Abstract
This study examined the relationship between exhaustive exercise in the heat at moderate and high intensities on the intracellular heat shock protein 72 (iHsp72) response. Twelve male subjects cycled to exhaustion at 60 and 75 % of maximal oxygen uptake in hot conditions (40 °C, 50 % RH). iHsp72 concentration was measured in monocytes before, at exhaustion and 24 h after exercise. Rectal temperature, heart rate and oxygen uptake were recorded during exercise. Volitional exhaustion occurred at 58.9 ± 12.1 and 27.3 ± 9.5 min (P < 0.001) and a rectal temperature of 39.8 ± 0.4 and 39.2 ± 0.6 °C (P = 0.002), respectively, for 60 and 75 %. The area under the curve above a rectal temperature of 38.5 °C was greater at 60 % (17.5 ± 6.6 °C min) than 75 % (3.4 ± 4.8 °C min; P < 0.001), whereas the rate of increase in rectal temperature was greater at 75 % (5.1 ± 1.7 vs. 2.2 ± 1.4 °C h−1; P < 0.001). iHsp72 concentration increased similarly at exhaustion relative to pre-exercise (P = 0.044) and then increased further at 24 h (P < 0.001). Multiple regression analysis revealed no predictor variables associated with iHsp72 expression; however, a correlation was observed between exercise intensities for the increase in iHsp expression at exhaustion and 24 h (P < 0.05). These results suggest that iHsp72 expression increased in relation to the level of hyperthermia attained and sustained at 60 % and the higher metabolic rate and greater rate of increase in core temperature at 75 %, with the further increase in iHsp72 concentration 24 h after exercise reinforcing its role as a chaperone and cytoprotective agent.
Keywords: Heat shock proteins, Hyperthermia, Core temperature, Intracellular Hsp, Extracellular Hsp, Fatigue
Introduction
Heat shock proteins (Hsps) are highly conserved proteins originally described for their role as chaperones in maintaining cellular conformation and homeostasis during heat stress (i.e. the heat shock response) (Locke 1997; Kregel 2002). Although Hsp expression is also known to increase in response to other stressors (e.g. hypoxia, ischemia, inflammation), the heat shock response induced during exposure to hot environmental conditions confers transient thermotolerance and protection against subsequent exposure. Based on their molecular mass (8 to 110 kDa), Hsps have different cellular locations and functions, processing stress-denatured proteins, managing protein fragments, maintaining structural proteins and chaperoning other proteins across cell membranes (Pockley 2003; Asea 2007; Noble et al. 2008).
Both passive heat exposure and exercise elicit Hsp synthesis (Febbraio and Koukoulas 2000; Oehler et al. 2001); however, the combination of exercise and heat stress elicits a greater Hsp response than either stressor independently (Skidmore et al. 1995). Hsp72 is particularly responsive to heat stress and exercise (Locke 1997). Recently, the extracellular expression of Hsp72 (eHsp72) during exercise-heat stress was shown to increase in relation to both the level of hyperthermia attained and sustained, and rate of rise in core temperature (Périard et al. 2012), possibly in response to surpassing a minimum endogenous criteria mediated by whole-body temperature and sympathetic activity (Gibson et al. 2013). Interestingly, the upregulation of eHsp72 is considered immuno-stimulatory, stimulates the synthesis of pro-inflammatory cytokines, augments chemokine synthesis, upregulates co-stimulatory molecules and enhances anti-tumour surveillance (Asea et al. 2000; Pockley 2003; Asea 2007). Conversely, an increase in intracellular Hsp72 (iHsp72) is cytoprotective as it induces anti-apoptosis, represses gene expression, modulates cell cycle progression and is anti-inflammatory (Locke 1997; Kregel 2002; Noble et al. 2008). Whilst iHsp72 expression has been demonstrated to increase during exercise-heat stress (Fehrenbach et al. 2001, 2003; Selkirk et al. 2009; Magalhaes et al. 2010), its magnitude of expression may differ during exercise at different intensities, given that this elicits distinctive metabolic demands and induces different rates of heat storage. Due to the dual role of Hsp72 in relation to its cellular location, the influence of exercise to exhaustion in the heat at distinct intensities may provide insight as to the role of iHsp72 based on its level of expression.
In human leucocytes, monocytes demonstrate a particularly strong responsiveness to stress, despite having a shorter life cycle than lymphocytes in the circulation (Oehler et al. 2001). These circulating cells are therefore ideal for investigating the influence of hyperthermia and exercise on Hsp expression, as they are exposed to similar temperatures and stressors as the various organs and tissues of the body (Sonna et al. 2001; Lovell et al. 2008). The purpose of this study was therefore to investigate the relationship between iHsp72 expression in monocytes extracted from venous blood samples before, immediately after and 24 h following exercise to exhaustion in a hot environment at moderate and high exercise intensities. It was hypothesised that similar to the expression of eHsp72 (Périard et al. 2012), iHsp72 concentration would increase to a comparable level following exercise at both intensities. This was anticipated in response to the level of hyperthermia attained and sustained above a core temperature of 38.5 °C in the moderate-intensity trial and the higher metabolic rate and greater rate of increase in core temperature at the higher intensity.
Results and discussion
Twelve healthy male subjects unacclimatised to heat participated in this study. Their physical characteristics for age, body mass, height and maximal oxygen uptake were 28 ± 6 years, 70.7 ± 3.9 kg, 180.3 ± 5.9 cm and 4.5 ± 0.7 l · min−1. Sixteen subjects were recruited and participated in the study; however, for technical reasons (i.e. insufficient monocytes isolated), data are presented for only 12. Experimental procedures and measures, along with physiological and plasma eHsp data for 16 subjects have been published previously (Périard et al. 2012). The expression of Hsp72 in monocytes was measured following isolation of monocytes from 12-ml EDTA-treated blood using Optiprep density-gradient medium (Axis-Shield, Oslo, Norway) as described by the manufacturer except that BSA was omitted from the solutions. Using a similar method, average purity ranged from 87.9 to 96.4 % (Graziani-Bowering et al. 1997). Monocytes were extracted as described previously for lymphocytes (Ruell et al. 2007). Monocyte extracts were analysed for total protein using a commercial method (Pierce, Rockford, IL, USA), and Hsp72 was measured using an R&D Elisa kit (DYC1663, Minneapolis, MN, USA) according to the manufacturer’s instructions. A two-way (time-by-trial) repeated-measures ANOVA (SPSS, Chicago, IL, US) was performed to test significance between and within trials for similar time points, whilst Student’s paired t tests were used to compare the final time point values and hydration data. Multiple regression analysis was used with the enter method to determine predictors (i.e. rectal temperature, rate in increase in rectal temperature, area under the curve (AUC), time and mean heart rate) of exercise-induced increases in iHsp72 expression.
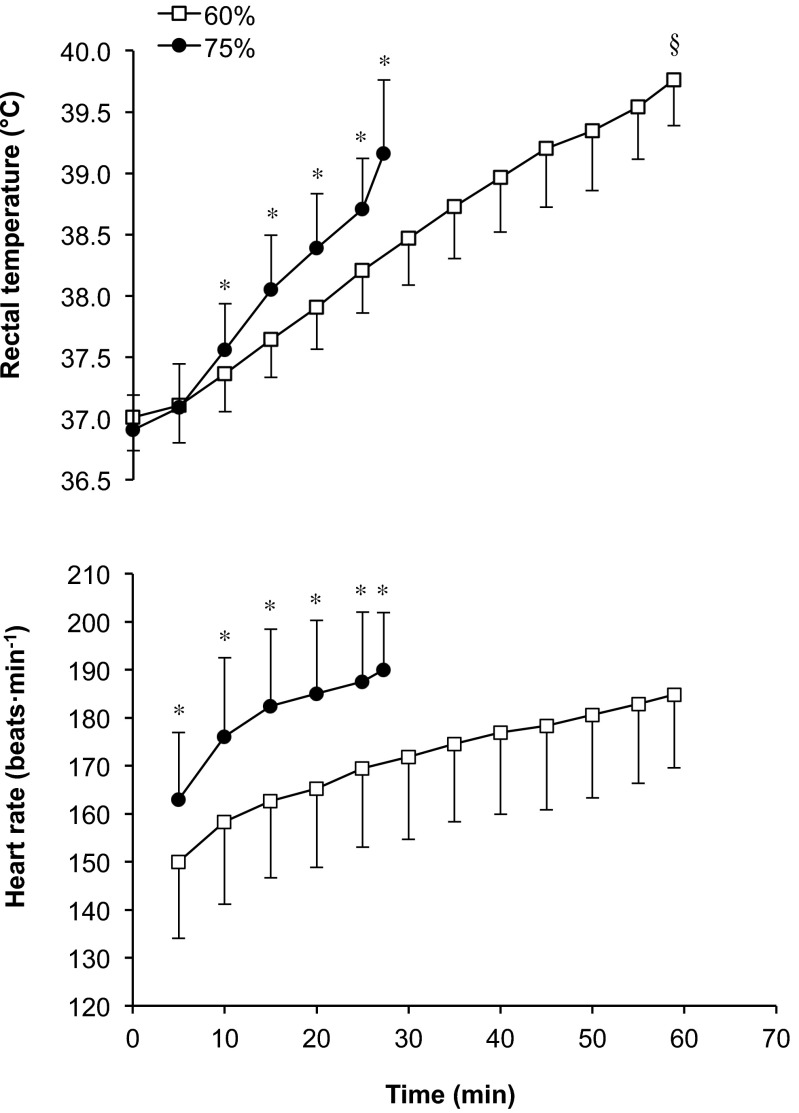
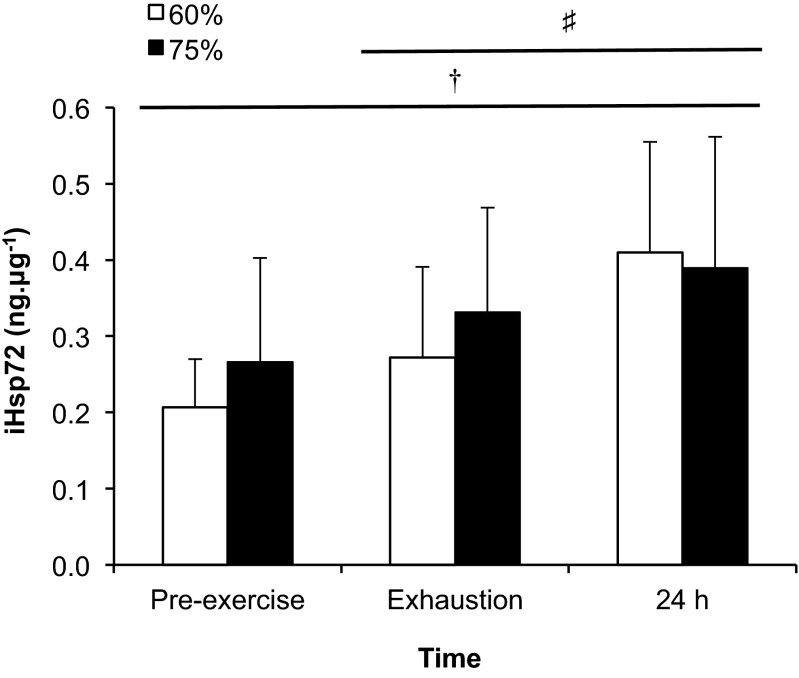
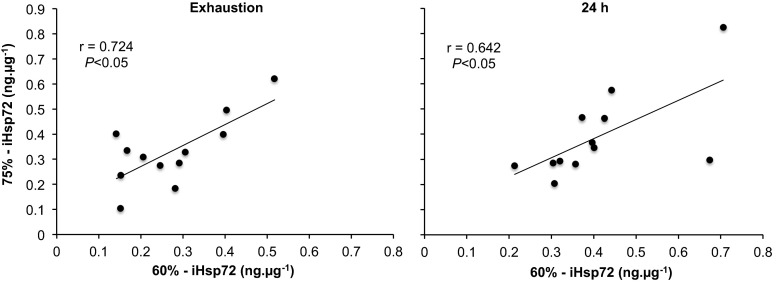
In the 60 % trial subjects reached volitional exhaustion at 58.9 ± 12.1 min, whereas in the 75 % trial it was attained at 27.3 ± 9.5 min (P < 0.001). There were significant condition (P = 0.001), time (P < 0.001) and interaction (P < 0.001) effects for rectal temperature (Fig. 1). The rate of increase in rectal temperature was greater during exercise at 75 % relative to 60 % (5.1 ± 1.7 vs. 2.2 ± 1.4 °C h−1; P < 0.001). The calculated AUC was significantly greater for the 60 % (17.5 ± 6.6 °C min) trial relative to 75 % (3.4 ± 4.8 °C min; P < 0.001). There were significant condition (P < 0.001) and time (P < 0.001) effects for heart rate, with a trend towards an interaction effect (P < 0.190; Fig. 1). There were also significant condition (P < 0.001) and time (P = 0.002) effects for , which was 2.6 ± 0.5 l min−1 (10 min), 2.7 ± 0.5 l min−1 (30 min) and 2.9 ± 0.4 l min−1 (exhaustion) in the 60 % trial, whilst it was 3.5 ± 0.6 l min−1 (10 min) and 3.6 ± 0.6 l min−1 (exhaustion) in the 75 % trial. Percent body mass losses were similar for exercise performed at 60 % (0.5 ± 0.8 %) and 75 % (0.5 ± 0.6 %). However, sweat production was slightly lower in the 60 % trial (1.5 ± 0.5 l.h−1) compared with 75 % (1.7 ± 0.4 l.h−1; P = 0.032), whilst fluid consumption was similar between the 60 % (1.1 ± 0.6 l.h−1) and 75 % (1.1 ± 0.5 l.h−1) trials. There was a significant time (P < 0.001) effect for iHsp72 expression (Fig. 2). The multiple regression analysis revealed no predictor variables associated with iHsp72 expression at either exercise intensity. However, a correlation was observed between exercise intensities for the increase in iHsp expression both at exhaustion and 24 h after exercise (P < 0.05; Fig. 3).
Fig. 1.
Rectal temperature and heart rate during exercise to volitional exhaustion at 60 and 75 % in hot (40 °C and 50 % relative humidity) conditions. Significant difference between 60 and 75 %, P < 0.05 (*). Significantly greater final temperature, P < 0.001 (§)
Fig. 2.
iHsp72 expression at rest, at volitional exhaustion during exercise at 60 and 75 % in hot (40 °C and 50 % relative humidity) conditions and 24 h after exercise termination. Pre-exercise significantly lower than exhaustion and 24 h, P < 0.05 (†). Exhaustion significantly lower than 24 h, P < 0.05 (♯)
Fig. 3.
Correlation between iHsp72 expression at exhaustion and 24 h after exercise to volitional exhaustion at 60 and 75 % in hot (40 °C and 50 % relative humidity) conditions
The novel findings of the study are that iHsp72 concentration increased similarly on reaching exhaustion during exercise at 60 and 75 % and then increased further 24 h after the termination of exercise at both intensities. Moreover, a correlation was observed in the increase in iHsp72 between conditions both at exhaustion and 24 h. The finding that a comparable increase in iHsp72 concentration occurred at exhaustion in the 60 and 75 % is similar to eHsp72 (Périard et al. 2012). However, in contrast to eHsp72, iHsp72 continued to increase during the 24-h period after exercise. Correspondingly, exercise-heat stress activates Hsp72 gene expression in various tissues, leading to enhanced iHsp72 expression in the hours and days following induction. This allows for iHsp72 to function as a chaperone and cytoprotective agent, should a subsequent stress occur (Locke 1997; Kregel 2002). By contrast, eHsp72 is acutely released from tissues into the circulation in response to cellular stress, acting as a danger signal (Fleshner and Johnson 2005; Heck et al. 2011). Circulating levels of eHsp72 usually return to basal levels when the physiological or biological stress terminates. Our results support this dual role for Hsp72, the expression of which is dependent on their location (intra vs. extracellular).
Hsp72 is the most abundant stress-induced protein, accounting for up to 20 % of total cellular protein expressed under stressful conditions (Donati et al. 1990). In the current study, the increase in iHsp72 noted at exhaustion (Fig. 2) corroborates previous findings in which an increase in iHsp72 was shown immediately following endurance exercise in temperate conditions (Fehrenbach et al. 2000a, b; Shin et al. 2004), as well as in the heat (Fehrenbach et al. 2001, 2003; Selkirk et al. 2009; Magalhaes et al. 2010). In animals, Hsp72 concentration in various tissues has been shown to correlate with the level of hyperthermia attained (Walters et al. 1998; Ruell et al. 2004), as well as the rate of rise in temperature (Flanagan et al. 1995). In the current study, the increase in rectal temperature to 39.8 °C (Fig. 1) along with the AUC at 38.5 °C (17.5 °C min) in the 60 % trial was sufficient to induce the heat shock response. In the 75 % trial, the attainment of a rectal temperature of 39.2 °C and the rate of increase in rectal temperature (5.1 °C h−1), along with the increased intensity (e.g. and heart rate; Fig. 1) were equally sufficient to stimulate an increase in iHsp72. The latter result is interesting given the short duration of the exercise bout. Indeed, few studies have examined iHsp72 in monocytes after short-duration exercise. In a study by Fehrenbach et al. (2001), athletes ran in warm conditions (28 °C and 50 % RH) for 60 min, which resulted in a 1.8-fold increase in monocyte Hsp72 immediately post-exercise. In a separate study by Whitham et al. (2004), although not significant, a 1.5-fold increase in monocyte Hsp72 was found immediately following a test (20–30 min). This was noted after the subjects had followed a 6-day intensified training programme. These observations reinforce the influence of exercise intensity and hyperthermia in increasing iHsp72 concentration, especially during the 75 % trial.
The increase in iHsp72 concentration observed at exhaustion reflects findings showing an increase in eHsp72 after exercise at the same intensities and environmental conditions (Périard et al. 2012). The similar pattern of expression in response to exhaustive exercise is not entirely unexpected, given that intracellular proteins are typically detected before or simultaneous to extracellular proteins (Kwak et al. 2000). Notwithstanding, changes in eHsp72 expression do not necessarily reflect changes in iHsp72 being released from leukocytes, as hepatosplanchnic and cerebral tissues also represent sources of Hsp72 release during exercise (Febbraio et al. 2002; Lancaster et al. 2004). Hence, eHsp72 concentration is representative of the net balance of the Hsp72 being released into and removed from the circulation (Whitham and Fortes 2008). Moreover, whilst the eHsp72 response was correlated with the temperature attained at exhaustion (60 %) and the rate of rise in core temperature during exercise (75 %) (Périard et al. 2012), the multiple regression analysis for iHsp72 in the current study did not reveal any predictor variables associated with the increase in expression. However, a significant correlation in the expression of iHsp72 at exhaustion and 24 h after exercise was observed between exercise intensities (Fig. 3). Given the similarity in response, it appears that iHsp72 expression is sensitive to exercise intensity, total time spent above a rectal temperature of 38.5 °C and the rate of rise in temperature.
Following the initial increase in iHsp72 concentration at exhaustion, a further rise was observed 24 h later (Fig. 2). This observation varies slightly from that of a recent study in which a 53 % increase in iHsp72 concentration was demonstrated immediately following exercise in the heat and accompanied by a 30 % elevation in the basal level 24 h after exercise (Lee et al. 2014). Previous studies examining the role of heat acclimation on the iHsp72 response have also shown an increase in the basal concentration of iHsp72 after 3–11 days of exposure to heat stress (Yamada et al. 2007; McClung et al. 2008; Magalhaes et al. 2010; Lee et al. 2015). Interestingly, Marshall et al. (2007) reported that basal iHsp72 concentration in human peripheral blood mononuclear cells was not elevated after 2 days of exercise-heat exposure; however, a large (3.5-fold) non-significant increase in iHsp72 was noted. In a study of passive heating where rectal temperature reached ∼39 °C, iHsp72 was elevated 1.6-fold from basal levels 22 h following the heat exposure (Oehler et al. 2001). In the present study, the combination of exercise and heat exposure resulted in similar 2-fold (60 %) and 1.6-fold (75 %) increases in iHsp72 24 h post-exercise.
The increase in basal iHsp72 concentration 24 h after exercise cessation is associated with the level of heat strain incurred, in response to the prevailing ambient conditions and the intensity of exercise performed. As highlighted by Hom et al. (2012), the greatest combination of ambient temperature and relative humidity above 40 °C resulting in the highest level of thermal strain during heat acclimation (Yamada et al. 2007; McClung et al. 2008; Magalhaes et al. 2010), resulted in the greatest post-acclimation increase in basal iHsp72. Accordingly, studies demonstrating no change in basal iHsp72 concentration following exercise in the heat induced only a moderate increase in core temperature (i.e. <38.5 °C) (Watkins et al. 2008; Hom et al. 2012). As such, it would appear that environmental conditions that induce only mild/moderate hyperthermia are associated with lower iHsp72 responses in humans. Notwithstanding, environmental conditions are not the only factor influencing iHsp72 expression, as exercise intensity and duration are known to exacerbate the rise in core temperature and physiological strain. In the current study, the combination of exercising at 60 and 75 % until exhaustion in the heat, along with exceeding a rectal temperature of 39 °C, resulted in a further rise in iHsp72 24 h after exercise. As with values measured at exhaustion, the rise in basal iHsp72 expression measured 24 h after the termination of exercise was correlated between exercise intensities (Fig. 3).
Although the current study demonstrates that the increase in iHsp72 following exhaustive exercise in the heat at 60 and 75 % occurs in a pattern similar to that of eHsp72, it also highlights a contrast in 24-h responses between iHsp and eHsp expressions. Indeed, eHsp72 decreased below basal (i.e. pre-exercise) levels 24 h after the completion of exercise (Périard et al. 2012), whereas iHsp72 concentration continued to increase during the 24-h period following the cessation of exercise (Fig. 2). This contrast underscores the dichotomous role of Hsp in relation to their relative location (i.e. intracellular vs. extracellular). Moreover, iHsp72 expression results from stimulation of its gene expression, whilst eHsp72 concentration increases following release into the circulation from tissues expressing Hsp72, or those with an initially elevated content. Hence, once the stress stimulus is abated, there is a progressive decrease in Hsp72 being released in the circulation. This is corroborated by findings that basal levels of eHsp72 decreased on days 2 and 3 following exercise-heat exposure (Marshall et al. 2006) and that similar interventions do not result in a sustained basal elevation (Yamada et al. 2007; Magalhaes et al. 2010). Notwithstanding, the post-exercise and 24-h magnitude of iHsp72 expression and the circulating levels of eHsp72 are likely dependent on the level of heat strain attained, as well as sustained.
In summary, the current study demonstrates that exercise to exhaustion at moderate (60 % ) and high (75 % ) intensities in the heat induces a similar increase in iHsp72 concentration in monocytes. Furthermore, our results indicate that an additional increase occurs in the 24 h following exercise termination at both intensities, which reinforces the role of iHsp72 as a chaperone and cytoprotective agent. Interestingly, a correlation was observed between exercise intensities for the increase in iHsp72 noted at exhaustion and 24 h after exercise. The similar increase in concentration noted at exhaustion between exercise intensities is comparable to results observed in eHsp72. However, in contrast to eHsp72, iHsp72 continued to increase during the 24-h period after exercise, highlighting the dichotomy between Hsp72 in relation to their location.
Acknowledgments
The authors thank all the subjects that participated in this investigation. The authors also thank Rhys Philips, Madeline Lynch, Colin Tuohy, Carl Cheah, Angelina Tan and Thomas Wüthrich for their help with the data collection. This work was supported by the University of Sydney Faculty of Health Sciences.
References
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft S-T, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Donati YR, Slosman DO, Polla BS. Oxidative injury and the heat shock response. Biochem Pharmacol. 1990;40:2571–2577. doi: 10.1016/0006-2952(90)90573-4. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol. 2000;89:1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Schlotz E, Passek F, Dickhuth H-H, Northoff H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol. 2000;89:704–710. doi: 10.1152/jappl.2000.89.2.704. [DOI] [PubMed] [Google Scholar]
- Fehrenbach E, Passek F, Niess AM, Pohla H, Weinstock C, Dickhuth H-H, Northoff H. HSP expression in human leukocytes is modulated by endurance exercise. Med Sci Sports Exerc. 2000;32:592–600. doi: 10.1097/00005768-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Veith R, Dickhuth H-H, Northoff H. Changes of HSP72-expression in leukocytes are associated with adaptation to exercise under conditions of high environmental temperature. J Leukoc Biol. 2001;69:747–754. [PubMed] [Google Scholar]
- Fehrenbach E, Veith R, Schmid M, Dickhuth HH, Northoff H, Niess AM. Inverse response of leukocyte heat shock proteins and DNA damage to exercise and heat. Free Radic Res. 2003;37:975–982. doi: 10.1080/10715760310001595748. [DOI] [PubMed] [Google Scholar]
- Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol. 1995;268:R28–R32. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperther. 2005;21:457–471. doi: 10.1080/02656730500088211. [DOI] [PubMed] [Google Scholar]
- Gibson OR, Dennis A, Parfitt T, Taylor L, Watt PW, Maxwell NS (2013) Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress Chaperones [DOI] [PMC free article] [PubMed]
- Graziani-Bowering GM, Graham JM, Filion LG. A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes. J Immunol Methods. 1997;207:157–168. doi: 10.1016/S0022-1759(97)00114-2. [DOI] [PubMed] [Google Scholar]
- Heck TG, Scholer CM, de Bittencourt PI. HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct. 2011;29:215–226. doi: 10.1002/cbf.1739. [DOI] [PubMed] [Google Scholar]
- Hom LL, Lee EC, Apicella JM, Wallace SD, Emmanuel H, Klau JF, Poh PY, Marzano S, Armstrong LE, Casa DJ, Maresh CM. Eleven days of moderate exercise and heat exposure induces acclimation without significant HSP70 and apoptosis responses of lymphocytes in college-aged males. Cell Stress Chaperones. 2012;17:29–39. doi: 10.1007/s12192-011-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol (1985) 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Kwak DJ, Augustine NH, Borges WG, Joyner JL, Green WF, Hill HR. Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect Immun. 2000;68:320–327. doi: 10.1128/IAI.68.1.320-327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GI, Moller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–280. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Emery-Sincliar EL, Mackenzie RWA, Hussain A, Taylor L, James RS, Thake CD. The impact of submaximal exercise during heat and/or hypoxia on the cardiovascular and monocyte HSP72 responses to subsequent (post 24 h) exercise in hypoxia. Extreme Physiol Med. 2014;3:1–16. doi: 10.1186/2046-7648-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Mackenzie RW, Cox V, James RS, Thake CD. Human monocyte heat shock protein 72 responses to acute hypoxic exercise after 3 days of exercise heat acclimation. BioMed Res Int. 2015;2015:849809. doi: 10.1155/2015/849809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M. The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev. 1997;25:105–136. [PubMed] [Google Scholar]
- Lovell R, Madden L, McNaughton LR, Carroll S (2008) Effects of active and passive hyperthermia on heat shock protein 70 (HSP70). Amino Acids 34:203–211 [DOI] [PubMed]
- Magalhaes FC, Amorim FT, Passos RL, Fonseca MA, Oliveira KP, Lima MR, Guimaraes JB, Ferreira-Junior JB, Martini AR, Lima NR, Soares DD, Oliveira EM, Rodrigues LO. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones. 2010;15:885–895. doi: 10.1007/s12192-010-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HC, Ferguson RA, Nimmo MA. Human resting extracellular heat shock protein 72 concentration decreases during the initial adaptation to exercise in a hot, humid environment. Cell Stress Chaperones. 2006;11:129–134. doi: 10.1379/CSC-158R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HC, Campbell SA, Roberts CW, Nimmo MA. Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J Therm Biol. 2007;32:341–348. doi: 10.1016/j.jtherbio.2007.04.003. [DOI] [Google Scholar]
- McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R185–R191. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol, Nutr Metab. 2008;33:1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- Oehler R, Pusch E, Zellner M, Dungel P, Hergovics N, Homoncik M, Eliasen MM, Brabec M, Roth E. Cell type-specific variations in the induction of hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperones. 2001;6:306–315. doi: 10.1379/1466-1268(2001)006<0306:CTSVIT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones. 2012;17:375–383. doi: 10.1007/s12192-011-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Hoffman KM, Chow CM, Thompson MW. Effect of temperature and duration of hyperthermia on HSP72 induction in rat tissues. Mol Cell Biochem. 2004;267:187–194. doi: 10.1023/B:MCBI.0000049382.63841.e4. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Thompson MW, Hoffman KM, Brotherhood JR, Richards DAB. Lymphocyte HSP72 following exercise in hyperthermic runners: the effect of temperature. J Therm Biol. 2007;32:406–412. doi: 10.1016/j.jtherbio.2007.07.001. [DOI] [Google Scholar]
- Selkirk GA, McLellan TM, Wright HE, Rhind SG. Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol. 2009;296:R575–R586. doi: 10.1152/ajpregu.90683.2008. [DOI] [PubMed] [Google Scholar]
- Shin Y-O, Oh J-K, Sohn H-S, Bae J-S, Lee M-Y, Lee J-B, Yang H-M, Min Y-K, Song H-Y, Ko K-K, Matsumoto T. Expression of exercise-induced HSP70 in long-distance runner’s leukocytes. J Therm Biol. 2004;29:769–774. doi: 10.1016/j.jtherbio.2004.08.053. [DOI] [Google Scholar]
- Skidmore R, Gutierrez JA, Guerriero V, Jr, Kregel KC. HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am J Physiol. 1995;268:R92–R97. doi: 10.1152/ajpregu.1995.268.1.R92. [DOI] [PubMed] [Google Scholar]
- Sonna LA, Gaffin SL, Pratt RE, Cullivan ML, Angel KC, Lilly CM (2001) Selected Contribution: effect of acute heat shock on gene expression by human peripheral blood mononuclear cells. J Appl Physiol 92:2208–2220. [DOI] [PubMed]
- Walters TJ, Ryan KL, Tehrany MR, Jones MB, Paulus LA, Mason PA. HSP70 expression in the CNS in response to exercise and heat stress in rats. J Appl Physiol. 1998;84:1269–1277. doi: 10.1152/jappl.1998.84.4.1269. [DOI] [PubMed] [Google Scholar]
- Watkins AM, Cheek DJ, Harvey AE, Blair KE, Mitchell JB. Heat acclimation and HSP-72 expression in exercising humans. Int J Sports Med. 2008;29:269–276. doi: 10.1055/s-2007-965331. [DOI] [PubMed] [Google Scholar]
- Whitham M, Fortes MB. Heat shock protein 72: release and biological significance during exercise. Front Biosci. 2008;1:1328–1339. doi: 10.2741/2765. [DOI] [PubMed] [Google Scholar]
- Whitham M, Halson SL, Lancaster GI, Gleeson M, Jeukendrup AE, Blannin AK. Leukocyte heat shock protein expression before and after intensified training. Int J Sports Med. 2004;25:522–527. doi: 10.1055/s-2004-820953. [DOI] [PubMed] [Google Scholar]
- Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider SM. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol (1985) 2007;103:1196–1204. doi: 10.1152/japplphysiol.00242.2007. [DOI] [PubMed] [Google Scholar]