Abstract
Chronic obstructive pulmonary disease (COPD) is a sustained blockage of the airways due to lung inflammation occurring with chronic bronchitis and/or emphysema. Progression of emphysema may be slowed by vascular endothelial growth factor A (VEGFA), which reduces apoptotic tissue depletion. Previously, authors of the present report demonstrated that cis-resveratrol (c-RSV)-induced heat-shock protein 70 (HSP70) promoter-regulated VEGFA expression promoted neovascularization of genetically modified mesenchymal stem cells (HSP-VEGFA-MSC) in a mouse model of ischemic disease. Here, this same stem cell line was evaluated for its protective capacity to alleviate elastase-induced pulmonary emphysema in mice. Results of this study showed that c-RSV-treatment of HSP-VEGFA-MSC exhibited synergy between HSP70 transcription activity and induced expression of anti-oxidant-related genes when challenged by cigarette smoke extracts. Eight weeks after jugular vein injection of HSP-VEGFA-MSC into mice with elastase-induced pulmonary emphysema followed by c-RSV treatment to induce transgene expression, significant improvement was observed in respiratory functions. Expression of VEGFA, endogenous nuclear factor erythroid 2-related factor (Nrf 2), and manganese superoxide dismutase (MnSOD) was significantly increased in the lung tissues of the c-RSV-treated mice. Histopathologic examination of treated mice revealed gradual but significant abatement of emphysema and restoration of airspace volume. In conclusion, the present investigation demonstrates that c-RSV-regulated VEGFA expression in HSP-VEGFA-MSC significantly improved the therapeutic effects on the treatment of COPD in the mouse, possibly avoiding side effects associated with constitutive VEGFA expression.
Keywords: Stem cell-based gene therapy, Resveratrol, Hsp70 promoter, VEGFA, Mouse model, Emphysema
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressively severe inflammation-associated airway blockage, often associated with bronchitis and/or emphysema (Vestbo et al. 2013). Reports have demonstrated that cigarette smoke activates neutrophils and macrophages and produces proteases and oxidants resulting in lung damage (Barnes 2004). The destruction of air space (alveolar) walls in the alveoli reduces available surface area leading to loss of capillary endothelial and alveolar epithelial cells through apoptosis (Chung and Adcock 2008; Crosby and Waters 2010). A previous study reported that in patients with severe COPD, total lung tissue extracts showed lower levels of vascular endothelial growth factor A (VEGFA) compared with healthy subjects (Henson et al. 2006). Alveolar septum thickness in COPD patients was significantly thinner, with diminished number of small capillaries relative to healthy tissue, features that contribute to reduced alveolar functional efficiency. Thus, all evidence supports a “vascular hypothesis” as a major feature of COPD pathophysiology (Henson et al. 2006). Moreover, VEGFA may be partially cleaved by neutrophil elastase (NE), generating a VEGFA fragment with altered activities (Kurtagic et al. 2009). The resulting binding by the altered receptor is translated to a loss of signaling potentials in endothelial cells and hinders lung vascular functions (Kurtagic et al. 2009). Moreover, intratracheal adenovirus-mediated VEGFA gene therapy has been shown to improve survival, formation of lung capillaries, and restoration of irreversible lung injuries in a hyperoxia model of alveolar development of bronchopulmonary dysplasia (Thébaud et al. 2005).
Reports have demonstrated increased concentrations of serum levels of heat-shock protein 70 (HSP70) in patients in early and late stages of COPD, and a four-fold increase in the COPD-II group compared to non-symptomatic smokers (Hacker et al. 2009). Thus, the serum contents of HSP70 are associated with the stage of COPD. Resveratrol (RSV) is a phytochemical inducer of heat-shock factor protein 1 (HSF-1) that activates HSP70 promoter activities (Putics et al. 2008). Our other study demonstrated that resveratrol-induced and HSP70 promoter-regulated VEGFA expression enhanced tube formation of human umbilical vein endothelial cell line (HUVEC) (Chen et al. 2015).
Trans-resveratrol (t-RSV) activation of nuclear factor erythroid 2-related factor (Nrf2) protects against oxidative stress induced by cigarette smoke extract (CSE)-mediated reduction of glutathione levels in lung epithelial cells (Kode et al. 2008). Studies have also indicated that t-RSV inhibits cytokines releases from both smokers and COPD patients via actions of anti-inflammatory and anti-oxidant properties (Culpitt et al. 2003). In contrast, there are few studies on the biological effects of cis-resveratrol (c-RSV) on anti-cancer and anti-platelet aggregation activities (Pettit et al. 2002; Bertelli et al. 1996). In a previous report, we showed that c-RSV reduced, whereas t-RSV increased, IL-1β secretion, and that the anti-inflammatory actions of c-RSV in human acute monocytic leukemia cells (THP-1)-differentiated macrophages could be associated with inhibition of the non-canonical inflammasome pathway (Huang et al. 2014).
Evidences have also shown that lung exposure to CSE could activate nuclear factor erythroid-2-related factor (Nrf2)-dependent anti-oxidant- and cytoprotective-related genes, contributing to resistance to CSE-induced emphysema by upregulating anti-oxidant defense systems, inhibition of lung inflammation, and alveolar cell apoptosis (Tuder et al. 2006; Rangasamy et al. 2004). Other evidences include observations that Nrf2-knockout mice were highly susceptible to risks of emphysema on CSE exposure, lack of Nrf2/anti-oxidant response element (ARE)-regulated anti-oxidant/anti-inflammatory responses, and maintenance of protease/anti-protease balance protecting against the development of pulmonary emphysema (Iizuka et al. 2005).
Several studies have demonstrated that mesenchymal stem cell (MSC)-based cell therapy results in improvement in the treatment of emphysema in rat models (Zhen et al. 2008; van Haaften et al., 2009). Furthermore, MSCs secrete anti-apoptotic and anti-inflammatory paracrine factors (Meirelles Lda et al. 2009; Akram et al. 2013), and reduce lung injuries (Katsha et al. 2011). Despite reported successes, the amount of engrafted MSCs decreased dramatically 24 h post-transplantation most likely due to exposure to toxic and oxidative microenvironments (Leblond et al. 2009; Lan et al. 2015). We have previously demonstrated in a mouse angiogenesis model that genetically modified MSCs that were conditionally expressing VEGFA under the control of a RSV-inducible HSP70 promoter showed maximized efficacy by spatial and temporal control of angiogenic abilities (Chen et al. 2015). In this study, we further investigated the protective effects of inducible expression of stem cell-based VEGFA gene therapy approach against elastase-induced pulmonary emphysema in a mouse model.
Materials and methods
Cell culture
Mouse bone marrow mesenchymal stem cells (MSC) were isolated from bone marrow of C57BL/6 female mice at ≤8 weeks after gestation. MSCs were purchased from Life Technologies (Carlsbad, CA) and stably HSP-VEGFA-transduced MSC (HSP-VEGFA-MSC) cells were cultured and prepared as previously described (Chen et al. 2015). Cells were maintained in Dulbecco’s modified Eagle’s Medium/Ham’s Nutrient Mixture F-12 (DMEM/F12; Life Technologies) supplemented with 10 % fetal bovine serum (FBS; Life Technologies), 2 mM l-glutamine (Life Technologies), and 1 % penicillin/streptomycin (Life Technologies).
Preparation of cigarette smoke extract
Cigarette smoke extract (CSE) was prepared as described (Cheng et al. 2009). Quality of the CSE solution was assessed based on absorbance at 302 nm, which is the specific absorption spectrum of peroxynitrite. The CSE was prepared as a stock solution (20 mg/ml of CSE containing 0.36 mg/ml of nicotine).
MTT cell viability assay
MSC cells were plated at a density of 1 × 105 cells/well in a 12-well culture plate (BD Biosciences) and were treated with CSE in the presence of mock or t-RSV or c-RSV (Cayman) for 48 h. Cell viability was detected using the MTT method at 540 nm by VersaMax spectrophotometry (Molecular Devices).
Luciferase activity assay
Luciferase activity was assayed using a Promega assay kit according to the manufacturer’s instructions. Cells were plated at a density of 1 × 105 in a 24-well plate, lysed with passive lysis buffer and assayed by the dual-luciferase reporter assay system (Hung et al. 2014a).
Quantitative real-time RT-PCR
Total RNA was prepared from the cell lines and was treated with DNase I (New England BioLabs, Ipswich, MA). RNAs were reverse transcribed into complementary DNAs (cDNAs) at 42 °C for 60 min using Moloney murine leukemia virus reverse transcriptase (Life Technologies). After the oligo (dT)-primed reverse transcription reaction, quantitative real-time RT-PCR was performed using LightCycler 480 SyberGreen I Master Mix and LightCycler® 480 Instrument (Roche, Mannheim, Germany) as previously described (Chong et al. 2015). Sequences of the mouse and human gene-specific primers used are listed in Table 1. For normalization, Gadph messenger RNA (mRNA) levels of each RNA preparation were determined. Relative gene expression was determined by the △△Ct method, where Ct is threshold cycle (Livak and Schmittgen 2001). The relative mRNA levels were normalized to the mRNA level of the reference Gadph gene. The melting curve of the amplification product was always checked to ensure a single clean peak that represented good quality quantitative real-time RT-PCR data.
Table 1.
PCR primers
| Gene name | Forwards primers 5′ → 3′ | Reverse primers 5′ → 3′ |
|---|---|---|
| Nrf 2 | TAGATGACCATGAGTCGCTTGC | GCCAAACTTGCTCCATGTCC |
| HO 1 | AAGCCGAGATAGCTGAGTTCA | GCCGTGTAGATATGGTACAAGGA |
| VEGFA | CTGTGCAGGCTGCTGTAAC | ACAGTGATTTTCTGGCTTTGTTC |
| MnSOD | ACAAACCTGAGCCCTAAGGGT | GAACCTTGGACTCCCACAGAC |
| GAPDH | TCCCACCCAGAGACAGCCGC | CCGTTCACACCGACCTTCAC |
Western blot analysis
Total cellular proteins were isolated from cell lines by the PRO-PREP™ Protein Extraction Solution (Intron Biotechnology, Kyonggi-do, Korea) and Western blot analysis was performed as described previously (Hung et al. 2014b). In brief, an amount of 25 or 50 μg of total proteins from cell lysates or conditioned media was loaded onto each lane and the proteins were separated in sodium dodecyl sulfate polyacrylamide electrophoresis (SDS-PAGE; Bio-Rad Laboratories, Hercules, CA). After electrophoresis, the resolved proteins were transferred to PVDF membrane (EMD Millipore, Billerica, MA). The membranes were blocked with 5 % skimmed milk powder (Anchor, Kowloon, Hong Kong) in phosphate-buffered saline-Tween (PBS-T): phosphate-buffered saline (PBS, Sigma-Aldrich) containing 0.1 % Tween-20 in (Sigma-Aldrich) for 1 h and probed overnight with the following antisera at appropriate dilutions: 1:1000 dilution of the anti-heme oxygenase 1 (HO-1) (MBL International, Woburn, MA), a 1:1000 dilution of the anti-Nrf2 (Santa Cruz Biotechnology Inc., Dallas, TX), and a 1:10,000 dilution of the anti β-actin (EMD Millipore) antisera in PBS-T. Identification of each protein was achieved with the Western Lighting Plus Reagent (PerkinElmer, Waltham, MA) using an appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson Immuno Research Laboratories, West Grove, PA). Protein levels in the Western blot analysis were detected and quantified by the LAS-3000 chemiluminescence detection device (Fujifilm, Valhalla, NY). To adjust for loading differences, the optical density of each protein was normalized to that of the β-actin band.
Construction of lentiviral vector and transduction into MSCs
The 8XARE sequence was composed of eight copies of the oligonucleotide sequence 5ʹ-GTGACAAAGCA-3ʹ; the minimal functional enhancer was assembled by PCR synthesis and cloning into pLenti-LT-hyg, resulting in p8XARE-Luc (Fig. 1a). Virus stocks were prepared by co-transfecting the p8XARE-Luc or pHSP-Luc (Fig. 1a) construct with three packaging plasmids, pMDLg/pRRE, CMV-VSVG, and RSV-Rev, into 293 T cells. The viral supernatants were harvested 36–48 h later, filtered, and centrifuged at 20,000×g for 90 min. The viral titer was determined by the method of end-point dilution through counting the number of infected red cells at ×100 magnification under a fluorescence microscope 96 h after transduction of 293 T cells. Titer in the transducing units was computed as follows: (TU)/mL = (the numbers of red fluorescent cells) × (dilution factor)/(volume of virus solution). Titers of the viral particles were quantified by an HIV quantification ELISA kit. MSCs were seeded in 12-well plate and the cells were transduced with an equal ratio of viral particle of virus particle following previous method (Chan et al. 2001), and the stably transduced cells were designated as ARE-Luc-MSC and HSP-Luc-MSC.
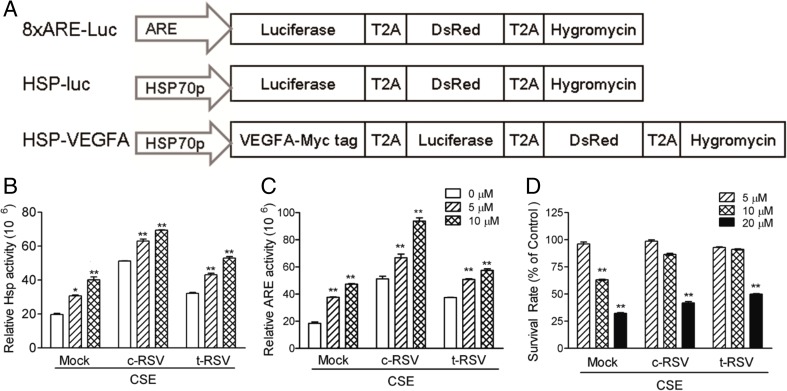
Fig. 1.
Resveratrol significantly induces transcription activities of the HSP70 promoter and ARE and enhances MSC survival on CSE challenge. a Schematic representation of the lentiviral vectors. The 2A peptide sequence (T2A) from the Thosea asigna virus was used as a linker connecting the red fluorescence protein (DsRed), hygromycin and the luciferase (Luc) gene to generate the lentiviral multi-cistronic expression vector, pLenti-LT-hyg. The pLenti-8XARE-Luciferase-DsRed-hygromycin (abbreviated as 8XARE-Luc), pLenti-HSPp-Luciferase-DsRed-hygromycin (HSP-Luc), and pLenti-HSP70p-VEGFA-Luciferase-DsRed-hygromycin (HSP-VEGFA). b HSP-Luc-MSC or c ARE-Luc-MSC was co-treated with the indicated concentrations of c-RSV or t-RSV and CSE for 12 h. At the end of the treatment, cells were harvested for luciferase assays. d HSP-VEGFA-MSC was similarly co-treated with c-RSV or t-RSV and CSE for 48 h, followed by cell viability MTT assay. Data represent means ± SD of three independent experiments. *P < 0.05 and **P < 0.01 indicate differences between the treated cells and the mock control
Porcine pancreatic elastase-induced emphysema mouse model
Eight-week-old female C57BL/6JNarl mice were purchased from the National Laboratory Animal Center (Taipei, Taiwan). The mice were maintained in an air-conditioned animal facility under constant temperature and humidity conditions with a 12:12 light/dark cycle and were allowed ad libitum diet and drinking water. Mice were randomly picked to different groups, and there were at least nine or more mice in each group. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Chang Gung University. The mice were anesthetized by isoflurane (Abbott Laboratories, Abbott Park, IL) and intratracheally instilled porcine pancreatic elastase (PPE) at 1.5 mg/kg body weight in 50-μl sterile PBS. On day 14 after administration, mice were randomly selected for intravenous administration of HSP-VEGFA-MSC or PBS via the jugular vein. The mice with or without stem cell transplantation were intraperitoneally injected 20 mg/kg c-RSV every other day. All animals were placed in the whole-body plethysmograph tanks for analysis of pulmonary functions using the whole-body plethysmography (WBP) system every other week. All mice were sacrificed at week 8 by an overdose of 2.5 % avertin following previous method (Lan et al. 2015).
Noninvasive measurement of pulmonary functions by WBP
The baseline-enhanced respiratory pause (Penh) in unrestrained conscious mice was measured by WBP (Buxco Electronic Inc, Wilmington, NC). Mice were placed in the chamber and allowed to equilibrate for approximately 10 min. According to the manufacturer’s instructions, Penh is calculated by the formula Penh = (Te/Rt − 1) × (PEF/PIF), where Te is expiratory time (s), Rt is relaxation time (s) defined as the time of “volume” decay to 35 % of the total expiratory pressure signal (area under the box pressure signal at expiration), PEF is peak expiratory flow (ml/s), and PIF is peak inspiratory flow (ml/s). Penh reflects changes in the waveform of the box pressure signal during both inspiration and expiration, and combines these changes with the timing comparison of early and late expiration during animal’s spontaneous breathing. Penh was measured for 3 min for each experiment following previous method (Lan et al. 2015).
Morphometric assessment
Left lungs were fixed with 10 % formalin and were embedded in paraffin sections before staining with hematoxylin and eosin following previous method (Tung et al. 2011). After fixation, 4-μm serial step sections were taken along the longitudinal axis of the lobe. The fixed distance between the sections was calculated to allow systematic sampling of 10 sections across the whole lobe. Alveolar size of the lung was assessed by determining the mean linear intercepts (Lm). Lm was calculated based on 20 randomly selected fields in each section (in total 80 fields/mouse) at ×200 magnification with two crossed test lines. The intercepts of alveolar walls with these lines were counted
Immunohistochemical staining for beta-galactosidase protein
Immunohistochemistry was performed to evaluate engraftment of transplanted beta-Gal-MSCs in vivo. Paraffin-fixed sections were microwave heated (750 W, three 5-min cycles) in 10 mmole/l citrate (pH 6.0) or 1 mmol/l EDTA (pH 8.0) solution. Immunostaining was performed with a 1:1000 dilution of the anti-beta-galactosidase antibody (ab9361; Abcam Inc., Cambridge, MA) using the Dako-REAL, Alkaline-Phosphatase/RED detection system (Dako, Glostrup, Denmark). Hematoxylin was used for counterstaining according to the manufacturer’s protocol. The stained sections were scanned by the HistoFAXS (TissueFAX plus; TissueGnostics, Vienna, Austria).
Statistical analysis
The surviving fractions and the relative luminescence units were measured in triplicates and expressed as the mean ± SD. Comparisons between two groups were analyzed using a two-tailed Student’s t test. The results were analyzed by one-way ANOVA followed by Tukey’s post hoc test for analyzing parametric data. All statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA). *P < 0.05 was considered statistically significant.
Results
Induction of transcription activities of HSP and ARE and enhanced cell survival in RSV-treated MSCs after CSE challenge
To examine if HSP70 promoter-driven transgene expression responded to CSE challenge, HSP-Luc-MSC was first treated with 5 or 10 μM c-RSV or t-RSV for 12 h. The transcriptional activity of the HSP70 promoter was found to be upregulated up to three- and fourfold, respectively, on c-RSV and t-RSV treatments (Fig. 1b). Simultaneous exposure of HSP-Luc-MSC to 5 or 10 μM c-RSV or t-RSV and CSE for 12 h resulted in significantly upregulated HSP70 promoter activities in a dose-dependent manner. Furthermore, HSP70 transcriptional activities were increased by up to 2.5-fold in c-RSV and 2-fold in t-RSV treatment groups on CSE challenge (Fig. 1b).
Regulation of oxidative stress response by anti-oxidant response element (ARE) activity in RSV-treated ARE-Luc-MSC under CSE challenge was investigated next. The transcriptional activity of the ARE promoter was found to increase by up to 3.5-fold on c-RSV and 1-fold in t-RSV treatment under CSE challenge in a dose-dependent manner (Fig. 1c). The results indicated that resveratrol and the CSE challenge exerted synergistic effects on activating HSP70 and ARE transcriptional activities.
More recently, attention has been given to cellular injuries due to oxidants in the cigarette smoke or oxidants induced by CSE. HSP-VEGFA-MSC stable cells previously generated in our laboratory were used in this study (Chen et al. 2015). As anticipated, CSE induced cell death in a dose-dependent manner in HSP-VEGFA-MSC in MTT assays (Fig. 1d). However, significant degrees of cytoprotection against CSE-induced cell death on treatments with both forms of RSV were also observed (Fig. 1d). Western blot results further showed that c-RSV upregulated expression of luciferase and VEGFA as well as heme oxygenase 1 (HO-1) in HSP-VEGFA-MSC. t-RSV treatment showed higher induction ability than c-RSV treatment as described in our previous report (Chen et al. 2015).
Induction of Nrf2 and HO-1 in response to c-RSV treatment
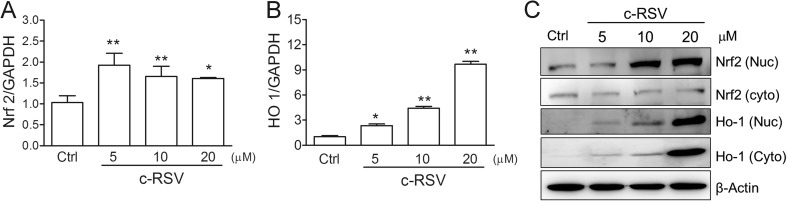
To investigate the responses of oxidative stress and anti-oxidant genes in c-RSV-treated HSP-VEGFA-MSC, different dosages of c-RSV was used to treat the cells for 8 h. Real-time PCR analysis showed that Nrf2 mRNA was increased twofold on c-RSV treatment in HSP-VEGFA-MSC (Fig. 2a). HO-1 mRNA also increased nine-fold on treatment with 20 μM c-RSV relative to the control (Fig. 2b). Nrf2 and HO-1 protein levels also increased in a dose-dependent manner on c-RSV treatment; at 20 μM, RSV significantly induced Nrf2 and HO-1 protein expression (Fig. 2c). When the HSP-VEGFA-MSC was treated with 5–20 μM c-RSV, Nrf2 protein levels increased and the phosphorylated Nrf2 was translocation into the nucleus.
Fig. 2.
c-RSV induces anti-oxidant-related genes expression in HSP-VEGFA-MSC. a Nrf2 or b HO-1 mRNA levels of HSP-VEGFA-MSC treated with c-RSV or t-RSV was determined by real-time PCR. Values are normalized to that of Gadph values and expressed relative to the control (Ctrl) group. c Cytoplasmic or nuclear Nrf2 and HO-1 protein levels were detected by Western blot analysis. Values were normalized to that of β-actin and were expressed relative to the control (Ctrl) group. Data represent means ± SD of three independent experiments. *P < 0.05 and **P < 0.01 indicate differences between the treated cells and the mock control
Improvement of pulmonary respiratory functions in the lungs of PPE-induced emphysema on c-RSV co-treatment of HSP-VEGFA-MSC transplant
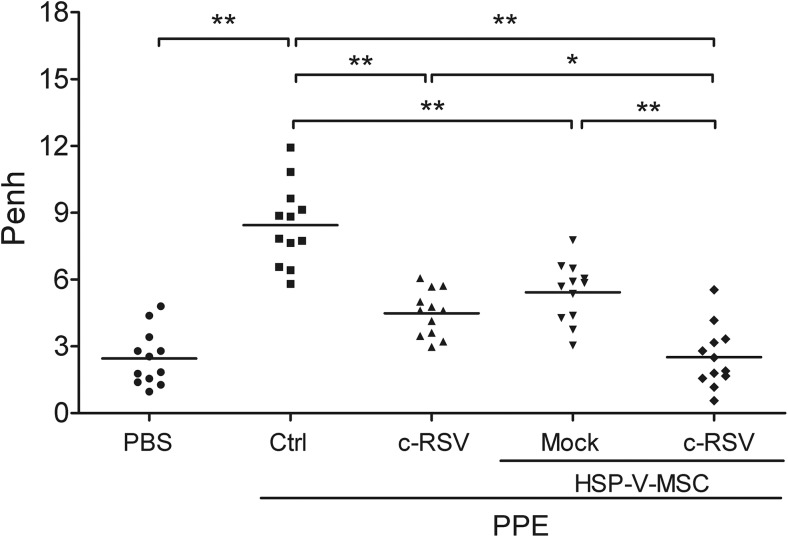
To evaluate the therapeutic efficacy of c-RSV co-treatment in HSP-VEGFA-MSC transplantation in the lungs of PPE-emphysema mouse model, whole-body barometric plethysmography (WBP) was used to monitor lung functions of the treated mice. In the experiments, the enhanced pause value (Penh) of respiratory parameter showed a fourfold increment at 8 weeks after PPE treatment compared with the PBS group. In addition, the PPE group with c-RSV treatment, or PPE with HSP-VEGFA-MSC, showed two-fold improved Penh values when compared with the PPE-only group (Fig. 3). Furthermore, combined c-RSV co-treatment and HSP-VEGFA-MSC had the lowest Penh value, which was approximately the same as the mock control (Fig. 3). Taken together, our results indicated that co-treatment of c-RSV with transplanted HSP-VEGFA-MSC showed better therapeutic effects and improved lung functions in mice with PPE-induced emphysema.
Fig. 3.
c-RSV-treated HSP-VEGFA-MSC improves pulmonary functions in PPE-induced emphysema mice. Pulmonary functions were evaluated as Penh values in animals that received the PBS control, PPE, or PPE group that received either c-RSV or HSP-VEGFA-MSC with or without c-RSV treatment for 8 weeks following PPE administration on day 14. Whole-body plethysmograph (WBP) was employed and Penh was used as a noninvasive index of airway dysfunction. *P < 0.05, **P < 0.01. Each dot represents an individual mouse with the mean shown for n ≥ 9 per group
Upregulated expression of anti-oxidant-related genes and VEGFA in c-RSV co-treatment of HSP-VEGFA-MSC transplantation in the lungs of PPE-induced emphysema
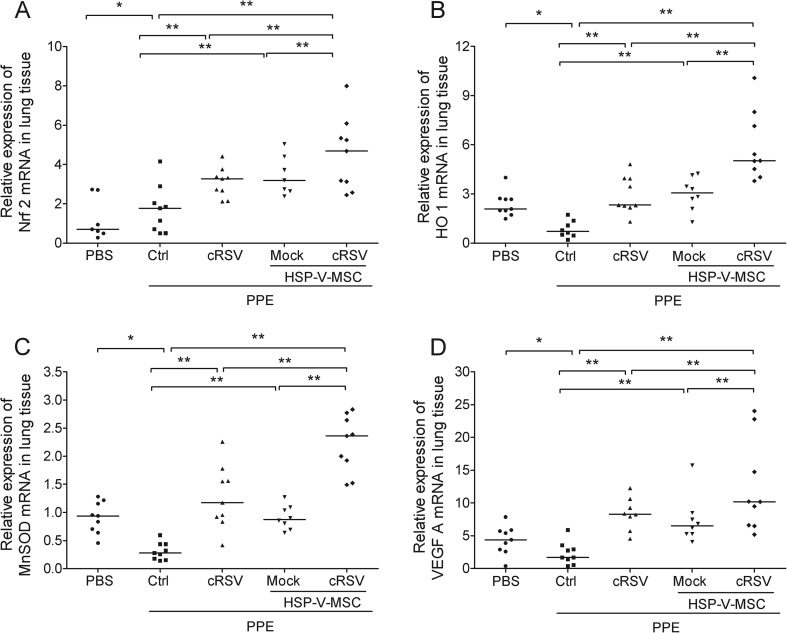
To determine the effects on expression of anti-oxidant-related genes, real-time PCR analysis showed that Nrf2 mRNA was upregulated four-fold in the PPE group when compared with the control group (Fig. 4a). PPE with c-RSV or PPE with HSP-VEGFA-MSC showed further four-fold increases when compared with the PPE control groups. HO-1 mRNA (Fig. 4b), manganese superoxide dismutase (MnSOD) mRNA and VEGFA, were found to be decreased in the PPE group when compared with the control group (Fig. 4). Interestingly, all the three anti-oxidant genes and VEGFA tested were significantly upregulated on c-RSV co-treatment in the HSP-VEGFA-MSC transplants in the lungs of PPE-induced emphysema. Our data indicated that the engrafted co-treatment of HSP-VEGFA-MSC had better therapeutic effects and improved lung functions in the PPE-induced emphysema, possible through overexpression of anti-oxidant-related genes and VEGFA, thus preventing continued exacerbation of pulmonary alveolar and lung parenchymal tissues.
Fig. 4.
c-RSV-treated HSP-VEGFA-MSC upregulates expression of anti-oxidant-related genes and VEGFA in PPE-induced emphysema mice. Real-time RT-PCR was performed to analyze the expression of the anti-oxidant-related genes Nrf 2 (a), HO-1 (b), MnSOD (c), and growth factor VEGFA (d) in the lung tissues of the animals that received PBS, the PPE group, and PPE group that received either c-RSV or HSP-VEGFA-MSC with or without c-RSV treatment for 8 weeks following PPE administration on day 14. Values are normalized to the Gapdh values and expressed relative to the PBS group. *P < 0.05, and **P < 0.01. Each dot represents an individual mouse with the mean shown for n ≥ 9 per group
c-RSV co-treatment of HSP-VEGFA-MSC transplantation alleviates histological changes in the lungs of PPE-emphysema
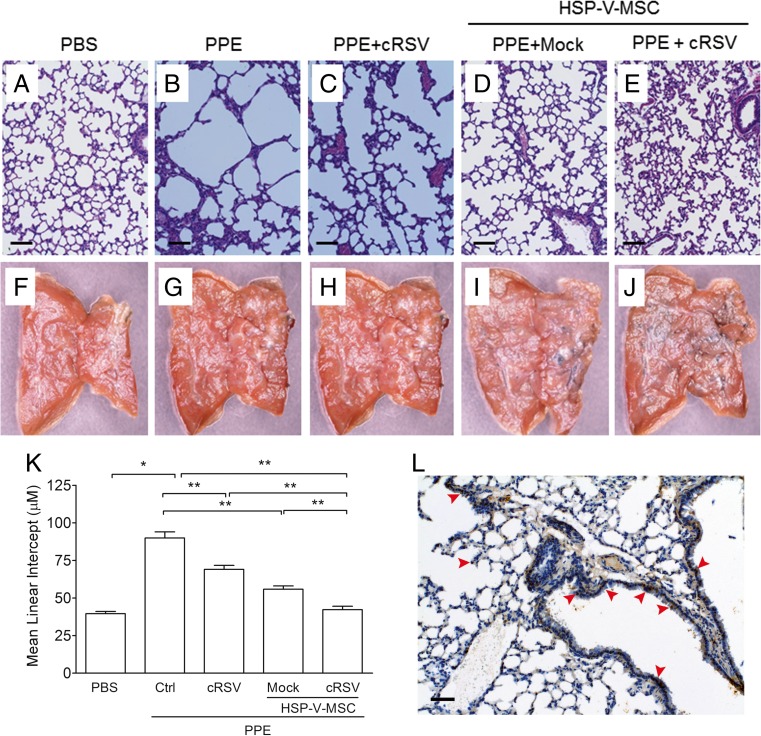
In lung injuries, airspace was enlarged, followed by accumulation of acute neutrophil and subacute macrophage within the lung. Additionally, more serious lung damage promotes increased expression of proinflammatory and necrotic mediators in lung tissues. Histopathologic observation of lung tissues from animals that received control, PPE only, PPE co-treated with c-RSV, or PPE with c-RSV and HSP-VEGFA-MSC co-treatment for 8 weeks is shown in Fig. 5. There were no obvious lesions or inflammatory effects in the lung of the control group (Fig. 5a). However, in the PPE groups, H&E staining showed obvious enlargement of the pulmonary alveolus cavity and airspace and breakdown of connective tissue components in the alveolar wall (Fig. 5b). In the PPE group that received c-RSV treatment, the lung interstitium was slightly improved (Fig. 5c). Furthermore, in the PPE group that received c-RSV with HSP-VEGFA-MSC treatment significantly improved (Fig. 5e) when compared with the PPE group without c-RSV treatment (Fig. 5d). The severity of emphysema assessed by measuring the mean linear intercepts (Lm) and destructive index (DI) in the lung interstitium was significantly reduced in HSP-VEGFA-MSC with or without c-RSV treatment (Fig. 5k).
Fig. 5.
c-RSV-treated HSP-VEGFA-MSC transplants reduces mean linear intercepts of histopathologic changes in PPE-induced emphysema mice Representative images at 8 weeks of a–e H&E- or f–j LacZ-stained lung tissues. a, f PBS control. b, g PPE. c, h PPE + c-RSV. d, i, PPE + mock-treated HSP-VEGFA-MSCs. e, j PPE + c-RSV treated HSP-VEGFA-MSCs. k Mean linear intercept (Lm) was calculated in H&E-stained lung sections. l Immunohistochemical staining to observe distribution of LacZ of MSC-treated lung tissues of mice. Scale bar 100 μm
To determine if the engrafted HSP-VEGFA-MSC survived and adapted to the environment in the damaged lungs 8 weeks after PPE treatment, LacZ reporter-transduced HSP-VEGFA-MSC (β-Gal-MSC) was used to monitor the cells after transplantation. No LacZ-labeled cells were observed in sections of lung tissues of either the normal, PPE, or PPE with c-RSV-treated groups under microscopy (data not shown). In contrast, LacZ-labeled cells were observed and distributed around the bronchi in the HSP-VEGFA-MSC group (Fig. 5l). Our data indicated that the engrafted co-treatment c-RSV and HSP-VEGFA-MSC showed better therapeutic effects and improved the lung functions in the PPE-induced emphysema.
Discussion
VEGFA plays significant physiologic roles in the pathogenesis of several lung disorders, including COPD and pulmonary hypertension, acting via non-inflammatory processes (Papaioannou et al. 2006). Studies have also indicated that VEGFA ameliorates the progression of emphysema and prevents subsequent apoptosis (Thébaud et al. 2005). Side effects of continued over-expression of VEGFA may result in vascularity, growth of lung tumor (Moon et al. 2003), increases in vascular permeability, and development of substantial tissue edema (Rissanen et al. 2004). To avoid complications associated with constitutive VEGFA expression, we are the first to demonstrate in this work, and a related work (Chen et al. 2015), that conditional expression of VEGFA under the regulation of a c-RSV-inducible HSP70 promoter leads to tight and comprehensive regulated VEGFA expression that promotes cell survival (Fig. 1d) and enhanced releases of anti-oxidant and growth factors (Fig. 2). Combined treatment of c-RSV and engraftment of HSP-VEGFA-MSC rendered better therapeutic effects than mock control in a PPE-induced emphysema mouse model (Figs. 3 and 5).
We have further shown in this work that the cis-form of RSV, c-RSV, also acts as an inducer of ARE and heat-shock response (HSE) (Fig. 1). Furthermore, c-RSV showed greater potency as a regulator than t-RSV-regulated HSP70 promoter activities (Fig. 1a). c-RSV also exhibited anti-oxidant activity in CSE-challenged MSC in micromolar concentration range, similar to rat aortic myocytes and human umbilical vein endothelial cell line (HUVEC) treated with t-RSV (Orallo 2006). Nrf2 and HO-1 have been shown to play a role in preventing cell or tissue apoptosis and necrosis in response to various insults such as inflammatory and oxidative stress (Messier et al. 2013). Our results also demonstrated that c-RSV induced Nrf2- and HO-1 expression in concentration- and time-dependent manners in HSP-VEGFA-MSC (Fig. 2), reminiscent of the inducer properties of t-RSV and curcumin (Juan et al. 2005).
CSE generates extracellular reactive oxygen species (ROS) during incubation with human monocytes (Pinot et al. 1999), and HSP70 and HO-1 play similar oxidant-dependent responses to CSE in monocytes and endothelial (Vayssier-Taussat et al. 2001). Our result demonstrated that both forms of RSV also protected HSP-VEGFA-MSC against CSE-induced cellular cytotoxic response of apoptosis and necrosis (Fig. 1d).
We showed previously that tubule formation of HUVEC significantly increased in HUVECs grown in conditional medium from c-RSV-treated HSP-VEGFA-MSC (Chen et al. 2015). The results suggest the release of VEGFA from c-RSV-treated MSCs. Furthermore, c-RSV-treated HSP-VEGFA-MSC was shown in this work to promote VEGFA release and regulate the local microenvironment of the lung of PPE-induced emphysema in the mouse. We further demonstrated that c-RSV-induced expression of anti-oxidant-related genes, including Nrf2 target genes, HO-1, and MnSOD (Fig. 4), and ameliorated pulmonary respiratory functions in the HSP-VEGFA-MSCs transplants in lung tissues of PPE-induced emphysema (Fig. 3). These data were consistent with the notion that HO-1 overexpression attenuates inflammatory response in PPE-induced emphysema in mice (Ishii et al. 2005; Shinohara et al. 2005).
Studies have clearly demonstrated that upregulation of Nrf2 and its down-stream effectors HO-1 and MnSOD significantly reduces CSE-induced release of ROS in lung epithelial cells (Kode et al. 2008; Foronjy et al. 2006) and protects against smoke- or elastase-generated pulmonary emphysema (Foronjy et al. 2006). In contrast, Nrf2-deficient mice without a defense system are highly susceptible to cigarette smoke-induced pulmonary emphysema (Iizuka et al. 2005).
Despite reported successes, possible adverse effects that may arise from clinical applications of VEGFA should be closely monitored. Possible carcinogenic transformation of MSC and tissue cells as a result of high-level VEGFA expression constitutes another source of risks which should also be addressed (Moon et al. 2003). It has been reported that VEGFA overexpression may lead to blood-brain barrier leakage after stroke (Wang et al., 2005), rapid microvessel enlargement, and increased vascular permeability in the heart (Rissanen et al. 2004). Furthermore, Nrf2 activity is also controlled by oncogenic signaling pathways (Geismann et al. 2014), metabolism, and cellular microenvironment (Moon and Giaccia 2015). Additional evidence has accumulated that the beneficial role of Nrf2 in cancer prevention essentially depends on the tight control of its activity (Geismann et al. 2014), Thus, resveratrol and other agents that upregulate Nrf2/ARE may promote harmful as well as cytoprotective effects. However, the genetically modified MSCs that conditionally expressed VEGFA under the control of a c-RSV-inducible HSP70 promoter described in this work should alleviate, or vastly reduce, possible clinical risks associated with constitutive VEGFA expression as described. In summary, we demonstrated in this work that c-RSV-treated MSCs exhibited beneficial effects such as upregulated expression of anti-oxidant-regulated Nrf2 and HO-1 genes, inhibition of inflammatory cytokine, replacement of dysfunctional endothelial cells, engraftment into the pulmonary wall and re-activation of lung regeneration signaling pathways.
Acknowledgments
This work was supported in part by the Ministry of Science and Technology-Taiwan, NSC 99-2632-B-182-001-MY3 and MOST 103-2320-B-182-021, Chang Gung Memorial Hospital Grant (CMRPD 34012, CMRPD 180133, CMRPD1B0471, CMRPD1B0472, and CMRPD1B0473), and the Ministry of Education-Taiwan (EMRPD1C0121). The authors thank Molecular Imaging Center, Chang Gung Memorial Hospital-Linkou, for tissue slide imaging by HistoFAXS), and Professor Mei-Ling Kuo, Department of Microbiology and Immunology, Chang Gung University, for technical advice of noninvasive measurement of pulmonary function by whole-body plethysmography (WBP).
Contributor Information
Hsuan-Shu Lee, Phone: +886-2-23123456, Email: benlee@ntu.edu.tw.
Kowit-Yu Chong, Phone: +866-3-2118393, Email: kchong@mail.cgu.edu.tw.
References
- Akram KM, Samad S, Spiteri MA, Forsyth NR. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir Res. 2013;14:9. doi: 10.1186/1465-9921-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- Bertelli AA, Giovannini L, Bernini W, Migliori M, Fregoni M, Bavaresco L, Bertelli A. Antiplatelet activity of cis-resveratrol. Drugs Exp Clin Res. 1996;22:61–63. [PubMed] [Google Scholar]
- Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G. Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science. 2001;291:309–312. doi: 10.1126/science.291.5502.309. [DOI] [PubMed] [Google Scholar]
- Chen YB, Lan YW, Hung TH, Chen LG, Choo KB, Cheng WTK, Lee HS, Chong KY. Mesenchymal stem cell-based HSP70 promoter-driven VEGFA induction by resveratrol alleviates elastase-induced emphysema in a mouse model. Cell Stress Chaperones. 2015;20:643–652. doi: 10.1007/s12192-015-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SE, Luo SF, Jou MJ, Lin CC, Kou YR, Lee IT, Hsieh HL, Yang CM. Cigarette smoke extract induces cytosolic phospholipase A2 expression via NADPH oxidase, MAPKs, AP-1, and NF-kappaB in human tracheal smooth muscle cells. Free Radic Biol Med. 2009;46:948–960. doi: 10.1016/j.freeradbiomed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Chong KY, Hsu CJ, Hung TH, Hu HS, Wang TH, Wang CH, Chen CM, Choo KB, Lai HC, Tseng CP. Wnt pathway activation and ABCB1 expression account for attenuation of proteasome Inhibitor-mediated apoptosis in multidrug-resistant cancer cells. Cancer Biol Ther. 2015;16:149–159. doi: 10.4161/15384047.2014.987093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt SV, Rogers DF, Fenwick PS, Shah P, De Matos C, Russell RE, Barnes PJ, Donnelly LE. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D'Armiento JM. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173:623–631. doi: 10.1164/rccm.200506-850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geismann C, Arlt A, Sebens S, Schäfer H. Cytoprotection "gone astray": Nrf2 and its role in cancer. Onco Targets Ther. 2014;7:1497–1518. doi: 10.2147/OTT.S36624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, Lichtenauer M, Mangold A, Niederpold T, Zimmermann M, Taghavi S, Klepetko W, Ankersmit HJ. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55:31–40. [PubMed] [Google Scholar]
- Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2006;3:713–717. doi: 10.1513/pats.200605-104SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Lai HC, Chen YB, Chen LG, Wu YH, Ko YF, Lu CC, Chang CJ, Wu CY, Martel J, Ojcius DM, Chong KY, Young JD. cis-Resveratrol produces anti-inflammatory effects by inhibiting canonical and non-canonical inflammasomes in macrophages. Innate Immun. 2014;20:735–750. doi: 10.1177/1753425913507096. [DOI] [PubMed] [Google Scholar]
- Hung TH, Chen CM, Tseng CP, Shen CJ, Wang HL, Choo KB, Chong KY. FZD1 activates protein kinase C delta-mediated drug-resistance in multidrug-resistant MES-SA/Dx5 cancer cells. Int J Biochem Cell Biol. 2014;53:55–65. doi: 10.1016/j.biocel.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Hung TH, Hsu SC, Cheng CY, Choo KB, Tseng CP, Chen TC, Lan YW, Huang TT, Lai HC, Chen CM, Chong KY. Wnt5A regulates ABCB1 expression in multidrug-resistant cancer cells through activation of the non-canonical PKA/beta-catenin pathway. Oncotarget. 2014;5:12273–12290. doi: 10.18632/oncotarget.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, Hegab AE, Hosoya T, Nomura A, Sakamoto T, Yamamoto M, Sekizawa K. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175:6968–6975. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- Juan SH, Cheng TH, Lin HC, Chu YL, Lee WS. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in humanaortic smooth muscle cells. Biochem Pharmacol. 2005;69:41–48. doi: 10.1016/j.bcp.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, Saijo Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19:196–203. doi: 10.1038/mt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- Kurtagic E, Jedrychowski MP, Nugent MA. Neutrophil elastase cleaves VEGF to generate a VEGF fragment with altered activity. Am J Physiol Lung Cell Mol Physiol. 2009;296:L534–L546. doi: 10.1152/ajplung.90505.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan YW, Choo KB, Chen CM, Huang TH, Chen YB, Hsieh CH, Kuo HP, Chong KY. Hypoxia-preconditioned mesenchymal stem cells attenuates bleomycin-Induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6:97. doi: 10.1186/s13287-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond AL, Naud P, Forest V, Gourden C, Sagan C, Romefort B, Mathieu E, Delorme B, Collin C, Pagès JC, Sensebé L, Pitard B, Lemarchand P. Developing cell therapy techniques for respiratory disease: intratracheal delivery of genetically engineered stem cells in a murine model of airway injury. Hum Gene Ther. 2009;20:1329–1343. doi: 10.1089/hum.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Messier EM, Bahmed K, Tuder RM, Chu HW, Bowler RP, Kosmider B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EJ, Giaccia A. Dual roles of NRF2 in tumor prevention and progression: possible implications in cancer treatment. Free Radic Biol Med. 2015;79:292–299. doi: 10.1016/j.freeradbiomed.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003;16:552–557. doi: 10.1097/01.MP.0000071841.17900.69. [DOI] [PubMed] [Google Scholar]
- Orallo F. Comparative studies of the antioxidant effects of cis- and trans-resveratrol. Curr Med Chem. 2006;13:87–98. doi: 10.2174/092986706775197962. [DOI] [PubMed] [Google Scholar]
- Papaioannou AI, Kostikas K, Kollia P, Gourgoulianis KI. Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir Res. 2006;7:128. doi: 10.1186/1465-9921-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit GR, Grealish MP, Jung MK, Hamel E, Pettit RK, Chapuis JC, Schmidt JM. Antineoplastic agents. 465. Structural modification of resveratrol: sodium resverastatin phosphate. J Med Chem. 2002;45:2534–2542. doi: 10.1021/jm010119y. [DOI] [PubMed] [Google Scholar]
- Pinot F, Bachelet M, François D, Polla BS, Walti H. Modified natural porcine surfactant modulates tobacco smoke-induced stress response in human monocytes. Life Sci. 1999;64:125–134. doi: 10.1016/S0024-3205(98)00542-6. [DOI] [PubMed] [Google Scholar]
- Putics A, Végh EM, Csermely P, Soti C. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antioxid Redox Signal. 2008;10:65–75. doi: 10.1089/ars.2007.1866. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI200421146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen TT, Rutanen J, Ylä-Herttuala S. Gene transfer for therapeutic vascular growth in myocardial and peripheral ischemia. Adv Genet. 2004;52:117–164. doi: 10.1016/S0065-2660(04)52004-7. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Kaneko T, Nagashima Y, Ueda A, Tagawa A, Ishigatsubo Y. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lungs attenuates elastase-induced pulmonary emphysema in mice. Hum Gene Ther. 2005;16:318–327. doi: 10.1089/hum.2005.16.318. [DOI] [PubMed] [Google Scholar]
- Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Yoshida T, Fijalkowka I, Biswal S, Petrache I. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc. 2006;3:673–679. doi: 10.1513/pats.200605-124SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung YT, Chen HL, Lai CW, Shen CJ, Lai YW, Chen CM. Curcumin reduces pulmonary tumorigenesis in vascular endothelial growth factor (VEGF)-overexpressing transgenic mice. Mol Nutr Food Res. 2011;55:1036–1043. doi: 10.1002/mnfr.201000654. [DOI] [PubMed] [Google Scholar]
- van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thébaud B (2009) Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 180:1131–1142. doi:10.1164/rccm.200902-0179OC [DOI] [PMC free article] [PubMed]
- Vayssier-Taussat M, Camilli T, Aron Y, Meplan C, Hainaut P, Polla BS, Weksler B. Effects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responses. Am J Physiol Heart Circ Physiol. 2001;280:H1293–H1300. doi: 10.1152/ajpheart.2001.280.3.H1293. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, Hermann DM (2005) VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain 128:52–63 [DOI] [PubMed]
- Zhen G, Liu H, Gu N, Zhang H, Xu Y, Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–3422. doi: 10.2741/2936. [DOI] [PubMed] [Google Scholar]