Abstract
Social stressors evolving from individual and population interactions produce stress reactions in many organisms (including humans), influencing homeostasis, altering the activity of the immunological system, and thus leading to various pathological states including cancer and their progression. The present study sought to validate the effectiveness of chronic unpredictable stress (CUS) in cancer promotion and to assess oxidative stress outcomes in terms of various in vivo biochemical parameters, oxidative stress markers, DNA damage, and the development of skin tumors in Swiss albino mice. Animals were randomized into different groups based on their exposure to CUS alone, 7,12-dimethylbenz(a)anthracene (DMBA) alone (topical), and DMBA-12-O-tetradecanoylphorbol-13-acetate (TPA) (topical) and exposure to CUS prior to DMBA or DMBA-TPA treatments and sacrificed after 16 weeks of treatment. Prior exposure to CUS significantly increased the pro-oxidant effect of carcinogen, depicted by compromised levels of antioxidants in the circulation and skin, accompanied by enhanced lipid peroxidation, plasma corticosterone, and marker enzymes as compared to DMBA-alone or DMBA-TPA treatments. DNA damage results corroborated the above biochemical outcomes. Also, the development of skin tumors (in terms of their incidence, tumor yield, and tumor burden) in mice in the presence and absence of stress further strongly supported our above biochemical measurements. CUS may work as a promoter of carcinogenesis by enhancing the pro-oxidant potential of carcinogens. Further studies may be aimed at the development of interventions for disease prevention by identifying the relations between psychological factors and DNA damage.
Keywords: Antioxidants; Corticosterone; Chronic unpredictable stress; 7,12-Dimethylbenz(a)anthracene; DNA damage; Skin tumors
Introduction
The influence of stress-inducing conditions on cancer development has been the subject of investigation, both at clinical and experimental levels (Pradhan and Prabhati 1974; Sklar and Anisman 1979). Carcinogenesis may either be inhibited (Steplewski et al. 1985, 1987) or be stimulated (Steplewski et al. 1985; Shavit et al. 1983) depending on the time relationships between the activity of the carcinogenic factor and that of the stressor, as well as the stressor type. Physical and psychological stress stimulates numerous pathways leading to increased production of reactive oxygen species (ROS), adding to the oxidant burden associated with normal aerobic metabolism, and consequently damaging cellular macromolecules (lipids, proteins, and DNA) (Liu and Mori 1999).
The skin is directly and frequently exposed to sunlight and is always in contact with oxygen, resulting in the production of ROS, implying that the skin is always prone to be attacked by ROS (Garmyn and Degreef 1997). Skin carcinogenesis is divisible into two main stages: initiation and promotion. Initiation involves the conversion of some basal epidermal cells into latent neoplastic cells. Promotion involves selective expansion of initiated cells into benign lesions, which is accomplished by repeated applications of promoter for extended periods of time (Kulesz-Martin 1997). ROS including superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (·OH), and singlet oxygen have been shown to be involved in both initiation and promotion stages of carcinogenesis. Indeed, convincing evidences indicate that ROS acts as an endogenous class of carcinogens by triggering the mutation of the cells (Guyton and Kensler 1993; Feig et al. 1994). Moreover, oxidative stress can stimulate the expansion of mutated cell clones by modulating genes related to proliferation and triggering redox-responsive signaling cascades (Droge 2002; Camhi et al. 1998). The effect of ROS on mutation is dose-dependent and in a dynamic equilibrium with antioxidant defenses and cellular repair systems (Dreher and Junod 1996).
Several chemical carcinogens seem to induce oxidative stress either directly or indirectly through modification of cellular antioxidant defense mechanisms. Dimethylbenz(a)anthracene (DMBA), a member of the polycyclic aromatic hydrocarbons, is present in the environment as a product of incomplete combustion of complex hydrocarbons. It is also present in some amount in cigarette smoke. Being a well-known cytotoxic, carcinogenic, mutagenic, and immunosuppressive agent, it is often used as a tumor initiator in animal models (Miyata et al. 2001; Wijnhoven et al. 2001). DMBA exposure results in a pronounced mutagenic response in several in vivo and in vitro mutation assay systems (Donovan et al. 2004; Wijnhoven et al. 2001; Arciszewska et al. 1982). Being an indirect carcinogen, DMBA requires metabolic activation by cytochrome P450 enzymes to acquire carcinogenicity (Conney 1982). This multistep activation cascade suggests that a number of intervening conditions or predispositions may affect the extent to which environmental carcinogens cause abnormal cell growth. This study examined the effect of one such mediator-chronic unpredictable stress, to mimic the daily stressful situations experienced by humans.
The present study was undertaken to address the effectiveness of chronic unpredictable stress (CUS) in the induction of tumor promotion and to assess oxidative stress outcomes in terms of various in vivo biochemical parameters, oxidative stress markers, and DNA damage in the skin and circulation of Swiss albino mice throughout a two-stage carcinogenesis protocol. Development of skin papillomas by various treatments was also monitored and used as a marker of carcinogenesis.
Materials and methods
Chemicals
DMBA, 12-O-tetradecanoylphorbol-13-acetate (TPA), and dexamethasone were purchased from Sigma-Aldrich Chemical Company, USA. All the other chemicals were of analytical grade, purchased from Qualigens, Fine Chemicals, and HiMedia Laboratories Pvt. Ltd., Mumbai, India.
Animals
Adult (6–8 weeks) male Swiss albino mice (45 ± 5 g) obtained from Jamia Hamdard University, Delhi, India, were used for the study. They were kept and treated under humane and hygienic conditions (25 ± 5 °C with 12-h day/night cycle). Animals were allowed to acclimatize for 10 days prior to the treatment on standard pellet mice diet (Ashirwad Industries, Chandigarh, India) and fresh drinking water available ad libitum. All the experimental protocols adhered to the guidelines of the Animal Welfare Committee of the University as per the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, India) norms.
CUS procedure
The CUS protocol was carried out according to the published procedures (Katz et al. 1981; Willner et al. 1987) with slight modifications as discussed in our earlier studies (Suhail et al. 2011). The CUS sequence consisted of different types of stressors presented randomly, two per day, over a period of 15 days as outlined in Table 1. All procedures were carried out in isolated rooms adjacent to the housing room, requiring minimal handling or transport of the mice. After each stressor, animals were placed in clean cages with fresh bedding and returned to the housing facility. Control animals were left undisturbed until the time of the experiment with the exception of daily cleaning of the cages and weekly weighing.
Table 1.
Chronic unpredictable stress procedure
| Days | Stressor | Time |
|---|---|---|
| Day 1 | 1-h restraint stress | 08:00 a.m. |
| 30-min cold room (4 °C) | 11: 00 p.m. | |
| Day 2 | 1-h shaking/crowding | 11:00 a.m. |
| 5-min cold water swim | 03:00 p.m. | |
| Day 3 | 4-h wet bedding | 09:00 a.m. |
| Lights on overnight | 06:00 p.m. | |
| Day 4 | 3-h high density housing | 10:00 a.m. |
| 5-min warm water swim | 06:00 p.m. | |
| Day 5 | 2-h restraint stress | 08:00 a.m. |
| 30-min cold room (4 °C) | 03:00 p.m. | |
| Day 6 | 6-h isolation | 10:00 a.m. |
| Lights on overnight | 07:00 p.m. | |
| Day 7 | 5-min cold water swim | 09:00 a.m. |
| 16-h food and water deprivation | 03:00 p.m. | |
| Day 8 | 2-h restraint stress | 10:00 a.m. |
| 1-h shaking/crowding | 02:00 p.m. | |
| Day 9 | 4-h high-density housing | 08:00 a.m. |
| Lights on overnight | 07:00 p.m. | |
| Day 10 | 4-h wet bedding | 09:00 a.m. |
| 16-h food and water deprivation | 04:00 p.m. | |
| Day 11 | 3-h restraint stress | 11:00 a.m. |
| 2-h isolation | 05:00 p.m. | |
| Day 12 | 5-min cold water swim | 08:00 a.m. |
| 1-h shaking/crowding | 11:00 a.m. | |
| Day 13 | 10-min tail pinch in restrainer | 09:00 a.m. |
| 1-h shaking/crowing | 04:00 p.m. | |
| Day 14 | 2-h restraint stress | 11:00 a.m. |
| 3-h high-density housing | 04:00 p.m. | |
| Day 15 | 24-h food and water deprivation | 08:00 a.m. |
Experimental design
Mice were randomly divided into seven groups of 12 mice each. The dorsal skin in the interscapular area was shaved with a surgical clipper 2 days before tumor initiation, and animals showing no hair regrowth were used in the experiment.
Group I: animals served as untreated controls.
Group II (stress alone): animals were exposed to CUS for consecutive 15 days just before the termination of the experiment.
Group III (vehicle control): animals received topical application of acetone (200 μL/mouse) on the shaved back and served as vehicle controls.
Group IV (DMBA alone): DMBA at a dose of 300 μg/200 μL acetone/mouse was applied topically to the shaved back once every week for 4 weeks.
Group V (pre-stress DMBA): animals were first exposed to CUS for consecutive 15 days followed by topical DMBA application as in group IV.
Group VI (DMBA-TPA): animals received the same DMBA dose and application as in group IV. One week after the last DMBA application, animals were further exposed to a weekly application of 5 μg TPA/200 μL acetone/mouse to the same site until the end of the experiment.
Group VII (pre-stress DMBA-TPA): animals were first exposed to CUS for consecutive 15 days followed by topical DMBA-TPA application as in group VI.
Papilloma counts in mice of all the above groups were recorded weekly until sacrifice. All the animals were sacrificed after 16 weeks of treatment. Blood and skin tissue of six mice from each group were collected for biochemical estimations. Remaining six mice from each group were sacrificed, and their blood and skin samples were subjected to DNA damage analysis by alkaline Comet assay.
Preparation of samples and biochemical analysis
The treatment regimen was scheduled in such a way that all the animals were sacrificed on the same day by decapitation, and blood was collected in heparinized tubes. Plasma was obtained by centrifugation at 3000 rpm for 5 min at 4 °C. Level of corticosterone (stress marker); the activities of free radical-metabolizing enzymes viz. superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), and glutathione reductase (GR); levels of malondialdehyde (MDA); reduced glutathione (GSH); and various biochemical markers {glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), alkaline phosphatase (ALP), uric acid, and glucose} were examined in the circulation of treated animals and controls.
A weighed portion of skin tissue was homogenized in chilled 0.1 M phosphate buffer pH 7.4 using glass teflon homogenizer and volume adjusted to give 10 % w/v homogenate. The homogenate was centrifuged at 10,000 rpm for 20 min at 4 °C. Clear supernatant thus obtained was subjected to the analysis of SOD, CAT, GST, GR, GSH, and MDA. All the samples were labeled properly and kept at −70 °C until analysis.
Estimation of corticosterone level by HPLC using UV detector
An HPLC/UV system (Waters, USA) was used for quantification of plasma corticosterone according to Woodward and Emery (1987) with minor modifications using dexamethasone as an internal standard. Briefly, 500 μL of plasma containing known quantity of dexamethasone was extracted with 5 mL of dichloromethane. The dichloromethane extract was evaporated to dryness and dissolved in 100 μL of mobile phase. Twenty microliters of extract was injected into HPLC system for quantification. Mobile phase consisted of methanol/water (70:30) at a flow rate of 1.2 mL/min, and corticosterone was detected at 250 nm using UV detector (Model 2487, Waters, USA). The chromatogram was recorded and analyzed with Breeze software (Version 3.2). Results were expressed as nanogram corticosterone per milliliter of plasma.
Estimation of enzymatic and non-enzymatic antioxidants
Determination of SOD activity
SOD activity in the plasma and skin homogenate was assayed by the method of Marklund and Marklund based on the ability of SOD to inhibit the auto-oxidation of pyrogallol at 412 nm (Marklund and Marklund 1974). Results were expressed as enzyme units per milligram protein. One enzyme unit is defined as the amount of enzyme required to cause 50 % inhibition of the rate of pyrogallol auto-oxidation.
Determination of CAT activity
CAT activity was determined by following the rate of decomposition of H2O2 at 240 nm (Claiborne 1985). Results were expressed as enzyme units per milligram protein. One unit is defined as nanomoles of H2O2 consumed per minute per milligram protein.
Determination of GST activity
GST activity in the plasma and skin homogenate was assayed by the method of Habig et al. using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate (Habig et al. 1974). Calculation was made using millimolar extinction coefficient value of 9.6 mM−1 cm−1, and results were expressed as enzyme units per milligram protein. One enzyme unit is defined as nanomoles CDNB conjugate formed per minute per milligram protein.
Determination of GR activity
GR activity was assessed according to the method of Carlberg and Mannervik (1975). This enzyme catalyzes the NADPH-dependent reduction of glutathione disulfide to glutathione. Oxidation of NADPH was followed spectrophotometrically at 340 nm after 30-s intervals for 3 min. Results were expressed as enzyme units per milligram protein. One enzyme unit is defined as nanomoles of NADPH oxidized per minute per milligram protein.
Estimation of total reduced glutathione
The level of GSH was determined by the method of Jollow et al. (1974) by using sulfosalicylic acid and 5-5′dithiobis-2-nitrobenzoic acid (DTNB). Results were expressed as micromoles GSH per milligram protein.
TBARS determination
Lipid peroxidation was assessed by determining MDA (a thiobarbituric acid-reactive species (TBARS)) spectrophotometrically following the thiobarbituric acid (TBA) test for the formation of TBARS during an acid-heating reaction (Buege and Aust 1978). The pink chromogen formed by MDA–TBA complex was detected at 535 nm and quantified using an extinction coefficient of 1.56 × 105 M/cm.
Estimation of various other biochemical parameters
Activity of GOT and GPT
Commercial kits (Span Diagnostics Ltd India) were used for the measurement of the transaminases (GOT and GPT) in the plasma.
ALP activity
ALP activity in the plasma was measured using p-nitrophenyl phosphate (pNPP) as the substrate (Shah et al. 1979).
Levels of uric acid and glucose
Levels of uric acid and glucose in the plasma were measured by using commercial kits (Span Diagnostic Ltd India).
Estimation of protein content
The protein content in the plasma and skin samples was estimated according to the method of Lowry et al. (1951) using bovine serum albumin as standard.
Preparation of cells for Comet assay
Lymphocyte isolation
Immediately after each sacrifice, the heparinized blood was suitably diluted in PBS (Ca++ and Mg++ free). Lymphocytes were isolated using Histopaque 1077. The isolated cells were finally suspended in RPMI 1640.
Isolation of skin cells
A small portion of skin was minced thoroughly with a blade, and the tissue mass was suspended in ice-cold solution of HBSS. After centrifugation, cells were suspended in RPMI 1640.
DNA damage studies by single-cell gel electrophoresis (Comet assay)
Comet assay was performed under alkaline conditions essentially according to the procedure of Singh et al. (1988) with slight modifications as discussed in our earlier studies (Muqbil et al. 2006). The parameter taken to assess cellular DNA damage was tail length (migration of DNA from the nucleus, μm) and was automatically generated by Komet 5.5 image analysis system.
Statistical analysis
All the data are expressed as mean ± standard error of mean (SEM) and analyzed by one-way ANOVA for differences among controls and treatment groups. P values less than 0.05 are considered statistically significant.
Results
Effect of CUS and topical treatments of DMBA and DMBA-TPA on the levels of plasma corticosterone (stress marker) of mice
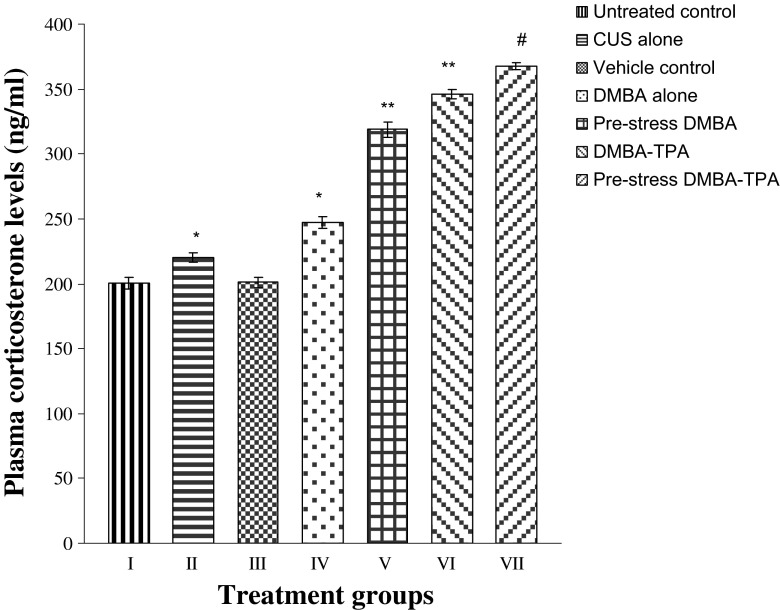
Plasma corticosterone levels were found to be increased significantly (P < 0.01) in the mice exposed to CUS alone, DMBA alone, and DMBA-TPA as compared to untreated and vehicle control groups. Exposure of stressed animals to DMBA or DMBA-TPA caused a further significant (P < 0.001) elevation in these levels, with almost comparable enhancements in both pre-stress DMBA- and DMBA-TPA-treated groups (Fig. 1).
Fig. 1.
Effect of CUS, DMBA-alone (topical), and DMBA-TPA (topical) treatments on the plasma corticosterone levels. Each value represents mean ± SEM of six animals. *P < 0.01 when compared to untreated and vehicle control groups. **P < 0.001 when compared to CUS-alone- and DMBA-alone-treated groups. #P < 0.001 when compared to DMBA-TPA-treated group
Effect of CUS and topical treatments of DMBA and DMBA-TPA on the in vivo antioxidant status and various other biochemical parameters in the skin and circulation of mice
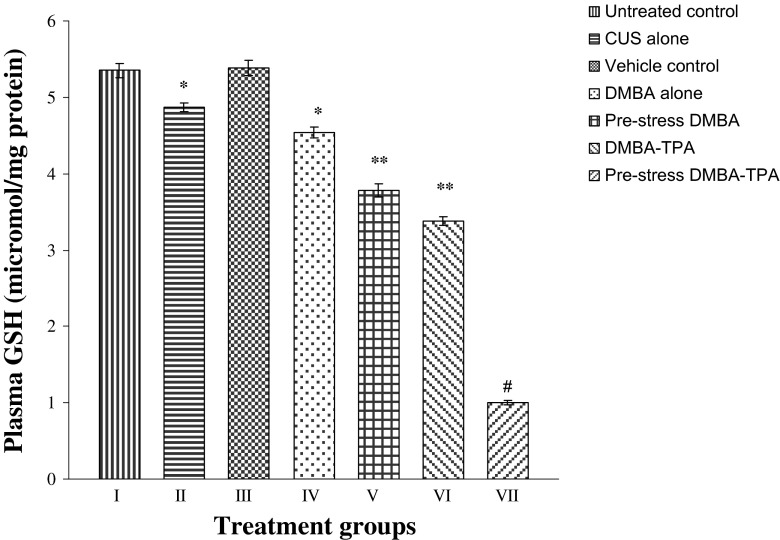
Exposure of mice to CUS-alone, DMBA-alone, and DMBA-TPA treatments caused significantly compromised activities of all the antioxidant enzymes (SOD, CAT, GST, and GR) in the plasma (P < 0.01) and skin (P < 0.05) of animals (Tables 2 and 3) as compared to untreated and vehicle controls. Diminished levels of GSH (Figs. 2 and 3) accompanied by a significant increase in MDA levels (Figs. 4 and 5) were observed in the plasma (P < 0.01) and skin (P < 0.05) of mice exposed to CUS-alone, DMBA-alone, and DMBA-TPA treatments as compared to untreated and vehicle controls. Circulatory levels of uric acid and glucose were also found to be decreased in the stress-alone-, DMBA-alone-, and DMBA-TPA-treated mice accompanied by a significant increase (P < 0.01) in the levels of marker enzymes GOT, GPT, and ALP when compared to untreated and vehicle controls (Table 4). Exposure to CUS prior to DMBA or DMBA-TPA application caused a similar but more pronounced pattern in all the above biochemical parameters. However, the levels of all the above biochemical parameters in the pre-stress DMBA group were found almost comparable to the DMBA-TPA-treated group.
Table 2.
Circulatory levels of various free radical-metabolizing enzymes in mice exposed to CUS alone, DMBA alone (topical), and DMBA-TPA (topical) and exposure to CUS prior to DMBA or DMBA-TPA application
| Groups | SOD (units/mg protein) | CAT (units/mg protein) | GST (units/mg protein) | GR (units/mg protein) |
|---|---|---|---|---|
| Untreated control | 27.32 ± 0.41 | 0.59 ± 0.01 | 0.48 ± 0.008 | 3.98 ± 0.06 |
| CUS alone | 20.51* ± 0.33 | 0.43* ± 0.01 | 0.41* ± 0.008 | 2.54* ± 0.09 |
| Vehicle control | 26.52 ± 0.91 | 0.57 ± 0.01 | 0.47 ± 0.008 | 3.99 ± 0.13 |
| DMBA alone | 15.84* ± 0.32 | 0.38* ± 0.007 | 0.34* ± 0.005 | 1.69* ± 0.05 |
| Pre-stress DMBA | 11.47** ± 0.11 | 0.23** ± 0.009 | 0.25** ± 0.006 | 0.98** ± 0.006 |
| DMBA-TPA | 10.34** ± 0.12 | 0.16** ± 0.003 | 0.17** ± 0.002 | 0.85** ± 0.006 |
| Pre-stress DMBA-TPA | 8.85# ± 0.15 | 0.12# ± 0.002 | 0.14# ± 0.003 | 0.48# ± 0.05 |
Each value represents mean ± SEM of six animals
*P < 0.01 when compared to untreated and vehicle control groups; **P < 0.001 when compared to CUS-alone and DMBA-alone groups; #P < 0.01 when compared to DMBA-TPA group
Table 3.
Levels of free radical scavenging enzymes in the skin of mice exposed to CUS alone, DMBA alone (topical), and DMBA-TPA (topical) and exposure to CUS prior to DMBA or DMBA-TPA application
| Groups | SOD (units/mg protein) | CAT (units/mg protein) | GST (units/mg protein) | GR (units/mg protein) |
|---|---|---|---|---|
| Untreated control | 107.4 ± 2.3 | 10.7 ± 0.3 | 68.6 ± 1.6 | 18.0 ± 0.1 |
| CUS alone | 98.1* ± 3.0 | 9.1* ± 0.1 | 63.7* ± 1.0 | 16.9* ± 0.2 |
| Vehicle control | 106.6 ± 1.6 | 10.8 ± 0.2 | 68.2 ± 1.2 | 18.1 ± 0.1 |
| DMBA alone | 88.0* ± 1.5 | 7.7* ± 0.1 | 59.2* ± 0.7 | 13.2* ± 0.2 |
| Pre-stress DMBA | 75.1** ± 1.6 | 6.4** ± 0.1 | 50.4** ± 1.0 | 11.1** ± 0.2 |
| DMBA-TPA | 68.3** ± 1.4 | 5.4** ± 0.1 | 47.1** ± 0.8 | 9.9** ± 0.1 |
| Pre-stress DMBA-TPA | 54.4# ± 0.5 | 3.4# ± 0.1 | 33.2# ± 1.0 | 7.2# ± 0.04 |
Each value represents mean ± SEM of six animals
*P < 0.05 when compared to untreated and vehicle control groups; **P < 0.01 when compared to CUS-alone and DMBA-alone groups; #P < 0.01 when compared to DMBA-TPA group
Fig. 2.
Effect of CUS, DMBA-alone (topical), and DMBA-TPA (topical) treatments on the circulatory levels of GSH. Each value represents mean ± SEM of six animals. *P < 0.01 when compared to untreated and vehicle control groups. **P < 0.001 when compared to CUS-alone and DMBA-alone-treated groups. #P < 0.001 when compared to DMBA-TPA-treated group
Fig. 3.
Effect of CUS, DMBA-alone (topical), and DMBA-TPA (topical) treatments on the levels of GSH in the skin of mice. Each value represents mean ± SEM of six animals. *P < 0.05 when compared to untreated and vehicle control groups. **P < 0.001 when compared to CUS-alone and DMBA-alone-treated groups. #P < 0.001 when compared to DMBA-TPA-treated group
Fig. 4.
Effect of CUS, DMBA-alone (topical), and DMBA-TPA (topical) treatments on the circulatory levels of MDA. Each value represents mean ± SEM of six animals. *P < 0.01 when compared to untreated and vehicle control groups. **P < 0.001 when compared to CUS-alone- and DMBA-alone-treated groups. #P < 0.001 when compared to DMBA-TPA-treated group
Fig. 5.
Effect of CUS, DMBA-alone (topical), and DMBA-TPA (topical) treatments on the levels of MDA in the skin of mice. Each value represents mean ± SEM of six animals. *P < 0.05 when compared to untreated and vehicle control groups. **P < 0.001 when compared to CUS-alone- and DMBA-alone-treated groups. #P < 0.001 when compared to DMBA-TPA-treated group
Table 4.
Circulatory levels of various biochemical parameters in mice exposed to CUS alone, DMBA alone (topical), and DMBA-TPA (topical) and exposure to CUS prior to DMBA or DMBA-TPA application
| Groups | SGPT (IU/L) | SGOT (IU/L) | ALP (mg/mL) | Glucose (mg/dL) | Uric acid (mg/dL) |
|---|---|---|---|---|---|
| Untreated control | 27.6 ± 1.71 | 28.8 ± 1.03 | 0.96 ± 0.04 | 126.8 ± 3.65 | 4.71 ± 0.09 |
| CUS alone | 59.2* ± 1.03 | 63.4* ± 1.02 | 1.65* ± 0.02 | 106.8* ± 1.42 | 3.12* ± 0.04 |
| Vehicle control | 28.0 ± 1.74 | 28.4 ± 1.0 | 0.95 ± 0.02 | 126.0 ± 2.63 | 4.79 ± 0.07 |
| DMBA alone | 74 .4* ± 2.17 | 79.4* ± 1.34 | 2.71* ± 0.09 | 84.0* ± 2.85 | 2.04* ± 0.02 |
| Pre-stress DMBA | 88.4** ± 1.23 | 91.8** ± 1.52 | 3.80** ± 0.07 | 65.2** ± 1.31 | 1.05** ± 0.008 |
| DMBA-TPA | 94.2** ± 2.34 | 97.6** ± 1.57 | 4.01** ± 0.06 | 57.8** ± 1.84 | 0.93** ± 0.006 |
| Pre-stress DMBA-TPA | 107.2# ± 1.80 | 112.6# ± 1.73 | 4.71# ± 0.04 | 37.2# ± 0.79 | 0.39# ± 0.009 |
Each value represents mean ± SEM of six animals
*P < 0.01 when compared to untreated and vehicle control groups; **P < 0.01 when compared to CUS-alone and DMBA-alone groups; #P < 0.01 when compared to DMBA-TPA group
Briefly, the observed alterations in the biochemical parameters during various treatments in comparison to controls were as follows:
Pre-stress DMBA-TPA > DMBA-TPA > pre-stress DMBA > DMBA alone > CUS alone
Effect of CUS and topical treatments of DMBA and DMBA-TPA on the DNA of lymphocytes and skin cells of mice
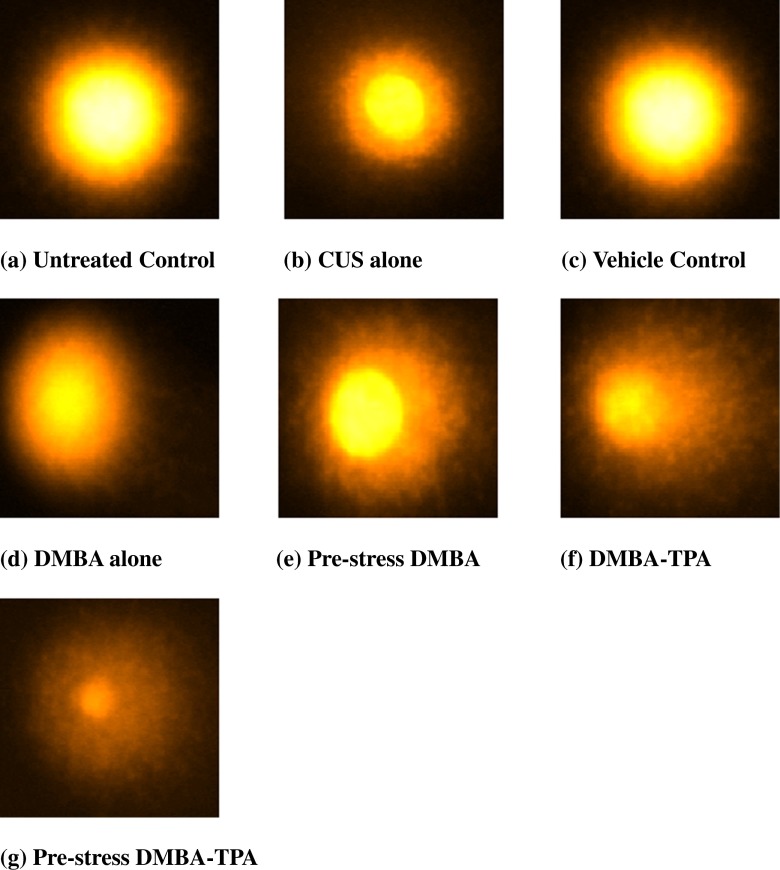
Significant DNA damage (P < 0.05) was observed in the lymphocytes (9.6 ± 0.05 vs. 2.2 ± 0.06; 9.6 ± 0.05 vs. 2.1 ± 0.04) and skin cells (9.41 ± 0.07 vs. 2.3 ± 0.07; 9.41 ± 0.07 vs. 2.4 ± 0.06) of CUS-exposed mice as compared to untreated and vehicle control groups. Treatment with DMBA alone (28.4 ± 0.1 in lymphocytes; 30.1 ± 0.32 in skin cells) and DMBA-TPA (40.24 ± 0.15 in lymphocytes; 43.2 ± 0.26 in skin cells) increased the extent of damage. Pre-exposure of mice to CUS further enhanced the DNA damaging potential of DMBA significantly (P < 0.01) as is evident by increased tail lengths in the pre-stress DMBA (38.81 ± 0.29 in lymphocytes; 41.56 ± 0.34 in skin cells) and pre-stress DMBA-TPA (49.4 ± 0.22 in lymphocytes; 52.8 ± 0.33 in skin cells) groups as compared to CUS-alone-, DMBA-alone-, or DMBA-TPA-treated groups. The extent of DNA damage observed in the lymphocytes (38.81 ± 0.29 vs. 40.24 ± 0.15) and skin cells (41.56 ± 0.34 vs. 43.2 ± 0.26) of pre-stress DMBA group was found almost comparable to the DMBA-TPA-treated group (Figs. 6 and 7).
Fig. 6.
Single-cell gel electrophoresis of mice lymphocytes showing Comets (×100) after treatment with CUS, DMBA-alone (topical), and DMBA-TPA (topical). a Untreated control. b CUS treated. c Vehicle control. d DMBA alone. e Pre-stress DMBA. f DMBA-TPA. g Pre-stress DMBA-TPA
Fig. 7.
Single-cell gel electrophoresis of mice skin cells showing Comets (×100) after treatment with CUS, DMBA alone (topical), and DMBA-TPA (topical). a Untreated control. b CUS treated. c Vehicle control. d DMBA alone. e Pre-stress DMBA. f DMBA-TPA. g Pre-stress DMBA-TPA
A comparison of tail lengths of the lymphocytes and skin cells revealed that skin cells, being the target organ of the carcinogen, exhibited the highest level of DNA damage on DMBA-alone, pre-stress DMBA, DMBA-TPA, and pre-stress DMBA-TPA treatments. The comparative DNA damage observed in the lymphocytes and skin cells by various treatments with respect to controls was comparable as observed for biochemical parameters and can be summarized as follows:
Pre-stress DMBA-TPA > DMBA-TPA > pre-stress DMBA > DMBA alone > CUS alone
Effect of CUS and topical treatments of DMBA and DMBA-TPA on the development of skin papillomas in Swiss albino mice
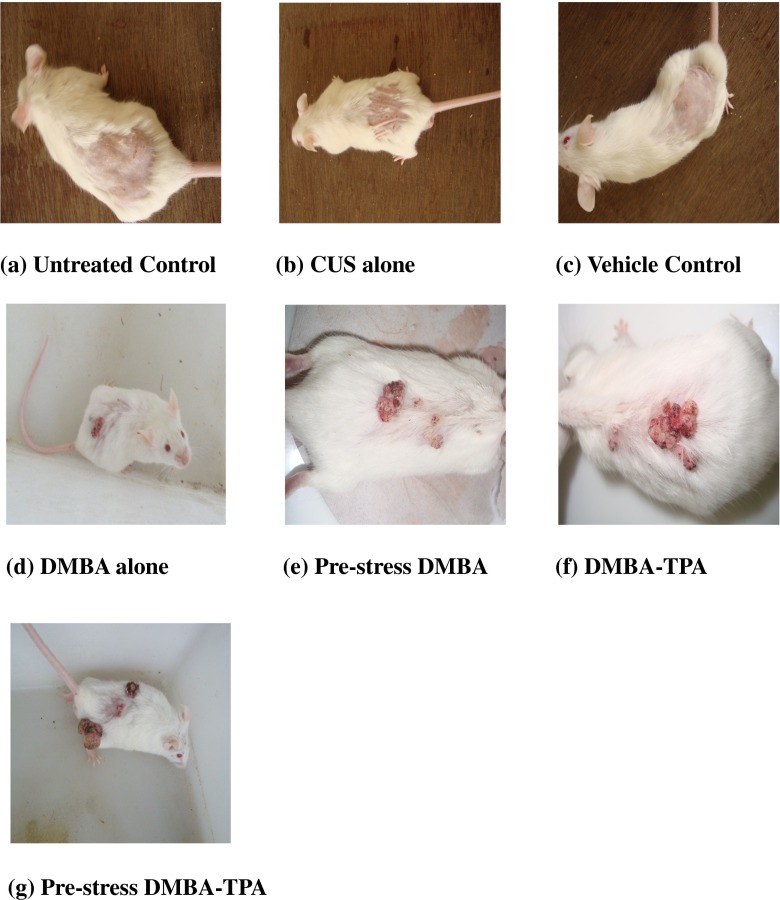
The untreated, vehicle control and CUS-alone-treated groups did not show any papillomas during the entire 16 weeks of observation (Fig. 8). Papillomas started to appear in mice receiving topical application of DMBA from 12th week and DMBA-TPA from week 7 onward, and the papillomas continued to increase until 16 weeks. The tumor incidence in the DMBA-alone-treated mice reached 58.3 % by the end of the experiment (16 weeks). Pre-exposure to CUS along with DMBA application increased the tumor incidence to 75 % and reduced the time of appearance of these papillomas. The cumulative number of papillomas in these mice was recorded as 19 which was almost comparable to DMBA-TPA group (22). Pre-stress DMBA- and DMBA-TPA-treated groups exhibited almost comparable values of tumor yield (1.58 vs. 1.83) and tumor burden (2.1 vs. 2.2). Pre-exposure to CUS along with DMBA-TPA treatment increased the tumor incidence to 100 % by the end of the experiment, and the cumulative number of papillomas was found to be 34. Tumor yield and tumor burden were also increased to 2.83 (Table 5).
Fig. 8.
Effect of CUS and topical treatments of DMBA and DMBA-TPA on the development of skin papillomas in Swiss albino mice. All the mice are approximately the same size (45 ± 5 g). . a Untreated control. b CUS treated. c Vehicle control. d DMBA alone. e Pre-stress DMBA. f DMBA-TPA. g Pre-stress DMBA-TPA
Table 5.
Different parameters observed during CUS, DMBA-alone (topical), and DMBA-TPA (topical) treatments on the development of skin papillomas in Swiss albino mice
| Groups (12 mice/group) | Cumulative number of tumors | Tumor incidence (%) | Tumor yield | Tumor burden |
|---|---|---|---|---|
| Untreated control | 0 | 0 | 0 | 0 |
| CUS alone | 0 | 0 | 0 | 0 |
| Vehicle control | 0 | 0 | 0 | 0 |
| DMBA alone | 11 | 58.33 | 0.91 | 1.57 |
| Pre-stress DMBA | 19 | 75.0 | 1.58 | 2.1 |
| DMBA-TPA | 22 | 83.33 | 1.83 | 2.20 |
| Pre-stress DMBA-TPA | 34 | 100 | 2.83 | 2.83 |
The development of papillomas (in terms of their incidence, tumor yield, and tumor burden) in mice during various treatments can be summarized as follows:
Pre-stress DMBA-TPA > DMBA-TPA > pre-stress DMBA > DMBA alone > CUS alone
Discussion
Oxidative stress, an imbalance in oxidant and antioxidant status that has been repeatedly addressed as an indicator of indirect genotoxicity, is considerably increased in certain pathological conditions including cancer.
Stress has a significant impact on some of the major drug-metabolizing enzyme systems. It alters both constitutive and induced cytochrome P450 expression. There is growing evidence that stress and the resulting changes in behavior, immune status, and stress hormone profiles (particularly catecholamines) significantly influence tumor initiation, progression, and metastasis (Powell et al. 2013). Following stress, various events appear to hold determinant roles in CYP regulation. These include activation of the hypothalamic–pituitary–adrenal (HPA) axis with the consequent release of glucocorticoids and epinephrine from adrenal glands, oxidative stress, increased release of cytokines/NF-κB, and the altered secretion of hormones, such as GH, thyroid hormones, and insulin (Dvorak and Pavek 2010).
Body’s neuroendocrine response has been shown to directly alter important cellular processes that help protect against the formation of cancer, such as DNA repair and the regulation of cell growth (Antoni et al. 2006). However, exposure to stress results in enhanced release of catecholamines and glucocorticoids due to the activation of sympathoadrenal and HPA axes (Joels et al. 2007), and chronic exposure to these mediators of the stress response has damaging effects, in which oxidative stress plays a major role (Simmons et al. 1991; Perez-Nievas et al. 2007). The present study showed a significant rise of the plasma corticosterone level in the animals treated with DMBA alone or DMBA-TPA as compared to controls or CUS alone, and pre-exposure to CUS caused a further elevation in these levels suggesting the possible role of stress in the promotion of carcinogenesis. Neurodegeneration caused by glucocorticoid stress hormones under stress conditions may be linked to an increase in the generation of ROS, which can directly damage cellular proteins, DNA, and lipids (McIntosh and Sapolsky 1996). Acute stress has immunoenhancing effects, while chronic stress suppresses or dysregulates innate and adaptive immune responses through mechanisms involving suppression of leukocyte numbers, trafficking, or changing the type 1–type 2 cytokine balance (Dhabhar and McEwen 2006; Glaser and Kiecolt-Glaser 2005; Ader 2006). Chronic stress increases the susceptibility to skin cancer by suppressing type 1 cytokines and protective T cells with an increase in suppressor T cell function (Saul et al. 2005). Stress-induced changes in rodent blood leukocyte numbers are characterized by a significant decrease in the numbers and percentages of lymphocytes and monocytes and an increase in the numbers and percentages of neutrophils (Dhabhar et al. 1994, 1995).
Heat shock proteins (Hsp) play a critical role in the body’s self-defense under a variety of stresses, including heat shock, oxidative stress, radiation, and wounds, through the regulation of folding and functions of relevant cellular proteins (Feder and Hofmann 1999; Morano and Thiele 1999). Exercise is associated with transient elevations of Hsp expression, body temperature, hormones, and oxidative stress, which may reduce inflammatory mediators (Locke et al. 1990). Hsps not only protect cells and organisms against proteotoxic stresses, but also are also critical in normal functioning of several cellular processes (Noble et al. 2008). Several signaling pathways involving Hsps are implicated in the regulation of immune and inflammatory systems (Jones et al. 2011; Shields et al. 2012). Though there is some controversy regarding their exact role (Eden et al. 2012), Hsps may not only activate the immune response (Radons and Multhoff 2005) but also dampen the inflammatory pathways (Jones et al. 2011). Chronic stress inhibits HSP70 activity in rat intestinal epithelial layer that is associated with intestinal epithelial barrier dysfunction (Yang et al. 2009).
During the multistage carcinogenesis process, the activities of antioxidant enzymes tend to decrease which leads to a pro-oxidant state of the cell, facilitating tumor promotion and progression (Oberley and Oberley 1993). To cope with the generation of ROS, GSH acts as an endogenous non-enzymatic biological antioxidant. In addition to its well-known antioxidant properties, GSH is emerging as a regulator of the expression of proteins (or molecules) involved in apoptosis (Friesen et al. 2004; Armstrong et al. 2002). A reduction in GSH level due to its utilization in the conjugation reaction is quite likely to affect several pathways and may further lead to a state of oxidative stress. This is evident in the present study from the diminished levels of circulatory GSH in the DMBA-alone- and DMBA-TPA-treated groups. Furthermore, because oxidative stress plays a major role in the induced adaptive expression of antioxidants, malignant cells are often characterized by differential expression in the levels of a number of antioxidant enzymes (Teoh et al. 2009; Pompella et al. 2007). In the present study, it was noted that the treatment to the initiator and promoter decreased the enzyme activities of not only SOD, but also other key antioxidant enzymes such as CAT, GST, and GR and the plasma levels of uric acid and glucose in stress-alone-, DMBA-alone-, and DMBA-TPA-treated groups which further confirms the state of oxidative stress. GSH in conjunction with GST detoxifies reactive intermediate species generated during DMBA metabolism, thereby enhancing resistance against oxidative stress (Locigno and Castronovo 2001). The decreased levels of GSH along with decreased activity of GST might contribute significantly in enhancing DMBA-TPA-induced carcinogenesis due to its reduced clearance from the system. Recent studies from our lab have documented oxidative damage to the liver and kidney of mice by prior exposure to CUS. In these organs, it was shown that CUS decreased the activities of SOD, CAT, GST, and GR and markedly reduced the degree of protection offered by the GSH system, thus aggravating oxidative damage (Suhail et al. 2011). The present study also demonstrates that pre-exposure to CUS further decreased the levels of all the above antioxidants by deranging the oxidative status of animals either by enhanced production or by decreased clearance of free radicals. Under such compromised state, the infusion of DMBA or DMBA-TPA further aggravated the situation which is observed in terms of significantly decreased activities of free radical scavenging enzymes SOD, CAT, GR, and GST in comparison to CUS-alone-, DMBA-alone-, and DMBA-TPA-treated groups.
Insufficiency in the antioxidant systems and consequent rise in free radical-mediated processes enhance the rate of lipid peroxidation as evidenced by the increased levels of MDA in the plasma and skin tissue of DMBA- and DMBA-TPA-treated animals in the present study. The levels were further significantly increased when the animals were subjected to CUS prior to DMBA or DMBA-TPA treatment as compared to stress-alone-, DMBA-alone-, and DMBA-TPA-treated groups, suggesting that already existing oxidative stress facilitates the effect of DMBA on lipid peroxidation and a cumulative effect is observed. This may be due to the generation of OH· radical through the metal-catalyzed Haber–Weiss reaction during the course of stress which plays a direct role in stress-induced oxidative damage (Das 2002). MDA is one of the most intensively studied lipid hydroperoxide-derived bifunctional electrophiles that forms adducts with nucleic acid bases at physiological pH, causing damage to the genetic material (Stone et al. 1990). Increased lipid peroxidation accompanied with compromised host antioxidant status may result in accumulation of free radicals, and as a consequence, the levels of marker enzymes, GOT, GPT, and ALP, in circulation were increased significantly following DMBA or DMBA-TPA treatments depicting an effect on liver cell integrity.
Topical treatment with DMBA alone leads to the formation of skin papillomas in mice as observed in the present study; however, application of TPA following DMBA treatment decreased the time of appearance (from 12 to 7 weeks) of the papillomas accompanied by their increased incidence. TPA-induced tumor promotion is accompanied by alterations in various cellular processes including cell growth, cell differentiation, and cell death (Kim et al. 2005; La et al. 1999). However, pre-exposure to CUS followed by DMBA or DMBA-TPA infusion resulted in significantly increased oxidative stress and enhanced the incidence of skin papillomas relative to their non-stressed counterparts, with comparable effects being observed in the pre-stress DMBA- and DMBA-TPA-treated groups, suggesting that the role of stress in enhancing the carcinogenic potential of DMBA is comparable to TPA. Further, a concordance exists between endocrine changes induced by large tumor burdens, consisting of increased corticosterone and growth hormone (Besedovsky et al. 2000). Thus, the present study further confirms that stress accelerates the onset of tumor formation produced in response to DMBA and significantly influences the type and location of tumors as reported earlier (Flint et al. 2011).
DNA damage has been implicated in the pathogenesis of various diseases including cancer. Stress affects DNA integrity by production of ROS resulting in oxidative stress and increased lipid peroxidation (Adachi et al. 1993; Guerin 1978). Previous studies from our laboratory have already reported that chronic restraint stress not only causes increased lipid peroxidation and compromised antioxidant status but also enhances the pro-oxidant effect of DMBA in rats (Muqbil et al. 2006; Muqbil and Banu 2006). The results of the present study showed that CUS caused significant damage to the DNA of lymphocytes and skin cells of mice as compared to controls by incurring DNA damage within the cell either by altering the ability of cells to repair DNA or by causing oxidative stress as enhanced lipid peroxidation is observed in the skin and plasma of stressed animals. The DNA damage was further enhanced in mice treated with DMBA or DMBA-TPA following exposure to stress as compared to those treated with DMBA alone or DMBA-TPA. ROS formed during DMBA metabolism produce deleterious effects by initiating lipid peroxidation directly or indirectly by acting as second messengers for the primary free radicals, and the toxic metabolites of DMBA bind to adenine residues of DNA causing damage (Guerin 1978). The DNA breakage may be the result of the generation of hydroxyl radicals and other ROS in situ. These concurrent interactions of initiating (adducts) and promoting (oxidized bases) processes may be key to complete carcinogenesis, and exposure to stress plays an important role from the very early stages of carcinogenesis right at the basic cellular level by causing oxidative stress and/or increasing the damaging potential of a carcinogen.
Alterations of various in vivo biochemical parameters and the extent of DNA damage in the pre-stress DMBA-treated group were found almost comparable to the DMBA-TPA-treated group, suggesting that exposure to stress prior to the carcinogen treatment may work as a promoter of carcinogenesis. Thus, in the absence of any promoter, stress alone can enhance the carcinogenic potential of a carcinogen.
In conclusion, the results of our study clearly indicate the influence of stress events, produced by various unpredictable psycho-social problems on carcinogenesis. Further studies may be aimed at the development of interventions for disease prevention by identifying the relations between psychological factors and DNA damage.
Acknowledgments
Thanks are due to University Grants Commission (UGC), New Delhi, for financial support to the author (N. Suhail) in the form of a scholarship. The authors are grateful to UGC-SAP (Special Assistance Programme), DST-FIST (Department of Science and Technology), and Aligarh Muslim University for the facilities.
References
- Adachi S, Kawamura K, Takemoto K. Oxidative damage of nuclear DNA in liver of rats exposed to psychological stress. Cancer Res. 1993;53:4153–4155. [PubMed] [Google Scholar]
- Ader R. Psychoneuroimmunology. 4. San Diego: Academic; 2006. [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciszewska LK, Martin SE, Milner JA. The antimutagenic effect of selenium on 7,12-dimethylbenz(a)anthracene and metabolites in the ames Salmonella/microsome system. Biol Trace Elem Res. 1982;4(4):259–267. doi: 10.1007/BF02786540. [DOI] [PubMed] [Google Scholar]
- Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, Normann S, Schardt M, Del RA. Endocrine host responses during early and late phases of tumor development. Int J Cancer. 2000;86:457–461. doi: 10.1002/(SICI)1097-0215(20000515)86:4<457::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Camhi SL, Alam J, Wiegand GW, Chin BY, Choi AMK. Transcriptional activation of the HO-1 gene by lipopolysaccharide is mediated by 5′ distal enhancers: role of reactive oxygen intermediates and AP-1. Am J Respir Cell Mol Biol. 1998;18:226–234. doi: 10.1165/ajrcmb.18.2.2910. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- Claiborne A. Catalase activity. In: Green Wald RA, editor. CRC handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1985. pp. 283–284. [Google Scholar]
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons, Clowes GHA memorial lecture. Cancer Res. 1982;4:4875–4917. [PubMed] [Google Scholar]
- Das UN. A radical approach to cancer. Med Sci Monit. 2002;8:79–92. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Bidirectional effects of stress on immune function: possible explanations for salubrious as well as harmful effects. In: Ader R, editor. Psychoneuroimmunology. 4. San Diego: Elsevier; 2006. [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution—dynamics and hormonal mechanisms. J Immunol. 1995;54:5511–5527. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Stein M, et al. Diurnal and stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- Donovan PJ, Smith GT, Nardone R. The mutagenic effects of 7,12-dimethylbenz[a]anthacene, 3-methylcholanthrene and benzo[a]pyrene to the developing Syrian hamster fetus measured by an in vivo/in vitro mutation assay. Mutat Res. 2004;554(1–2):111–120. doi: 10.1016/j.mrfmmm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer. 1996;32A:30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dvorak Z, Pavek P. Regulation of drug-metabolizing cytochrome P450 enzymes by glucocorticoids. Drug Metab Rev. 2010;42:621–635. doi: 10.3109/03602532.2010.484462. [DOI] [PubMed] [Google Scholar]
- Eden WV, Spiering R, Broere F, van der Zee R. A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones. 2012;17(3):281–292. doi: 10.1007/s12192-011-0311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feig DI, Reid TM, Loeb LA. Reactive oxygen species in tumorigenesis. Cancer Res. 1994;54:1890s–1894s. [PubMed] [Google Scholar]
- Flint M, McCarty K, Jenkins F, Conrads T, Sun M, Baum A. Psychological stress accelerates the onset of tumor formation and alters the type and location of tumors in a DMBA mouse carcinogenesis model. Stress Health. 2011;27:e129–e138. doi: 10.1002/smi.1343. [DOI] [Google Scholar]
- Friesen C, Kiess Y, Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- Garmyn M, Degreef H. Suppression of UVB-induced c-fos and c-jun repression in human keratinocytes by N-acetylcysteine. J Photochem Photobiol. 1997;B37:125–130. doi: 10.1016/S1011-1344(96)07340-X. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Guerin MR. Energy sources of polycyclic aromatic hydrocarbons. In: Gelboin HV, Ts’o POP, editors. Polycyclic hydrocarbons and cancer: chemistry, molecular biology and environment. New York: Academic; 1978. pp. 1–42. [Google Scholar]
- Guyton KZ, Kensler TW. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49:523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic intermediate. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Jones Q, Voegeli TS, Li G, et al. Heat shock proteins protect against ischemia and inflammation through multiple mechanisms. Inflamm Allergy Drug Targets. 2011;10(4):247–259. doi: 10.2174/187152811796117726. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, Surh YJ. Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24:2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- Kulesz-Martin MF (1997) Biological aspects of multistage carcinogenesis as studied in experimental animals and in cell culture models, In: Sipes IG, Charlene A, McQueen AJ (ed) Chemical carcinogens and anticarcinogens. Comprehensive Toxocology 12:7–30
- La E, Muga SJ, Locniskar MF, Fischer SM. Altered expression of interleukin-1 receptor antagonist in different stages of mouse skin carcinogenesis. Mol Carcinog. 1999;24:276–286. doi: 10.1002/(SICI)1098-2744(199904)24:4<276::AID-MC5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Liu J, Mori A. Stress, aging and oxidative damage. Neurochem Res. 1999;24:1479–1497. doi: 10.1023/A:1022597010078. [DOI] [PubMed] [Google Scholar]
- Locigno R, Castronovo V. Reduced glutathione system; role of cancer development, prevention and treatment (Review) Int J Oncol. 2001;19:221–236. doi: 10.3892/ijo.19.2.221. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG, Atkinson BG. Exercising mammals synthesize stress proteins. Am J Physiol. 1990;258(4):C723–C729. doi: 10.1152/ajpcell.1990.258.4.C723. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marklund S, Marklund G. The involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996;141:201–206. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- Miyata M, Furukawa M, Takahashi K, Gonzales FJ, Yamazoe Y. Mechanism of 7,12-dimethylbenz(a)anthracene-induced immunotoxicity: role of metabolic activation at the target organ. Jpn J Pharmacol. 2001;86:302–309. doi: 10.1254/jjp.86.302. [DOI] [PubMed] [Google Scholar]
- Morano KA, Thiele DJ. Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 1999;7(4–6):271–282. [PMC free article] [PubMed] [Google Scholar]
- Muqbil I, Azmi AS, Banu N. Prior exposure to restraint stress enhances 7,12-dimethylbenz(a)anthracene (DMBA) induced DNA damage in rats. FEBS Lett. 2006;580:3995–3999. doi: 10.1016/j.febslet.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Muqbil I, Banu N. Enhancement of pro oxidant effect of dimethylbenz (a) anthracene (DMBA) in rats by pre exposure to restraint stress. Cancer Lett. 2006;240:213–220. doi: 10.1016/j.canlet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Noble EG, Milne KJ, Melling CWJ. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33(5):1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- Oberley TD, Oberley LW. Oxygen radicals and cancer. In: Yu BP, editor. Free radicals in aging. Boca Raton: CRC Press; 1993. pp. 247–267. [Google Scholar]
- Perez-Nievas BG, Garcia-Bueno B, Caso JR, Menchen L, Leza JC. Corticosterone as a marker of susceptibility to oxidative/nitrosative cerebral damage after stress exposure in rats. Psychoneuroendocrinology. 2007;32:703–711. doi: 10.1016/j.psyneuen.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol. 2007;7:360–366. doi: 10.1016/j.coph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30(Suppl):S41–S47. doi: 10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Pradhan SN, Prabhati R. Effects of stress on growth of transplanted and 7,12-dimethylbenz (a) anthracene induced tumors and their modification by psychotropic drugs. J Natl Inst. 1974;53:1241–1245. doi: 10.1093/jnci/53.5.1241. [DOI] [PubMed] [Google Scholar]
- Radons J, Multhoff G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev. 2005;11:17–33. [PubMed] [Google Scholar]
- Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SV, Kempson SA, Northrup TE, Dousa TP. Renal adaptation to low phosphate diet in rats. J Clin Invest. 1979;64:955–966. doi: 10.1172/JCI109562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit J, Lewis LW, Ferman GW, Gale RP, Liebeskind JC. Endogenous opioids may mediate the effects of stress on tumor growth and immune function. Proc West Pharmacol Soc. 1983;26:53–56. [PubMed] [Google Scholar]
- Shields AM, Panayi GS, Corrigall VM. A new-age for biologic therapies: long-term drug-free therapy with BiP? Front Immunol. 2012;3:17. doi: 10.3389/fimmu.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons HF, James RC, Harbison RD, Patel DG, Roberts SM. Examination of the role of catecholamines in hepatic glutathione suppression by cold-restraint in mice. Toxicology. 1991;67:29–40. doi: 10.1016/0300-483X(91)90161-S. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Sklar LS, Anisman H. Stress and coping factors influence tumor growth. Science. 1979;205:513–515. doi: 10.1126/science.109924. [DOI] [PubMed] [Google Scholar]
- Steplewski Z, Goldman PR, Vogel WH. Effect of housing stress on the formation and development of tumors in rats. Cancer Lett. 1987;34:257–261. doi: 10.1016/0304-3835(87)90175-3. [DOI] [PubMed] [Google Scholar]
- Steplewski Z, Vogel WH, Ehya H, Poropatich C, McDonald-Smith J. Effect of restraint stress on inoculated tumor growth and immune response in rats. Cancer Res. 1985;45:5128–5133. [PubMed] [Google Scholar]
- Stone K, Ksebati M, Marnett LJ. Investigation of the adducts formed by reaction of malondialdehyde with adenosine. Chem Res Toxicol. 1990;3:33–38. doi: 10.1021/tx00013a006. [DOI] [PubMed] [Google Scholar]
- Suhail N, Bilal N, Hasan S, Banu N. Chronic unpredictable stress exacerbates 7,12-dimethylbenz (a) anthracene induced hepatotoxicity and nephrotoxicity in Swiss albino mice. Mol Cell Biochem. 2011;355:117–126. doi: 10.1007/s11010-011-0845-y. [DOI] [PubMed] [Google Scholar]
- Teoh ML, Fitzgerald MP, Oberley LW, Domann FE. Over expression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res. 2009;69:6355–6363. doi: 10.1158/0008-5472.CAN-09-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnhoven SW, Kool HJ, Mullenders LHF, Slater R, Van Zeeland AA, Vrieling H. DMBA-induced toxic and mutagenic response vary dramatically between NER-deficient Xpa, Xpc and Csb mice. Carcinogenesis. 2001;22:1099–1106. doi: 10.1093/carcin/22.7.1099. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Woodward CJ, Emery PW. Determination of plasma corticosterone using high-performance liquid chromatography. J Chromatogr. 1987;419:280–284. doi: 10.1016/0378-4347(87)80287-6. [DOI] [PubMed] [Google Scholar]
- Yang PC, Tu YH, Perdue MH, Oluwole C, Struiksma S. Regulatory effect of heat shock protein 70 in stress-induced rat intestinal epithelial barrier dysfunction. N Am J Med Sci. 2009;1(1):9–15. [PMC free article] [PubMed] [Google Scholar]