Abstract
Background
Breast-conserving therapy (BCT) and mastectomy (MTX) has been considered to have a similar long-time survival. However, better survival in women undergoing BCT compared with MTX is found in two recent register studies from the United States. The purpose of this study was to compare survival after BCT and MTX for women with early-stage breast cancer in Norway.
Methods
Women with invasive, early-stage breast cancer (1998–2008) where BCT and MTX were considered as equally beneficial treatments were included for a total of 13,015 women. Surgery was divided in two main cohorts (primary BCT, primary MTX) and five subcohorts. Analyses were stratified into T1N0M0, T2N0M0, T1N1M0, T2N1M0, and age groups (<50, 50–69, ≥70). Overall survival and breast cancer-specific survival (BCSS) were calculated in life tables, hazard ratios by Cox regression, and sensitivity analyses.
Results
Five-year BCSS for women who underwent primary BCT or primary MTX was 97 and 88 %, respectively. Women who underwent primary MTX had a hazard ratio of 1.64 (95 % confidence interval 1.43–1.88) for breast cancer death compared with women who underwent primary BCT after adjusting for the year of diagnosis, age at diagnosis, stage, histology, and grade.
Conclusions
Survival was better or equal after breast-conserving therapy than mastectomy in all early stages, surgical subcohorts, and age groups. This advantage could not only be attributed to differences in tumor biology.
Introduction
The clinical trials comparing breast-conserving therapy (BCT) with mastectomy (MTX) were done decades ago.1–6 Since these studies were conducted, treatment and especially adjuvant therapy have changed and survival improved.7,8
In 2013, Hwang et al. published a paper reporting better survival among patients undergoing BCT compared with MTX, challenging the notion of equality in survival between BCT and MTX.9 They suggested that differences in tumor biology might have contributed to survival differences between BCT and MTX. In January 2014, Agarwal et al. published a paper corroborating the results of Hwang.10 They assumed that a difference in the breast cancer-specific survival rate between BCT and MTX might be due to differences in compliance to adjuvant therapy or tumor biology. Because these two studies were not randomized trials but observational studies, more studies on this topic are needed, especially outside the United States.
In Norway, all cancer cases have to be reported to the Cancer Registry of Norway, making this a complete register for the whole population of Norway with the possibility to form a cohort where, according to national guidelines, BCT and MTX are considered as equal treatment options.11 The purpose of this study was to compare differences in survival after BCT and MTX for women with early-stage breast cancer in Norway.
Materials and Methods
In this study, data from the Cancer Registry of Norway containing information on diagnosis, time of diagnosis, surgery type, surgery month, morphology, tumor grade, and TNM classification (done according to Union of International Cancer Control) were used.12
Cohort Selection
A total of 27,182 female residents of Norway were diagnosed with invasive, primary, early-stage breast cancer during the period January 1, 1998 to December 31, 2008. In this study, early-stage breast cancer is defined as T1–2 N0–1 M0 and stratified into T1N0M0, T2N0M0, T1N1M0, and T2N1M0 (tumor size ≤ 5 cm and 0–3 ipsilateral axillary nodes with metastasis). From these women, a cohort who, according to the Norwegian Breast Cancer Group (NBCG) recommendations, could be offered either MTX or BCT was selected.7
The women excluded were as follows: women with previous cancer (2501), women diagnosed with more than one primary breast cancer in same or contralateral breast within 3 months (840), women who did not undergo surgery or information about the operation was missing (2153 of these 41 % were aged ≥ 80 years), missing information about metastasis status (4919 of these 35 % underwent BCT as primary and 65 % underwent MTX as primary), unknown size of tumor or unknown nodal status (2196), final BCT (BCT operated once and BCT with reoperation) not received or missing information on RT (1073), final BCT receiving RT more than 365 days after diagnosis (62), women who received radiotherapy after MTX when nodal axillary status was negative (399), and women who died within 3 months after primary operation (24). The final cohort consists of 13,015 women.
Surgical Cohorts
Surgery was divided into two main cohorts: primary BCT and primary MTX. Primary BCT was further divided into three subcohorts: BCT operated once, BCT with reoperation, and BCT followed by MTX. Primary MTX was divided into two subcohorts: MTX operated once and MTX with reoperation. Division of surgical main and subcohorts was done 3 months after primary operation. If no further operation was done 3 months after primary operation, the operation was defined as one operation (BCT operated once and MTX operated once). If the women underwent two or more surgeries within 3 months after primary the operation, the operation was defined as several (BCT with reoperation, BCT followed by MTX, and MTX with reoperation).
Treatment Recommendations from the Norwegian Breast Cancer Group Between 1998 and 2008
NBCG criteria to accept BCT as final result of surgery was as follows: free margin should be at least 5 mm from 1998 to 2003 and 3 mm from 2003 to 2008; an acceptable cosmetic result obtained; tumor size < 5 cm from 2003; multifocal tumors were not accepted from 1998 to 2003; multifocal tumors < 1 cm apart were accepted for BCT from 2003.
Radiation therapy: all women undergoing BCT as final treatment should receive RT. Women younger than 55 years undergoing MTX with one to three positive nodes in axilla were recommended RT to chest wall and axilla from year 1998 to 2003; the age was increased to 70 years from 2003. Women undergoing MTX also were recommended RT if margins were not free. Radiation therapy was deemed given if the patient received a total dose of 47 Gy or more and start of treatment was no more than 365 days from date of diagnosis.
Neoadjuvant treatment is not recommended for early-stage breast cancer. Furthermore, choice of surgery did not influence recommendations of adjuvant chemotherapy or antiestrogen therapy.
Statistical Analyses
Life tables for 5-year overall survival (OS) and breast cancer-specific survival (BCSS) were stratified by primary BCT, primary MTX, and the following age groups: <50 years, 50–69 years, and ≥70 years. Furthermore, the surgical main cohorts were stratified in grade 1–3, ductal carcinoma, T1N0M0, T2N0M0, T1N1M0, T2N1M0, age < 50, age 50–69, and age ≥ 70 years. Kaplan–Meier curves were stratified in T1N1M0, grade 3, ductal carcinoma, and age 50–69 years in the surgical main and sub cohorts.
Cox proportional hazards were performed to estimate crude and adjusted hazard ratios for OS and BCSS between BCT and MTX in the surgical main and subcohorts. Cox analyses were performed in the following strata: surgical main cohorts; surgical sub cohorts; first 3 and last 3 years of the study period; women aged < 50 years; women aged 50–69 years; women aged ≥ 70 years; T1N0M0 grade1; T1-2N1M0 where primary MTX received RT and T1-2N1M0 where primary MTX did not receive RT. Furthermore, multivariate analysis was performed were all women receiving RT after MTX were excluded from the cohort. The multivariate analysis was adjusted in the surgical subcohorts for the year of diagnosis, stage, age, histology, and grade. Deterministic sensitivity analyses were performed on misclassification of surgery, selection bias, and uncontrolled confounding according to Greenland.13 Statistical analyses were conducted in STATA version 13.1 (StataCorp, Texas, USA).
Results
Of the 13,015 women with early-stage breast cancer, 8065 (62 %) underwent primary BCT and 4950 (38 %) underwent primary MTX. Table 1 shows clinical characteristics of the patient cohort.
Table 1.
Baseline characteristics
| Surgical main cohorts | Surgical sub cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number 13,015 | Reoperateda | Number 13,015 | |||||||
| PrimaryBCT | PrimaryMTX | BCT | MTX | BCTonce | BCTreop. | BCT-MTX | MTX once | MTX reop. | |
| Number of patients | 8065 | 4950 | 1481 | 164 | 6583 | 1287 | 194 | 4786 | 164 |
| Proportion of patients | 62 | 38 | 50.6 | 9.9 | 1.5 | 36.7 | 1.3 | ||
| Median follow-up time | 7.3 years | 7.0 years | 7.3 years | 7.5 years | 8.7 years | 7.0 years | 7.0 years | ||
| Proportion RT | 99.3 | 30.7 | 100 | 100 | 70.1 | 30.3 | 43.3 | ||
| Year of diagnosis | Annual proportion (100 %) | Reoperateda | Annual proportion (100 %) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BCT | MTX | ||||||||
| 1998 | 34 % (264) | 66 % (512) | 16 % | 3.3 % | 29 % | 3.1 % | 2.2 % | 63.8 % | 2.2 % |
| 1999 | 35 % (279) | 65 % (520) | 18 % | 6.3 % | 29 % | 3.9 % | 2.4 % | 61.0 % | 4.1 % |
| 2000 | 41 % (364) | 59 % (519) | 14 % | 3.5 % | 35 % | 4.0 % | 1.8 % | 56.7 % | 2.0 % |
| 2001 | 53 % (482) | 47 % (422) | 21 % | 4.3 % | 42 % | 9.2 % | 1.9 % | 44.7 % | 2.0 % |
| 2002 | 63 % (736) | 37 % (436) | 23 % | 3.9 % | 49 % | 11.9 % | 2.2 % | 35.8 % | 1.5 % |
| 2003 | 72 % (967) | 28 % (384) | 23 % | 3.6 % | 55 % | 14.9 % | 1.6 % | 27.4 % | 1.0 % |
| 2004 | 73 % (1005) | 26 % (368) | 20 % | 2.2 % | 58 % | 13.9 % | 1.0 % | 26.2 % | 0.6 % |
| 2005 | 72 % (1042) | 28 % (407) | 17 % | 2.7 % | 60 % | 10.8 % | 1.2 % | 27.3 % | 0.8 % |
| 2006 | 71 % (962) | 29 % (388) | 18 % | 2.8 % | 59 % | 11.0 % | 1.6 % | 27.9 % | 0.8 % |
| 2007 | 68 % (990) | 32 % (465) | 17 % | 1.3 % | 57 % | 10.5 % | 0.9 % | 31.5 % | 0.4 % |
| 2008 | 65 % (974) | 35 % (529) | 14 % | 2.1 % | 56 % | 8.3 % | 0.9 % | 34.5 % | 0.7 % |
| Age at diagnosis (years) | |||||||||
| <30 | 61 % (31) | 39 % (20) | 26 % | 5.0 % | 45 % | 16 % | 0 % | 37 % | 2.0 % |
| 30–39 | 56 % (287) | 44 % (222) | 26 % | 5.0 % | 42 % | 11 % | 3.9 % | 41 % | 2.2 % |
| 40–49 | 66 % (1467) | 34 % (761) | 20 % | 4.5 % | 53 % | 11 % | 2.1 % | 33 % | 1.5 % |
| 50–59 | 72 % (3024) | 29 % (1203) | 19 % | 3.7 % | 58 % | 12 % | 1.5 % | 27 % | 1.0 % |
| 60–69 | 72 % (2515) | 29 % (1024) | 17 % | 2.5 % | 59 % | 11 % | 1.1 % | 28 % | 0.7 % |
| 70–79 | 38 % (651) | 62 % (1062) | 16 % | 2.9 % | 32 % | 5 % | 1.3 % | 60 % | 1.8 % |
| ≥80 | 12 % (90) | 88 % (658) | 13 % | 2.6 % | 10 % | 1 % | 0.5 % | 86 % | 2.3 % |
| TNM stage | |||||||||
| T1N0 | 75 % (5165) | 25 % (1686) | 17 % | 2.4 % | 63 % | 11 % | 1.7 % | 24 % | 0.6 % |
| T2N0 | 44 % (888) | 56 % (1140) | 20 % | 2.4 % | 35 % | 8 % | 1.2 % | 55 % | 1.3 % |
| T1N1 | 60 % (1340) | 40 % (893) | 22 % | 4.4 % | 47 % | 11 % | 2.0 % | 38 % | 1.7 % |
| T2N1 | 35 % (672) | 65 % (1231) | 20 % | 4.6 % | 28 % | 7 % | 0.5 % | 62 % | 3.0 % |
| Histology | |||||||||
| Ductal c. | 62 % (6618) | 38 % (3981) | 18 % | 3.5 % | 51 % | 10 % | 1.5 % | 36 % | 1.3 % |
| Lobular c. | 58 % (756) | 42 % (549) | 24 % | 2.6 % | 44 % | 12 % | 2.0 % | 41 % | 1.1 % |
| Other c. | 62 % (691) | 38 % (420) | 19 % | 2.6 % | 51 % | 10 % | 1.3 % | 37 % | 1.0 % |
| Grade | |||||||||
| I | 73 % (2261) | 27 % (844) | 16 % | 2.7 % | 61 % | 11 % | 1.1 % | 26 % | 0.7 % |
| II | 61 % (3600) | 39 % (2259) | 17 % | 3.3 % | 51 % | 9 % | 1.3 % | 37 % | 1.3 % |
| III | 54 % (1650) | 46 % (1382) | 23 % | 4.1 % | 42 % | 10 % | 2.3 % | 44 % | 1.8 % |
| Unknown | 54 % (554) | 46 % (465) | 22 % | 2.4 % | 42 % | 10 % | 1.7 % | 45 % | 1.1 % |
BCT once BCT operated once, BCT reop. BCT followed by reoperation, BCT-MTX BCT followed by MTX, MTX once MTX operated once, MTX reop. MTX with reoperation
aBCT reoperated is calculated by number of primary BCT undergoing BCT reoperation and BCT followed by MTX. MTX reoperated is calculated by number of primary MTX undergoing MTX with reoperation
RT was given to 99.3 % in primary BCT and 30.7 % in primary MTX. In the subcohorts, RT was given to 100 % in BCT operated once, 100 % in BCT with reoperation, 70 % in BCT followed by MTX, 30 % in MTX operated once, and 43 % in MTX with reoperation. The proportion of women who underwent primary BCT is highest among women aged 50–69 years. Of women aged 70–79 years, 62 % were operated with primary MTX. At age 80 years and older, 88 % were operated with primary MTX.
Impact of Surgery Type on Overall and Breast Cancer-Specific Survival
A total of 2,475 deaths were identified in the cohort during the study period, including 1,132 (1,083 after 10 years) due to breast cancer. The 5-year OS was 89 %, and BCSS was 94 % (Table 2). Life tables showed better survival for women undergoing BCT compared with MTX. For women who underwent primary BCT or primary MTX, the 5-year BCSS was 97 and 88 %, respectively. In the age group 50–69 years, the 5-year BCSS for those who underwent primary BCT was 98 and 90 %, respectively.
Table 2.
Survival by surgical main and subcohorts
| Median age | Total number at start | Number of overall deaths in 5-year period | Overall survival | Number of breast cancer deaths | Breast cancer survival | |
|---|---|---|---|---|---|---|
| Survival | ||||||
| 5-year | 59.0 | 13,015 | 1334 | 89 % | 742 | 94 % |
| 10-year | 59.0 | 9814 | 2260 | 78 % | 1083 | 89 % |
| Surgical main cohorts | ||||||
| 5-year survival BCT | 56.9 | 8065 | 412 | 95 % | 225 | 97 % |
| 5-year survival MTX | 62.4 | 4950 | 922 | 80 % | 517 | 88 % |
| 10-year survival BCT | 56.9 | 6370 | 796 | 86 % | 384 | 93 % |
| 10-year survival MTX | 62.4 | 3444 | 1464 | 64 % | 699 | 82 % |
| Age < 50 years | ||||||
| 5-year survival BCT | 43.6 | 1785 | 89 | 95 % | 72 | 96 % |
| 5-year survival MTX | 42.7 | 1003 | 120 | 87 % | 111 | 88 % |
| Age 50–69 years | ||||||
| 5-year survival BCT | 58.8 | 5539 | 232 | 95 % | 115 | 98 % |
| 5-year survival MTX | 59.0 | 2227 | 296 | 86 % | 201 | 90 % |
| Age ≥ 70 years | ||||||
| 5-year survival BCT | 74.6 | 741 | 91 | 87 % | 38 | 94 % |
| 5-year survival MTX | 78.3 | 1720 | 506 | 69 % | 205 | 86 % |
| Surgical subcohorts, 5-year survival | ||||||
| BCT operated once | 57.0 | 6583 | 324 | 95 % | 163 | 97 % |
| BCT with reoperation | 56.2 | 1287 | 67 | 94 % | 48 | 96 % |
| BCT followed by MTX | 55.2 | 195 | 21 | 89 % | 14 | 92 % |
| MTX operated once | 62.5 | 4786 | 884 | 80 % | 484 | 89 % |
| MTX with reoperation | 59.3 | 164 | 38 | 76 % | 33 | 79 % |
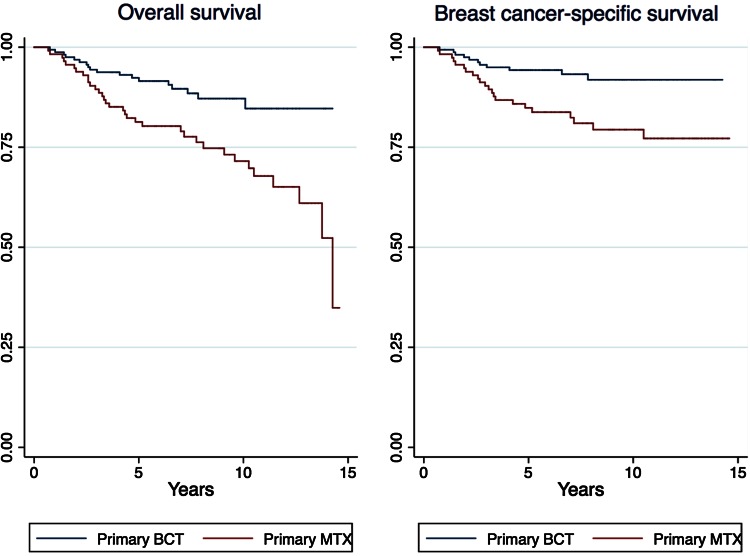
The main and surgical subcohorts stratified in stage T1N1M0, grade 3, and ductal carcinoma showed better survival among women undergoing BCT compared with MTX (Kaplan–Meier curves in Fig. 1)
Fig. 1.
Kaplan–Meier, surgical main cohorts stratified in T1N1M0, grade 3, ductal carcinoma, and age 50–69
Furthermore, the two main surgical cohorts, primary BCT and primary MTX, were stratified in grade 1–3, ductal carcinoma, and stage (T1N0M0, T2N0M0, T1N1M0, T2N1M0), and none of these strata showed a significant benefit of MTX over BCT; i.e., in all these analyses, BCT was better or equal compared with MTX regarding survival (result not shown in table). Women who underwent MTX with reoperation had the worst prognosis, 79 % 5-year BCSS (Table 2).
In the adjusted Cox analysis, women who underwent primary MTX had a hazard ratio [HR] of 1.64 (95 % confidence interval [CI] 1.43–1.88) for breast cancer death compared with women who underwent primary BCT (Table 3). Adjusted analysis in the beginning of study period (1998–2001) showed HR 1.76 (95 % CI 1.02–3.05) compared with the end of study period (2006–2008) with HR 1.88 (95 % CI 1.23–2.87). Results not shown in table.
Table 3.
Crude and adjusted HR on overall and breast cancer death in women with early-stage breast cancer
| Crude | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall death | Breast cancer death | Overall death | Breast cancer death | |||||
| HR | 95 % CI | HR | 95 % CI | HR | 95 % CI | HR | 95 % CI | |
| Surgical main cohorts | ||||||||
| Primary BCT | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| Primary MTX | 3.11 | (2.86–3.38) | 3.16 | 2.79–3.57 | 1.65 | 1.50–1.82 | 1.64 | 1.43–1.88 |
| Surgical subcohorts | ||||||||
| BCT once | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| BCT reop. | 1.05 | 0.87–1.26 | 1.38 | 1.07–1.77 | 1.04 | 0.86–1.25 | 1.28 | 0.99–1.64 |
| BCT-MTX | 1.90 | 1.39–2.58 | 2.92 | 1.97–4.33 | 1.69 | 1.24–2.31 | 2.19 | 1.47–3.26 |
| MTX once | 3.90 | 2.89–3.47 | 3.35 | 2.92–3.85 | 1.68 | 1.51–1.86 | 1.72 | 1.48–2.01 |
| MTX reop. | 4.56 | 3.60–5.78 | 7.93 | 5.94–10.58 | 2.50 | 1.96–3.19 | 3.40 | 2.53–4.58 |
| Year of diagnosis | ||||||||
| 1998 | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| 1999 | 0.93 | 0.79 | 0.89 | 0.73 | ||||
| 2000 | 0.87 | 0.78 | 0.9 | 0.77 | ||||
| 2001 | 0.87 | 0.75 | 1.05 | 0.83 | ||||
| 2002 | 0.69 | 0.53 | 0.89 | 0.63 | ||||
| 2003 | 0.77 | 0.58 | 0.99 | 0.76 | ||||
| 2004 | 0.64 | 0.45 | 0.89 | 0.62 | ||||
| 2005 | 0.69 | 0.34 | 0.87 | 0.44 | ||||
| 2006 | 0.6 | 0.29 | 0.75 | 0.39 | ||||
| 2007 | 0.59 | 0.22 | 0.77 | 0.30 | ||||
| 2008 | 0.53 | 0.17 | 0.66 | 0.23 | ||||
| Age categories (years) | ||||||||
| <30 | 2.15 | 3.12 | 1.51 | 1.78 | ||||
| 30–39 | 1.59 | 2.42 | 1.15 | 1.46 | ||||
| 40–49 | 0.98 | 1.21 | 0.82 | 0.91 | ||||
| 50–59 | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| 60–69 | 1.42 | 0.96 | 1.52 | 1.11 | ||||
| 70–79 | 3.71 | 2.15 | 2.98 | 1.66 | ||||
| ≥80 | 8.38 | 2.92 | 6.04 | 2.13 | ||||
| TNM stage | ||||||||
| T1N0M0 | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| T2N0M0 | 2.38 | 2.95 | 1.41 | 1.83 | ||||
| T1N1M0 | 1.56 | 2.86 | 1.92 | 2.18 | ||||
| T2N1M0 | 3.34 | 6.57 | 3.37 | 3.95 | ||||
| Histology | ||||||||
| Ductal c. | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| Lobular c. | 1.04 | 0.88 | 0.93 | 0.91 | ||||
| Other | 1.03 | 0.89 | 1.04 | 1.02 | ||||
| Grade | ||||||||
| I | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
| II | 1.69 | 2.61 | 1.41 | 1.98 | ||||
| III | 2.47 | 5.65 | 1.92 | 3.53 | ||||
| Unknown | 1.73 | 2.84 | 1.30 | 1.99 | ||||
Numbers in italic are not significant (p > 0.05)
Women younger than 50 years who underwent primary MTX had HR 1.58 (95 % CI 1.22–2.04) for breast cancer death compared with women who underwent primary BCT (Table 4). A stratified adjusted analysis performed for women aged 50–69 years (screening age) who underwent primary MTX showed an HR of 1.64 (95 % CI 1.35–1.99) for breast cancer death compared with women who underwent primary BCT with base HR 1.00.
Table 4.
Crude and adjusted HR on overall and breast cancer death in women with early-stage breast cancer stratified in women <50 years, women aged 50–69 years, women aged ≥70 years, and T1N0M0 grade 1
| Crude | Adjusted | ||||
|---|---|---|---|---|---|
| Overall death | Breast cancer death | Overall death | Breast cancer death | ||
| HR | HR | HR | HR | ||
| Women aged < 50 years | |||||
| Surgery | Nr | ||||
| Primary BCT | 1785 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Primary MTX | 1003 | 2.10 (1.71–2.59) | 2.51 (1.98–3.18) | 1.43 (1.15–1.80) | 1.58 (1.22–2.04) |
| Surgical subcohorts | |||||
| BCT once | 1415 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| BCT reop. | 304 | 0.88 (0.57–1.35) | 0.84 (0.50–1.41) | 0.85 (0.54–1.31) | 0.80 (0.48–1.35) |
| BCT-MTX | 66 | 1.51 (0.79–2.87) | 1.50 (0.70–3.23) | 1.45 (0.75–2.79) | 1.32 (0.60–2.87) |
| MTX once | 957 | 1.96 (1.56–2.46) | 2.32 (1.79–3.01) | 1.35 (1.06–1.72) | 1.46 (1.11–1.93) |
| MTX reop. | 46 | 5.42 (3.42–8.61) | 6.41 (3.87–10.62) | 2.95 (1.83–4.75) | 3.12 (1.86–5.25) |
| Women aged 50–69 years | |||||
| Surgery type | |||||
| Primary BCT | 5539 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Primary MTX | 2227 | 2.40 (2.12–2.72) | 3.26 (2.74–3.88) | 1.74 (1.52–2.00) | 1.64 (1.35–1.99) |
| Surgical subcohorts | |||||
| BCT once | 4546 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| BCT reop. | 891 | 1.14 (0.90–1.43) | 1.83 (1.34–2.49) | 1.09 (0.87–1.39) | 1.71 (1.25–2.33) |
| BCT-MTX | 102 | 2.24 (1.47–3.40) | 3.95 (2.32–6.71) | 2.03 (1.33–3.09) | 2.91 (1.70–6.28) |
| MTX once | 2157 | 2.42 (2.13–2.78) | 3.65 (3.99–4.45) | 1.77 (1.35–2.05) | 1.85 (1.49–2.30) |
| MTX reop. | 70 | 5.35 (3.77–7.59) | 11.70 (7.77–17.62) | 3.32 (2.31–4.78) | 4.09 (2.66–6.28) |
| Women aged ≥ 70 years | |||||
| Surgery type | |||||
| Primary BCT | 741 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Primary MTX | 1720 | 2.15 (1.84–2.51) | 2.23 (1.67–2.96) | 1.56 (1.31–1.85) | 1.50 (1.10–2.05) |
| Surgical subcohorts | |||||
| BCT once | 622 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| BCT reop. | 92 | 1.16 (0.77–1.76) | 1.05 (0.47–2.32) | 1.01 (0.72–1.66) | 0.83 (0.34–1.84) |
| BCT MTX | 27 | 1.42 (0.75–2.69) | 2.68 (1.06–6.76) | 1.30 (0.68–2.47) | 1.92 (0.75–4.90) |
| MTX once | 1672 | 2.25 (1.89–2.67) | 2.36 (1.72–3.23) | 1.61 (1.33–1.93) | 1.52 (1.08–2.13) |
| MTX reop. | 48 | 1.67 (1.06–2.64) | 2.82 (1.43–5.62) | 1.36 (0.85–2.16) | 2.14 (1.06–4.31) |
| T1N0M0 grade 1 | |||||
| Surgery type | |||||
| Primary BCT | 1451 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Primary MTX | 366 | 2.61 (1.91–3.56) | 3.52 (1.80–6.90) | 1.77 (1.22–2.56) | 2.07 (0.94–4.56) |
| Surgical sub cohorts | |||||
| BCT once | 1245 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| MTX once | 358 | 2.61 (1.88–3.61) | 4.73 (2.22–10.10) | 1.80 (1.23–2.63) | 2.80 (1.18–6.61) |
In the T1N0M0 grade 1 strata, 17 primary BCT died and 18 primary MTX died
Numbers in italics are not significant (p > 0.05)
Women not recommended to receive chemotherapy or antiestrogen therapy, i.e., T1N0M0 grade 1, who underwent primary MTX had an HR of 2.07 (95 % CI 0.94–6.61) compared with women who underwent primary BCT.
Women with node-positive disease (T1-2N1M0) where all in the primary MTX strata received RT gave a primary MTX HR of 2.13 (95 % CI 1.52–2.98) compared with primary BCT with base HR 1.00 (result not shown in table). Respectively, women with node-positive disease (T1-2N1M0) where no one in the primary MTX received RT gave a primary MTX HR of 2.16 (95 % CI 1.78–2.61) compared with primary BCT with base HR 1.00 (result not shown in table). MTX stage T1-2N1M0 shows no advantage of receiving RT. Multivariate analysis where all women receiving RT after MTX were excluded (1,521 women) gave a primary MTX HR of 1.51 (95 % CI 1.27–1.80) compared with primary BCT with base HR 1.00 (result not shown in table).
Women operated with BCT followed by MTX were found to have a worse prognosis compared with women who underwent BCT operated once, BCT with reoperation, and MTX operated once in the adjusted analysis. Nevertheless, only 0.7 % of the primary BCT cohort underwent BCT followed by MTX without receiving RT.
After MTX, 164 underwent reoperation. This group had the worst prognosis in the cohort; 34 % (55) died of breast cancer during a median follow-up time of 6.9 years.
Sensitivity Analyses
When assuming a dichotomous unmeasured confounder to present in 20, 40, 60, and 90 % of the women undergoing MTX, and 10 % in among women undergoing BCT, the rate ratios were of 2.57, 1.78, 1.36, and 1.01, respectively. In these analyses, we assumed the relative risk of confounding to be 5. Sensitivity analyses of misclassification of surgery and selection bias did not show lower risk between MTX and BCT than in the crude analysis.
Discussion
The main finding of this study is that both OS and BCSS were better in women with early-stage breast cancer undergoing BCT compared with MTX. This is contrary to the general consensus that MTX and BCT patients have a similar long-time survival, but corresponds well with the two studies done in the United States by Whang and Agarwal, who found better survival in women undergoing BCT compared with MTX.1–3,5,10,14–16
Possible Selection Effects
The present study is an observational study, and several possible selection effects might explain the observed differences; i.e., the observed differences might be due to other than the surgical procedures. In the following, we discuss the most probable selection effects that might have influenced the observed results.
Completeness
The Cancer Registry of Norway during the period 2001–2005 had an overall completeness on cancer estimated at 98.8 %.11 Selection bias due to missing registration is thus unlikely. There was a higher proportion of patients undergoing primary MTX without known distant metastasis status than primary BCT, 65 versus 35 %, before cohort selection. Nevertheless, information on distant metastasis status was available for all patients in the analyzed cohorts; i.e., they were metastasis-free at the time of diagnosis (M0).
Access to Health Care
Almost every inhabitant in Norway receives the same health care offer regardless of private insurance, and only public hospitals provide treatment of breast cancer. This might be in contrast to the United States, where women with a higher socioeconomic status are more likely to undergo BCT.17,18
Comorbidity
Some of the women underwent MTX due to an overall judgment of their health situation. We have no information on comorbidity; however, the difference between OS and BCSS in women younger than aged 50 years was 1 % in both the primary BCT and primary MTX strata, 3 % for women undergoing BCT, and 4 % for women undergoing MTX at age 50–69 years. This indicates that there are small differences in serious comorbidity in women younger than age 70 years between the two cohorts. However, comorbidity has probably influenced the choice of MTX among the older women.
Hereditary Breast Cancer
We are not able to stratify for women with hereditary breast cancer, because BRCA1/2 or prophylactic MTX is not recorded in the Cancer Registry. However, in a population-based incidence study in one of the counties in Norway, it was shown that 2.5 % of the women studied were mutation carriers.19 This fraction might have a slight detrimental effect on survival in the MTX cohort.
Patients Own Choice
In a hospital in Norway, 14 % of the women operated for breast cancer underwent MTX because of the patient’s own request, or the cancer had preoperatively been considered more prevalent than at the final histological examination.20 This might seem like a low proportion. However, a study from the United States regarding involvement in decision making about surgery for early-stage breast cancer showed that 9 % underwent primary MTX based on patient preference.21
Tumor Biology
When surgery is decided, results from cytology or biopsy together with mammogram and ultrasound normally give information on morphology, grade, and tumor size. Details on tumor biology, such as lymph vascular invasion, are normally not known when the decision on type of surgery is made, and therefore do not explain the difference between BCT and MTX. Furthermore, routine examination on HER2 was recommended from June 2005, late in the study period; therefore, triple-negative disease cannot explain the difference in survival between BCT and MTX.
Radiation Therapy
Today’s guidelines from NBCG differ from the guidelines in our study period, and today fewer patients would receive RT based on axillary node positive disease (1–3 lymph nodes). MTX with RT and MTX without RT are not directly comparable in our study, based on different recommendations for RT during the study period, but RT given to women with node-positive disease does not seem to increase the survival benefit of the MTX cohort.
Women undergoing MTX with RT likely represent a high-risk disease. Multivariate analysis where none of the patients in the MTX group received RT showed the benefit of BCT compared with MTX (HR 1.51; 95 % CI 1.27–1.80).
Adjuvant Therapy
The Cancer Registry is incomplete when it comes to chemotherapy and antiestrogen therapy given. However, recommendations for chemo and antiestrogen therapy are identical for patients undergoing BCT and MTX. In this study it was not possible to see whether women undergoing MTX have less compliance to recommended therapy.
Sensitivity Analysis of Misclassification, Selection Bias, and Unmeasured Confounder
Sensitivity analyses were done under several different assumptions within the following three areas: misclassification of surgery; selection bias, and unmeasured confounder. However, the larger the difference in the proportion of unmeasured confounding in the two cohorts, the lesser the rate ratio adjusted for unmeasured confounding. In the present study, first when assuming that as much as 90 % of women undergoing MTX had uncontrolled confounding (e.g., compliance to adjuvant therapy), and only 10 % in the BCT cohort, did we find a rate ratio of 1.0. We find it unlikely that the difference in adjuvant therapy was more than 10–30 % between the surgical groups (both surgical groups have the same recommendations to adjuvant therapy), and thus the adjusted rate ratio for unmeasured confounding was found to be 1.78, when assuming 10 and 40 % unmeasured confounding in BCT and MTX, respectively, compared with an unadjusted rate ratio of 3.31.
Proportion of Women Undergoing BCT Compared with MTX Changed During Study Period
The proportion of BCT at the beginning of study period was lower than at the end of study period, but the benefit of BCT compared with MTX did not seem to change during the study period.
Strengths and Weaknesses of the Study
The major strength of our study is that the results are based on the whole population of women diagnosed with early-stage breast cancer in Norway during the period January 1, 1998 to December 31, 2008. Dividing the surgical main cohort into five surgical subcohorts made it possible to include women initially treated with BCT followed by MTX without receiving RT. If this had not been done, women initially treated with BCT would have been regarded as BCT without RT and excluded from the cohort.
The weaknesses are that the Cancer Registry lacks information on hormone receptor status and information on given adjuvant therapy. However, neither of these factors determines whether a patient should undergo BCT or MTX.7 Observational studies, such as this, are prone to selection effects. However, as discussed above, we find it unlikely that this can explain all of the observed differences in survival among women undergoing BCT compared with MTX.
Conclusions
This study corroborates the findings of two studies from the United States showing better survival for women undergoing BCT compared with MTX. This advantage could not be attributed to differences in tumor biology. Further studies are necessary to determine whether this benefit is caused by variation in adjuvant therapy or by type of surgery.
Acknowledgment
This study was supported by Oslo University Hospital by giving the first author 50 % of the working time for research.
Disclosure
All authors have declared: no support from any organization for the submitted work; no financial relationships with any organization for the submitted work; no financial relationships in the previous three years with any organization that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Blichert-Toft M, Nielsen M, During M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol (Stockholm, Sweden). 2008;47(4):672–81. [DOI] [PubMed]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Litiere S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13(4):412–419. doi: 10.1016/S1470-2045(12)70042-6. [DOI] [PubMed] [Google Scholar]
- 4.Simone NL, Dan T, Shih J, et al. Twenty-five year results of the national cancer institute randomized breast conservation trial. Breast Cancer Res Treat. 2012;132(1):197–203. doi: 10.1007/s10549-011-1867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson JB, Vicini FA, Shah C, et al. Twenty-year outcomes after breast-conserving surgery and definitive radiotherapy for mammographically detected ductal carcinoma in situ. Ann Surg Oncol. 2012;19(12):3785–3791. doi: 10.1245/s10434-012-2412-5. [DOI] [PubMed] [Google Scholar]
- 7.Norwegian breast cancer group (www.nbcg.no).
- 8.Cancer Registry of Norway (www.kreftregisteret.no) Cancer in Norway 2010.
- 9.Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119(7):1402–1411. doi: 10.1002/cncr.27795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. EFfect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014. [DOI] [PubMed]
- 11.Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 12.(UICC) UfICC. The TNM classification of Malignant Tumors, 7th edn; 2009.
- 13.Orsini N. A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies. Stata J. 2008;8(1):29–48. [Google Scholar]
- 14.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 15.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98(4):697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 16.Hwang ES, Lichtensztajn D, Gomez S, Fowble B, Clarke C. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. [DOI] [PMC free article] [PubMed]
- 17.Mac Bride MB, Neal L, Dilaveri CA, et al. Factors associated with surgical decision making in women with early-stage breast cancer: a literature review. J Women’s Health (2002). 2013;22(3):236–42. [DOI] [PubMed]
- 18.Albain KS, Green SR, Lichter AS, et al. Influence of patient characteristics, socioeconomic factors, geography, and systemic risk on the use of breast-sparing treatment in women enrolled in adjuvant breast cancer studies: an analysis of two intergroup trials. J Clin Oncol. 1996;14(11):3009–3017. doi: 10.1200/JCO.1996.14.11.3009. [DOI] [PubMed] [Google Scholar]
- 19.Moller P, Hagen AI, Apold J, et al. Genetic epidemiology of BRCA mutations–family history detects less than 50% of the mutation carriers. Eur J Cancer. 2007;43(11):1713–1717. doi: 10.1016/j.ejca.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Opsahl EM, Westre B, Samset JH, Olafsson S, Michelsen K, Varhaug JE. Breast cancer: diagnosis and treatment in a Alesund hospital. Tidsskrift Norske Laegeforen. 2010;130(7):724–728. doi: 10.4045/tidsskr.09.0155. [DOI] [PubMed] [Google Scholar]
- 21.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]