090 | R | 0.000 | 91.1 |
| Caucasian | 2 | 230 | 188 | 1.214 | 0.881–1.672 | 0.237 | F | 0.615 | 0 | |
| Non-Caucasian | 4 | 455 | 591 | 0.550 | 0.342–0.884 | 0.214 | R | 0.005 | 76.6 | |
| GG + GC vs. CC (dominant) | All subjects | 7 | 714 | 861 | 0.794 | 0.542–1.163 | 0.263 | F | 0.145 | 37.1 |
| Caucasian | 3 | 259 | 270 | 1.128 | 0.670–1.897 | 0.650 | F | 0.329 | 0 | |
| Non-Caucasian | 4 | 455 | 591 | 0.528 | 0.302–0.925 | 0.026 | F | 0.200 | 35.4 | |
| GG vs. GC + CC (recessive) | All subjects | 6 | 685 | 779 | 0.648 | 0.392–1.072 | 0.091 | R | 0.001 | 76.5 |
| Caucasian | 2 | 230 | 188 | 1.234 | 0.796–1.913 | 0.347 | F | 0.570 | 0 | |
| Non-Caucasian | 4 | 455 | 591 | 0.504 | 0.296–0.860 | 0.012 | R | 0.016 | 70.9 | |
| GG vs. CC | All subjects | 6 | 685 | 779 | 0.698 | 0.458–1.065 | 0.096 | R | 0.011 | 66.1 |
| Caucasian | 2 | 230 | 188 | 1.062 | 0.693–1.627 | 0.784 | F | 0.818 | 0 | |
| Non-Caucasian | 4 | 455 | 591 | 0.586 | 0.344–0.996 | 0.048 | R | 0.019 | 69.6 | |
| IL-6 −634 G/C G vs. C allele | All subjects | 3 | 267 | 251 | 0.586 | 0.414–0.829 | 0.003 | F | 0.405 | 0 |
| Middle Eastern | 2 | 105 | 95 | 0.760 | 0.388–1.456 | 0.422 | F | 0.312 | 1.65 | |
| Asian | 1 | 162 | 156 | 0.532 | 0.354–0.799 | 0.002 | NA | NA | NA | |
| GG + GC vs. CC (dominant) | All subjects | 4 | 343 | 344 | 0.556 | 0.383–0.806 | 0.002 | F | 0.793 | 0 |
| Middle Eastern | 2 | 105 | 95 | 0.737 | 0.171–3.131 | 0.680 | F | 0.439 | 0 | |
| Asian | 2 | 238 | 249 | 0.545 | 0.371–0.800 | 0.002 | F | 0.598 | 0 | |
| GG vs. GC + CC (recessive) | All subjects | 3 | 267 | 251 | 0.632 | 0.302–1.322 | 0.223 | F | 0.113 | 54.0 |
| Middle Eastern | 2 | 105 | 95 | 0.773 | 0.360–1.661 | 0.510 | F | 0.534 | 0 | |
| Asian | 1 | 162 | 156 | 0.039 | 0.002–0.665 | 0.025 | NA | NA | NA | |
| GG vs. CC | All subjects | 3 | 267 | 251 | 0.386 | 0.106–1.404 | 0.148 | F | 0.131 | 50.8 |
| Middle Eastern | 2 | 105 | 95 | 0.720 | 0.169–3.072 | 0.657 | F | 0.434 | 34.0 | |
| Asian | 1 | 162 | 156 | 0.035 | 0.002–0.600 | 0.021 | NA | NA | NA | |
F fixed-effect model, R random-effect model, NA not available
Fig. 1.
Odds ratios (ORs) and 95 % confidence intervals (CIs) from individual studies and pooled data for the association between the GG + GC genotype of the IL-6 −174 G/C (a) and −634 G/C (b) polymorphisms and RPL in Caucasian and non-Caucasian populations
Meta-analysis of the association between the IFN-γ +874 A/T polymorphism and RPL
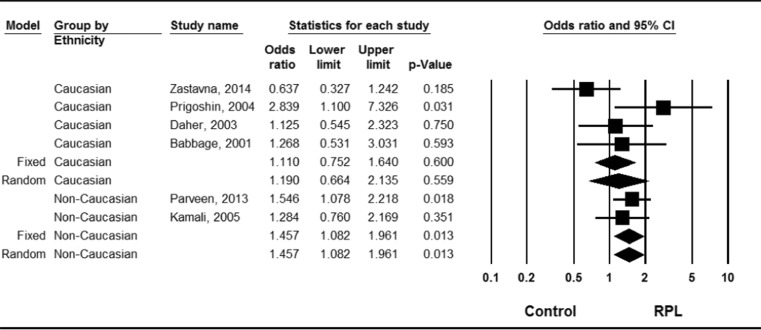
No allelic association between the IFN-γ +874 A/T polymorphism and RPL was discerned in the data taken as a whole (OR = 1.188, 95 % CI = 0.905–1.559, p = 0.214; Table 3). However, ethnicity-specific meta-analysis indicated an association between the +874 A allele and RPL in non-Caucasians (OR = 1.403, 95 % CI = 1.135–1.734, p = 0.002), but not in Caucasians (OR = 1.073, 95 % CI = 0.702–1.640, p = 0.746; Fig. 2, Table 3). Use of the recessive model and homozygote contrast resulted in the same pattern, that is, a significant association between the +874 A allele and RPL in non-Caucasian study subjects.
Table 3.
Meta-analysis of the association between the IFN-γ +874 A/T polymorphism and RPL
| Polymorphism | Population | No. of studies | Subject no. | Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | OR | 95 % CI | p value | Model | p value | I 2 | |||
| IFN-γ +874 T vs. A allele | All subjects | 6 | 622 | 712 | 1.188 | 0.905–1.559 | 0.214 | R | 0.029 | 59.8 |
| Caucasian | 4 | 290 | 280 | 1.073 | 0.702–1.640 | 0.746 | R | 0.041 | 63.7 | |
| Non-Caucasian | 2 | 459 | 432 | 1.403 | 1.135–1.734 | 0.002 | F | 0.499 | 0 | |
| TT + AA vs. AA (dominant) | All subjects | 6 | 622 | 712 | 1.318 | 1.041–1.670 | 0.022 | R | 0.155 | 37.6 |
| Caucasian | 4 | 290 | 280 | 1.190 | 0.664–2.135 | 0.559 | R | 0.089 | 53.9 | |
| Non-Caucasian | 2 | 459 | 432 | 1.457 | 1.082–1.961 | 0.013 | F | 0.567 | 0 | |
| TT vs. TA + AA (recessive) | All subjects | 6 | 622 | 712 | 1.200 | 0.716–2.013 | 0.489 | R | 0.013 | 65.4 |
| Caucasian | 4 | 290 | 280 | 0.981 | 0.428–2.197 | 0.962 | R | 0.017 | 70.4 | |
| Non-Caucasian | 2 | 459 | 432 | 1.658 | 1.114–2.468 | 0.013 | F | 0.697 | 0 | |
| TT vs. AA | All subjects | 6 | 622 | 712 | 1.373 | 0.802–2.351 | 0.248 | R | 0.035 | 58.3 |
| Caucasian | 4 | 290 | 280 | 1.120 | 0.481–2.610 | 0.792 | R | 0.042 | 63.5 | |
| Non-Caucasian | 2 | 459 | 432 | 1.895 | 1.226–2.928 | 0.004 | F | 0.621 | 0 | |
F fixed-effect model, R random-effect model
Fig. 2.
Odds ratios (ORs) and 95 % confidence intervals (CIs) from individual studies and pooled data for the association between the GG + GC genotype of the IFN-γ +874 A/T polymorphism and RPL in Asian and Middle Eastern populations
Heterogeneity and publication bias
Some between-study heterogeneity was observed in our meta-analyses; however, none was evident under the dominant model in non-Caucasian and Asian subgroups (Tables 2 and 3). In addition, excluding studies containing control groups that deviated from HWE did not affect our results [15, 22]. Funnel plots show no asymmetry, and Egger’s regression test showed no evidence of publication bias (Egger’s regression p values > 0.1).
Discussion
In this meta-analysis, we combined data from published studies to evaluate genetic associations between IL-6 −174 G/C, IL-6 −634 G/C, and IFN-γ +874 A/T polymorphisms and RPL. Our results show that IL-6 −174 G/C and −634 G/C variants are associated with susceptibility to RPL in non-Caucasian and Asian populations, and a negative association was detected between the GG + GC genotypes of these polymorphisms and this disease. In addition, IFN-γ +874 A/T polymorphism is associated with RPL risk in non-Caucasian population. The reason behind this disparity between ethnic groups is unclear but may be due in part to the frequency at which these polymorphisms are present in each population. For example, the IL-6 −174 C allele has not been observed in Japanese subjects [19] and is known to be rare in Chinese and Korean populations [40, 41], while this allele is common in Caucasians. The studies in Korean and Chinese populations did not examine other alleles that may be associated with RPL [40, 41], but the study in Japanese population has shown that women carrying the −634G allele of the IL-6 gene have a decreased risk of RPL [19]. In addition, the IL-6 −634 C allele is not widespread in Middle Eastern groups [22] but is prevalent among Asians [23], a population in which the IFN-γ +874 AA genotype is also more common compared to other non-Caucasians [17]. The results of our study are consistent with findings regarding the functional significance of IL-6 and IFN-γ polymorphisms [13, 42]. Thus, IL-6 −174 G/C, IL-6 −634 G/C, and IFN-γ +874 A/T variants may contribute to RPL risk by affecting the expression of their corresponding proteins.
This investigation differs from previous meta-analyses assessing the relationship between the aforementioned polymorphisms and RPL risk performed by Medica et al. [43] and Bombell et al. [44] in several ways. In the present study, an extra nine studies [14–17, 22–25], 1097 RPL patients, and 1192 controls were included, and additional meta-analyses were performed to examine the potential association between the −634 G/C IL-6 polymorphism and RPL. Moreover, we carried out further ethnicity-specific analysis. Previous meta-analyses have failed to establish an association between the IL-6 −174 G/C and IFN-γ +874 A/T polymorphisms and RPL risk [43, 44]. However, our investigation revealed a relationship between these polymorphisms and RPL in non-Caucasian populations, but not in Caucasians.
The present study has some limitations that should be noted. First, heterogeneity, confounding factors, and publication bias might have distorted the analysis. Although Egger’s regression test showed no evidence of publication bias (Egger’s regression p values > 0.1), it is possible that it does not have enough power due to the sample size. However, funnel plots show no asymmetry, suggesting no evidence of publication bias. Second, since our ethnicity-specific meta-analyses only included data from Caucasian, Middle Eastern, and Asian patients, our findings may not be applicable beyond these groups. Further studies would be needed to gain an understanding of disease risk associations in other ethnicities. Finally, the number of available studies was too small to draw definitive conclusions regarding the association between the polymorphisms under investigation and RPL, particularly in specific ethnic groups.
In conclusion, this meta-analysis demonstrates that IL-6 −174 G/C, IL-6 −634 G/C, and IFN-γ +874 A/T polymorphisms are associated with susceptibility to RPL, especially in non-Caucasian populations. The relative importance of these polymorphisms in the development of RPL may thus be dependent on ethnicity. These findings suggest a need for further investigations into the association between IL-6 and IFN-γ polymorphisms and RPL susceptibility in different ethnic groups, with a view to elucidating their roles in the pathogenesis of this disease.
Acknowledgments
This research received no specific grant from any public, commercial, or not-for-profit sectors funding agency.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Capsule
Our meta-analysis demonstrates that IL-6 −174 G/C, −634 G/C, and IFN-γ +874 A/Tpolymorphisms are associated with susceptibility to RPL, particularly in non-Caucasians.
References
- 1.Stirrat GM. Recurrent miscarriage I: definition and epidemiology. Lancet. 1990;336(8716):673–5. doi: 10.1016/0140-6736(90)92159-F. [DOI] [PubMed] [Google Scholar]
- 2.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Review article: tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens*. Am J Reprod Immunol. 2010;63(2):93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 3.Saini V, Arora S, Yadav A, Bhattacharjee J. Cytokines in recurrent pregnancy loss. Clin Chim Acta. 2011;412(9):702–8. doi: 10.1016/j.cca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Kwak‐Kim J, Park JC, Ahn HK, Kim JW, Gilman‐Sachs A. Review article: immunological modes of pregnancy loss. Am J Reprod Immunol. 2010;63(6):611–23. doi: 10.1111/j.1600-0897.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- 5.Raghupathy R (ed) Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Seminars in immunology. Elsevier;2001. [DOI] [PubMed]
- 6.Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod. 2001;16(10):2219–26. doi: 10.1093/humrep/16.10.2219. [DOI] [PubMed] [Google Scholar]
- 7.Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15(8):819–35. doi: 10.1016/S0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- 8.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta (BBA)-Mol Cell Res. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1-and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196(2):122–30. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 10.Daher S, Denardi KDAG, Blotta MHSL, et al. Cytokines in recurrent pregnancy loss. J Reprod Immunol. 2004;62(1):151–7. doi: 10.1016/j.jri.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Morse HR, Olomolaiye OO, Wood NA, Keen LJ, Bidwell JL. Induced heteroduplex genotyping of TNF-alpha, IL-1beta, IL-6 and IL-10 polymorphisms associated with transcriptional regulation. Cytokine. 1999;11(10):789–95. doi: 10.1006/cyto.1999.0491. [DOI] [PubMed] [Google Scholar]
- 12.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren’s syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford) 2001;40(6):656–61. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]
- 13.Pravica V, Perrey C, Stevens A, Lee J-H, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human IFN-γ gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-γ production. Hum Immunol. 2000;61(9):863–6. doi: 10.1016/S0198-8859(00)00167-1. [DOI] [PubMed] [Google Scholar]
- 14.C LB, V ER. Interleukin-6 and interleukin-10 gene polymorphisms and recurrent pregnancy loss in Romanian population. Iranian journal of reproductive medicine. 2014;12(9):617–22. [PMC free article] [PubMed]
- 15.Demirturk F, Ates O, Gunal O, Bozkurt N, Aysal T, Nacar MC. IL-6 gene promoter polymorphisms: genetic susceptibility to recurrent pregnancy loss. Bratisl Lek Listy. 2014;115(8):479–82. doi: 10.4149/bll_2014_092. [DOI] [PubMed] [Google Scholar]
- 16.Bahadori M, Zarei S, Zarnani AH, et al. IL-6, IL-10 and IL-17 gene polymorphisms in Iranian women with recurrent miscarriage. Iran J Immunol: IJI. 2014;11(2):97–104. [PubMed] [Google Scholar]
- 17.Parveen F, Shukla A, Agarwal S. Cytokine gene polymorphisms in northern Indian women with recurrent miscarriages. Fertil Steril. 2013;99(2):433–40. doi: 10.1016/j.fertnstert.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 18.von Linsingen R, Bompeixe EP, Bicalho MG. A case-control study in IL6 and TGFB1 gene polymorphisms and recurrent spontaneous abortion in southern Brazilian patients. Am J Reprod Immunol. 2005;53(2):94–9. doi: 10.1111/j.1600-0897.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 19.Saijo Y, Sata F, Yamada H, Kondo T, Kato EH, Kishi R. Single nucleotide polymorphisms in the promoter region of the interleukin-6 gene and the risk of recurrent pregnancy loss in Japanese women. Fertil Steril. 2004;81(2):374–8. doi: 10.1016/j.fertnstert.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Daher S, Shulzhenko N, Morgun A, et al. Associations between cytokine gene polymorphisms and recurrent pregnancy loss. J Reprod Immunol. 2003;58(1):69–77. doi: 10.1016/S0165-0378(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 21.Unfried G, Bocskor S, Endler G, Nagele F, Huber JC, Tempfer CB. A polymorphism of the interleukin-6 gene promoter and idiopathic recurrent miscarriage. Hum Reprod. 2003;18(2):267–70. doi: 10.1093/humrep/deg094. [DOI] [PubMed] [Google Scholar]
- 22.Alkhuriji AF, Alhimaidi AR, Babay ZA, Wary AS. The relationship between cytokine gene polymorphism and unexplained recurrent spontaneous abortion in Saudi females. Saudi Med J. 2013;34(5):484–9. [PubMed] [Google Scholar]
- 23.Ma X, Xu LJ, Wang J, Xian MM, Liu M. Association of IL-1beta and IL-6 gene polymorphisms with recurrent spontaneous abortion in a Chinese Han population. Int J Immunogenet. 2012;39(1):15–9. doi: 10.1111/j.1744-313X.2011.01049.x. [DOI] [PubMed] [Google Scholar]
- 24.El-Hamid SA, El-Khayat W. Relationship of the protein Z intron F G79A and IL6 C634G gene polymorphisms with the risk of recurrent pregnancy loss in Egyptian women. J Investig Med Off Publ Am Fed Clin Res. 2011;59(4):655–60. doi: 10.2310/JIM.0b013e31820c9c90. [DOI] [PubMed] [Google Scholar]
- 25.Zastavna D, Sosnina K, Terpylyak O, et al. Cytogenetic and immunogenetic analysis of recurrent pregnancy loss in women. Tsitol Genet. 2014;48(4):44–50. [PubMed] [Google Scholar]
- 26.Kamali-Sarvestani E, Zolghadri J, Gharesi-Fard B, Sarvari J. Cytokine gene polymorphisms and susceptibility to recurrent pregnancy loss in Iranian women. J Reprod Immunol. 2005;65(2):171–8. doi: 10.1016/j.jri.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Prigoshin N, Tambutti M, Larriba J, Gogorza S, Testa R. Cytokine gene polymorphisms in recurrent pregnancy loss of unknown cause. Am J Reprod Immunol. 2004;52(1):36–41. doi: 10.1111/j.1600-0897.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 28.Babbage SJ, Arkwright PD, Vince GS, et al. Cytokine promoter gene polymorphisms and idiopathic recurrent pregnancy loss. J Reprod Immunol. 2001;51(1):21–7. doi: 10.1016/S0165-0378(01)00069-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Bae SC, Choi SJ, Ji JD, Song GG. Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep.38(6):3643–51. [DOI] [PubMed]
- 30.Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Associations between osteoprotegerin polymorphisms and bone mineral density: a meta-analysis. Mol Biol Rep.37(1):227–34. [DOI] [PubMed]
- 31.Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010;30(3):357–63. doi: 10.1007/s00296-009-0969-5. [DOI] [PubMed] [Google Scholar]
- 32.Davey Smith G, Egger M. Meta-analyses of randomised controlled trials. Lancet. 1997;350(9085):1182. doi: 10.1016/S0140-6736(05)63833-0. [DOI] [PubMed] [Google Scholar]
- 33.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bombell S, McGuire W. Cytokine polymorphisms in women with recurrent pregnancy loss: meta-analysis. Aust NZ J Obstet Gynaecol. 2008;48(2):147–54. doi: 10.1111/j.1479-828X.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 38.Choi YK, Kwak-Kim J. Cytokine gene polymorphisms in recurrent spontaneous abortions: a comprehensive review. Am J Reprod Immunol. 2008;60(2):91–110. doi: 10.1111/j.1600-0897.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 39.Medica I, Ostojic S, Pereza N, Kastrin A, Peterlin B. Association between genetic polymorphisms in cytokine genes and recurrent miscarriage—a meta-analysis. Reprod Biomed Online. 2009;19(3):406–14. doi: 10.1016/S1472-6483(10)60176-9. [DOI] [PubMed] [Google Scholar]
- 40.Lim CS, Zheng S, Kim YS, et al. The −174 G to C polymorphism of interleukin-6 gene is very rare in Koreans. Cytokine. 2002;19(1):52–4. doi: 10.1006/cyto.2002.1951. [DOI] [PubMed] [Google Scholar]
- 41.Zhai R, Liu G, Yang C, Huang C, Wu C, Christiani DC. The G to C polymorphism at -174 of the interleukin -6 gene is rare in a Southern Chinese population. Pharmacogenet Genomics. 2001;11(8):699–701. doi: 10.1097/00008571-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102(7):1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medica I, Ostojic S, Pereza N, Kastrin A, Peterlin B. Association between genetic polymorphisms in cytokine genes and recurrent miscarriage—a meta-analysis. Reprod Biomed Online. 2009;19(3):406–14. doi: 10.1016/S1472-6483(10)60176-9. [DOI] [PubMed] [Google Scholar]
- 44.Bombell S, McGuire W. Cytokine polymorphisms in women with recurrent pregnancy loss: meta‐analysis. Aust NZ J Obstet Gynaecol. 2008;48(2):147–54. doi: 10.1111/j.1479-828X.2008.00843.x. [DOI] [PubMed] [Google Scholar]