Abstract
Mycotoxins are considered to be significant contaminants of food and animal feed. Zearalenone (ZEN) is a non-steroidal estrogenic mycotoxin produced by several species of Fusarium in cereals and agricultural products. ZEN has been shown to be cytotoxic, genotoxic, and mutagenic in different cell types. In the present study, we investigated the involvement of endoplasmic reticulum (ER) stress in ZEN-mediated toxicity in human intestine (HCT116) and kidney (HEK293) cells and evaluated the effects of the two common dietary compounds Quercetin (QUER) and Crocin (CRO). We show that ZEN treatment induces ER stress and activates the unfolded protein response (UPR) as evidenced by XBP1 mRNA splicing and upregulation of GRP78, ATF4, GADD34, PDIA6, and CHOP. Activation of the ER stress response is associated with activation of the mitochondrial pathway of apoptosis. This apoptotic process is characterized by an increase in ROS generation and lipid peroxidation, a loss of mitochondrial transmembrane potential (ΔΨm), and an activation of caspases and DNA damages. We also demonstrate that the antioxidant properties of QUER and CRO help to prevent ER stress and reduce ZEN-induced apoptosis in HCT116 and HEK293 cells. Our results suggest that antioxidant molecule might be helpful to prevent ZEN-induced ER stress and toxicity.
Keywords: Zearalenone, Crocin, Quercetin, Oxidative stress, Endoplasmic reticulum stress, Apoptosis
Introduction
Molds synthesize secondary metabolites, which are toxic compounds known as mycotoxins. The global contamination by these natural products in food, feed, and environment, including indoor surfaces and particles, form a health risk to both animals and humans (Zain 2011). Zearalenone (ZEN) [6-(10-hydroxy-6-oxo-trans-1-undecenyl)-bresorcyclic acid lactone] is a mycotoxin produced by various Fusarium fungi, including Fusarium graminearum, Fusarium culmorum, Fusarium equiseti, and Fusarium cerealis (Habschied et al. 2011; Rodrigues and Naehrer 2012) and found in grains and animal feeds (Kuiper-Goodman et al. 1987; Bryden 2012). ZEN occurs naturally all over the world in a variety of food products designed for human and animal consumption, and potentially high concentrations are encountered as contamination in many important crops (Bennett and Klich 2003). The main route of human exposure to ZEN is through ingestion of contaminated food products, such as maize, wheat, rye, and other cereals (Zinedine et al. 2007). Spontaneous outbreaks of Fusarium mycotoxicosis in humans and animals have been reported worldwide (Fung and Clark 2004).
ZEN and its metabolites exhibit potent estrogenic activity and are thus often referred to as mycoestrogens. ZEN is implicated in reproductive disorders of farm animals and occasionally in hypoestrogenic syndromes in human (Zinedine et al. 2007). In addition, cytotoxic and genotoxic activities of ZEN, which are independent of its binding affinity to estrogen receptor, have also been reported. For instance, ZEN has been demonstrated to be hepatotoxic (Maaroufi et al. 1996; Obremski et al. 1999; Conkova et al. 2001; Bouaziz et al. 2008), hematotoxic (Maaroufi et al. 1996; Murata et al. 2003; Abbes et al. 2006a, b), nephrotoxic (Ouanes et al. 2003; Abbes et al. 2006a; Liang et al. 2010), and toxic toward the intestinal tract (Abid-Essefi et al. 2003). Different reports have shown in vitro and in vivo that ZEN induces cytotoxicity through reactive oxygen species (ROS) production leading to lipid peroxidation, DNA damages, and apoptosis by the mitochondrial pathway (Abid-Essefi et al. 2004; Hassen et al. 2007; Abbes et al. 2007; Bouaziz et al. 2008). ZEN was also shown to induce endoplasmic reticulum (ER) stress-mediated apoptosis in leukemic cells (Banjerdpongchai et al. 2010).
The ER plays crucial roles in various cellular processes including protein folding, protein trafficking, and intracellular Ca2+ regulation. Impairment of the physiological function of the ER, such as accumulation of unfolded proteins, disturbance of luminal calcium homeostasis, and disruption of redox status, initiates ER stress which in turn triggers the unfolded protein response (UPR) (Szegezdi et al. 2006). The UPR is an adaptive response that acts to restore ER homeostasis by activating the three proximal sensors IRE1α (inositol-requiring enzyme 1α), PERK (PKR-like endoplasmic reticulum kinase), and ATF6 (activating transcription factor 6). Nevertheless, if ER stress is too severe or prolonged, the UPR leads to apoptosis by activating downstream effectors including CHOP (C/EBP homologous protein), JNK, caspases, and members of Bcl2 family (Ron and Walter 2007; Akazawa et al. 2004).
The prevention of ZEN toxicity involves reduction of mycotoxin levels in foodstuffs and increasing the intake of diet components such as vitamins and antioxidants. We have demonstrated that almost all ZEN toxic effects are prevented in vitro and in vivo using vitamin E (Abid-Essefi et al. 2003; Ouanes et al. 2003; 2005; El Golli et al. 2006; Hassen et al. 2007). Furthermore, a strong protection against ZEN-induced toxicity has been demonstrated to be conferred by extracts from cactus cladodes (Zourgui et al. 2008; 2009), radish (Raphanus sativus) (Ben Salah-Abbes et al. 2009), or garlic (Allium sativum) (Abid-Essefi et al. 2012). Studies on the effect of antioxidants, especially those consumed in food, thus appear of great interest to prevent ZEN-induced toxicity.
Crocin is a pharmacologically active compound of Crocus sativus L. (saffron) (Rios et al. 1996). The high antioxidant capacity of Crocin has been reported in vitro and in vivo (Ochiai et al. 2004a, b, 2007; Hosseinzadeh et al. 2009, 2010; Mousavi et al. 2010). For example, Crocin can decrease lipid peroxidation in kidney (Hosseinzadeh et al. 2005) and skeletal muscle (Hosseinzadeh et al. 2009) during ischemia-reperfusion-induced oxidative damage in rats. In addition, this carotenoid increases cell viability in PC12 cells upon serum deprivation by inducing glutathione (GSH) synthesis, increasing glutathione reductase (GR), and c-glutamylcysteinyl synthase (c-GCS) activities, and decreasing ceramide formation (Ochiai et al. 2004a, b).
Quercetin (3,5,7,3′4′-Pentahydroxy flavon), a typical member of the flavonoid family, is one of the most widely recognized dietary polyphenolic compounds. It is ubiquitously present in foods and exhibits a broad spectrum of properties, i.e., antioxidant, anti-inflammatory, and immunomodulatory (Kobylińska and Janas 2015). The protective activity of Quercetin has been largely associated with its antioxidant and anti-inflammatory properties. Indeed, within the flavonoid family, Quercetin is proven to be the most potent scavenger of free radicals (Hanasaki et al. 1994). There is evidence that Quercetin reduces low-density lipoprotein oxidation (Loke et al. 2008) and prevents the development of atherosclerotic lesions (Loke et al. 2010). It has also been reported that Quercetin inhibits the production of superoxide anion (O2·−) in rat aorta and decreases protein expression of the NADPH oxidase subunit, p47phox (Sanchez et al. 2006; Romero et al. 2009).
The present study was designed to investigate whether ZEN toxicity involves ER stress induction in human large intestine (HCT116) and kidney (HEK293) cells and to determine the effect of the antioxidant molecules Crocin and Quercetin.
Materials and methods
Chemicals
Zearalenone, Crocin, and Quercetin were purchased from Sigma–Aldrich (St. Louis, MO, USA). Dulbecco’s modified eagle medium-F12 (DMEM-F12), fetal bovine serum (FBS), phosphate buffer saline (PBS), trypsin–EDTA, penicillin and streptomycin mixture, dichlorodihydrofluorescein diacetate (DCFH-DA), propidium iodide (PI), fluorescein diacetate (FDA), 30-dihexyloxacarbocyanin iodode (DiOC6(3)), MitoSOX™ Red and Hanks’ balanced salt solution (HBSS) were supplied by Invitrogen (Saint Aubin, France). All other compounds were purchased from Sigma–Aldrich, and all the used chemicals were of analytical grade.
Cell culture and treatment
Human colon carcinoma cells HCT116 and embryonic kidney cells HEK293 were cultured in DMEM-F12 and supplemented with 10 % FBS, 1 % l-glutamine (200 mM), 1 % of mixture penicillin (100 IU/ml), and streptomycin (100 lg/ml) at 37 °C with 5 % CO2. ZEN was dissolved in pure DMSO. To obtain the studied concentrations in the cell culture media, the mycotoxin treatment volume was negligible and represented about 0.025 % of the total medium volume. In these conditions, untreated cells and cells receiving this low vehicle volume responded in the same manner. For this reason, we have chosen untreated cells as control.
Flow cytometry analysis
For flow cytometry analysis of mitochondrial transmembrane potential (ΔΨm), cells were stained with 10-nM DiOC6(3) for 20 min at 37 °C. Necrosis was estimated by adding 10 μg/ml of propidium iodide (PI) just before analysis. The fluorescent probe fluorescein diacetate (FDA) was used to assess cell viability. After treatment, cells were incubated for 5 min at 37 °C with FDA at 0.2 μg/ml. To measure the relative levels of mitochondrial superoxide anion, O2·−, the probe MitoSOX™ Red was used. Once in the mitochondria, MitoSOX™ Red reagent is oxidized by superoxide anion and exhibits red fluorescence detected by flow cytometry. Cells were centrifuged, washed with PBS, and incubated with 2 μM of MitoSOX™ Red for 10 min at 37 °C. MitoSOX™ Red fluorescence was immediately analyzed by flow cytometry (10,000 cells). Caspase-3 activation was assessed using DEVD-NucView 488 caspase-3 substrate. After treatment, cells were incubated with 5 μM DEVD-NucView 488 for 30 min at RT before cytometric analysis. Fluorescence of cells was analyzed on a Cell Lab Quanta MPL cytometer (Beckman Coulter, Villepinte, France).
Western blot analysis
Cells were lysed in RIPA lysis buffer (50 mM Tris–HCl pH 8, 150 mM NaCl, 1 % Triton, 1 mM EDTA, 0.1 % SDS, 0.5 % deoxycholic acid) plus a cocktail of protease inhibitors (Roche) and PMSF for 30 min at 4 °C. Proteins (30 μg) were separated by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Merck Millipore). Membranes were incubated overnight at 4 °C with the following antibodies: anti-GRP78 (sc-1050), anti-GADD34 (sc-8327), anti-PDIA6 (sc-107533), and anti-β-actin (sc-47778) from Santa Cruz. Proteins were detected on a ChemiDoc XRS (BioRad) by using the ECL method according to the manufacturer’s instructions (Merck Millipore). To calculate the relative density (RD), ImageJ software was used and the intensity of each protein was normalized to β-actin. The data obtained were then expressed as the ratio of the intensity of the protein in treated cells to that of the corresponding protein in untreated cells.
RNA isolation and real-time reverse transcription polymerase chain reaction
RNA was isolated from cultured cells using Zymo Research QuickRNA MiniPrep according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed using BioRad iScript reverse transcription kit. For real-time PCR, cDNA was amplified by the “two-step” SsoFast EvaGreen supermix (BioRad), heated at 95 °C for 30 s, then 50 cycles of denaturation at 95 °C during 2 s and hybridization/elongation at 60 °C during 5 s. The PCR primers were obtained from Eurofins: 5′GTCCCTCCAACAACAGCAAG3′(F) and 5′ AGGTCATCTGGCATGGTTTC3′(R) for ATF4; 5′TGCTGAGTCCGCAGCAGGTG3′(F) and 5′GCTGGCAGGCTCTGGGGAAG3′(R) for spliced XBP1 as described by van Schadewijk et al. (2012); 5′AGCGACAGAGCCAAAATCAG3′(F) and 5′ACAAGTTGGCAAGCTGGTCT 3′(R) for CHOP. The results were quantified according to the Cq value method, where Cq is defined as the quantification cycle of PCR at which the amplified product is detected. The ratio (1 + Etarget gene)^−(Cq sample−Cq control)target gene/(1+Ereference gene)^−(Cq sample−Cq control) reference gene was calculated, where E represents the efficiency of the quantitative PCR reaction. With this calculation, expression of control was equivalent to one.
Measurement of reactive oxygen species production
The intracellular amounts of ROS were measured by a fluorometric assay with 2,7-dichlorofluorescein diacetate (DCFH-DA) to detect intracellular ROS. The probe, after diffusing in the cell membrane, is hydrolyzed by intracellular esterases to non-fluorescent dichlorofluorescein (DCFH), which is trapped inside the cells then oxidized to fluorescent DCF through the action of peroxides in the presence of ROS (Le Bel et al. 1992). HCT116 and HEK293 cells were seeded on 24-well culture plates (Polylabo, France) at 105 cells/well for 24 h. After incubation, cells were treated with 20 μM DCFH-DA. Intracellular production of ROS was measured after 30 min incubation at 37 °C by fluorometric detection of DCF oxidation on a fluorimeter (Biotek FL 800×) with an excitation wavelength of 485 nm and emission wavelength of 522 nm. The DCF fluorescence intensity is proportional to the amount of ROS formed intracellularly.
Lipid peroxidation
Lipid peroxidation was assayed by the measurement of malondialdehyde (MDA) according to the method of Ohkawa et al. (1979). Cells were seeded on 6-well plates at 7.5 × 105 cells/well. After 24 h of incubation, they were exposed to ZEN in the absence or the presence of CRO and QUER for 24 h at 37 °C. The cells were then washed with cold PBS, scraped, and lysed by homogenization in ice-cold 1.15 % KCl. Samples containing 0.1 ml of cell lysates were combined with 0.2 ml of 8.1 % SDS, 1.5 ml of 20 % acetic acid adjusted to pH 3.5, and 1.5 ml of 0.8 % thiobarbituric acid. The mixture was brought to a final volume of 4 ml with distilled water and heated to 95 °C for 120 min. After cooling to room temperature, 5 ml of mixture of n-butanol and pyridine (15:1, v/v) was added to each sample and the mixture was shaken vigorously. After centrifugation at 4000 rpm for 10 min, the supernatant fraction was isolated and the absorbance measured at 546 nm. The concentration of MDA was determined according to a standard curve.
DNA damage assessed by the comet assay
Single cell gel electrophoresis (SCGE) is a visual and sensitive technique for measuring DNA breakage in individual mammalian cells. HCT116 and HEK293 cells were seeded on 6-well culture plates (Polylabo, France) at 7.5 × 105 cells/well for 24 h of incubation and were re-incubated as described above in the presence of ZEN in the absence or the presence of QUER/CRO for 24 h at 37 °C. Approximately 2 × 104 cells were mixed with 1 % low melting point (LMP) agarose in PBS and spread on a microscope slide previously covered with a 1 % normal melting agarose (NMP) in PBS layer. After agarose solidification, cells were treated with an alkaline lysis buffer (2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, pH 10, 1 % (v/v) Triton X-100, and 10 % (v/v) DMSO) for 1 h at 4 °C, then the DNA was allowed to unwind for 40 min in the electrophoresis buffer (0.3 M NaOH, 1 mM EDTA, pH>13). The slides were then subjected to electrophoresis in the same buffer for 30 min at 25 V and 300 mA. Slides were then neutralized using a Tris buffer solution (0.4 M Tris, pH 7.5) for 15 min. After staining the slides with ethidium bromide (20 μg/ml), the comets were detected and scored using a fluorescence microscope. The experiment was done in triplicate. The damage is represented by an increase of DNA fragments that have migrated out of the cell nucleus during electrophoresis and formed an image of a “comet” tail. A total of 100 comets on each slide were visually scored according to the intensity of fluorescence in the tail and classified by one of five classes as described by Collins et al. (1996). The total score was evaluated according to the following equation: (% of cells in class 0 × 0) + (% of cells in class 1 × 1) + (% of cells in class 2 × 2) + (% of cells in class 3 × 3) + (% of cells in class 4 × 4).
Statistical analysis
Each experiment was done three times separately. Values were presented as means ± SD One-way ANOVA was used to assess differences among groups followed by Dunnett’s post hoc test. When two groups were compared, differences were assessed by Student’s t test. Differences were considered significant at P < 0.05.
Results
ZEN induces cell death and ER stress in HCT116 and HEK293 cells
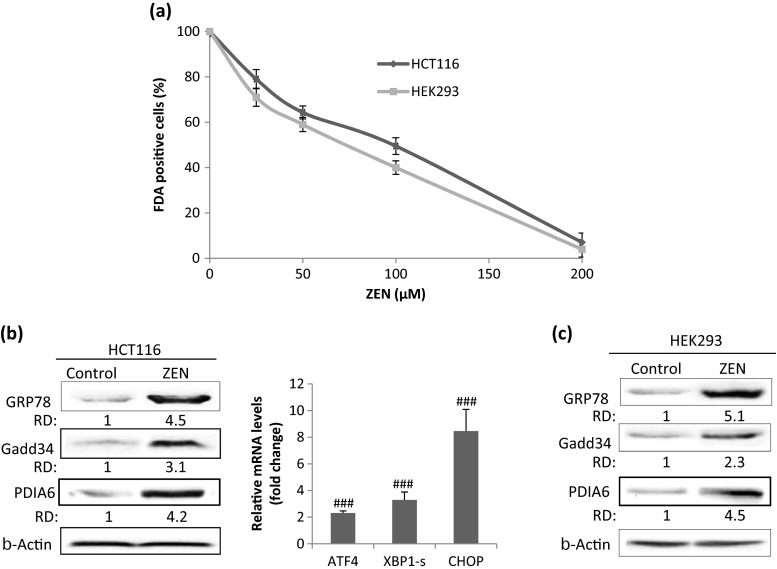
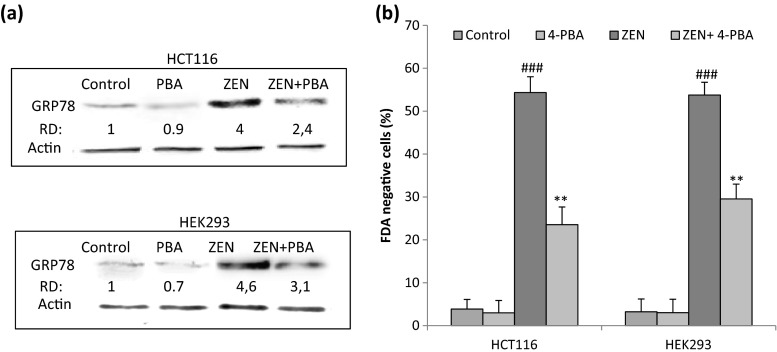
To determine the cytotoxicity of Zearalenone, HCT116 and HEK293 cell lines were exposed to increasing concentrations of ZEN ranging from 25 to 200 μM for 24 h and cell viability was determined by FDA assay. Our results indicate that ZEN induced a marked decrease in cell viability in a dose-dependent manner in the two cell lines (Fig. 1a). The IC50 values, determined after 24 h of cell treatment, were about 100 and 75 μM, respectively, in HCT116 and HEK293 cells. We next investigated whether ER stress and UPR are activated in response to ZEN. HCT116 cells were incubated with ZEN, and the expression of ER stress markers and UPR target genes was measured by western blot and quantitative RT-PCR (Fig. 1b). After treatment with ZEN, an increase in the level of GRP78 and GADD34, two well-known ER stress markers was observed (Fig. 1b, left panel). ZEN also induced the upregulation of ATF4, involved in the PERK branch of the UPR, increased the expression of PDIA6, suggesting the induction of ATF6 branch and induced the splicing of XBP1 (XBP1-s), indicative of the activation of IRE1 branch (Fig. 1b, right panel). In addition, ZEN increased by about 8.45-fold the expression of CHOP, a major component of the ER stress-mediated apoptosis pathway. Taken together, these results indicate that ZEN induces ER stress and activates different branches of the UPR in HCT116 cells. Similarly, ZEN-induced ER stress in HEK293 cells as shown by the increase in the level of GRP78 and GADD34 (Fig. 1c). To confirm the relationship between ZEN-induced ER stress and cell death, cells were pre-incubated for 2 h with the chemical chaperone 4-phenylbutyric acid (PBA) before ZEN treatment and cell mortality (FDA negative cells) and expression of GRP78 were analyzed after 24 h. By stabilizing protein conformation, PBA is known to alleviate ER stress (Morris et al. 1997; Ozcan et al. 2006). As expected, we observed that PBA greatly decreased protein level of the ER stress marker GRP78 (Fig. 2a) in HCT116 and HEK293 cells. In addition, PBA significantly decreased cell mortality induced in response to ZEN (Fig. 2b).
Fig. 1.
ZEN induces ER stress and cell death in HCT116 and HEK293 cells. (a) Cells were treated with ZEN at the indicated concentrations for 24 h. Cell viability was determined using the FDA assay and expressed as percentages of FDA positive cells (viable cells). Data are expressed as the mean ± SD of three independent experiments. Values are significantly different (P < 0.05) from control. (b) HCT116 cells were incubated for 24 h with ZEN (100 μM), and protein levels of the ER stress markers GRP78, GADD34, and PDIA6 were analyzed by western blot. The relative mRNA levels of the UPR target genes ATF4, spliced XBP1 (XBP1-s), and CHOP were quantified by qRT-PCR and expressed as fold change over untreated controls. Data are expressed as the mean ± SD of three separate experiments. ### P<0.001 vs. control (c) HEK293 cells were incubated for 24 h with ZEN (75 μM), and protein levels of the ER stress markers GRP78 and GADD34 were analyzed by western blot. β-actin was used as a loading control. RD relative density
Fig. 2.
PBA inhibits ZEN-induced ER stress and cell death. Cells were pretreated for 2 h with 1 mM PBA before treatment with ZEN at the indicated concentrations for 24 h. (a) The protein level of the ER stress marker GRP78 was analyzed by western blot. (b) Cell death was measured using FDA assay. Data are expressed as the mean ± SD of three separate experiments. ### P≤0.001 vs. control, **P≤0.01 vs. ZEN alone
Effect of Quercetin and Crocin on ZEN-induced ROS generation and lipid peroxidation
The toxicity of ZEN has been linked to the generation of oxidative stress in different cells (Hassen et al. 2007). Therefore, we evaluate whether Quercetin (QUER) and Crocin (CRO) exert their proposed antioxidant properties and modulate the level of ROS induced by ZEN in HCT116 and HEK293 cells. The level of intracellular ROS was measured after ZEN treatment in the absence or presence of QUER (5 μM) or CRO (250 μM) by recording the fluorescence of DCF, which is the result of DCFH oxidation mainly by H2O2. As shown in Fig. 3a, ZEN treatment induced an important increase in ROS production to about 2.57- and 1.74-fold to control, respectively, in HCT116 and HEK293 cells. Pretreatment with QUER or CRO totally abolished the intracellular ROS generated by ZEN in the two cell lines. Mitochondrial superoxide anion (O2·−) levels were examined by staining cells with the MitoSOX™ Red probe. Mitochondrial O2·− level increased in HCT116 cells from 5.64 ± 1.12 % in control to 40.85 ± 3.5 % in the presence of ZEN and from 4.65 ± 1.32 % in control to 42.32 ± 3.37 % in HEK293-treated cells. The presence of QUER or CRO significantly reduced the percentage of MitoSOX high cells induced by ZEN (Fig. 3b), reflecting a marked decrease in the level of O2·−. Lipid peroxidation was also measured by MDA assay in response to ZEN. After 24 h incubation with ZEN, the MDA level was 1.20 ± 0.11 μmol MDA/mg of protein and 0.88 ± 0.081 μmol MDA/mg of protein, respectively in HCT116 and HEK293 cells as compared to the control (Fig. 3c), indicating that ZEN treatment significantly increased the peroxidation of lipids. The addition of QUER or CRO decreased the level of MDA induced by ZEN both in HCT116 and HEK293 cell lines. Altogether, these data indicate that QUER and CRO exert antioxidant activity and limit ZEN-induced oxidative stress in intestinal and renal cells.
Fig. 3.
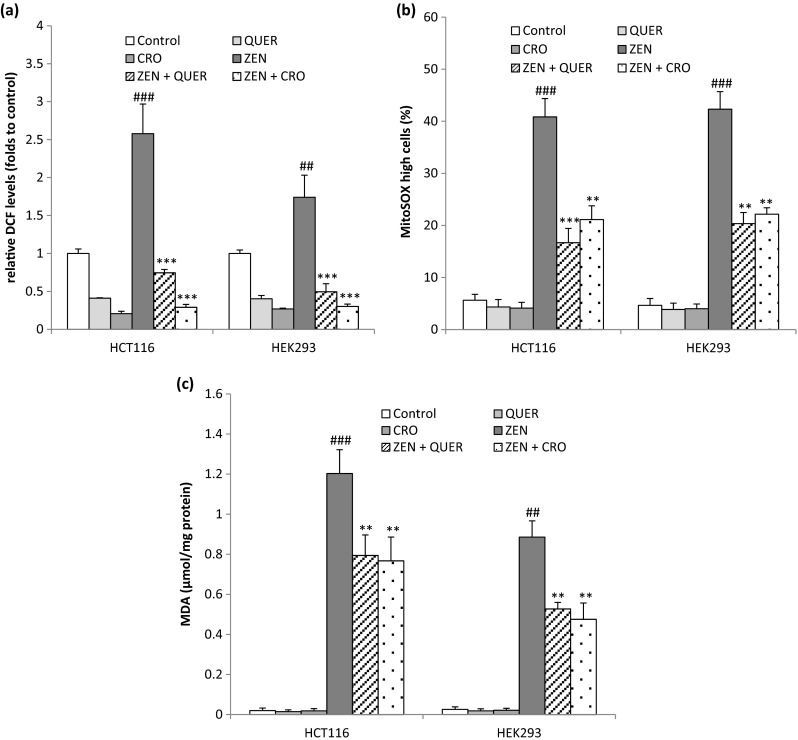
Effects of Quercetin and Crocin on ZEN-induced ROS generation. HCT116 and HEK293 cells were pretreated with QUER (5 μM) or CRO (250 μM) for 2 h before ZEN treatment for 24 h (100 μM for HCT116, 75 μM for HEK293) (a) The relative intracellular ROS production was evaluated by recording the fluorescence of DCF, the product of DCFH oxidation mainly by H2O2. (b) The production of mitochondrial anion superoxide was analyzed by flow cytometry using MitoSOX™ Red probe. MitoSOX high cells represent cells with a high level of mitochondrial anion superoxide. (c) The peroxidation of lipids was recorded by measuring the accumulation of MDA. Data are expressed as the mean ± SD of three separate experiments. ### P<0.001 and ## P<0.01 vs. control, ***P<0.001 and **P<0.01 vs. ZEN alone
Quercetin and Crocin inhibit ER stress and cell death induced by ZEN
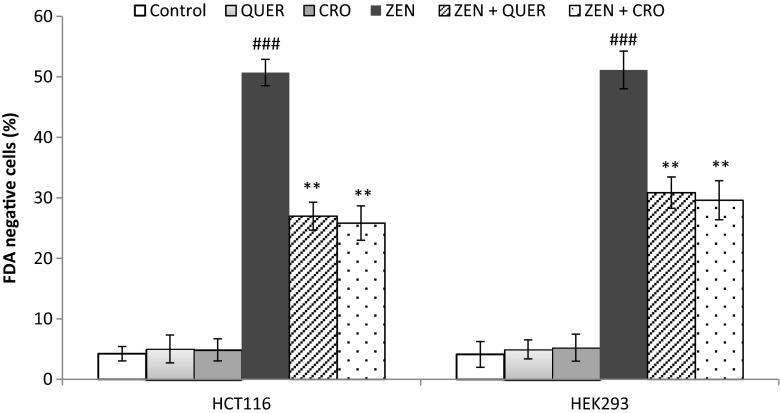
ER stress response has been suggested to be a consequence of ROS generation (Santos et al. 2009). We thus investigated whether the antioxidant activity of QUER and CRO modulates ER stress and cell death induced by ZEN. As shown in Fig. 4, pretreatment of cells with QUER or CRO prevented the increase in the level of the ER stress markers GRP78 and GADD34 induced by ZEN in the two tested cell lines. In addition, while QUER and CRO alone exhibited no toxicity towards HCT116 or HEK293 cells, pretreatment with these antioxidant molecules significantly decreased ZEN-mediated cytotoxicity. Indeed, in HCT116, cell mortality was decreased from 50.71 ± 2.19 % with ZEN alone to 26.97 ± 2.3 % and 25.83 ± 2.84 %, in the presence of QUER or CRO, respectively (Fig. 5). Similarly, in HEK293, cell viability was increased from 51.14 ± 3.12 % after treatment by ZEN to 30.86 ± 2.58 % in the presence of QUER and to 29.61 ± 3.23 % in the presence of CRO (Fig. 5). Collectively, our results indicate that QUER and CRO inhibit ER stress and protect HCT116 and HEK293 cells from cell death.
Fig. 4.
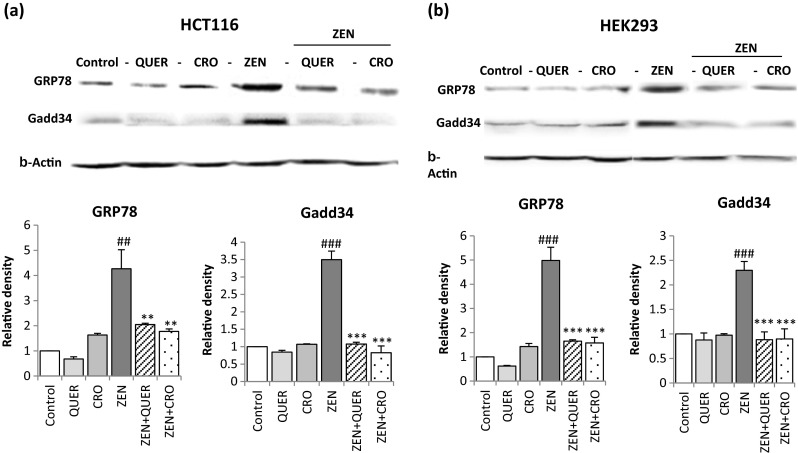
Quercetin and Crocin inhibits ZEN-induced ER stress. Cells were pretreated with QUER (5 μM) or CRO (250 μM) for 2 h before ZEN treatment for 24 h (100 μM for HCT116, 75 μM for HEK293). Protein levels of the ER stress markers GRP78 and GADD34 were detected by western blot in HCT116 (a) and HEK293 cells (b). β-actin was used as a loading control. Values represent mean ± SD of three independent experiments. ### P<0.001 and ## P<0.01 vs. control, ***P < 0.001 and **P<0.01 vs. ZEN alone
Fig. 5.
Quercetin and Crocin reduce ZEN-induced cytotoxicity in HCT116 and HEK293 cells. Cells were pretreated for 2 h with QUER (5 μM) or CRO (250 μM) before ZEN treatment for 24 h (100 μM for HCT116, 75 μM for HEK293). Cell viability was determined using the FDA assay and expressed as percentages of FDA positive cells (viable cells). Data are expressed as the mean ± SD of three separate experiments. ### P<0.001 vs. control, **P<0.01 vs. ZEN alone
Quercetin and Crocin decrease mitochondrial alterations induced by ZEN
Severe ER stress is known to induce apoptosis through the mitochondrial pathway to eliminate damaged cells (Sharaf el dein et al., 2009). In addition, we have previously reported that ZEN induces cell death by activating the mitochondrial pathway of apoptosis (Bouaziz et al., 2008). We thus assessed the effect of QUER and CRO on the mitochondrial alterations induced by ZEN. To this end, cells were analyzed by flow cytometry after staining with propidium iodide (PI) to evaluate necrosis and DiOC6(3) to measure mitochondrial transmembrane potential (ΔΨm). As shown in Fig. 6, the percentage of PI high cells remained very low (less than 20 %), excluding the involvement of necrosis in ZEN-induced cell death. The percentage of DIOC6(3) low cells, after 24 h of mycotoxin exposure, reached about 57.74 ± 3.12 % and 55.33 ± 3.05 % in HCT116 (Fig. 6a) and HEK293 (Fig. 6b) cells, respectively. Pretreatment of cells by QUER or CRO significantly decreases the percentage of DIOC6(3) low cells as compared to cells treated by ZEN alone. These results indicate that QUER and CRO reduce the mitochondrial alterations induced by ZEN.
Fig. 6.

Quercetin and Crocin protect cells from ZEN-induced loss of mitochondrial membrane potential. HCT116 (a) and HEK293 (b) cells were pretreated for 2 h with QUER (5 μM) or CRO (250 μM) before ZEN treatment for 24 h (100 μM for HCT116, 75 μM for HEK293). After treatment, cells were stained with the mitochondrial transmembrane potential (Δψm)-sensitive dye DiOC6(3) and the necrosis/late apoptosis probe propidium iodide (PI) and analyzed by flow cytometry. The data are expressed as the mean ± SD of three separate experiments. ## P < 0.01 and # P < 0.05 vs. control, *P < 0.05 vs. ZEN alone
Quercetin and Crocin reduce ZEN-induced caspase activation and DNA fragmentation
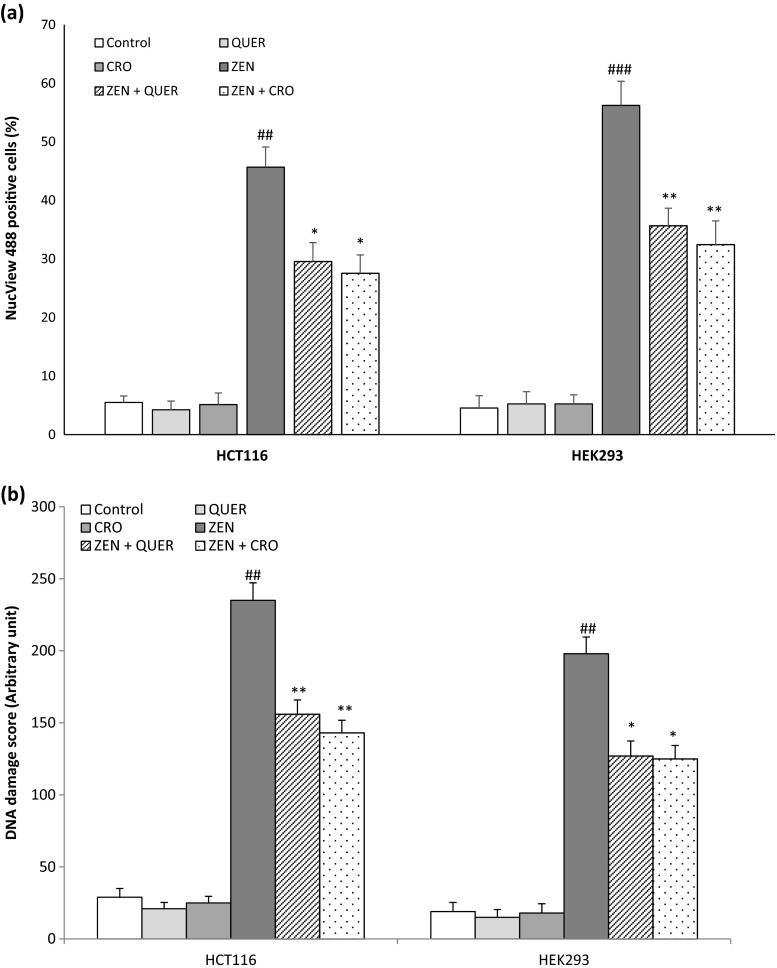
We next examined the ability of Quercetin and Crocin to modulate ZEN-induced caspase activation and DNA fragmentation, two apoptotic hallmarks. Cells were incubated with ZEN for 24 h, labeled with the DEVD-NucView 488 caspase-3 substrate, which becomes fluorescent when cleaved by active caspase-3, and analyzed by flow cytometry. We observed that ZEN increases the percentage of cells with activated caspase-3, suggesting that caspases are involved in the apoptotic process triggered by ZEN. Pretreatment of cells with QUER or CRO reduced caspase-3-activation induced by ZEN both in HCT116 and HEK293 cells (Fig. 7a). DNA damages were analyzed using the alkaline Comet assay. The high sensitivity of the Comet assay allows measurement of DNA fragmentation in individual cells. As shown in Fig. 7b, ZEN induced 235.8 ± 12.2 and 198 ± 11.57 DNA fragmentations, as compared to 29 ± 6 and 19 ± 6.3 DNA fragmentations in controls, in HCT116 and HEK293 cells, respectively. The addition of QUER or CRO significantly decreased the DNA fragmentation induced by ZEN in the two tested cell lines. Together, these data demonstrate that the antioxidant molecules Quercetin and Crocin protect HCT116 and HEK293 cells from ZEN-triggered caspase activation and DNA fragmentation.
Fig. 7.
(a) Quercetin and Crocin reduce caspase 3 activation induced by ZEN. HCT116 and HEK293 cells were pretreated for 2 h with QUER (5 μM) or CRO (250 μM) before ZEN treatment for 24 h (100 μM for HCT116, 75 μM for HEK293). The percentage of cells with activated caspase-3 was analyzed by flow cytometry after staining with the NucView 488 caspase-3 substrate. Data are expressed as the mean ± SD of three separate experiments. ### P < 0.001 and ## P < 0.01 vs. control, **P < 0.01 and *P < 0.05 vs. ZEN alone. (b) Quercetin and Crocin reduce ZEN-induced DNA fragmentation in HCT116 and HEK293. Cells were pretreated for 2 h with QUER (5 μM) or CRO (250 μM) before ZEN treatment for 24 h (100 μM for HCT116, 75 μM for HEK293). DNA damages were analyzed by the comet assay as described in the “Materials and methods”. Data are expressed as the mean ± SD of three separate experiments. ## P < 0.01 vs. control, **P < 0.01, and *P < 0.05 vs. ZEN alone
Discussion
The main focus of this study was to explore the involvement of ER stress in ZEN-induced apoptosis and to evaluate the protective effects of the two common food components Quercetin and Crocin in HCT116 and HEK293 cells. Our results show that ZEN induces cell death in the two human cell lines tested, at least in part, by inducing ER stress-associated activation of the mitochondrial pathway of apoptosis. In addition, we demonstrate that Quercetin and Crocin reduce the level of ROS produced by ZEN, inhibit ER stress, and protect cells from apoptosis.
Our data show that cell treatment with ZEN results in induction of ER stress as demonstrated by robust increase in the expression of the ER resident chaperone GRP78. ER stress is sensed by three upstream proteins IRE1, ATF6, and PERK, which activate different signaling pathways collectively termed UPR (Tabas and Ron 2011). In HCT116 cells, we showed that ZEN induces an increase in the expression of ATF4 and GADD34, two markers of the PERK pathway, and the splicing of XBP1 (XBP1-s). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress (Yoshida et al. 2001), and ATF6 is required for optimal expression of GRP78 (Baumeister et al. 2005). Our results thus indicate that PERK/ATF4, IRE1, and ATF6 arms of the UPR are activated upon ZEN treatment. Different reports have shown that treatment with other mycotoxins such as deoxynivalenol and trichodermin also induce ER stress in different cell systems (Yang et al. 2010; Su et al. 2013). This suggests that induction of ER stress may be a common feature of mycotoxin toxicity. In response to ER stress, the UPR is initially induced to restore ER homeostasis by inhibiting general protein translation, increasing chaperones and folding enzymes in the ER, and eliminating unfolded proteins. In the case of severe or sustained ER stress, the pro-survival function of the UPR turns into an apoptotic signal to eliminate stressed cells (Tabas and Ron 2011). Our results show that the expression of the pro-apoptotic factor CHOP is increased after ZEN treatment and that reduction of ER stress by PBA reduces GRP78 expression and ZEN-induced cell mortality indicating that the ER stress induced by this mycotoxin is sufficient to activate the pro-apoptotic pathway of the UPR. The transcription factor CHOP was the first protein identified as a key mediator of ER stress-induced apoptosis and is upregulated by the three branches of the UPR (Zinszner et al. 1998). CHOP acts through downregulation of the anti-apoptotic protein Bcl-2 and upregulation of the BH3-only protein Bim, which is necessary for Bax-mediated mitochondrial permeabilization and apoptosis (Tabas and Ron 2011). In agreement, we observed in HCT116 and HEK293 cells that ZEN induces the dissipation of ΔΨm, indicative of mitochondrial membrane permeabilization, the activation of caspases, and the fragmentation of DNA with a relative persistence of plasma membrane integrity. These data indicate that ER stress elicited by ZEN is associated with an induction of apoptosis through a mitochondria-mediated caspase-dependent pathway.
ZEN is known as a potent inducer of ROS in mammalian system. Indeed, different reports have demonstrated that ZEN induces ROS production (Bouaziz et al. 2009; Jia et al. 2014; Zhou et al. 2015) and lipid peroxidation in vitro and in vivo and can engender oxidized DNA bases (Abid-Essefi et al. 2004; Hassen et al. 2007; Abbes et al. 2007). Results of our study provide evidence that ZEN triggers ROS generation in HCT116 and HEK293 cells. ZEN-induced oxidative stress was evidenced by production of intracellular and mitochondrial ROS and accumulation of MDA, an end product of lipid peroxidation, considered as a late biomarker of oxidative stress and cellular damages (Vaca et al. 1988). The toxicity of ZEN thus appears to be related to the substantial degree of intracellular oxidative stress generated. Therefore, preventing ROS production or scavenging of free radicals may be an effective strategy for limiting the toxicity of ZEN.
We demonstrate here that Quercetin and Crocin decrease the level of ROS and the peroxidation of lipids induced by ZEN and reduce the toxicity of this mycotoxin in HCT116 and HEK293 cells. Quercetin is one of the most frequently studied dietary flavonoids, distributed in vegetables, fruits, and many other dietary sources (Bhatt and Flora 2009). This flavonoid has been reported to scavenge free radicals directly to inhibit lipid peroxidation and to strengthen antioxidant defense pathway in vivo and in vitro (Anjaneyulu and Chopra 2004). Crocin, a major carotenoid of saffron, exhibits high antioxidant and scavenging properties in vitro and in vivo (Ochiai et al. 2004a, b, 2007; Hosseinzadeh et al. 2009, 2010; Mousavi et al. 2010). For instance, Crocin has been shown to attenuate lipid peroxidation in kidney (Hosseinzadeh et al. 2005) and skeletal muscle (Hosseinzadeh et al. 2009) during ischemia-reperfusion-induced oxidative damage in rats and to promote cell viability in PC12 cells (Mousavi et al. 2010). Our results indicate that, as effective free-radical scavengers and antioxidants, Quercetin and Crocin reduce ZEN toxicity in HCT116 and HEK293 cells.
An excess ROS production can induce ER stress (Santos et al. 2009) and initiate the mitochondrial pathway of apoptosis (Le Bras et al. 2005). Indeed, even if the ER is one of the most oxidizing intracellular compartments to allow disulfide formation and proper protein folding, an oxidative stress will lead to excessive oxidative modifications to proteins and hence to ER stress and UPR activation. Here, we report for the first time that scavenging of ROS by the antioxidants Quercetin and Crocin inhibits the ER stress response and protects cells from ZEN-induced mitochondrial apoptosis. These results suggest that ROS are initiators of ER stress and mitochondrial damages in the signalling cascade induced by ZEN.
In conclusion, this study provides insight into ways to reduce the toxicity of the commonly encountered mycotoxin Zearalenone. We show that ZEN-induced mitochondrial apoptotic cell death is associated with ER stress in HCT116 and HEK293 cells. We also demonstrated that the two common antioxidant food components Quercetin and Crocin protect cells from ZEN by inhibiting ER stress, suggesting that antioxidant treatment might be helpful to prevent ZEN-related toxicity.
Acknowledgments
This study was supported by “Le Ministère Tunisien de l’Enseignement Supérieur, de la Recherche Scientifique et de la Technologie” and by grants from LabEx LERMIT. A. Prola received a fellowship from GRRC. A. Guilbert received a fellowship from Région Ile de France CORDDIM.
Conflict of interest
The authors declare that they have no competing interests.
Abbreviations
- ZEN
Zearalenone
- CRO
Crocin
- QUER
Quercetin
- ER
Endoplasmic reticulum
- ΔΨm
Mitochondrial transmembrane potential
- mtO2·−
Mitochondrial superoxide anion
Footnotes
Highlights
Zearalenone triggers ER stress in human intestinal and kidney cells
Zearalenone induces apoptosis through ER stress and activation of the mitochondrial pathway
Crocin and Quercetin protect cells against ZEN-induced ER stress and apoptosis
References
- Abbes S, Ouanes Z, Ben Salah-Abbes J, Houas Z, Oueslati R, Bacha H. The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by zearalenone in mice. Toxicon. 2006;47:567–574. doi: 10.1016/j.toxicon.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Abbes S, Salah-Abbes JB, Ouanes Z, Houas Z, Othman O, Bacha H. Preventive role of phyllosilicate clay on the immunological and biochemical toxicity of zearalenone in Balb/c mice. Int Immunopharmacol. 2006;6:1251–1258. doi: 10.1016/j.intimp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Abbes S, Ouanes Z, Ben Salah-Abbes J, Abdel-Wahhab MA, Oueslati R, Bacha H. Preventive role of aluminosilicate clay against induction of micronuclei and chromosome aberrations in bone-marrow cells of Balb/c mice treated with zearalenone. Mutat Res. 2007;631:85–92. doi: 10.1016/j.mrgentox.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Abid-Essefi S, Baudrimont I, Hassen W, Ouanes Z, Mobio TA, Anane R. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: prevention by vitamin E. Toxicology. 2003;192:237–248. doi: 10.1016/S0300-483X(03)00329-9. [DOI] [PubMed] [Google Scholar]
- Abid-Essefi S, Ouanes Z, Hassen W, Baudrimont I, Creppy EE, Bacha H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol In Vitro. 2004;4:467–474. doi: 10.1016/j.tiv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Abid-Essefi S, Zaied C, Bouaziz C, Ben Salem I, Kaderi R, Bacha H. Protective effect of aqueous extract of Allium sativum against zearalenone toxicity mediated by oxidative stress. Exp Toxicol Pathol. 2012;64:689–695. doi: 10.1016/j.etp.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31:244–248. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31:244–248. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- Banjerdpongchai R, Kongtawelert P, Khantamat O, Srisomsap C, Chokchaichamnankit D, Subhasitanont P, Svasti J. Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone-induced apoptosis of human leukemic cells. J Hematol Oncol. 2010;3:1–16. doi: 10.1186/1756-8722-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister P, Luo S, Skarnes WC, Sui G, Seto E, Shi Y, Lee AS. Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol Cell Biol. 2005;25:4529–4540. doi: 10.1128/MCB.25.11.4529-4540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Salah-Abbes J, Abbes S, Abdel-Wahhab M, Oueslati R. Raphanus sativus extract protects against zearalenone induced reproductive toxicity, oxidative stress and mutagenic alterations in male Balb/c mice. Toxicon. 2009;53:525–533. doi: 10.1016/j.toxicon.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K, Flora SJS. Oral cp-administration of α-lipoic acid, quercetin and captopril prevents gallium arsenide toxicity in rats. Environ Toxicol Pharmacol. 2009;28:140–146. doi: 10.1016/j.etap.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Bouaziz C, Sharaf El Dein O, El Golli E, Abid-Essefi S, Brenner C, Lemaire C, Bacha H. Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology. 2008;254:19–28. doi: 10.1016/j.tox.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Bouaziz C, Martel C, Sharaf OD, Abid-Essefi S, Brenner C, Lemaire C, Bacha H. Fusarial toxin-induced toxicity in cultured cells and in isolated mitochondria involves PTPC-dependent activation of the mitochondrial pathway of apoptosis. Toxicol Sci. 2009;110:363–375. doi: 10.1093/toxsci/kfp117. [DOI] [PubMed] [Google Scholar]
- Bryden WL. Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Anim Feed Sci Technol. 2012;173:134–158. doi: 10.1016/j.anifeedsci.2011.12.014. [DOI] [Google Scholar]
- Collins AR, Dusinska M, Gedik CM, Stetina R. Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect. 1996;104:465–469. doi: 10.1289/ehp.96104s3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkova E, Laciakova A, Pastorova B, Seidel Kovac G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol Lett. 2001;121:145–149. doi: 10.1016/S0378-4274(01)00312-5. [DOI] [PubMed] [Google Scholar]
- El Golli E, Hassen W, Bouslimi A, Bouaziz C, Ladjimi MM, Bacha H. Induction of Hsp 70 in Vero cells in response to mycotoxins cytoprotection by sub-lethal heat shock and by vitamin E. Toxicol Lett. 2006;166:122–130. doi: 10.1016/j.toxlet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Fung F, Clark R. Health effects of mycotoxins: a toxicological overview. I Toxicol. 2004;42:217–234. doi: 10.1081/clt-120030947. [DOI] [PubMed] [Google Scholar]
- Habschied K, Sarkanj B, Klapec T, Krstanovic V. Distribution of zearalenone in malted barley fractions dependent on Fusarium graminearum growing conditions. Food Chem. 2011;129:329–332. doi: 10.1016/j.foodchem.2011.04.064. [DOI] [PubMed] [Google Scholar]
- Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Hassen W, Ayed-Boussema I, Oscoz AA, De Cerain LA, Bacha H. The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: oxidative DNA damage, glutathione depletion and stress proteins induction. Toxicology. 2007;232:294–302. doi: 10.1016/j.tox.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Sci. 2005;8(3):387–393. [PubMed] [Google Scholar]
- Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6(3):343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents crocin and safranal. Pharmacogn Mag. 2010;5(20):419–424. [Google Scholar]
- Jia Z, Liu M, Qu Z, Zhang Y, Yin S, Shan A. Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ Toxicol Pharmacol. 2014;37:580–591. doi: 10.1016/j.etap.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Kobylińska A, Janas KM. Health-promoting effect of quercetin in human diet. Postepy Hig Med Dosw. 2015;69:51–62. doi: 10.5604/17322693.1135423. [DOI] [PubMed] [Google Scholar]
- Kuiper-Goodman T, Scoot PM, Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul Toxicol Pharmacol. 1987;7:253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Le Bel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2-,7-dichlorofluorescein as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Le Bras M, Clément MV, Pervaiz S, Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol. 2005;20:205–220. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- Liang ZS, MA YJ, Liu CY, Deng XB, Fan XL, Yan HK, Hu QX (2010). In 583 vivo toxicity of zearalenone on liver and kidney in mice. Chinese Journal of 584 Veterinary Science. 30:673–676
- Loke WM, Proudfoot JM, McKinley AJ. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J Agric Food Chem. 2008;56:3609–3615. doi: 10.1021/jf8003042. [DOI] [PubMed] [Google Scholar]
- Loke WM, Proudfoot JM, Hodgson JM. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2010;30:749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- Maaroufi K, Chekir L, Creppy EE, Ellouz F, Bacha H. Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon. 1996;34:535–540. doi: 10.1016/0041-0101(96)00008-6. [DOI] [PubMed] [Google Scholar]
- Morris JA, Dorner AJ, Edwards CA, Hendershot LM, Kaufman RJ. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J Biol Chem. 1997;272(7):4327–4334. doi: 10.1074/jbc.272.7.4327. [DOI] [PubMed] [Google Scholar]
- Mousavi SH, Tayarani NZ, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. 2010;30(2):185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H, Sultana P, Shimada N, Yashioka M. Structure activity relationships among zearalenone and its derivatives based on bovine neutrophil chemiluminescence. Vet Hum Toxicol. 2003;1:18–20. [PubMed] [Google Scholar]
- Obremski K, Zielonka L, Zaluska G, Zwierzchowski W, Pirus K, Gajecki M (1999) The influence of low doses of zearalenone on liver enzyme activities in gilts. In: Proceedings of the X conference “Microscopy Fungi – plant pathogens and their metabolites”: 66
- Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of a-tocopherol. Neurosci Lett. 2004;362(1):61–64. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Ochiai T, Soeda S, Ohno S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem Int. 2004;44(5):321–330. doi: 10.1016/S0197-0186(03)00174-8. [DOI] [PubMed] [Google Scholar]
- Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, Shoyama Y, Toda A, Eyanagi R, Soeda S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770(4):578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ouanes Z, Abid S, Ayed I, Anane R, Mobio T, Creppy EE, Bacha H. Induction of micronuclei by zearalenone in Vero monkey kidney cells and in bone marrow cells of mice: protective effect of vitamin E. Mutation Research/Genetic Toxicology and Environ Mutagenesis. 2003;538:63–70. doi: 10.1016/S1383-5718(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Ouanes Z, Ayed-Boussema I, Baati T, Creppy EE, Bacha H. Zearalenone induces chromosome aberrations in mouse bone marrow: preventive effect of 17betaestradiol, progesterone and vitamin E. Mutat Res. 2005;565:139–149. doi: 10.1016/j.mrgentox.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JL, Recio MC, Giner RM, Manez S. An update review of saffron and its active constituents. Phytother Res. 1996;10(3):189–193. doi: 10.1002/(SICI)1099-1573(199605)10:3<189::AID-PTR754>3.0.CO;2-C. [DOI] [Google Scholar]
- Rodrigues I, Naehrer K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. X. 2012;4:663–675. doi: 10.3390/toxins4090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M, Jiménez R, Sánchez M. Quercetin inhibits vascular superoxide production induced by endothelin-1: role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis. 2009;202:58–67. doi: 10.1016/j.atherosclerosis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Galisteo M, Vera R. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- Santos XC, Tanaka LY, Wosniak JJ, Laurindo FRM. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxidants & Redox Signaling. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Sharaf el dein O, Gallerne C, Deniaud A, Brenner C, Lemaire C. Role of the permeability transition pore complex in lethal inter-organelle crosstalk. Front Biosci. 2009;14:3465–3482. doi: 10.2741/3465. [DOI] [PubMed] [Google Scholar]
- Su CM, Wang SW, Lee TH, Tzeng WP, Hsiao CJ, Liu SC, Tang CH. Trichodermin induces cell apoptosis through mitochondrial dysfunction and endoplasmic reticulum stress in human chondrosarcoma cells. Toxicol Appl Pharmacol. 2013;272:335–344. doi: 10.1016/j.taap.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca CE, Wilhelm J, Harms-Ringdahl M. Interaction of lipid peroxidation product with DNA. Mutat Res. 1988;195:137–149. doi: 10.1016/0165-1110(88)90022-X. [DOI] [PubMed] [Google Scholar]
- Van Schadewijk A, Van’t Wout EF, Stolk J, Hiemstra PS. A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress. Cell Stress Chaperones. 2012;17:275–279. doi: 10.1007/s12192-011-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Park SH, Choi HJ, Do KH, Kim J, An TJ, Lee SH, Moon Y. Mechanism-based alternative monitoring of endoplasmic reticulum stress by 8-keto-trichothecene mycotoxins using human intestinal epithelial cell line. Toxicol Lett. 2010;198:317–323. doi: 10.1016/j.toxlet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Zain ME. Impact of mycotoxins on humans and animals. J Soudi Chem Soc. 2011;15:129–144. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- Zhou C, Zhang Y, Yin S, Jia Z, Shan A. Biochemical changes and oxidative stress induced by zearalenone in the liver of pregnant rats. Hum Exp Toxicol. 2015;34:65–73. doi: 10.1177/0960327113504972. [DOI] [PubMed] [Google Scholar]
- Zinedine A, Jose MS, Juan CM, Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol. 2007;45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorgui L, Ayed-Boussema I, Ayed Y, Bacha H, Hassen W. The antigenotoxic activities of cactus (Opuntia ficus-indica) cladodes against the mycotoxin zearalenone in Balb/c mice: prevention of micronuclei, chromosome aberrations and DNA fragmentation. Food Chem Toxicol. 2009;47:662–667. doi: 10.1016/j.fct.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Zourgui L, El Golli E, Bouaziz C, Bacha H, Hassen W. Cactus (Opuntia ficus-indica) cladodes prevent oxidative damage induced by the mycotoxin zearalenone in Balb/C mice. Food Chem Toxicol. 2008;46:1817–1824. doi: 10.1016/j.fct.2008.01.023. [DOI] [PubMed] [Google Scholar]