Abstract
Acute stress alters anti-bacterial defenses, but the neuroimmunological mechanisms underlying this association are not yet well understood. Metallothionein (MT), a cysteine-rich protein, is a stress response protein that is induced by a variety of chemical, biological, and psychological stressors, and MT has been shown to influence immune activities. We investigated MT’s role in the management of anti-bacterial responses that occur during stress, using a C57BL/6 (B6) strain that has targeted disruptions of the Mt1 and Mt2 genes (B6-MTKO), and a B6 strain that has additional copies of Mt (B6-MTTGN). The well-characterized listeriosis model was used to examine immune mechanisms that are altered by a 1-h stress treatment (cold-restraint, CR) administered just prior to bacterial infection. Intriguingly, MT gene doses both greater and lower than that of wild-type (WT) B6 mice were associated with improved host defenses against Listeria monocytogenes (LM). This augmented protection was diminished by CR stress in the MTKO mice, but transgenic mice with additional MT copies had no CR stress-induced increase in their listerial burden. During the transition from innate to adaptive immunity, on day 3 after infection, oxidative burst and apoptosis were assessed by flow cytometric methods, and cytokine transcription was measured by real-time quantitative PCR. MT gene expression and CR-stress affected the expression of IL-6 and TNFα. Additionally, these genetic and environmental modulations altered the generation of ROS responses as well as the number of apoptotic cells in livers and spleens. Although the level of MT altered the listerial response, MT expression was equally elevated by listerial infection with or without CR stress. These results indicate the ability of MT to regulate immune response mechanisms and demonstrate that increased amounts of MT can eliminate the immunosuppression induced by CR.
Keywords: Metallothionein, Infection, Listeria, Apoptosis, Oxidative stress
Introduction
The concept of stress-induced immune suppression is widely acknowledged, yet the molecular underpinnings of this complex physiologic phenomenon remain ill defined. The current analysis focused on the immunomodulatory role of the stress protein, metallothionein (MT), in a well-defined model of bacterial infection. MT synthesis is associated with early fetal development (Webb 1987), and it is inducible with a wide range of stressful conditions, including exposure to metal toxicants and reactive oxygen species (Ghoshal and Jacob 2001), infection and inflammation (Manuel et al. 1992), and psychological stress (Hidalgo et al. 1986). In previous studies, MT has been shown to modulate in vitro immune activities, such as lymphoproliferation (Borghesi et al. 1996) and chemotaxis (Yin et al. 2005), and to modify the severity of chronic inflammatory disease (Laukens et al. 2009; Devisscher et al. 2014) and autoimmune disease (Lynes et al. 2006). We have demonstrated that manipulations of MT expression (MT deficiency or excess) can enhance host defenses against Listeria monocytogenes (LM) (Emeny et al. 2009), yet the functional contributions made by MT to bacterial resistance during stress have not been explored.
Primary infection with the Gram-positive bacterium LM activates both innate and adaptive immune cells to produce cytokines required for bacterial clearance (Poston and Kurlander 1992). Successful host resistance to listeriosis, as measured by a decline in viable LM, is mediated by NK and CD8+ T cells (Unanue 1997) and usually begins 3 days after infection with a relatively low (<104 cfu) inoculum of LM (Cao et al. 2003a). Day 3 of infection is the time point when innate immunity begins to transition to adaptive immune mechanisms (Unanue 1997).
The experimental model of 1 h cold-restraint (CR, 4 °C) is known to elicit both physical and psychological stress (Pare and Glavin 1986). CR was chosen as the additional stressor, because it is known to elicit oxidative stress (Simmons et al. 1991), and oxidative stress affects MT expression (Ghoshal and Jacob 2001). We have shown that mice subjected to CR prior to LM infection become sicker than those infected without CR. Neither cytokine profiles nor depletion of B or T cells can explain the stress-induced inhibition of host defenses (Cao et al. 2003b); however, some stress-induced immunomodulation appears to act through the beta1-adrenoceptor (β1AR) (Cao et al. 2003b; Emeny et al. 2007).
In the present study, we evaluated the in vivo effects of MT gene dose with regard to host defense against LM when infection is preceded by the additional stress of CR. The LM burden was measured in livers and spleens, RT-PCR analyses were used to detect changes in IL-6 and TNFα expression, and flow cytometric analyses were used to assess oxidative burst and apoptosis.
Materials and methods
Mice and listeria monocytogenes
All of the in vivo studies were performed using congenic mice bred at the University of Connecticut vivarium and housed at the AAALAC-approved Wadsworth Center animal facility, Albany NY, in accordance with the Institutional Animal Care and Use Committee guidelines. The congenic MT-I/II knockout mice and the congenic MT-I transgenic mice were derived from the original strain constructs (129S7/SvEvBrd-Mt1tm1BriMt2tm1Bri/J (Masters et al. 1994) and STOCK Tg(Mt1)174Bri/J (Palmiter et al. 1993), respectively) that had been obtained from the Jackson Laboratory (Bar Harbor, ME). The Mt1tm1BriMt2tm1 construct carries targeted disruptions in both the Mt-1 and Mt-2 genes while the Tg(Mt1) construct carries an additional 56 copies of the Mt1 gene. This transgene construct includes both a minimally marked MT-1 gene and the flanking 10 and 7 kb regions that are found on the 5’ and 3’ ends of the endogenous gene. The flanking regions retain responsiveness to standard inducing signals, including divalent heavy metal cations, endotoxin, and dexamethasone, and confer the same tissue specificity that has been observed with endogenous MT-1, though the increased copy number enables higher mRNA and protein production. The flanking regions also confer responsiveness to the developmental regulatory signals that are associated with fetal liver expression of MT (Palmiter et al. 1993). Genotyping protocols for the selection of heterozygotes at each generation were provided by the Jackson Laboratory. Each congenic strain was produced by 8 to 9 generations of backcrossing to the C57BL/6 J background strain, followed by incrossing of the heterozygotes to produce the new congenic strains. New strain production was performed at the University of Connecticut, where these strains are bred and maintained. Age-matched male mice (C57BL/6-Mt1tm1BriMt2tm1Bri, abbreviated B6-MTKO, and C57BL/6-Tg(Mt1)174Bri, abbreviated B6-MTTGN) were received from the colony at the University of Connecticut and allowed at least 1 week of habituation in the Wadsworth Center animal facility before use in experiments. Age and sex matched wild-type controls (B6-WT) were obtained directly from Jackson Laboratory.
Our LM stock was originally isolated from a meningitis patient, and it has been maintained as previously described (Schell and Lawrence 1977). Six mice per strain were infected intravenously with LM (14,000 cfu), half of each group received CR stress treatment prior to infection. LM burden, apoptosis, and oxidative burst were examined on day 3 of the infection.
Determination of listeria burden in liver and spleen
To determine the bacterial load in mice following inoculation, enumeration of viable LM was performed as previously described (Cao et al. 2002). Mice were sacrificed by lethal CO2 anesthesia, and the spleen and liver were removed aseptically and homogenized in sterile 0.9 % NaCl. Immediately after aseptic removal, a portion of the liver and spleen homogenates was aliquoted and stored in 1 % NP40 (Sigma) at −80 °C until mRNA analysis. For the enumeration of viable LM, serial dilutions of organ homogenates were plated in quadruplicate on blood-agar plates and cultured overnight. Bacterial colonies were counted in the following day. Bacterial burdens are expressed as the estimated number of viable LM colony forming units (cfu) per organ.
RT-PCR of cytokine production in liver and spleen
An RNeasy RNA isolation kit and protocol from Qiagen, Inc. (Valencia, CA) was used to obtain RNA from the liver and spleen homogenates. RNA was eluted from midi columns with two 250 μl aliquots of RNase-free water. The concentration and purity of the RNA were determined by measuring the absorbance at 260 nm and the 260/280 nm ratio using a Beckman DU 640 spectrophotometer (Fullerton, CA). RNA integrity was further assessed by agarose gel electrophoresis.
A two-step process was employed for cytokine mRNA quantification. First, cDNA was prepared from 2 μg of total RNA using a cDNA archive kit from Applied Biosystems, Inc. (Foster City, CA) according to the manufacturer’s directions. Relative cytokine gene expression was achieved by the TaqMan method with primers, probes, and master mixes from Applied Biosystems. Gene expression TaqMan kits used for these experiments were for mouse IL-6 (Mm00446190_m1), TNF-α (Mm00443258_m1), IFN-γ (Mm01168134_m1), and GADPH (Mm99999915_g1). The gapdh (GenBank: NM_008084) reference gene was used as the endogenous control for normalization of the cytokine mRNA quantities; GAPDH was run in quadruplicate for each RNA sample. The overall % CV for GAPDH was 1.44, which indicates that GADPH was a good control for the treatment groups. We performed the amplifications in an Applied Biosystems 7500 real-time PCR instrument under the following conditions: 50 °C for 2 min, then 95 °C for 10 min followed by 40 cycles of 95 °C for 30 s and 60 °C for 1 min. Readings were taken during the second step of each cycle. Relative cytokine gene expression was calculated according to the formula: gene expression = 2-(ExpCt-GAPDH Ct) × 1000, where Ct refers to cycle threshold number. The real-time PCR analysis conformed to general MIQE guidelines (Bustin et al. 2009).
Flow cytometric analysis of lymphocytes
On day 3 of the LM infection (injection = day 1), spleens and livers were removed aseptically from three mice per strain for use in flow cytometry assays for analysis of oxidative burst and apoptosis. A portion of livers and spleens was placed into digestion buffer containing collagenase IV (Sigma; 200 μg/ml) and DNase I (Sigma; 50 μg/ml) and was coarsely chopped with a sterile razor blade before 37 °C incubation for 20 min. Splenocytes were then homogenized as described above, while livers were pipetted through a Teflon mesh. Red blood cells were lysed, cells were washed twice with PBS, and total cell numbers were determined. Leukocytes (1 × 106 per tube) were used for flow cytometric staining procedures. The acquisition and analysis of all specimens were performed on a BD FACS Calibur using CellQuest software. Lymphocyte and macrophage populations were selected for analysis based on forward and side scatter parameters.
Oxidative burst assay
Uncultured leukocytes were used to measure oxidative burst using dihydrorhodamine 123 (DHR-123, Molecule Probes, Eugene, OR) to quantify the presence of oxidants based on the conversion of fluorogenic DHR 123 to fluorescent rhodamine 123. Briefly, cells were resuspended in 0.5 ml sample buffer (SB; HBSSS without Ca2+, Mg2+ and phenol red, Sigma) containing 0.1 % BSA and 1 mM EDTA and 18 μl of a 1:10 dilution (with SB) of stock DHR (29 mM in DMSO). Following a 15-min incubation in a 37 °C water bath, cells were centrifuged at 800×g and resuspended in 1 ml PharmLyse (BD BioSciences) and incubated for 10 min at room temperature. After centrifugation and one wash with SB, cells were resuspended in 0.5 ml SB and analyzed by flow cytometry.
Apoptosis assay
Splenocytes isolated from three mice per strain on day 3 post-inoculation were assayed for apoptosis and cell death using Annexin V (BD Biosciences) and 7-amino-actinomysin (7-AAD; Sigma) as described previously (Mondal et al. 2005).
Measurement of MT
The amount of MT in spleen and liver homogenates was measured by an inhibition ELISA, as previously described (Emeny et al. 2009).
Statistical analysis
LM burdens and cytokine levels were log transformed to achieve normal distributions for statistical analyses. Differences in mean levels of bacterial burden, cytokine transcript, cell death, and oxidative burst between stress treated or control conditions within each strain were determined using the t test. Error bars represent the standard error of the mean (SEM). Comparisons between B6-MTTGN, B6-MTKO, and B6-WT strains under each experimental condition were analyzed using one-way ANOVA and Tukey’s Multiple Comparison Post Test. Statistical analyses were performed with the software package SAS (Version 9.1 for Windows, SAS-Institute, Cary, North Carolina, USA). Differences were considered significant if p < 0.05.
Results
Altered MT gene dose enhances resistance to LM infection
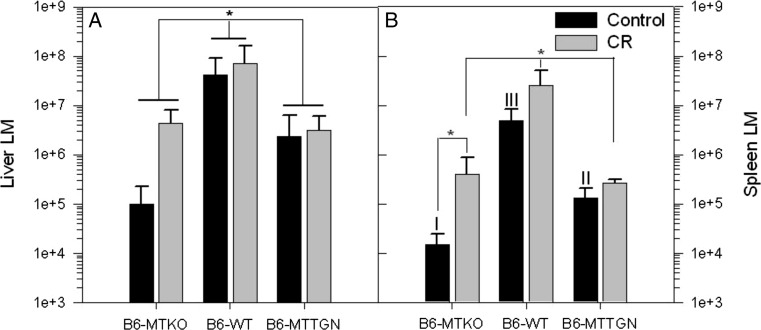
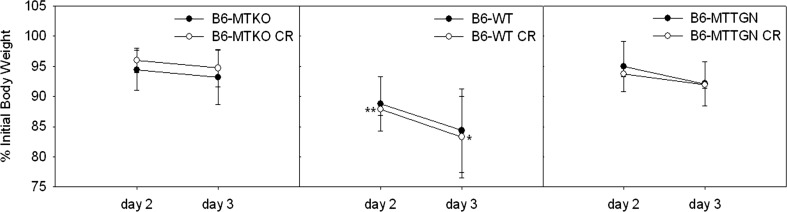
On day 3 post-infection bacterial burdens in livers of B6-WT mice were significantly higher than those of B6-MTTGN or B6-MTKO mice, when comparisons were made without treatment stratification (Fig 1a). Bacterial burdens in the spleens of infected, non-CR-stressed B6-WT mice were highest, followed by the LM burdens of B6-MTTGN mice, and were lowest in B6-MTKO mice (Fig. 1b). Similarly, CR-stressed B6-WT mice had significantly higher LM burdens in spleens compared to those of either the CR-stressed B6-MTTGN or B6-MTKO mice. CR stress-treated B6-MTKO mice had significantly increased LM burdens in the spleen compared to untreated B6-MTKO mice; whereas B6-MTTGN mice displayed the least effects of CR stress on the LM burden. LM infection caused significant wasting (loss of body weight expressed as a percentage of initial body weight) of B6-WT mice compared to that of either MT congenic strain on both day 2 and day 3 (Fig. 2). There were no differences due to CR stress in any of the three strains. The B6-WT mice had the greatest body burden of LM, and they experienced the greatest wasting effect, which supports the influence of infection on the neuroimmune induction of sickness behavior (Kelly et al. 2003).
Fig. 1.
CR stress impairs host defenses against LM in MTKO mice. Six mice from each strain were infected intravenously with 14,000 cfu LM, three mice per strain received CR stress prior to LM inoculation. Livers (a) and spleens (b) were collected 3 days post-inoculation, and organ homogenates were plated to determine total bacterial burden (cfu) in each tissue. Statistical comparisons of log transformed LM cfu levels between strains were analyzed by ANOVA. Between treatment group comparisons within each strain were measured by t tests. Error bars represent the standard error of the mean (SEM). In the liver, statistically significant differences were observed between strains, without stratification of CR stress treated or control groups, *p < 0.05. In the spleen, I versus II versus III indicate significantly different cfu levels between all strains p < 0.001
Fig. 2.
Weight loss induced by LM infection. Six mice from each strain were infected intravenously with LM at a standard inoculum of 14,000 cfu. Half of each group was treated with CR stress prior to bacterial infection. Significant wasting (loss of body weight expressed as a percentage of initial body weight) was measured on day 2 and 3 after LM inoculation. Irrespective of stress treatment, B6-WT mice exhibited greater wasting on both days 2 and 3 compared to mice of either MT congenic strain, *p < 0.05 and **p < 0.001 as assessed by one - way ANOVA
Cytokine production differs by strain and treatment
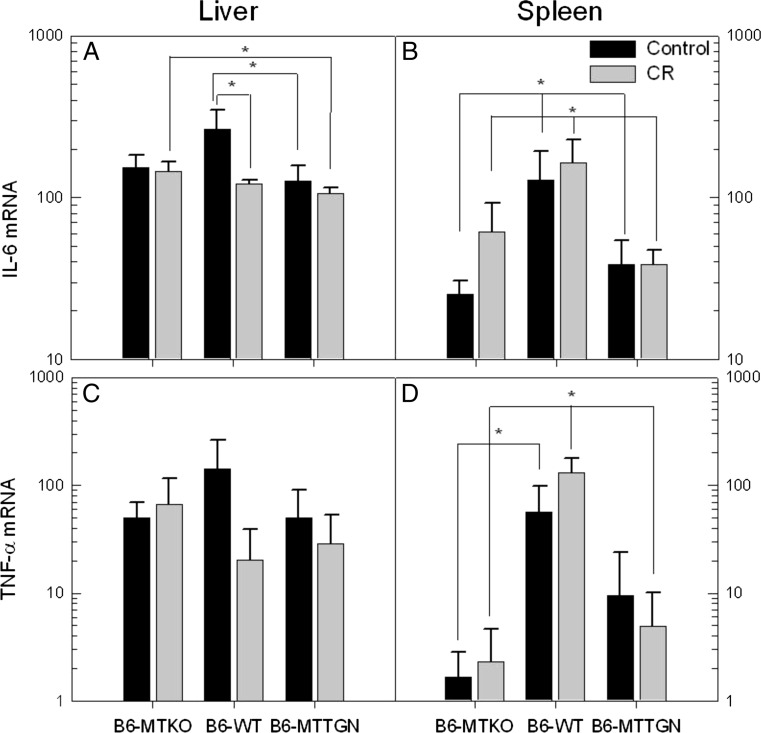
IL-6 production in livers from control B6-WT mice was significantly higher than that of infected control B6-MTTGN mice (Fig. 3a). CR stress reduced IL-6 mRNA expression of infected B6-WT mice. IL-6 expression was lower in the CR-stressed B6-MTTGN mice than in the CR-stressed B6-MTKO mice. No differences in liver expression of either TNFα (Fig. 3c) or IFNγ (data not shown) were observed.
Fig. 3.
MT levels and CR stress cause differential expression of IL-6 and TNFα. On day 3 post inoculation, RNA from isolated livers (a, c) and spleens (b, d) was assayed for inflammatory cytokines. IFNγ levels also were measured but no differences between strains or treatment groups were observed (data not shown). Statistical comparisons of log transformed mRNA cytokine levels between strains were analyzed by ANOVA. Comparisons between treatment groups within each strain were measured by t tests. Error bars represent the standard error of the mean (SEM), *p < 0.05
Cytokine expression in the infected spleens differed significantly between B6-WT mice and the MT congenic strains (Fig. 3b, d). IL-6 expression in infected B6-WT spleens was significantly higher than the levels of either B6-MTTGN or B6-MTKO infected mice, under both CR-stressed and control conditions. CR stress appeared to cause an increase in IL-6 expression in the spleens of B6-MTKO infected mice; however, this difference was not significant (p = 0.0568, Fig. 3b). A similar pattern for another acute phase cytokine, TNFα, was also observed in these strains under infection and stress (Fig. 3d). TNFα expression caused by infection alone was greater in B6-WT mice than in B6-MTKO mice and B6-MTTGN, but the latter comparison did not achieve significance. In CR stress-treated animals, TNFα was higher in B6-WT mice compared to either congenic strains. Within each strain, no differences were observed in TNFα expression due to the CR stress treatment.
ROS levels are altered by MT gene dose and CR stress treatment in the liver and spleens of LM-infected mice
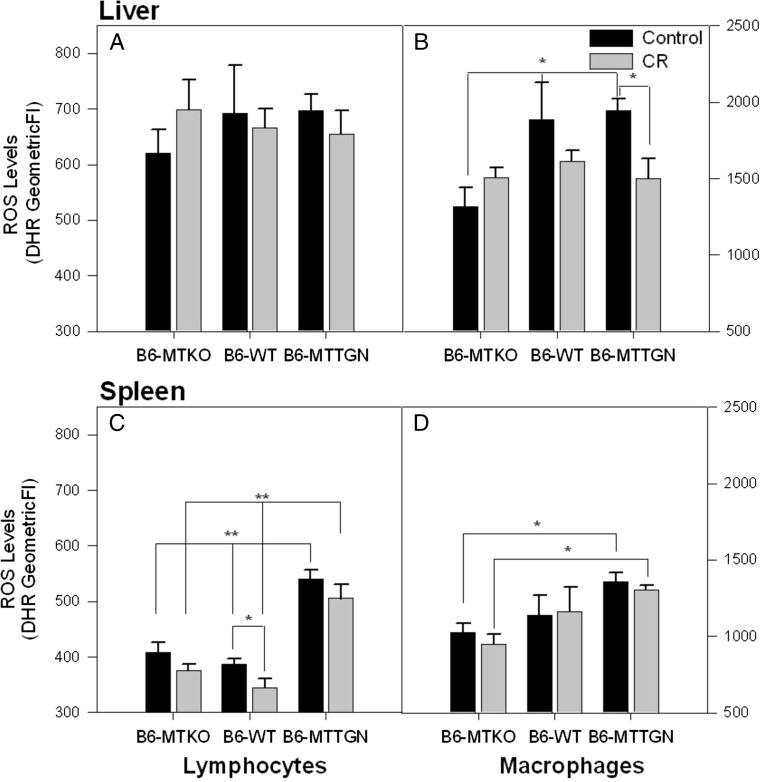
There were no differences in reactive oxygen species (ROS) produced by hepatic lymphocytes in any of the LM-infected mice, irrespective of genotype or CR stress (Fig. 4a). Kupffer cell (hepatic macrophage) ROS production was lower in infected B6-MTKO mice when compared to either B6-WT or B6-MTTGN mice (Fig. 4b). CR stress significantly lowered the ROS burst in Kupffer cells of infected B6-MTTGN mice compared to control B6-MTTGN mice, and it diminished ROS production by Kupffer cells from infected B6-WT mice, but this change did not reach statistical significance.
Fig. 4.
ROS levels in Kupfer cells and splenic lymphocytes and macrophages are altered by MT gene dose and CR stress treatment. On day 3 post inoculation, hepatic leukocytes (a Lymphocytes and b Kupffer cells) and splenocytes (c Lymphocytes and d macrophages) were tested for reactive oxygen species (ROS) levels (n = 3 per group). ROS was measured by levels of oxidized dihydrorhodamine 123 (DHR-123). Statistical comparisons of ROS levels between strains were analyzed by ANOVA. Comparisons between treatment groups within each strain were measured by t tests. Error bars represent the standard error of the geometric mean of the fluorescent intensity (GeomeanFI), *p < 0.05 and **p < 0.001
A striking result was the significantly (p < 0.001) higher levels of ROS observed in splenic lymphocytes harvested from infected B6-MTTGN mice compared to the other two strains, under both CR stress-treated and control conditions (Fig. 4c). In all strains, CR stress reduced the levels of ROS produced by splenic lymphocytes, albeit to a statistically significant level in only the infected WT mice; with B6-MTKO mice, the effect was nearly significant (p = 0.056). A similar ROS production pattern was observed in splenic macrophages. Splenic macrophages from infected B6-MTTGN mice produced significantly higher levels of ROS than from B6-MTKO mice under either control or CR stress-treated conditions. The ROS production by splenic macrophages was not altered by CR stress (Fig. 4d).
Splenic apoptosis is increased by a lack of MT
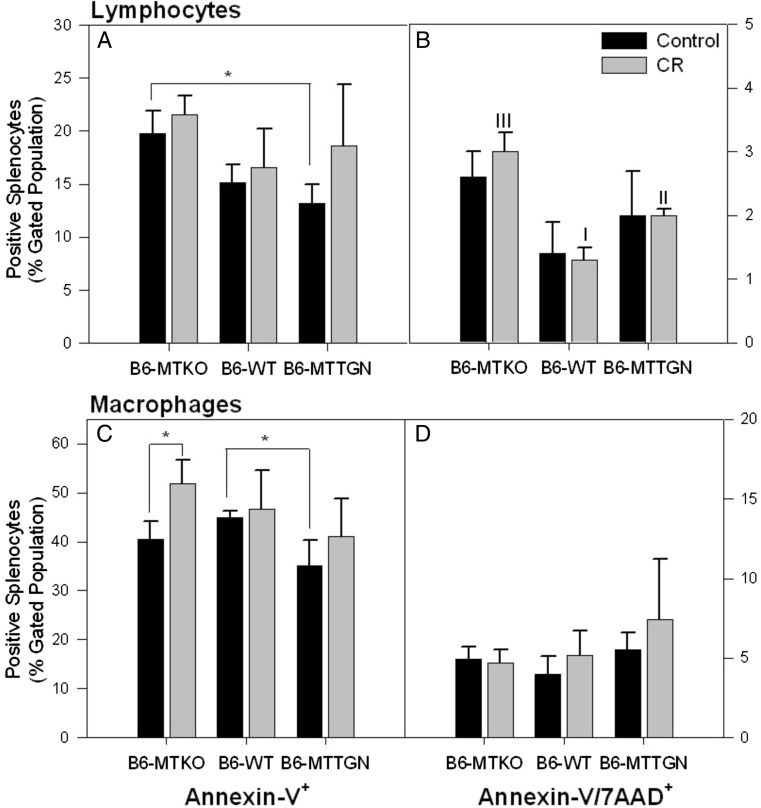
Early apoptosis (measured by Annexin-V labeling) of splenic lymphocytes was higher for cells from infected B6-MTKO mice compared to those from infected B6-MTTGN mice under control conditions (Fig. 5a). CR stress significantly increased late apoptosis (measured by Annexin-V+/7AAD+ labeling) in lymphocytes from infected B6-MTKO mice to levels higher than those found in cells from infected B6-MTTGN or B6-WT mice (Fig. 5b). Less early-apoptosis was observed in macrophages from B6-MTTGN mice than in macrophages from B6-WT mice (Fig. 5c). A significant stress-induced increase in early apoptosis was only observed in macrophage populations from B6-MTKO. No differences were observed in late apoptosis of splenic macrophages due to either genetic or stress conditions (Fig. 5d).
Fig. 5.
CR stress treatment differently affects apoptosis of spleen immune cells in MT altered strains. Apoptosis was measured on day 3 post inoculation in isolated splenic lymphocyte (a, b) and macrophage (c, d) populations (n = 3 per group). Surface expression of phosphatidylserine by binding of fluorescented-Annexin V is a flow cytometric measurement of early apoptosis (a and c). Loss of viability is measured by uptake of 7-AAD and in combination with Annexin V expression indicates late apoptosis (b and d). The percentage of leukocyte populations with positive expression of apoptotic markers was compared. Statistical comparisons of frequencies between strains were analyzed by ANOVA. Comparisons between treatment groups within each strain were measured by t tests. Error bars represent the standard error of the mean (SEM), * indicates significance p < 0.05. I versus II versus III indicate significantly different expression levels between all strains p < 0.001
MT protein expression
The expression of MT protein in spleen and liver homogenates was measured by a competition ELISA, as previously described (Emeny et al. 2009). Although the B6-MTTGN mice display improved host defenses against LM in comparison to B6-WT mice, their splenic and hepatic baseline MT levels were similar, and after LM infection, while splenic MT levels increased in both strains, the levels still did not differ between the strains. However, hepatic MT levels of B6-MTTGN mice were significantly greater than those of B6-WT mice after LM and LM + CR (Table 1). Interestingly, due to the slight (non-significant) decline of MT in the B6-WT spleens due to LM + CR and the slight (non-significant) elevation of MT in the spleens of the B6-MTTGN mice, there was a significant difference in spleens between these strains with LM + CR. Not surprisingly, the hepatic levels of MT were greater than the splenic levels. Although the non-treated B6-WT and B6-MTTGN mice had equivalent levels of hepatic MT, listeria-infected B6-MTTGN mice had greater increases of hepatic MT (about 4 fold) than the B6-WT mice (<3 fold). Similar to observations in spleen, CR did not significantly increase the hepatic MT levels of LM-infected B6-WT or B6-MTTGN mice above those infected without CR.
Table 1.
MT protein expression 3 days after LM infection with and without CR treatment‡
| Strain | Spleen | Liver | ||||
|---|---|---|---|---|---|---|
| Control* | LM | LM + CR | – | LM | LM + CR | |
| B6-WT | ND** | 4.1 ± 0.5 | 3.1 ± 0.9 | 2.5 ± 0.5 | 7.4 ± 3.3 | 6.3 ± 2.8 |
| B6-MTTGN | ND | 4.8 ± 0.9 | 6.0 ± 0.5a | 2.2 ± 0.4 | 20.4 ± 3.0b,d | 24.4 ± 2.3c,d |
‡Mean ± SD of μg MT per mg protein; *Non-infected mice; ND not detectable
aSpleens from B6-TTGN mice differed (p = 0.0082) from those of B6-WT mice after LM + CR
bLivers from B6-TTGN mice differed (p = 0.0072) from those of B6-WT mice after LM
cLivers from B6-TTGN mice differed (p < 0.001) from those of B6-WT mice after LM + CR
dLivers from LM and LM + CR treated B6-TGGN mice differed (p < 0.001) from non-treated (control) B6-MTTGN mice
Discussion
The results of these experiments indicate that the levels of MT expression differentially affect LM infections as well as infections that occur in the presence of additional physical/psychological stress from CR. Paradoxically, both greater and lesser amounts of MT than those of B6-WT mice can be associated with lower LM burdens even with additional CR stress. This correlates with other observations of ours: that MTKO mice have heightened humoral responses (Crowthers et al 2000) while injections of MT can suppress a humoral response (Lynes et al. 1993).
Influences of MT expression were observed for IL-6 and TNFα production, ROS generation, and levels of apoptosis in immune cells of the liver and spleen. Interestingly, enhanced MT expression of B6-MTTGN mice prevented an increased LM burden with LM + CR, and even though host defenses were improved in B6-MTKO mice, there was still an increased LM body burden with CR. This suggests that there are differential immune response mechanisms ongoing in the three strains, and MT levels likely are affecting both host defenses and LM pathogenesis. As previously reported (Emeny et al. 2007; Cao et al. 2002), catecholamine release from the sympathetic nervous system suppresses host defenses, and CR exacerbates this suppression. CR influences catecholamine effects on hepatic glutathione expression (Simmons et al. 1991). Since MT affects oxidative stress, it is posited that the CR induced elevation of LM burden in the livers of B6-MTKO and B6-WT mice that have less MT than the B6-MTTGN mice is due to elevated oxidative stress in the B6-KO and B6-WT strains.
The level of the acute phase cytokine IL-6 was significantly higher in spleens from B6-WT mice than in mice with manipulations of MT gene dose, which reflects their differences in LM burdens. For all three strains, the splenic levels of IL-6 were correlated well with the LM burden. The higher levels of IL-6 in the B6-WT mice also correlated well with their greater losses of body weight, which is a good indicator of the degree of sickness behavior (Kent et al. 1992). IL-6 expression is implicated in the establishment of sickness behavior, and increased levels of IL-6 have been reported to increase sickness behavior (Dyatlov and Lawrence 2002; Harden et al. 2008). It has been reported that with greater LM burdens the levels of IL-6 rise in conjunction with the levels of corticosterone, as indicators of the occurrence of elevated stress (Kim et al. 2001). Surprisingly, IL-6 mRNA expression in the liver did not correlate well with the LM burden. In the liver, mice expressing excess MT had the lowest levels of IL-6, implicating a role for MT in anti-inflammatory regulation. Interestingly, IL-6 has been shown to be an inducer of MT, and this mechanism (in association with glucocorticoid-mediated control) can be responsible for increases in MT synthesis during restraint stress (Hernández et al. 2000). Although the MT levels modified host defenses against LM, the infection with or without the extra stress from CR significantly increased the expression of MT only in the B6-TGGN mice. Our results further underscore the close interplay between redox regulation and inflammation (Loukili et al. 2010).
With CR, which was administered prior to LM inoculation, the bacterial burden was significantly increased in spleens of B6-MTKO, suggesting that MT is required during an enhanced stress response to facilitate optimal antibacterial defenses. This was observed in B6-MTKO liver as well, but the difference did not achieve significance. Stress-induced increases in IL-6 production were only observed in B6-WT mice. The MT changes in the liver were in reasonable agreement with the changes of LM burden, cytokines, and ROS; however, there was less consistency with the MT expression in the spleens and the biomarkers assessed after LM and LM + CR. The major source of MT comes from the liver, not the spleen, so more inconsistency in the spleen seems reasonable. The LM and CR effects need to be considered in a more systems biology manner. Only spleens and livers were assayed, because these are the two organs with the greatest LM burden after the intravenous injection of LM. The MT levels may be more fluid along with cytokines, and thus, the spleen levels may not reflect the localized level along with the LM burden. The ROS expression would be expected to be mainly local to the spleen and liver but their levels as well as those of MT and cytokine may not be temporally positively correlated, that is, as the LM burden continues to rise the MT levels may be already declining in the spleen.
ROS production was enhanced in splenic macrophages of B6-MTTGN mice compared to B6-MTKO mice regardless of stress treatment. This apparently beneficial consequence of an increased amount of MT on ROS production was abrogated by the stress from CR, as ROS production was diminished in the B6-MTTGN Kupffer cells. The ROS production of splenic lymphocytes in WT mice also was diminished by CR, which supports the suggestion that CR, which has been shown to influence the redox state of immune cells (Emeny and Lawrence 2007) prior to infection, may limit the antimicrobial capacities of immune cells needing to generate ROS to aid intracellular killing of LM. CR induced immediate enhancement of perforin expression in the liver indicating early innate immunity, which may interfere with later phagocyte functions (Emeny et al. 2007). CR also seems to enhance early production of nitric oxide within 2 h of LM infection (work in progress). Thus, the suggestion is that early innate immunity may delay onset of acquired immunity or lower adaptive killing potential similar to compensatory anti-inflammatory response (CARS) inhibiting systemic inflammatory response syndrome (SIRS) as can occur with sepsis (Shubin et al. 2011).
Analyses of apoptosis corroborate previous reports, in that apoptosis was increased in mice lacking MT (Emeny et al. 2009; Ren et al. 2014; Yang et al. 2015). Increased apoptosis in MTKO mice may be due to a lack of zinc caused by a deficit in its carrier protein MT (Zalewski et al. 1993). Alternatively, zinc deficiency has been shown to cause increased susceptibility to LM by diminishing DTH responses without altering bacterial clearance (Carlomagno et al. 1986). The stress-associated increased apoptosis observed in splenic macrophages of B6-MTKO mice may explain the stress-associated increase in splenic LM burden.
Overall, these results provide evidence for a role of MT in generating an efficient oxidative burst in macrophages and lymphocytes and in preventing the initiation of apoptosis in macrophages. In addition to the MT level affecting the redox status of cells (Maret 2011; Vašák and Meloni 2011; Torreggiani et al. 2013), MT may be affecting cation levels, including Ca2+, which directly and indirectly can affect oxidative and ER stress (Dai et al. 2012; Hu et al. 2013) as well as immunity.
There is a growing body of data to suggest that MT plays many and varied roles in the mounting of an effective immune response. In addition to the data discussed above, MT has been found to stimulate lymphocyte proliferation and chemotaxis (Lynes et al. 1990; Yin et al 2005). MT has also been shown to influence the progression of bacterial and viral infections (Emeny et al. 2009; Raymond et al. 2010), to influence the progression of autoimmune disease and the onset and severity of inflammation (Youn et al. 2002; Lynes et al. 1999; Inoue et al 2009; Waeytens et al. 2009). Alteration of MT gene dose or the extracellular pool of MT proteins can also influence the progression of an immune response (Emeny et al. 2009; Canpolat and Lynes 2001; Devisscher et al. 2014). Taken together, these results suggest that MT will be an important target for manipulation of immune function in instances where overexpression is deleterious, and where inadequate MT is not conducive to an effective immune response.
Acknowledgments
This work was supported, in part, by U01 ES016014 (DAL) and R01 ES007408 (MAL).
References
- Borghesi LA, Youn J, Olson EA, Lynes MA. Interactions of metallothionein with murine lymphocytes: plasma membrane binding and proliferation. Toxicology. 1996;108(1-2):129–140. doi: 10.1016/S0300-483X(95)03243-9. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cao L, Filipov NM, Lawrence DA. Sympathetic nervous system plays a major role in acute cold/restraint stress inhibition of host resistance to Listeria monocytogenes. J Neuroimmunol. 2002;125:94–102. doi: 10.1016/S0165-5728(02)00039-5. [DOI] [PubMed] [Google Scholar]
- Cao L, Hudson CA, Lawrence DA. Immune changes during acute cold/restraint stress-induced inhibition of host resistance to Listeria. Toxicol Sci. 2003;74:325–334. doi: 10.1093/toxsci/kfg146. [DOI] [PubMed] [Google Scholar]
- Cao L, Hudson CA, Lawrence DA. Acute cold/restraint stress inhibits host resistance to Listeria monocytogenes via beta1-adrenergic receptors. Brain Behav Immun. 2003;17:121–133. doi: 10.1016/S0889-1591(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Canpolat E, Lynes MA. In vivo manipulation of endogenous metallothionein with a monoclonal antibody enhances a T-dependent humoral immune response. Toxicol Sci. 2001;62:61–70. doi: 10.1093/toxsci/62.1.61. [DOI] [PubMed] [Google Scholar]
- Carlomagno MA, Coghlan LG, McMurray DN. Chronic zinc deficiency and listeriosis in rats: acquired cellular resistance and response to vaccination. Med Microbiol Immunol. 1986;175:271–280. doi: 10.1007/BF02126048. [DOI] [PubMed] [Google Scholar]
- Crowthers KC, Kline V, Giardina C, Lynes MA. Augmented humoral immune function in metallothionein-null mice. Toxicol Appl Pharmacol. 2000;166:161–172. doi: 10.1006/taap.2000.8961. [DOI] [PubMed] [Google Scholar]
- Dai ZK, Qin JK, Huang JE, Luo Y, Xu Q, Zhao HL. Tanshinone IIA activates calcium-dependent apoptosis signaling pathway in human hepatoma cells. J Nat Med. 2012;66(1):192–201. doi: 10.1007/s11418-011-0576-0. [DOI] [PubMed] [Google Scholar]
- Devisscher L, Hindryckx P, Lynes M, Waeytens A, Cuvelier C, De Vos F, Vanhove C, De Vos M, Laukens D. Role of metallothioneins as danger signals in the pathogenesis of colitis. J Pathol. 2014;233:89–100. doi: 10.1002/path.4330. [DOI] [PubMed] [Google Scholar]
- Dyatlov VA, Lawrence DA. Neonatal lead exposure potentiates sickness behavior induced by Listeria monocytogenes infection of mice. Brain Behav Immun. 2002;16:477–492. doi: 10.1006/brbi.2001.0641. [DOI] [PubMed] [Google Scholar]
- Emeny RT, Gao D, Lawrence DA. Beta1-adrenergic receptors on immune cells impair innate defenses against Listeria. J Immunol. 2007;178:4876–4884. doi: 10.4049/jimmunol.178.8.4876. [DOI] [PubMed] [Google Scholar]
- Emeny RT, Lawrence DA. Cold-restraint-induced immune and biochemical changes inhibit host resistance to Listeria. In: Ader R, editor. Psychoneuroimmunology, Vol 2. 4. London: Elsevier Academic Press; 2007. pp. 1035–1053. [Google Scholar]
- Emeny RT, Marusov G, Lawrence DA, Pederson-Lane J, Yin X, Lynes MA. Manipulations of metallothionein gene dose accelerate the response to Listeria monocytogenes. Chem Biol Interact. 2009;181(2):243–253. doi: 10.1016/j.cbi.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Jacob ST. Regulation of metallothionein gene expression. Prog Nucleic Acid Res Mol Biol. 2001;66:357–384. doi: 10.1016/S0079-6603(00)66034-8. [DOI] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008;22:838–849. doi: 10.1016/j.bbi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Hernández J, Carrasco J, Belloso E, Giralt M, Bluethmann H, Kee Lee D, Andrews GK, Hidalgo J. Metallothionein induction by restraint stress: role of glucocorticoids and IL-6. Cytokine. 2000;12:791–796. doi: 10.1006/cyto.1999.0629. [DOI] [PubMed] [Google Scholar]
- Hidalgo J, Armario A, Flos R, Dingman A, Garvey JS. The influence of restraint stress in rats on metallothionein production and corticosterone and glucagon secretion. Life Sci. 1986;39(7):611–616. doi: 10.1016/0024-3205(86)90041-X. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Shimada A, Satoh M. Metallothionein as an anti-inflammatory mediator. Mediators Inflamm. 2009;2009:101659. doi: 10.1155/2009/101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Han X, Lane EK, Gao F, Zhang Y, Ren J. Cardiac-specific overexpression of metallothionein rescues against cigarette smoking exposure-induced myocardial contractile and mitochondrial damage. PLoS One. 2013;8(2):e57151. doi: 10.1371/journal.pone.0057151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-U. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–S118. doi: 10.1016/S0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kim D, Reilly A, Lawrence DA. Relationships between IFNgamma, IL-6, corticosterone, and Listeria monocytogenes pathogenesis in BALB/c mice. Cell Immunol. 2001;207:13–18. doi: 10.1006/cimm.2000.1749. [DOI] [PubMed] [Google Scholar]
- Laukens D, Waeytens A, De Bleser P, Cuvelier C, De Vos M. Human metallothionein expression under normal and pathological conditions: mechanisms of gene regulation based on in silico promoter analysis. Crit Rev Eukaryot Gene Expr. 2009;19(4):301–317. doi: 10.1615/CritRevEukarGeneExpr.v19.i4.40. [DOI] [PubMed] [Google Scholar]
- Loukili N, Rosenblatt-Velin N, Rolli J, Levrand S, Feihl F, Waeber B, Pacher P, Liaudet L. Oxidants positively or negatively regulate nuclear factor kappaB in a context-dependent manner. J Biol Chem. 2010;285:15746–15752. doi: 10.1074/jbc.M110.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MA, Borghesi LA, Youn J, Olson EA. Immunomodulatory activities of extracellular metallothionein. I. Metallothionein effects on antibody production. Toxicology. 1993;85:161–177. doi: 10.1016/0300-483X(93)90040-Y. [DOI] [PubMed] [Google Scholar]
- Lynes MA, Garvey JS, Lawrence DA. Extracellular effects of metallothionein on lymphocyte activities. Mol Immunol. 1990;27:211–219. doi: 10.1016/0161-5890(90)90132-J. [DOI] [PubMed] [Google Scholar]
- Lynes MA, Richardson CA, McCabe R, Crowthers KC, Lee JC, Youn J, Schweitzer IB, Shultz LD. In: Metallothionein-mediated changes in cell populations of autoimmune mice. MT IV Klaassen C, editor. Basel: Birkhauser Verlag; 1999. pp. 437–444. [Google Scholar]
- Lynes MA, Zaffuto K, Unfricht DW, Marusov G, Samson JS, Yin X. The physiological roles of extracellular metallothionein. Exp Biol Med (Maywood) 2006;231(9):1548–1554. doi: 10.1177/153537020623100915. [DOI] [PubMed] [Google Scholar]
- Manuel Y, Thomas Y, Pellegrini O. Metallothionein and tissue damage. IARC Sci Publ. 1992;118:231–237. [PubMed] [Google Scholar]
- Maret W. Redox biochemistry of mammalian metallothioneins. J Biol Inorg Chem. 2011;16(7):1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, Brinster RL, Palmiter RD. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci. 1994;14:5844–5857. doi: 10.1523/JNEUROSCI.14-10-05844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal TK, Li D, Swami K, Dean JK, Hauer C, Lawrence DA. Mercury impairment of mouse thymocyte survival in vitro: involvement of cellular thiols. J Toxicol Environ Health A. 2005;68:535–556. doi: 10.1080/15287390590909706. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Sandgren EP, Koeller DM, Brinster RL. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol Cell Biol. 1993;13:5266–5275. doi: 10.1128/MCB.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP, Glavin GB. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev. 1986;10:339–370. doi: 10.1016/0149-7634(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Poston RM, Kurlander RJ. Cytokine expression in vivo during murine listeriosis. Infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J Immunol. 1992;149:3040–3044. [PubMed] [Google Scholar]
- Raymond AD, Gekonge B, Giri MS, Hancock A, Papasavvas E, Chehimi J, Kossenkov AV, Nicols C, Yousef M, Mounzer K, Shull J, Kostman J, Showe L, Montaner LJ. Increased metallothionein gene expression, zinc, and zinc-dependent resistance to apoptosis in circulating monocytes during HIV viremia. J Leukoc Biol. 2010;88(3):589–596. doi: 10.1189/jlb.0110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Wang YM, Zhao J, Zhao J, Zhao ZM, Zhang TF, He J, Ren SP, Peng SQ. Metallothioneins attenuate paraquat-induced acute lung injury in mice through the mechanisms of anti-oxidation and anti-apoptosis. Food Chem Toxicol. 2014;73:140–147. doi: 10.1016/j.fct.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Schell RF, Lawrence DA. Differential effects of concanavalin A and phytohemagglutinin on murine immunity. Suppression and enhancement of cell-mediated immunity. Cell Immunol. 1977;31:142–154. doi: 10.1016/0008-8749(77)90013-2. [DOI] [PubMed] [Google Scholar]
- Shubin NJ, Monaghan SF, Ayala A. Anti-inflammatory mechanisms of sepsis. Contrib Microbiol. 2011;17:108–124. doi: 10.1159/000324024. [DOI] [PubMed] [Google Scholar]
- Simmons HF, James RC, Harbison RD, Patel DG, Roberts SM. Examination of the role of catecholamines in hepatic glutathione suppression by cold-restraint in mice. Toxicology. 1991;67(1):29–40. doi: 10.1016/0300-483X(91)90161-S. [DOI] [PubMed] [Google Scholar]
- Torreggiani A, Chatgilialoglu C, Ferreri C, Melchiorre M, Atrian S, Capdevila M. Non-enzymatic modifications in metallothioneins connected to lipid membrane damages: structural and biomimetic studies under reductive radical stress. J Proteomics. 2013;92:204–215. doi: 10.1016/j.jprot.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Unanue ER. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/S0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- Vašák M, Meloni G. Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem. 2011;16(7):1067–1078. doi: 10.1007/s00775-011-0799-2. [DOI] [PubMed] [Google Scholar]
- Waeytens A, De Vos M, Laukens D. Evidence for a potential role of metallothioneins in inflammatory bowel diseases. Mediat Inflamm. 2009;2009:729172. doi: 10.1155/2009/729172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. Metallothionein in regeneration, reproduction and development. Experientia Suppl. 1987;52:483–498. doi: 10.1007/978-3-0348-6784-9_49. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang J, Yang J, Schamber R, Hu N, Nair S, Xiong L, Ren J. Antioxidant metallothionein alleviates endoplasmic reticulum stress-induced myocardial apoptosis and contractile dysfunction. Free Radic Res. 2015;12:1–33. doi: 10.3109/10715762.2015.1013952. [DOI] [PubMed] [Google Scholar]
- Yin X, Knecht DA, Lynes MA. Metallothionein mediates leukocyte chemotaxis. BMC Immunol. 2005;6:21. doi: 10.1186/1471-2172-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J, Hwang S-H, Ryoo Z-Y, Lynes MA, Paik DJ, Chung HS, Kim H-Y. Metallothionein suppresses collagen-induced arthritis via induction of TGF-β and downregulation of proinflammatory mediators. Clin Exp Immunol. 2002;129:232–239. doi: 10.1046/j.1365-2249.2002.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II) Biochem J. 1993;296(Pt 2):403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]