Abstract
Selenium (Se) is necessary for the immune system in chicken and mediates its physiological functions through selenoproteins. Heat shock proteins (Hsps) are indispensable for maintaining normal cell function and for directing the immune response. The aim of the present study was to investigate the effects of Se deficiency on the messenger ribonucleic acid (mRNA) expression levels of selenoproteins and Hsps as well as immune functions in the chicken bursa of Fabricius. Two groups of chickens, namely the control and Se-deficient (L group) groups, were reared for 55 days. The chickens were offered a basal diet, which contained 0.15 mg Se/kg in the diet fed to the control group and 0.033 mg Se/kg in the diet fed to the L group. We performed real-time quantitative polymerase chain reactionto detect the mRNA expression levels of selenoproteins and Hsps on days 15, 25, 35, 45 and 55. Western blotting was used to determine the protein expression levels of Hsps on days 35, 45 and 55, and immune functions were assessed through an enzyme-linked immunosorbent assay on days 15, 35, and 55. The data showed that the mRNA expression levels of selenoproteins, such as Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, GPx1, GPx2, GPx3 GPx4, Sepp1, Selo, Sel-15, Sepx1, Sels, Seli, Selu, Selh, and SPS2, were significantly lower (P < 0.05) in the L group compared with the control group. Additionally, the mRNA and protein expression levels of Hsps (Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90) were also significantly higher (P < 0.05) in the L group. The expression levels of IL-2, IL-6, IL-8, IL-10, IL-17, IL-1β, IFN-α, IFN-β, and IFN-γ were significantly lower (P < 0.05) and TNF-α was significantly higher (P < 0.05) in the L group compared with the control group. Our results show that immunosuppression was accompanied by a downregulation of mRNA expression levels of selenoproteins and an upregulation of the Hsp mRNA expression levels. Thus, Se deficiency causes defects in the chicken bursa of Fabricius, and selenoproteins and Hsps play important roles in immunosuppression in the bursa of Fabricius of chickens with Se deficiency.
Keywords: Selenium, Selenoproteins, Heat shock proteins, Cytokine, Bursa of Fabricius, Chicken
Introduction
The nutritional trace element selenium (Se) is a key component of various biological functions in animals and chickens (Kohrle et al. 2005; Liang et al. 2014; Lin et al. 2014). However, Se deficiency causes inhibition of the immune system (Demirci et al. 2013; Lei et al. 2011), inflammation, and oxidative stress in chickens (Ghazi Harsini et al. 2012; Xu et al. 2013). Previous studies have shown that Se deficiency inhibits the proliferation of T and B lymphocytes (Wilson et al. 2014). The chicken bursa of Fabricius is a distinct prime lymphoid organ that plays an important role in the development of B cells and the production of the immunoglobulin repertoire (Cooper et al. 1966). Low Se levels cause injuries in the mitochondria and a decrease in S-phase cells in the chicken bursa of Fabricius. Furthermore, cell cycle arrest, apoptosis, and restrained development of the bursa of Fabricius occur in response to a low Se diet (Peng et al. 2011).
Se mediates its physiological functions through selenoproteins (Stadtman 2000). In chicken, 25 selenoproteins have been identified (Mariotti et al. 2012). Selenoproteins are recognized by their different characteristics and functions (Bellinger et al. 2009; Pappas et al. 2008). Se deficiency induces pancreatic injury due to decreased mRNA levels of Txnrd2, GPx1, GPx3, Seli, Dio1, Sepp1, Sepw1, Selo, Selt, Selm, Sepx1, and SPS2 in chicken (Zhao et al. 2014b). In the chicken muscle, Se deficiency causes lipid peroxidation, decreases glutathione peroxide, and accelerates cell apoptosis due to a decrease in the expression of four endoplasmic reticulum-resident selenoproteins (Sepn1, Selk, Sels, and Selt) (Yao et al. 2013). The selenoproteins SelT, SecS, and SPS1 are decreased in chickens with Se deficiency, which induces oxidative stress and confirms the negative effects observed in the immune organs of chickens (You et al. 2014). However, no information regarding selenoprotein expression in the bursa of Fabricius of chickens with Se deficiency has been reported to date.
Molecular chaperone heat shock proteins (Hsps) engage in the assembly and disassembly of other proteins and assist in their folding (Wu et al. 2011). Hsps localize to different cellular compartments; their expression is also different in these compartments. They are synthesized rapidly under stress conditions (Al-Aqil and Zulkifli 2009) and induce an anti-inflammatory response through T cells (Van Eden et al. 2007). Testicular germ cells generate a high level of stress response proteins, including Hsps, which protect the cells against the free radical insult that occurs under oxidative stress (Patrick 1999). Hsps are related to immune system functions (Tsan and Gao 2009). Hsps present a connection between the innate and adaptive immune systems (Guo et al. 2007). Hsps maintain a protective role in the immune system in animals (Ausiello et al. 2005; Habich and Burkart 2007). The Hsp27, Hsp70, and Hsp90 mRNA levels are increased in the bursa of Fabricius and the spleen through the use of resveratrol in black bone chicken, and a low expression of Hsp27 and Hsp90 mRNA has been observed in the thymus (Liu et al. 2014c). A study of the heart and blood vessels of heat-stressed broilers showed that Hsp70 and Hsp90 are lower, whereas Hsp60 is higher, which indicates a protective role for Hsp60 in an unfavorable environment. The reduction in Hsp70 and 90 implies the formation of a myocardial lesion under heat stress, and the localization of Hsp70 to the blood vessels forms part of the protective mechanism (Yu et al. 2008).
The primary significance of Se to optimal immune function has been generally established. Appropriate Se intake is necessary for a suitable immune response to various infectious diseases (Broome et al. 2004). Cytokines are typically linked to the control of the host immune response to different foreign antigens (Berndt et al. 2007; Shaughnessy et al. 2009). Particular types of cytokines have autocrine, paracrine, and/or endocrine actions, and receptor binding can elicit a range of responses depending on the cytokine and the target cell. However, there are no reliable data available on the effects of Se deficiency on selenoproteins, Hsps, and cytokines in the chicken bursa of Fabricius. The present results show that Se deficiency decreases selenoprotein expression and affects cytokines, which play key roles in the immunosuppression response. Moreover, Se deficiency also increases the expression of a number of Hsps in the chicken bursa of Fabricius, indicating a protective role.
Materials and methods
Chickens were fed a Se-deficiency diet for 55 days, and the expression levels of selenoproteins, Hsp mRNA, and cytokines in the chicken bursa of Fabricius were investigated. A total of 180 chickens (1 day old; Weiwei Co. Ltd., Harbin, China) were randomly separated into two groups (90 chickens per group): the control group and the Se-deficiency group (L group). The chickens in the control group were fed 0.15 mg/kg (sodium selenite) through a basal diet, and chickens in the L group were fed 0.033 mg of Se/kg through a basal diet (Table 1). The basal diet consisted of corn and soy that were produced in the Se-deficient region of Heilongjiang Province, China. The chickens in each group were housed in three pens (30 chickens in each pen). The chickens were given free access to food and water. The chickens were checked on a daily basis, and their body weights were recorded weekly. The chicken bursa of Fabricius tissues were collected on days 15, 25, 35, 45, and 55 to analyze the parameters of interest. The collected tissue samples were blotted, rinsed with ice-cold sterile deionized water, frozen immediately in liquid nitrogen, and stored at −80 °C. All of the procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (Table 2).
Table 1.
The diet with Se deficiency is consisting as following
| Feedstock | Percent (%) |
|---|---|
| Corn | 64.73 |
| Mountain flour | 1.3 |
| Salt | 0.27 |
| Lysine | 0.4 |
| Choline chloride | 0.07 |
| Vitamins | 0.04 |
| Soyabean | 28 |
| Calcium hydrophosphate | 2.3 |
| Soybean oil | 2.5 |
| DL-methionine | 0.19 |
| Complexminerals | 0.2 |
| Total | 100 |
Table 2.
Gene-special primers used in the qPCR
| Gene | Serial number | Direction | Primer sequence (5, → 3) |
|---|---|---|---|
| Txnrd1 | NM_001030762.2 | Forward | TACGCCTCTGGGAAATTCGT |
| Reverse | CTTGCAAGGCTTGTCCCAGTA | ||
| Txnrd2 | NM_001122691.1 | Forward | GCTCTTAAAGATGCCCAGCACTAC |
| Reverse | GAACAGCTTGAGCCATCACAGA | ||
| Txnrd3 | NM_001122777.1 | Forward | CCTGGCAAAACGCTAGTTGT G |
| Reverse | CGCACCATTACTGTGACATCTAGAC | ||
| Dio1 | Y11110.1 | Forward | GCGCTATACCACAGGCAGTA |
| Reverse | GGTCTTGCAAATGTCACCAC | ||
| Dio2 | AF125575.1 | Forward | ATTTGCTGATCACGCTTCAG |
| Reverse | GCTCAGAAACAGCACCATGT | ||
| Dio3 | Y11273.1 | Forward | CTGTGCATTCGCAAGAAGAT |
| Reverse | GCCGACTTGAAGAAGTCCAG | ||
| GPx1 | BX935086.2 | Forward | ACGGCGCATCTTCCAAAG |
| Reverse | TGTTCCCCCAACCATTTCTC | ||
| GPx2 | NM_001277854.1 | Forward | ATCGCCAAGTCCTTCTACGA |
| Reverse | ACGTTCTCGATGAGGACCAC | ||
| GPx3 | NM_001163232.1 | Forward | CCTGCAGTACCTCGAACTGA |
| Reverse | CTTCAGTGCAGGGAG GATCT | ||
| GPx4 | AF498316 | Forward | CTTCGTCTGCATCATCACCAA |
| Reverse | TCGACGAGCTGAGTGTAATTCC | ||
| Sepp1 | NM_001031609 | Forward | CCAAGTGGTCAGCATTCACATC |
| Reverse | ATGACGACCACCCTCACGAT | ||
| Selo | NM_001115017.1 | Forward | CCAGCGTTAACCGGAATGAT |
| Reverse | ATGCGCCTCCTGGATTTCT | ||
| Selk | NM_001025441.2 | Forward | GAAGAGGGCCTCCAGGAAAT |
| Reverse | CAGCCATTGGTGGTGGACTAG | ||
| Sels | NM_001024734.2 | Forward | CCGACATGGTGGTAAGAAGACA |
| Reverse | GCTTGTGCATTCAACTCCTCTTG | ||
| Sepx1 | NM_001135558.1 | Forward | TGGCAAGTGTGGCAATGG |
| Reverse | GAATTTGAGCGAGCTGCTGAAT | ||
| Sel-15 | NM_001012926.2 | Forward | ACTTGGCTTCTCCAGTAACTTGCT |
| Reverse | GCCTACAGAATGGATCCAACTA | ||
| Seli | XM_426086.1 | Forward | TGCCAGCCTCTGAACTGGAT |
| Reverse | TGCAAACCCAGACATCACCAT | ||
| Selu | NM_001193518.1 | Forward | GATGCTTTCAGGCTTCTTCC |
| Reverse | CTGTCTTCCTGCTCCAATCA | ||
| Selh | BX932816.2 | Forward | CATCGAGCACTGCCGTAG |
| Reverse | GACACCTCGAAGCTGTTCCT | ||
| SPS2 | NM_001187543.1 | Forward | CGTTGGGTATCGGAACTGAC |
| Reverse | CGTCCACCAGAGGGTAGAAA | ||
| GAPDH | K01458 | Forward | AGAACATCATCCCAGCGT |
| Reverse | AGCCTTCACTACCCTCTTG |
Quantitative real-time polymerase chain reaction (qPCR)
According to the manufacturer’s instructions (Invitrogen, China), the total RNA from the tissue samples (50 mg tissue; n = 3/diet group) was isolated using the TRIzol reagent. The primer specificity was checked through standard PCR. The PCR products were electrophoresed on a 2 % agarose gel. A pMD18-T vector (TaKaRa, China) was used for extraction, cloning, and sequencing. Oligo dT primers and superscript II reverse transcriptase were used to synthesize the complimentary deoxyribonucleic acid (cDNA; Invitrogen, China). Sterile water was added to dilute the cDNA, and the diluted cDNA was stored at −80 °C until use. The mRNA expression levels were detected using an ABI PRISM 7500 real-time PCR system (Applied Biosystems, USA). The PCR solution consisting of 10 μl of 2× SYBR Green I PCR Master Mix (TaKaRa, China), 2 μl of diluted cDNA, 0.4 μl of each primer (10 μM), 0.4 μl of 50× ROX reference Dye II, and 6.8 μl of PCR-grade water was generated. The PCR program used consisted of 1 cycle of 95 °C for 30 s and 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The gene amplification efficiencies were obtained using the DART-PCR program. The mRNA expression levels were determined based on the Ct values of the PCR products and are expressed as the fold-change relative to the level of the GADPH gene, as determined using the 2−△△CT method.
Cytokine determination
To determine the cytokine content, tissues were collected on days 15, 35, and 55 from both groups. After tissue collection, 100 mg of the chicken bursa of Fabricius was homogenized with 1 ml of 1× PBS and stored at −20 °C. After thawing twice, the supernatant was obtained through centrifugation for 5 min at 5,000×g and 4 °C. An enzyme-linked immunosorbent assay (ELISA) kit provided by the manufacturer (CUSABIO BIOTECH Co., Ltd.) was used. A pre-diluted standard (50 μl) was added to each well of the plate, and the plate was incubated for 2 h at room temperature. The supernatants were discarded, and each well was washed five times with a washing solution. After washing, 100 μl of a pre-diluted detector solution was added to each well, and the plate was incubated for 1 h at room temperature. After incubation, the pre-diluted solution was discarded, and each well was washed five times with a washing solution. The TMB (100 μl) substrate solution was then added, and the plate was incubated for 30 min in a dark room. Finally, 50 μl of a stop solution was added to each well. A calibrated plate reader was used to determine the absorbance of each well at 450 nm. For analysis, the change in the readings at two wavelengths was used.
Western blot
The total protein from the chicken bursa of Fabricius tissues was extracted. An equivalent amount of tissue (between 50 and150 mg) was used. The proteins were extracted using a cell lysis buffer with phenylmethanesulfonyl fluoride (PMSF; 100 mM) for western blotting and IP and measured according to Bradford’s procedure (Bradford 1976). For western blotting, the proteins were separated on 12 % gels using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were then transferred to nitrocellulose membranes in Tris–glycine buffer containing 20 % methanol with the transfer tank method. The membranes were blocked with 5 % powdered skim milk solution for 2 h at 37 °C and then incubated with primary chicken antibodies against Hsp27 (1:800), Hsp40 (1:800), Hsp60 (1:800), Hsp70 (1:800), and Hsp90 (1:800; Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 polyclonal antibodies were produced by our lab) and then with a horseradish peroxidase (HRP)-conjugated secondary antibody against rabbit IgG (1:1,500, Santa Cruz, CA, USA) overnight. Incubation with a monoclonal β-actin antibody (1:1,000, Santa Cruz, CA, USA) followed by a HRP-conjugated goat anti-rabbit IgG (1:1,500) antibody was used to verify equal loading of the samples. The signal was detected with a ChemiScope 5300 chemiluminescence system (Clinx Science Instruments, Shanghai, China). The optical density (OD) of each band was determined with an Image VCD gel imaging system. The Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 expression levels were presented as the ratio of the OD of THE Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 to the OD of β-actin, respectively.
Statistical analysis
Statistical analyses of the selenoprotein expression levels, Hsps mRNA levels, and cytokine contents were performed using the SPSS statistical software for Windows (version 13; SPSS Inc., Chicago, IL, USA). A significant value (P < 0.05) was determined using a two-tailed paired Student’s t test. The data are expressed as the mean ± the standard deviations.
Results
Clinical data
The chicken room was maintained at 24 ± 2 °C and 50 % humidity with controlled lighting (12 h per day) throughout the duration of the experiment. None of the experimental animals suffered from other diseases or Se deficiency-induced lethality. The chickens in the control group presented good physical developmental growth, normal drinking and feeding, bright feathers, and normal feces. Compared with the control group, the chickens in the L group did not show any specific abnormalities in the early phase. However, after 2 weeks, the chickens with Se deficiency showed signs of depression, such as reduced activity and food intake and increased drinking. At day 50, the chickens in the L group exhibited signs of depression, anorexia, reduced movement, growth retardation, and a bluish-green color on the face, chest and wings. Additionally, some of these chickens could not stand steadily, experienced slight ataxia, and had feces that appeared green or brown.
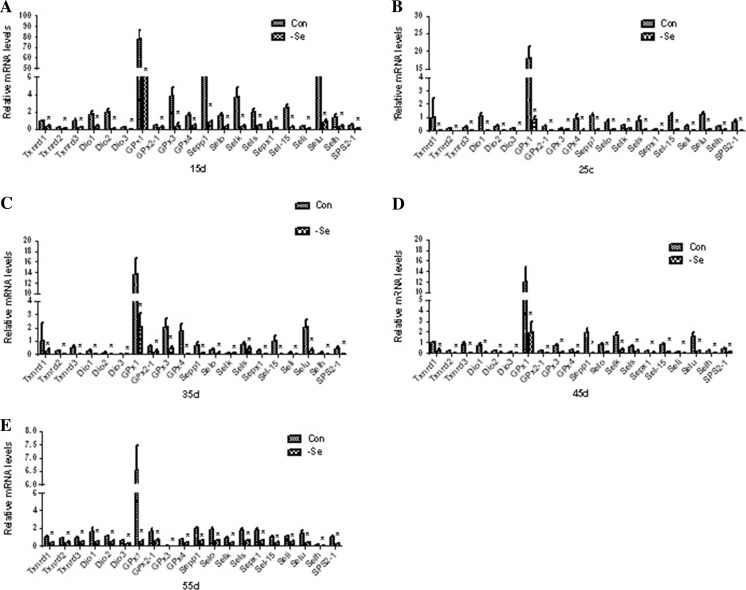
Effect of Se deficiency on the selenoprotein mRNA levels in the chicken bursa of Fabricius
To evaluate the effects of Se deficiency on the selenoprotein mRNA levels in the chicken bursa of Fabricius, we analyzed the collected tissue using the real-time qPCR technique. The expression levels of the selenoproteins are shown in Fig. 1a, b, c, d, e. The percent expression of the selenoproteins Txnrd1 37, Txnrd2 37, Txnrd3 24, Dio1 25, Dio2 6, Dio3 15, GPx1 24, GPx2 45, GPx3 11, GPx4 30, Selo 25, Seli 34, Sepp1 9, Selk 11, Sepx1 11, Sel-15 9, Sels 25, Selu 11, Selh 29, and SPS2 25 obtained for the chickens in the L group was significantly lower (P < 0.05) on day 15 compared with the levels observed in the control group (Fig. 1a). The percent expression of the selenoproteins Txnrd1 4, Txnrd2 3, Txnrd3 7, Dio1 0.989, Dio2 3, Dio3 1, GPx1 4, GPx2 12, GPx3 54, GPx4 5, Selo 11, Seli 3, Sepp1 7, Selk 42, Sepx1 16, Sel-15 8, Sels 11, Selu 4, Selh 20, and SPS2 2 was significantly lower (P < 0.05) on day 25 in the L group compared with the levels observed in the control group (Fig. 1b). The percent expression of the selenoproteins Txnrd1 25, Txnrd2 12, Txnrd3 2, Dio1 6, Dio2 8, Dio3 40, GPx1 15, GPx2 38, GPx3 19, GPx4 1, Selo 32, Seli 13, Sepp1 8, Selk 66, Sepx1 5, Sel-15 2, Sels 52, Selu 13, Selh 9, and SPS2 6 was significantly lower (P < 0.05) on day 35 in the L group compared with the levels observed in the control group (Fig. 1c). The percent expression of the selenoproteins Txnrd1 27, Txnrd2 4, Txnrd3 7, Dio1 8, Dio2 31, Dio3 9, GPx1 16, GPx2 17, GPx3 14, GPx4 35, Selo 10, Seli 50, Sepp1 1, Selk 19, Sepx1 34, Sel-15 15, Sels 41, Selu 12, Selh 9, and SPS2 20 was significantly lower (P < 0.05) on day 45 in the L group compared with the control group (Fig. 1d). The percent expression of the selenoproteins Txnrd1 38, Txnrd2 48, Txnrd3 50, Dio1 30, Dio2 50, Dio3 43, GPx1 7, GPx2 40, GPx3 6, GPx4 50, Selo 32, Seli 47, Sepp1 29, Selk 41, Sepx1 33, Sel-15 45, Sels 35, Selu 26, Selh 35, and SPS2 28 was significantly lower (P < 0.05) on day 55 in the L group compared with the levels obtained in the control group (Fig. 1e). Our results show that selenoprotein expression levels were lower in the L group at all of the investigated time points compared with the levels found in the control group.
Fig. 1.
Effect of Se deficiency on selenoprotein mRNA expression levels in the chicken bursa of Fabricius. a mRNA expression levels of selenoproteins on day 15. b mRNA expression levels of selenoproteins on day 25. c mRNA expression levels of selenoproteins on day 35. d mRNA expression levels of selenoproteins on day 45. e mRNA expression levels of selenoproteins on day 55. *P < 0.05 indicates significant differences between the control group and the L group at the same time point. Each value indicates the mean ± SD, n = 3
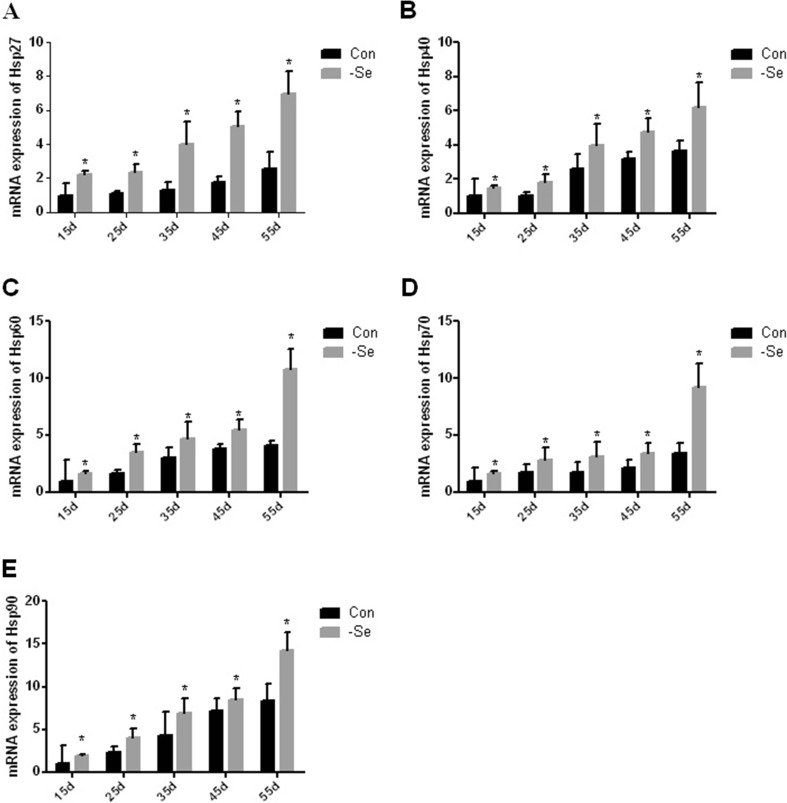
Effect of Se deficiency on Hsp mRNA levels in the chicken bursa of Fabricius
To determine the effects of Se deficiency on the Hsp mRNA levels in the chicken bursa of Fabricius, we analyzed Hsp expression using real-time qPCR. The expression levels of the Hsps are shown in Fig. 2a, b, c, d, e. The mRNA expression levels of Hsp27 were significantly higher (P < 0.05) on day 15, 25, 35, 45, and 55 by 220, 218, 303, 280, and 271 %, respectively, in the L group compared with the control group (Fig. 2a). The mRNA expression levels of Hsp40 were significantly higher (P < 0.05) on day 15, 25, 35, 45, and 55 by 152, 182, 150, 149, and 170 %, respectively, in the L group compared with the control group (Fig. 2b). The mRNA expression levels of Hsp60 were significantly higher (P < 0.05) on day 15, 25, 35, 45, and 55 by 162, 210, 158, 144, and 264 %, respectively, in the L group compared with the control group (Fig. 2c). The mRNA expression levels of Hsp70 were significantly higher (P < 0.05) on day 15, 25, 35, 45, and 55 by 153, 165, 175, 159, and 273 %, respectively, in the L group compared with the control group (Fig. 2d). The mRNA expression levels of Hsp90 were significantly higher (P < 0.05) on day 15, 25, 35, 45, and 55 by 195, 171, 160, 118, and 170 %, respectively, in the L group compared with the control group (Fig. 2e). These results indicate that the mRNA expression levels of many Hsps were higher in the L group, which may cause an improvement in the protective effect in the chicken bursa of Fabricius.
Fig. 2.
Effect of Se deficiency on the mRNA expression levels of Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 in the chicken bursa of Fabricius. a mRNA expression levels of Hsp27. b mRNA expression levels of Hsp40. c mRNA expression levels of Hsp60. d mRNA expression levels of Hsp70. e mRNA expression levels of Hsp90. *P < 0.05 indicates significant differences between the control group and the L group at the same time point. Each value represents the mean ± SD, n = 3
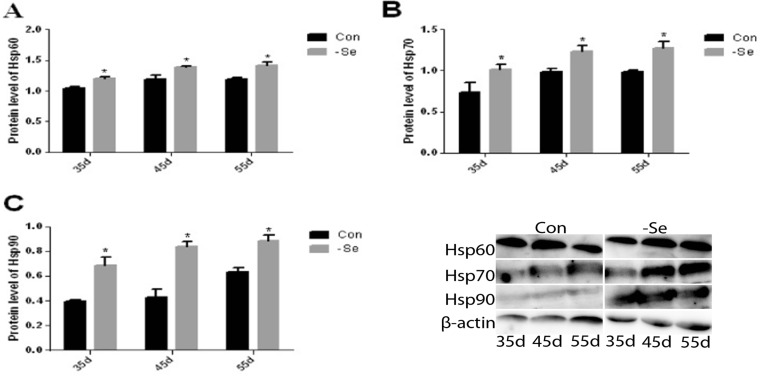
Effect of Se deficiency on Hsps protein levels in the chicken bursa of Fabricius
To assess the effects of Se deficiency on the Hsp protein levels in the chicken bursa of Fabricius, we used the western blot technique. The expression levels of the Hsps are shown in Fig. 3a, b, c. The protein expression level of Hsp60 was significantly higher (P < 0.05) on day 35, 45, and 55 by 116, 117, and 118 %, respectively, in the L group compared with the control group (Fig. 3a). The protein expression level of Hsp70 was significantly higher (P < 0.05) on day 35, 45, and 55 by 137, 129, and 125 %, respectively, in the L group compared with the control group (Fig. 3b). The protein expression level of Hsp90 was significantly higher (P < 0.05) on day 35, 45, and 55 by 173, 197, and 139 %, respectively, in the L group compared with the control group. These results show that Hsp protein levels were higher in the L group, which may also have a protective effect in the chicken bursa of Fabricius.
Fig. 3.
Effect of Se deficiency on the protein expression levels of Hsp60, Hsp70, and Hsp90 in the chicken bursa of Fabricius. a Protein expression levels of Hsp60. b Proteins expression levels of Hsp70. c Protein expression levels of Hsp90. *P < 0.05 indicates significant differences between the control group and the L group at the same time point. Each value represents the mean ± SD, n = 3
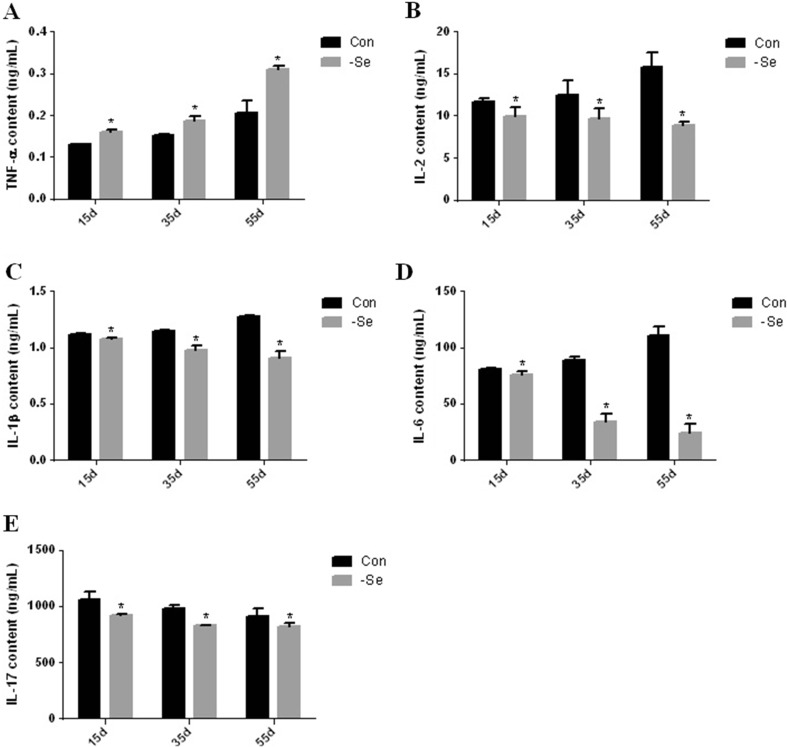
Effect of Se deficiency on cytokines in the chicken bursa of Fabricius
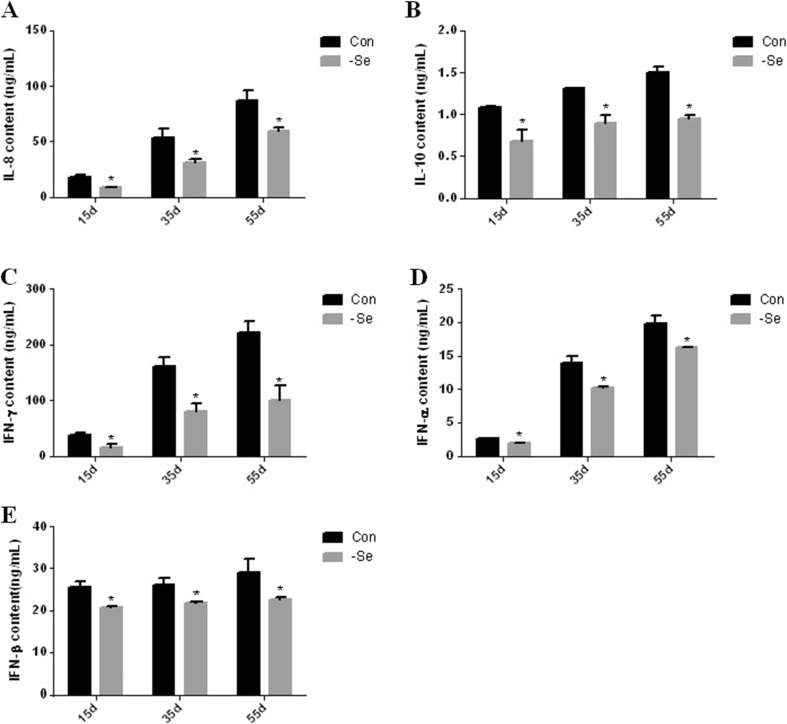
To highlight the effects of Se deficiency on the cytokine contents in the chicken bursa of Fabricius, we detected the cytokine contents by ELISA. The cytokine contents are shown Figs. 4a, b, c, d, e and 5a, b, c, d, e. TNF-α expression was significantly higher (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 122, 124, and 150 %, respectively (Fig. 4a). IL-2 expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 85, 78, and 56 %, respectively (Fig. 4b). The IL-1β expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 96, 85, and 70 %, respectively (Fig. 4c). IL-6 expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 94, 38, and 21 %, respectively (Fig. 4d). IL-17 expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 86, 84, and 89 %, respectively (Fig. 4e). IL-8 expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 49, 58, and 68 %, respectively (Fig. 5a). IL-10 expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 92, 68, and 48 %, respectively (Fig. 5b). IFN-γ expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 41, 49, and 45 %, respectively (Fig. 5c). IFN-α expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 74, 73, and 82 %, respectively (Fig. 5d). IFN-β expression was significantly lower (P < 0.05) in the L group compared with the control group on day 15, 35, and 55 by 87, 82, and 70 %, respectively (Fig. 5e).
Fig. 4.
Effect of Se deficiency on TNF-α, IL-2, IL-1β, IL-6, and IL-17 expression in the chicken bursa of Fabricius. a TNF-α expression. b IL-2 expression. c IL-1β expression. d IL-6 expression. e IL-17 expression. *P < 0.05 indicates significant differences between the control group and the L group at the same time point. Each value indicates the mean ± SD, n = 3
Fig. 5.
Effect of Se deficiency on IL-8, IL-10, IFN-γ, INF-α, and IFN-β expression in the chicken bursa of Fabricius. a IL-8 expression. b IL-10 expression. c IFN-γ expression. d INF-α expression. e IFN-β expression. *P < 0.05 indicates significant differences between the control group and the L group at the same time point. Each value indicates the mean ± SD, n = 3
Discussion
Se is incorporated into selenoproteins as the amino acid selenocysteine, and selenoproteins play important roles in animals and chickens (Kryukov et al. 2003). Previous studies have shown that different selenoproteins have different characteristics and functional activities in the body. The selenoproteins belonging to the GPx family (GPx1–GPx6), Txnrd family (Txnrd1, Txnrd2, and Txnrd3), and the Dio family (Dio1, Dio2, and Dio3) play vital roles in redox regulation, antioxidant defense, the conversion of T4 to T3, and immune regulation in human diseases (Bellinger et al. 2009; Pappas et al. 2008). Other selenoproteins have also been identified in mammals and chickens, such as SPS2, Seli, Selk, Selm, Sel-15, Sepw1, Selh, Selv, Sepn1, Selo, Sepp1, Seli, Sels, and Selt (Mariotti et al. 2012; Novoselov et al. 2007). SPS2 selenophosphate and Sepp1 transport Se in the body (Pappas et al. 2008). Sel-15, Sels, and Selk control protein folding in the endoplasmic reticulum (ER; Du et al. 2010a, 2010b). Sepw1, Selk, Selm, Sepn1, and Selt work to regulate intracellular calcium (Lescure et al. 2009; Reeves and Hoffmann 2009). The normal expression of selenoproteins depends on the Se level, and their expression levels decrease with Se deficiency. In the muscle and liver of broiler chickens, the expression levels of GPx1, GPx4, Sepw1, Sepn1, Sepp1, Selo, and Selk are lower due to Se deficiency (Huang et al. 2011). We found that Se deficiency decreased the Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, GPx1, GPx2, GPx3, GPx4, Sepp1, Selo, Sel-15, Seli, Sepx1, Sels, Selk, and SPS2 mRNA levels in the chicken bursa of Fabricius. A study of the chicken liver showed that Se regulates the selenoprotein and mRNA expression levels of Txnrd1, Txnrd2, Txnrd3, GPx1, GPx2, GPx3, GPx4, Sepn1, Sepp1, Selo, Sepx1, Selu, Dio1, Dio2, SPS2, Selm, SelPb, Sel-15, and Sels, which were found to be lower under Se-deficiency conditions (Liu et al. 2014b). Additionally, HepG2 and H4IIE cells have shown that GPxs (Hill et al. 1992) and Txnrd expression is lower with a Se-deficient diet (Hill et al. 1997). Se deficiency decreases the mRNA levels of Dio1, Dio2, and Dio3 in chickens, which causes inhibition of the conversion of T4 to T3 and decreases the levels of critical metabolic enzymes of the thyroid hormones (Lin et al. 2014). Se deficiency causes a decrease in metabolic energy and kidney tissue damage due to a decrease in the Selo, Selk, Sepn, Sel-15, and Sepx1 expression levels (Sunde et al. 2009). In chicken, Seli and Selu are decreased, which is associated with an antioxidant function (Liang et al. 2014). Se deficiency also causes muscle atrophy in chicken via a decrease in the expression of Selh, Selu, SPS2, and other selenoproteins. We also determined that the Seli, Selu, Selh, and SPS2 expression levels are decreased in this study.
Hsps are stress-responsive proteins that are found in different species. Hsps are named and classified according to their molecular mass, and these proteins include Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 (Lindquist and Craig 1988). Hsps help maintain the metabolic and structural integrity of cells and induce a protective response to external stresses (Parsell and Lindquist 1993). Hsp27 is a marker of cell damage in a pediatric population on chronic dialysis (Musial and Zwolinska 2012). Strengthening of the cytoskeleton and an increased tolerance to injury induced by stress depend on variations in the expression of Hsp27 in cells (Guay et al. 1997). Hsp27 helps organize the cytoskeleton and stabilize intracellular actin filaments (Hino et al. 2000). Hsp27 also protects cardiac myocytes against ischemic injury. Hsp40 is a eukaryotic homologue of a bacterial protein, which, with Hsp70, facilitates protein folding (Li et al. 2009). Cold and oxidative stress causes higher levels of Hsp40 and Hsp70 in chicken hearts (Zhao et al. 2014a). Hsp60 is higher under heat stress with an increased requirement of ATP (Koelkebeck and Odom 1995). Additionally, Hsp60 is also higher during heart failure (Lin et al. 2007; Wang et al. 2010). Hsp70 elicits rapid calcium flux, binds with high affinity in the plasma membrane, and activates nuclear factor (NF)-κB (Asea et al. 2000). Dietary Se deficiency causes oxidative stress in the liver due to higher Hsp70 mRNA expression (Kaushal and Bansal 2009). Hsps protects the organism from metal stress through their anti-stress response (Chen et al. 2014; Zhang et al. 2011). Hsp90 is involved in the antioxidation response in Pacific abalone (H. discus hannai) affected by lower Se levels (Zhang et al. 2011). We determined that Se deficiency causes an increase in the mRNA levels of Hsps (Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90) and protein levels of Hsps (Hsp60, Hsp70, and Hsp90) in the chicken bursa of Fabricius. Hsps help maintain the metabolic and structural integrity of cells and play a role in the protective response to external stresses. Se deficiency was found to increase Hsp expression levels, which helps protect the cells from damage in the chicken bursa of Fabricius. The increase in the mRNA expression levels of Hsps (Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90) plays an important role in the protective response to oxidative stress in Se-deficient livers (Liu et al. 2015). Hsp27, Hsp70, and Hsp90 also protect cardiac muscle cells from transport stress. When exposed to cold stress, the Hsps (Hsp90, Hsp70, Hsp60, Hsp40, and Hsp27) expression levels were increased, which protects immune organs (Zhao et al. 2014a).
Se is required for the proper functioning of the immune system, particularly macrophages, T lymphocytes, and other immune cells (Hoffmann 2007; Kiremidjian-Schumacher and Roy 1998). A study of blood lymphocytes of streptozotocin-induced diabetic C57BL mice showed that supplementation with Se and other micronutrients decreases the expression of TNF-α (Chang et al. 2005). We found that Se deficiency increased the TNF-α level in the chicken bursa of Fabricius. This increase in TNF-α under Se deficiency may cause tissue damage in the chicken bursa of Fabricius. A previous study of the heart showed that the NF-κB and TNF-α mRNA expression levels are significantly higher under stress (Zhao et al. 2013a). IL-2 is known to participate in the growth, activation and function of immune effectors cells. IL-2 expression is high with dietary supplementation of Se, and Se was found to effectively restore the age-related decline of IL-2 expression in lymphocyte proliferative responses to mitogen stimulation in mice (Roy et al. 1995). IL-1β is an important mediator of the inflammatory response and is involved in a variety of cellular processes, including apoptosis, proliferation and differentiation (Brigelius-Flohe et al. 2004). We found that Se deficiency caused a decrease in IL-2 and IL-1β expression in the chicken bursa of Fabricius. Se deficiency causes damage to immune organs via higher TNF-α and lower IL-2 and IL-1β expression (Zhang et al. 2012). The expression levels of IL-6 are considered the primary marker of disease severity, and the upregulation of IL-6 decreases pathogenicity. A deficiency in IL-6 causes severe disease and produces high levels of TNF-α (Horai et al. 2000). We found that the IL-6 expression level was lower under Se-deficient conditions, which may also be an indication of the inflammation observed in the chicken bursa of Fabricius. IL-17 is associated with the mucosal surfaces of the intestinal tract and lungs (Curtis and Way 2009; Maloy 2008). IL-17 expression is lower in the intestine, which causes intestinal injury in broiler chicken under cold stress (Zhao et al. 2013b). We determined that IL-17 expression was also lower in chickens with Se deficiency, which may indicate a risk of certain damage in the chicken bursa of Fabricius. IL-8 functions as a mitogen of fibroblasts and exhibits chemotactic functions toward peripheral blood mononuclear cells (Wuyts et al. 1998). IL-8 expression is high in the chicken intestine after treatment with Eimeria spp. (Cheeseman et al. 2008; Hong et al. 2006). Additionally, unvaccinated chickens challenged with infectious bursal diseases show an upregulation of IL-8 and other cytokines (Lee et al. 2014). We found that IL-8 was lower in Se-deficient chickens. It has been reported that interleukin IL-10 production is higher under chronic cold stress conditions in BALB/c mice (Sesti-Costa et al. 2012). We found that IL-10 was lower in response to Se deficiency in the chicken bursa of Fabricius. IFN-γ is essential for the protection of numerous intracellular microorganisms. It is also a major cytokine in the pathogenesis of several autoimmune diseases. IFN-γ is primarily produced by Th1 cells, and it is an indicator of immunity and rapidly activated host macrophages, which results in the enhancement of the immune response (Nakamura et al. 1984). We observed that IFN-γ was lower in the chicken bursa of Fabricius. Our results are consistent with those reported by Liu et al., who showed that IFN-γ was lower in the spleen of pigeons exposed to avermectin (Liu et al. 2014a). Another study showed that the downregulation of IFN-γ causes a decrease in the immune function of chickens (Fleshner et al. 1998). We observed that IFN-γ was lower in the bursa of Fabricius of chickens fed a Se-deficient diet, which exhibited poor immune function and may cause bursal injury. A previous study showed that IFN-α participates in the correct induction of NK cells against infectious agents and tumor cells (Terme et al. 2008). IFN-α and IFN-β expression was higher at the site of infection and also aided the production of secondary cytokines. This cytokine production implies the activation of the host response to infection through both direct and indirect mechanisms (Dinarello 2009). A previous study in the chicken thymus showed a relatively low level of IFN-α and IFN-β expression in chickens fed a Se-deficient diet. We observed a low expression of IFN-α and IFN-β in the bursa of Fabricius in chickens with Se deficiency.
Conclusion
Se deficiency caused defects in the chicken bursa of Fabricius. We found that immunosuppression was accompanied by a downregulation in selenoprotein expression and an upregulation of Hsp expression. This study provides evidence supporting the essential involvement of selenoproteins and the protective role of Hsps in the bursa of Fabricius of chickens with Se deficiency.
Acknowledgments
The authors thank the members of the Veterinary Internal Medicine Laboratory, College of Veterinary Medicine, Northeast Agriculture University for their help with chicken bursa of Fabricius sample collection.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 31472161).
Conflict of interest
There is no conflict of interest.
References
- Al-Aqil A, Zulkifli I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult Sci. 2009;88:1358–1364. doi: 10.3382/ps.2008-00554. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Ausiello CM, Palazzo R, Spensieri F, et al. 60-kDa heat shock protein of Chlamydia pneumoniae is a target of T-cell immune response. J Biol Regul Homeost Agents. 2005;19:136–140. [PubMed] [Google Scholar]
- Bellinger FP, Raman AV, Reeves MA, et al. Regulation and function of selenoproteins in human disease. Biochem J. 2009;422:11–22. doi: 10.1042/BJ20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Wilhelm A, Jugert C, et al. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect Immun. 2007;75:5993–6007. doi: 10.1128/IAI.00695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Banning A, Kny M, et al. Redox events in interleukin-1 signaling. Arch Biochem Biophys. 2004;423:66–73. doi: 10.1016/j.abb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Broome CS, McArdle F, Kyle JA, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80:154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- Chang Y, Piao SL, Gao S, et al. Regulatory effects of micronutrient complex on the expression of Th1 and Th2 cytokines in diabetic C57BL mice. Wei Sheng Yan Jiu. 2005;34:64–66. [PubMed] [Google Scholar]
- Cheeseman JH, Levy NA, Kaiser P, et al. Salmonella Enteritidis-induced alteration of inflammatory CXCL chemokine messenger-RNA expression and histologic changes in the ceca of infected chicks. Avian Dis. 2008;52:229–234. doi: 10.1637/8156-102307-Reg.1. [DOI] [PubMed] [Google Scholar]
- Chen X, Yao H, Yao L, et al. Selenium deficiency influences the gene expressions of heat shock proteins and nitric oxide levels in neutrophils of broilers. Biol Trace Elem Res. 2014;161:334–340. doi: 10.1007/s12011-014-0150-1. [DOI] [PubMed] [Google Scholar]
- Cooper MD, Raymond DA, Peterson RD, et al. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966;123:75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci S, Kutluhan S, Naziroglu M, et al. Effects of selenium and topiramate on cytosolic Ca(2+) influx and oxidative stress in neuronal PC12 cells. Neurochem Res. 2013;38:90–97. doi: 10.1007/s11064-012-0893-z. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Du S, Liu H, Huang K. Influence of SelS gene silence on beta-Mercaptoethanol-mediated endoplasmic reticulum stress and cell apoptosis in HepG2 cells. Biochim Biophys Acta. 2010;1800:511–517. doi: 10.1016/j.bbagen.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Du S, Zhou J, Jia Y, et al. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys. 2010;502:137–143. doi: 10.1016/j.abb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Nguyen KT, Cotter CS, et al. Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity. Am J Physiol. 1998;275:R870–878. doi: 10.1152/ajpregu.1998.275.3.R870. [DOI] [PubMed] [Google Scholar]
- Ghazi Harsini S, Habibiyan M, Moeini MM, et al. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res. 2012;148:322–330. doi: 10.1007/s12011-012-9374-0. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, et al. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Guo S, Wharton W, Moseley P, et al. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones. 2007;12:245–254. doi: 10.1379/CSC-265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habich C, Burkart V. Heat shock protein 60: regulatory role on innate immune cells. Cell Mol Life Sci. 2007;64:742–751. doi: 10.1007/s00018-007-6413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KE, Lyons PR, Burk RF. Differential regulation of rat liver selenoprotein mRNAs in selenium deficiency. Biochem Biophys Res Commun. 1992;185:260–263. doi: 10.1016/S0006-291X(05)80984-2. [DOI] [PubMed] [Google Scholar]
- Hill KE, McCollum GW, Boeglin ME, et al. Thioredoxin reductase activity is decreased by selenium deficiency. Biochem Biophys Res Commun. 1997;234:293–295. doi: 10.1006/bbrc.1997.6618. [DOI] [PubMed] [Google Scholar]
- Hino M, Kurogi K, Okubo MA, et al. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun. 2000;271:164–169. doi: 10.1006/bbrc.2000.2553. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR. Mechanisms by which selenium influences immune responses. Arch Immunol Ther Exp (Warsz) 2007;55:289–297. doi: 10.1007/s00005-007-0036-4. [DOI] [PubMed] [Google Scholar]
- Hong YH, Lillehoj HS, Lee SH, et al. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol. 2006;114:209–223. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Horai R, Saijo S, Tanioka H, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JQ, Li DL, Zhao H, et al. The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common Selenoprotein genes in liver and muscle. J Nutr. 2011;141:1605–1610. doi: 10.3945/jn.111.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Bansal MP. Diminished reproductive potential of male mice in response to selenium-induced oxidative stress: involvement of HSP70, HSP70-2, and MSJ-1. J Biochem Mol Toxicol. 2009;23:125–136. doi: 10.1002/jbt.20276. [DOI] [PubMed] [Google Scholar]
- Kiremidjian-Schumacher L, Roy M. Selenium and immune function. Z Ernahrungswiss. 1998;37(Suppl 1):50–56. [PubMed] [Google Scholar]
- Koelkebeck KW, Odom TW. Laying hen responses to acute heat stress and carbon dioxide supplementation: II. Changes in plasma enzymes, metabolites and electrolytes. Comp Biochem Physiol A Physiol. 1995;112:119–122. doi: 10.1016/0300-9629(95)00081-H. [DOI] [PubMed] [Google Scholar]
- Kohrle J, Jakob F, Contempre B, et al. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Lee CC, Kim BS, Wu CC, et al. Bursal transcriptome of chickens protected by DNA vaccination versus those challenged with infectious bursal disease virus. Arch Virol. 2014;160:69–80. doi: 10.1007/s00705-014-2232-y. [DOI] [PubMed] [Google Scholar]
- Lei C, Niu X, Ma X, et al. Is selenium deficiency really the cause of Keshan disease? Environ Geochem Health. 2011;33:183–188. doi: 10.1007/s10653-010-9331-9. [DOI] [PubMed] [Google Scholar]
- Lescure A, Rederstorff M, Krol A, et al. Selenoprotein function and muscle disease. Biochim Biophys Acta. 2009;1790:1569–1574. doi: 10.1016/j.bbagen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Li J, Qian X, Sha B. Heat shock protein 40: structural studies and their functional implications. Protein Pept Lett. 2009;16:606–612. doi: 10.2174/092986609788490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Lin SL, Wang CW, et al. Effect of selenium on selenoprotein expression in the adipose tissue of chickens. Biol Trace Elem Res. 2014;160:41–48. doi: 10.1007/s12011-014-0024-6. [DOI] [PubMed] [Google Scholar]
- Lin L, Kim SC, Wang Y, et al. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238–2247. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- Lin SL, Wang CW, Tan SR, et al. Selenium deficiency inhibits the conversion of thyroidal thyroxine (T4) to triiodothyronine (T3) in chicken thyroids. Biol Trace Elem Res. 2014;161:263–271. doi: 10.1007/s12011-014-0083-8. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu C, Li M, Cao Y, et al. Effects of avermectin on immune function and oxidative stress in the pigeon spleen. Chem Biol Interact. 2014;210:43–50. doi: 10.1016/j.cbi.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Liu CP, Fu J, Lin SL, et al. Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res. 2014;159:192–198. doi: 10.1007/s12011-014-0005-9. [DOI] [PubMed] [Google Scholar]
- Liu LL, He JH, Xie HB, et al. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Liu CP, Fu J, Xu FP, et al. The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. Biometals. 2015;28:163–173. doi: 10.1007/s10534-014-9812-x. [DOI] [PubMed] [Google Scholar]
- Maloy KJ. The Interleukin-23/Interleukin-17 axis in intestinal inflammation. J Intern Med. 2008;263:584–590. doi: 10.1111/j.1365-2796.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- Mariotti M, Ridge PG, Zhang Y et al (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One 7 [DOI] [PMC free article] [PubMed]
- Musial K, Zwolinska D. Hsp27 as a marker of cell damage in children on chronic dialysis. Cell Stress Chaperones. 2012;17:675–682. doi: 10.1007/s12192-012-0339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Manser T, Pearson GD, et al. Effect of IFN-gamma on the immune response in vivo and on gene expression in vitro. Nature. 1984;307:381–382. doi: 10.1038/307381a0. [DOI] [PubMed] [Google Scholar]
- Novoselov SV, Kryukov GV, Xu XM, et al. Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. J Biol Chem. 2007;282:11960–11968. doi: 10.1074/jbc.M701605200. [DOI] [PubMed] [Google Scholar]
- Pappas AC, Zoidis E, Surai PF, et al. Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:361–372. doi: 10.1016/j.cbpb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Patrick L. Nutrients and HIV: part one—beta carotene and selenium. Altern Med Rev. 1999;4:403–413. [PubMed] [Google Scholar]
- Peng X, Cui Y, Cui W, et al. The cell cycle arrest and apoptosis of bursa of Fabricius induced by low selenium in chickens. Biol Trace Elem Res. 2011;139:32–40. doi: 10.1007/s12011-010-8639-8. [DOI] [PubMed] [Google Scholar]
- Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Kiremidjian-Schumacher L, Wishe HI, et al. Supplementation with selenium restores age-related decline in immune cell function. Proc Soc Exp Biol Med. 1995;209:369–375. doi: 10.3181/00379727-209-43909. [DOI] [PubMed] [Google Scholar]
- Sesti-Costa R, Ignacchiti MD, Chedraoui-Silva S, et al. Chronic cold stress in mice induces a regulatory phenotype in macrophages: correlation with increased 11beta-hydroxysteroid dehydrogenase expression. Brain Behav Immun. 2012;26:50–60. doi: 10.1016/j.bbi.2011.07.234. [DOI] [PubMed] [Google Scholar]
- Shaughnessy RG, Meade KG, Cahalane S, et al. Innate immune gene expression differentiates the early avian intestinal response between Salmonella and Campylobacter. Vet Immunol Immunopathol. 2009;132:191–198. doi: 10.1016/j.vetimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Stadtman TC. Selenium biochemistry. Mammalian selenoenzymes. Ann N Y Acad Sci. 2000;899:399–402. doi: 10.1111/j.1749-6632.2000.tb06203.x. [DOI] [PubMed] [Google Scholar]
- Sunde RA, Raines AM, Barnes KM, et al. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep. 2009;29:329–338. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terme M, Ullrich E, Delahaye NF, et al. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- Van Eden W, Wick G, Albani S, et al. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen L, Hagiwara N, et al. Regulation of heat shock protein 60 and 72 expression in the failing heart. J Mol Cell Cardiol. 2010;48:360–366. doi: 10.1016/j.yjmcc.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Mann J, Walsh M et al (2014) Quiescent hepatic stellate cells functionally contribute to the hepatic innate immune response via TLR3. PLoS One 9 [DOI] [PMC free article] [PubMed]
- Wu Y, Pei Y, Qin Y. Developmental expression of heat shock proteins 60, 70, 90, and A2 in rabbit testis. Cell Tissue Res. 2011;344:355–363. doi: 10.1007/s00441-011-1151-4. [DOI] [PubMed] [Google Scholar]
- Wuyts A, Proost P, Lenaerts JP, et al. Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur J Biochem. 1998;255:67–73. doi: 10.1046/j.1432-1327.1998.2550067.x. [DOI] [PubMed] [Google Scholar]
- Xu SW, Yao HD, Zhang J, et al. The oxidative damage and disbalance of calcium homeostasis in brain of chicken induced by selenium deficiency. Biol Trace Elem Res. 2013;151:225–233. doi: 10.1007/s12011-012-9552-0. [DOI] [PubMed] [Google Scholar]
- Yao HD, Wu Q, Zhang ZW, et al. Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of Se-deficient chicks. J Nutr. 2013;143:613–619. doi: 10.3945/jn.112.172395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, Liu C, Yang ZJ, et al. Prediction of selenoprotein T structure and its response to selenium deficiency in chicken immune organs. Biol Trace Elem Res. 2014;160:222–231. doi: 10.1007/s12011-014-0049-x. [DOI] [PubMed] [Google Scholar]
- Yu J, Bao E, Yan J, et al. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones. 2008;13:327–335. doi: 10.1007/s12192-008-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhou X, Wang X, et al. Characterization of two heat shock proteins (Hsp70/Hsc70) from grass carp (Ctenopharyngodon idella): evidence for their differential gene expression, protein synthesis and secretion in LPS-challenged peripheral blood lymphocytes. Comp Biochem Physiol B Biochem Mol Biol. 2011;159:109–114. doi: 10.1016/j.cbpb.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Wang QH, Zhang JL, et al. Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res. 2012;149:352–361. doi: 10.1007/s12011-012-9439-0. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Zhang ZW, Wang C, et al. The role of heat shock proteins in inflammatory injury induced by cold stress in chicken hearts. Cell Stress Chaperones. 2013;18:773–783. doi: 10.1007/s12192-013-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FQ, Zhang ZW, Yao HD, et al. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res Vet Sci. 2013;95:146–155. doi: 10.1016/j.rvsc.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Zhang ZW, Qu JP, et al. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones. 2014;19:635–648. doi: 10.1007/s12192-013-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yao H, Fan R, et al. Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res. 2014;161:341–349. doi: 10.1007/s12011-014-0139-9. [DOI] [PubMed] [Google Scholar]