Abstract
Purpose
To assess the grading of Crohn’s disease activity using CT, MRI, US and scintigraphy.
Materials and methods
MEDLINE, EMBASE and Cochrane databases were searched (January 1983–March 2014) for studies evaluating CT, MRI, US and scintigraphy in grading Crohn’s disease activity compared to endoscopy, biopsies or intraoperative findings. Two independent reviewers assessed the data. Three-by-three tables (none, mild, frank disease) were constructed for all studies, and estimates of accurate, over- and under-grading were calculated/summarized by fixed or random effects models.
Results
Our search yielded 9356 articles, 19 of which were included. Per-patient data showed accurate grading values for CT, MRI, US and scintigraphy of 86 % (95 % CI: 75–93 %), 84 % (95 % CI: 67–93 %), 44 % (95 % CI: 28–61 %) and 40 % (95 % CI: 16–70 %), respectively. In the per-patient analysis, CT and MRI showed similar accurate grading estimates (P = 0.8). Per-segment data showed accurate grading values for CT and scintigraphy of 87 % (95 % CI: 77–93 %) and 86 % (95 % CI: 80–91 %), respectively. MRI and US showed grading accuracies of 67–82 % and 56–75 %, respectively.
Conclusions
CT and MRI showed comparable high accurate grading estimates in the per-patient analysis. Results for US and scintigraphy were inconsistent, and limited data were available.
Key Points
• CT and MRI have comparable high accuracy in grading Crohn’s disease.
• Data on US and scintigraphy is inconsistent and limited.
• MRI is preferable over CT as it lacks ionizing radiation exposure.
Electronic supplementary material
The online version of this article (doi:10.1007/s00330-015-3737-9) contains supplementary material, which is available to authorized users.
Keywords: Crohn’s disease, X-ray computed tomography, Magnetic resonance imaging, Ultrasound, Radionuclide imaging
Introduction
Cross-sectional imaging techniques are widely used for diagnosis and evaluation of Crohn’s disease. Numerous studies have evaluated the diagnostic accuracy of cross-sectional imaging techniques in patients with Crohn’s disease, and a meta-analysis was published that investigated the diagnostic accuracy of computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US) and scintigraphy [1]. However, clinical monitoring and choice of therapy largely rely on grading of disease activity.
Clinical symptoms and inflammatory lesions can exist independently, so assessment of the bowel is essential in guiding therapy decisions [2]. If inflammation is present, it is important to distinguish between mild, moderate and severe disease, as medical management differs among these stages [3]. Ileocolonoscopy, the current reference standard for luminal Crohn’s disease, is accurate for assessing mucosal abnormalities, but it has several drawbacks, as it is an invasive technique, is associated with the risk of bowel perforation, is incapable of assessing trans- and extraluminal disease, and is limited to the colon and terminal ileum [4]. Video capsule endoscopy (VCE) is a well-tolerated and accurate alternative to ileocolonoscopy that allows assessment of the whole gastrointestinal tract, although it has shown lower specificity and bears the risk of capsule retention, which occurs in up to 13 % of patients with Crohn’s disease [5].
Cross-sectional imaging techniques that could accurately grade disease severity would be preferable to ileocolonoscopy, as they are non-invasive and not limited to the colon and terminal ileum. Several studies have looked at the use of cross-sectional imaging for assessing the severity of Crohn’s disease, but offered no comparison between imaging techniques, as no meta-analysis was performed [2, 6]. To our knowledge, only one such meta-analysis has been performed, but it evaluated only MRI and used a search period that ended in April 2007 [7]. This study showed that MRI correctly graded disease activity in 91 % of patients with frank (moderate-to-severe) disease. However, correct grading was limited in patients with disease in remission and with mild disease (62 % for both). Furthermore, no comparison with other imaging techniques was made and numerous articles on the grading of Crohn’s disease using MRI have been published since 2007.
Our purpose was to systematically review and compare the accuracy of CT, MRI, US, scintigraphy and positron emission tomography–computed tomography (PET-CT) in grading Crohn’s disease activity on a per-patient or per-segment basis as compared to endoscopy, biopsies or intraoperative findings by performing a meta-analysis. Furthermore, we aimed to investigate the degree of over- and under-grading for these imaging techniques.
Material and methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8]. The review protocol was not published or registered in advance.
Literature search and strategy
We performed an electronic search in MEDLINE, EMBASE and Cochrane databases for studies examining the accuracy of CT, MRI, US, scintigraphy and PET (-CT) for grading Crohn’s disease activity in human subjects. Search terms ‘Crohn’s disease’ and ‘inflammatory bowel disease’ were combined using ‘OR’ and search terms for imaging modalities were combined using ‘OR’ as well. These two groups were combined using ‘AND’. The search period was limited from January 1983 to March 2014. Details of the search strategy are provided in the electronic supplementary material (Appendix E1).
Study selection on title and abstract
All articles retrieved from the electronic search were assessed by one observer (CP). Non-relevant articles and articles in the form of a review, case report, comment or letter were excluded. Subsequently, the remaining titles and abstracts were independently assessed by two observers (CP, JT) to identify potentially eligible articles. In cases of uncertainty, articles were deemed potentially eligible and retrieved as full text.
Study selection on full text
The full texts of the remaining articles were retrieved. Two observers (CP, JT) independently reviewed all eligible articles for the following inclusion criteria: (a) ten or more patients were included (fewer were considered case-series); (b) CT, MRI, US, scintigraphy or PET (-CT) was used to grade Crohn’s disease activity; (c) patients with clinically suspected inflammatory bowel disease (IBD) or known IBD/Crohn’s disease were included; (d) endoscopy, biopsies or intraoperative findings were used as a reference test; (e) imaging features used for grading disease activity were defined; (f) raw data were available to construct 3 × 3 tables; (g) articles were written in English, Italian, Spanish, French, German or Dutch; and (h) patients with Crohn’s disease could be analysed separately from other IBD patients. No patient age limits were applied. Articles in the form of a review, case report, conference abstract, comment or letter were excluded. In the case of duplicate publications, we excluded the studies with the lower number of patients. Disagreement regarding potential eligibility and inclusion was resolved by consensus. The observers were not blinded to author and journal names.
Study characteristics
Methodological characteristics
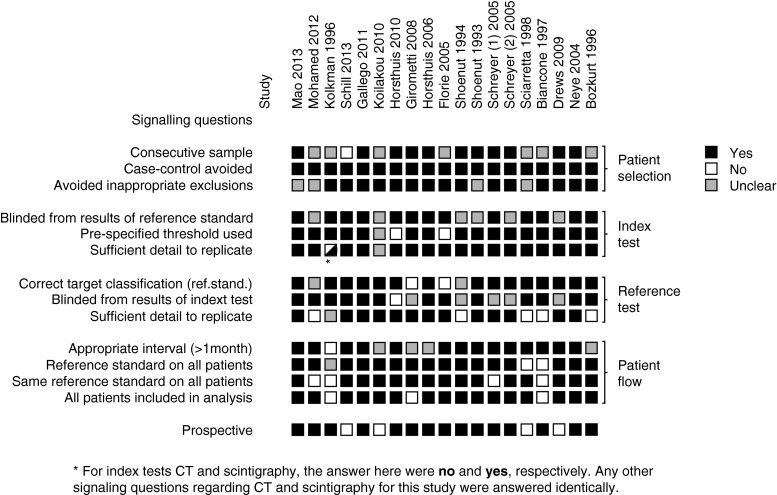
Both reviewers extracted study characteristics independently for all included articles using a standardized form. To assess the quality of the study design, we used a modified Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS 2) tool [9, 10], as it separately assesses risk of bias in several methodological domains (patient selection, index test, reference test and patient flow) using a number of signalling questions (Table 1). Risk of bias for each domain was described as high, low or unclear. In addition, concerns regarding the applicability of the patient population, index and reference test to the review question were rated by the observers as high, low or unclear. Disagreements were resolved by discussion.
Table 1.
Methodological characteristics from the QUADAS tool and their corresponding signalling questions [9, 10]. The risk of bias is determined for every domain using the signalling questions
| Modified QUADAS Methodological Characteristics | |
|---|---|
| Domain | Signalling questions (Yes, no, unclear) |
| Patient selection | Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? Did the study avoid inappropriate exclusions? |
| Index test | Were the index test results interpreted without knowledge of the results of the reference test? If a threshold was used, was it pre-specified? Was the execution and interpretation (expertise, image analysis) of the index test described in sufficient detail to permit its replication? a |
| Reference test | Is the reference test likely to correctly classify the target condition? Were the reference test results interpreted without knowledge of the results of the index test? Was the execution and interpretation of the reference test described in sufficient detail to permit its replication? a |
| Patient flow | Was there an appropriate interval between index test(s) and reference test (>1 month)? Did all patients receive a reference test? Did all patients receive the same reference test? Were all patients included in the analysis? |
| Prospective / Retrospective b | Was the data collected after the research question was defined? |
Patient characteristics
The following patient characteristics were recorded: number of patients included, number of patients in the analysis, whether patients were recruited consecutively, age characteristics, gender ratio, patient spectrum (i.e. known or suspected IBD or Crohn’s disease) and other selection criteria for patient inclusion.
Imaging characteristics
Imaging characteristics concerning type of equipment and basic specifications (type of scanner for CT, field strength and coil type for MRI, and transducer type for US), techniques used for evaluation (sequences for MRI, use of Doppler for US, labelling target and tracer type for scintigraphy), bowel preparation (fasting and/or laxatives), use of luminal and/or intravenous contrast medium, timing of post-contrast scans and use of spasmolytic drugs were extracted.
Reference test
All reference tests (i.e. endoscopy, biopsies or intraoperative findings) used for analysis were recorded.
Imaging and reference test interpretation
We recorded the following information regarding interpretation of imaging and reference tests: the interval in days between index and reference tests, bowel segments that were examined, grading criteria used for imaging and reference tests, imaging features used for evaluation of disease activity, and whether grading was performed on a per-patient and/or per-bowel-segment basis.
Data extraction
Grading results for imaging and reference tests were extracted with the grading scales used in individual studies (i.e. three-, four-, or five-grade scales). From this data, three-by-three contingency tables comparing results from index and reference tests categorized as none, mild or frank disease were constructed for each study. These categories did not use predefined criteria, but were formed either by using the original grading from each study (in the case of a three-grade scale) or by merging certain grades to form a three-grade scale. If a four-grade scale was used (none, mild, moderate or severe disease), groups with moderate and severe disease were merged into frank disease. For five-grade scales, the second and third scales were grouped into mild disease and the fourth and fifth were grouped into frank disease. When studies used multiple reference tests, we used intraoperative findings as the reference standard. In other cases, histological findings from biopsies were preferred over endoscopic findings. Because the imaging results in these studies were based on the most severe lesion, we considered histological data from biopsies as more lesion-specific and better resembling imaging results than endoscopic results.
Publication bias
To study publication bias, we followed the method by Deeks et al., as recommended in the Cochrane handbook for DTA reviews [11]. We first calculated effective sample sizes (ESS) for each study. We then performed linear regression analyses if enough datasets were available in a group (n > 5), with the proportion of accurate grading per study as the independent variable and 1/√ESS as the dependent variable. A significant regression coefficient (P < 0.05) was deemed sufficient to indicate publication bias.
Data analysis
For each study, we constructed three proportions: ‘accurate grading’, defined as the number of correctly graded patients or segments; ‘under-grading’, defined as the number of patients or segments on which the index test graded lower than the reference test; and ‘over-grading’, defined as the number of patients or segments on which the index test graded disease activity higher than the reference test. Datasets were sorted into groups by type of imaging, which were then subdivided by target of evaluation (per-patient or per-segment). To quantify heterogeneity we calculated the I2-statistic for each group. Data were pooled if more than one dataset was available in a group and the data were not too heterogeneous (I2 < 75 %) [12].
For the pooled data, we calculated mean logit accurate grading and under- and over-grading values with corresponding standard errors using non-linear fixed or random effects models based on the Akaike information criterion (AIC) statistic (a lower AIC value indicates a better fit) [13, 14]. Using anti-logit transformation, we obtained summary estimates with 95 % confidence intervals (95 % CI) for accurate grading and over- and under-grading. In several studies, multiple datasets were available (i.e. multiple readers). Because we used all datasets for analysis, we adjusted the correlation between datasets from the same study by adding the same number for each study in the subject statement of the random effects approach.
Comparison of CT, MRI, US and scintigraphy was performed with Z-tests using the logit values of the pooled data. For data that was not pooled, we performed logit transformation using proportion and sample size (n) to enable comparison. To calculate logit values for proportions of 0 or 100, we added 0.5 to the number of events [15]. P values less than 0.05 indicated a statistically significant difference. All data analyses were performed using Excel 2010 (Microsoft Corporation, Redmond, WA, USA), SPSS 22.0 (IBM SPSS Statistics for Macintosh, Version 22.0; IBM Corp., Armonk, NY, USA), and SAS 9.3 (SAS Institute, Cary, NC, USA) software programs.
Results
Search and study selection
The search yielded 9356 articles. After selection on title and/or abstract, 149 articles remained and were retrieved as full-text articles (Fig. 1). Of these remaining articles, 130 did not fulfil the eligibility criteria (Appendix E2). Nineteen articles met all inclusion criteria and were included for further data extraction. CT was evaluated in 3 [16–18], MRI in 11 [19–29], US in 3 [30–32], and scintigraphy in 3 [18, 33, 34]. No articles evaluating PET-CT were found that met our criteria.
Fig. 1.
Flow diagram showing study selection
Study characteristics
Methodological characteristics
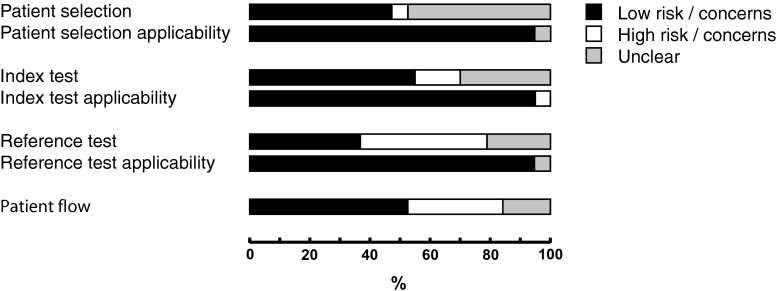
Evaluation of the imaging tests was performed blinded from the reference test in 13 studies [17, 18, 21, 22, 24–30, 33, 34]. The reference test was performed blinded to the imaging results in 12 studies [16, 17, 19, 21, 24, 26–30, 33, 34]. The remaining studies did not specify whether observers were blinded to other results [20, 23, 31, 32]. Fifteen of the studies included patients prospectively [16–26, 28, 30, 31, 34]. Signalling questions for the QUADAS tool were answered with ‘yes’ in 78.9 % of cases (Fig. 2). Patient selection and index test domains showed less risk of bias than reference test and patient flow domains. Concern about applicability of patient selection and index and reference tests was generally low (Fig. 3).
Fig. 2.
QUADAS signalling questions (Table 1) per domain (from up to down: patient selection, index test, reference test and patient flow). The last column shows whether studies included patients prospectively.
Fig. 3.
QUADAS risk of bias per domain and concerns regarding applicability for domains of patient selection, index test and reference test
Patient characteristics
A total of 549 patients were included (75 for CT, 347 for MRI, 86 for US, and 58 for scintigraphy). The mean study size was 29 patients (range, 10–76). Study characteristics are presented in Table 2. In ten of the studies, patients were recruited consecutively [17, 19, 20, 22–26, 28, 31]. Studies included patients with clinically suspected IBD, known IBD/Crohn’s disease, or a combination of both (12, 4, and 3 studies, respectively).
Table 2.
Study characteristics
| Study | Imaging modality | No. of patients included | No. of patients in analysis | Consecutive | Age, mean (range) or mean ± SD | Male/female ratio | Patient spectrum | Inclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Mao 2013 [17] | CT | 32 | 32 | Y | 30 (18–51) b | 22:10 | Known CD | Suspected recurrence after ileocolic resection |
| Mohamed 2012 [16] | CT | 26 | 26 | ? | 43.4 (19–69) | 18:8 | Known CD | Referred to further assessment with CTE |
| Kolkman 1996 [18] | CT, SG | 32 | 17 | ? | 36 (17–65) | 11:6 | Known/suspected IBD | Suspected IBD or IBD exacerbations or suspected abdominal complications |
| Schill 2013 [29] | MRI | 76 | 76 | N | 31.5 (16–76) b | 40:36 | Known CD | Patients scheduled for CD surgery |
| Gallego 2011 [28] | MRI | 61 | 61 | Y | 36.1 (14–65) | 29:32 | Known CD | NA |
| Koilakou 2010 [27] | MRI | 26 | 26 | ? | 36.5 (22–69) b | 16:13 | Known CD | Patients with previous ileocolic resection |
| Horsthuis 2010 [26] | MRI | 33 | 15 | Y | 14 (8–17) a, b | 15:18 a | Suspected IBD | Age 8–18 years |
| Girometti 2008 [25] | MRI | 52 | 45 | Y | 42.5 (18–67) | 23:29 a | Known/suspected CD | Referred for CC with biopsy and MRI for relapse or suspected onset of CD |
| Horsthuis 2006 [24] | MRI | 20 | 20 | Y | 36 ± 13 | 7:13 | Known CD | Scheduled for CC |
| Florie 2005 [21] | MRI | 31 | 31 | ? | 36 ± 12 | 22:9 | Known CD | Scheduled for ICC because of clinical suspicion of relapsing CD |
| Shoenut 1994 [20] | MRI | 20 | 12 | Y | 42.6 (20–70) a | 12:8 a | Suspected IBD | NA |
| Shoenut 1993 [19] | MRI | 28 | 19 | Y | 34.1 (20–58) | 17:11a | Known IBD | Referred to MRI for evaluation and on medical therapy |
| Schreyer 2005 [22] | MRI | 30 | 30 | Y | 29 (18–65) | 8:22 | Known CD | Routine small bowel MRI |
| Schreyer 2005 [23] | MRI | 22 | 12 | Y | 33.4 (19–55) | 5:7 | Known/suspected IBD | NA |
| Drews 2009 [32] | US | 32 | 32 | N | 38.8 (17–71) | 14:18 | Known CD | NA |
| Neye 2004 [31] | US | 22 | 22 | Y | 33.7 (16–56) | 9:13 | Known CD | Referred to gastroenterologist |
| Bozkurt 1996 [30] | US | 88 | 32 | ? | 39 (16–87) a | 48:40 a | Suspected IBD | NA |
| Biancone 1997 [34] | SG | 17 | 10 | ? | 43 ± 11 a | 9:8 a | Known CD | Patients 6–12 months after ileocecal resection |
| Sciarretta 1998 [33] | SG | 103 | 31 | ? | 38.3 (15–78) a | 54:49 a | Suspected IBD | NA |
CC colonoscopy, CD Crohn’s disease, CTE computed tomography enterography, IBD inflammatory bowel disease, ICC ileocolonoscopy, MRI magnetic resonance imaging, NA not applicable, Y yes, N no, ? unclear
aValues reflect the total number of patients included in their respective studies, not only the patients used in this analysis
bMedian (range)
Imaging characteristics
Imaging equipment and specifications are presented in Tables 3, 4, 5 and 6. Bowel preparation (fasting and/or laxatives) was used in eight studies (1 CT, 7 MRI) [17, 21–26, 28]. Luminal contrast medium was used in ten studies (3 CT, 7 MRI) [16–18, 21–23, 25, 27–29], of which one used enteroclysis [27]. Intravenous contrast medium was used in 13 studies (2 CT, 11 MRI) [16, 17, 19–29].
Table 3.
CT characteristics
| Study | Type of scanner | Bowel preparation | Luminal contrast | Enterography (EG) / enteroclysis (EC) | I.V. contrast | Post-contrast scan timing |
|---|---|---|---|---|---|---|
| Mao 2013 [17] | Multiple-slice helical CT with 64 detector rings | 1 night fasting | 2000 mL 2.5 % Mannitol solution 1 hr prior | EG | 100 mL Iopramide | 28 s and 60 s |
| Mohamed 2012 [16] | Multiple-slice helical CT | NS | 1500–2000 mL water | EG | 100–150 mL Iopamiro 300 | 60 s |
| Kolkman 1996 [18] | Siemens Somatom Plus 4 | NS | 500 ml water with 15 ml Rayvist 60 % both on the evening prior to CT and immediately preceding scan. 500 mL with 30 mL Rayvist 1 hr prior to CT | EG | NS | NS |
CT computed tomography, NS not specified
Table 4.
MRI characteristics
| Study | Field strength | Coil | Bowel preparation | Luminal contrast | Enterography (EG) / enteroclysis (EC) | I.V. contrast | Post-contrast sequence timing | Spasmolytic agent | Sequences |
|---|---|---|---|---|---|---|---|---|---|
| Schill 2013 [29] | 1.5 T | Body and spine array coils | NS | 1.5–2 L Mannitol solution orally 45 min prior and 0.5–1 L 0.9 % NaCl rectally | EG | 0.2 mL/kg Gd-DTPA | 70 s | 40 mg Buscopan iv | 3D T2-SPACE, bSSFP, RARE, T1-FLASH, T1-FLASH (post-contrast), T1-FLASH with fat suppression (post-contrast), |
| Gallego 2011 [28] | 1.0 T | Body coil | 8 hrs fasting | 1.5 L PEG and mineral salts | EG | 0.1 mmol/kg Gd-DTPA | 40 s, 70 s (used for RCE), 120 s, 180 s | Buscopan iv | bSSFP, interpolated 3D T1 with fat suppression (pre-/post-contrast), RARE |
| Koilakou 2010 [27] | 1.5 T | NS | NS | 100–150 mL/min 0.5 % methylcellulose solution | EC | 0.1 mmol/kg Gd-DTPA | NS | 20 mg Buscopan iv | Interpolated 3D T1 with fat suppression (post-contrast), SSFP, T2 with fat suppression, |
| Horsthuis 2010 [26] | 3.0 T | Phased array coil | Metamucil in 250 mL water 4 hrs prior | NS | EG | 0.1 mL/kg Gadodiamide | NS | Buscopan iv | Interpolated 3D T1 with fat suppression (post-contrast), RARE |
| Girometti 2008 [25] | 1.5 T | Phased array coil | 8 hrs fasting | 2 L PEG | EG | 0.2 mL/kg Gd-DTPA | 30 s, 45 s, 60 s, 75 s, 90 s, 150 s | 10 mg Buscopan iv | bSSFP, cine, interpolated 3D T1 with fat suppression (pre-/post-contrast), RARE |
| Horsthuis 2006 [24] | 3.0 T | Phased array body coil | Metamucil in 250 mL water 4 hrs prior | NS | EG | 0.05 mmol/kg Gadodiamide | 70 s | 20 mg Buscopan iv or 1 mg glucagon hydrochloride | bFFE, T2-TSE, T1-FFE with fat suppression (post-contrast) |
| Florie 2005 [21] | 1.5 T | NS | 4 hrs fasting | 1 L water 2 hrs prior | EG | 0.1 mmol/kg Gd-DTPA | NS | 20 mg Buscopan iv or 1 mg glucagon hydrochloride | bSSFP, interpolated 3D T1 (pre-/post-contrast), out-of-phase fast low angle shot, RARE |
| Shoenut 1994 [20] | 1.5 T | NS | NS | None | NA | 0.1 mmol/kg Gd-DTPA | 5 s, 30 s (used for RCE), 5 min, 10 min | NS | T1-FLASH (pre-/post-contrast), T1 spin echo with fat suppression (post-contrast) |
| Shoenut 1993 [19] | 1.5 T | NS | NS | NS | NA | 0.1 mmol/kg Gd-DTPA | 5 s, 30 s (used for RCE), 10 min | NS | T1-FLASH, T1-FLASH (post-contrast), T1 with fat suppression (pre-/post-contrast) |
| Schreyer 2005 [22] | 1.5 T | Phased array body coil | 12 hrs fasting | 2 L Mannitol solution with carob seed 1 hr prior orally and 0.4–1.0 L 0.9 % NaCl rectally | NA | 0.2 mmol/kg Gd-DTPA | 70 s | 40 mg Buscopan iv | 2D T1-FLASH, 2D & 3D T1-FLASH with fat suppression (post-contrast), bSSFP, RARE |
| Schreyer 2005 [23] | 1.5 T | Phased array body coil | Macrogol 3350 | 1.5 L Gd (5 mmol/L) mixture with water rectally | NA | 0.1 mmol/kg Gd-DTPA | NS | 40 mg Buscopan iv | 2D T1-FLASH, 2D T1-FLASH fat sat (post-contrast), 3D T1-FLASH, bSSFP, RARE, |
bFFE balanced fast-field echo, (b)SSFP (balanced) steady-state free precession, Gd(-DTPA) gadolinium(-diethylenetriaminepentaacetic acid), iv intravenous, MRI magnetic resonance imaging, NaCl sodium chloride, NS not specified, PEG polyethylene glycol, RARE rapid acquisition with refocusing echoes, RCE relative contrast enhancement, T Tesla, T1-FLASH T1-weighted fast low-angle shot, TSE turbo spin-echo
Table 5.
US characteristics
| Study | Transducer type + frequency | Bowel preparation | Luminal contrast | I.V. contrast | Doppler + type |
|---|---|---|---|---|---|
| Drews 2009 [32] | Linear 5–12 MHz (neoterminal ileum) and convex 2–5 MHz (entire abdomen) | NS | NS | NS | Power Doppler |
| Neye 2004 [31] | Linear 5–12 Mhz and dynamic sector scanner 4–7 MHz | NS | NS | NS | Pulsed Doppler and Power Doppler |
| Bozkurt 1996 [30] | Linear 7.5 MHz | NS | NS | NS | NA |
MHz megahertz, NA not applicable, NS not specified, US ultrasound
Table 6.
Scintigraphy characteristics
| Study | Labelling target | Tracer | Amount of tracer | Scans | Criteria used for image analysis |
|---|---|---|---|---|---|
| Kolkman 1996 [18] | Antigranulocyte antibodies | Tc-99 m HMPAO | NS | 2 scans (at 1 hrs and 4 hrs) | Uptake of tracer compared to bone marrow and liver |
| Biancone 1997 [34] | Leukocytes | Tc-99 m HMPAO | 185 MBq | 2 scans (at 30 min and 3 hrs) | Uptake of tracer compared to bone marrow and liver |
| Sciarretta 1998 [33] | Leukocytes | Tc-99 m HMPAO | 370–555 MBq | 3 scans (at 30 min, 2–2.5 hrs and 24 hrs) | Uptake of tracer compared to bone marrow and liver |
MBq megabecquerel, NS not specified, Tc-99 m HMPAO technetium hexamethylpropyleneamine oxime
Reference test
Endoscopy, biopsies and intraoperative findings were used in 11, 8 and 4 studies, respectively (Table 7). Three studies recorded results for both endoscopy and histology from biopsies, for which we used the histological data in our analysis [30, 33, 34].
Table 7.
Imaging and reference test interpretation
| Study | Imaging modality | Reference test used in analysis | Analysis per patient/ per segment | Time interval (days) index & reference test | Part of GI tract examined | Grading scale index test | Grading scale reference test | Imaging features used for grading disease activity |
|---|---|---|---|---|---|---|---|---|
| Mao 2013 [17] | CT | ICC | Patient | <=7 | Neoterminal ileum | 0–3 | i0–i4 (Rutgeerts score) | Bowel wall thickness, post-contrast enhancement, mucosal irregularities/hyperdensities, mural stratification, stenosis and prestenotic dilatation and extraluminal findings (lymph nodes, abscesses, fistulas, comb sign, creeping fat) |
| Mohamed 2012 [16] | CT | B, SS | Patient | <=7 a | Colon and TI | Mild, moderate, severe | Mild, moderate, severe | Bowel wall thickness, post-contrast enhancement, extraluminal findings (lymph nodes, abscesses, fistulas, comb sign, creeping fat, edema) |
| Kolkman 1996 [18] | CT, SG | B, ICC, SS | Segment | 1–50 | Colon and TI | 0–3 (CT), 0–4 (SG) | 0–3 | Bowel wall thickness, T1 enhancement and pattern, ulceration, double-halo sign and extraluminal findings (creeping fat, mesenteric fibrovascular strands) (CT). Uptake of tracer compared to bone marrow and liver (SG) |
| Schill 2013 [29] | MRI | SS | Patient | <=28 | NS | B1, B2, B3 (Montreal class.) | B1, B2, B3 (Montreal class.) | Target sign, T2 mural signal intensity, inflammatory mass, stenosis with prestenotic dilatation and extraluminal findings (lymph nodes, abscesses, fistulas, comb sign) |
| Gallego 2011 [28] | MRI | ICC | Patient | <=15 | Ileum | None, mild, moderate/severe | 0–3 (SES-CD) | Bowel wall thickness and edema, T1 enhancement, mucosal abnormalities, inflammatory mass, motility, stenosis and extraluminal findings (lymph nodes, fistulas) |
| Koilakou 2010 [27] | MRI | ICC | Patient | NS | Neoterminal ileum | 0–3 | i0–i4 (Rutgeerts score) | Bowel wall thickness, T1 enhancement, T2 mural signal, mucosal irregularities, infiltrate, edema, stenosis and prestenotic dilatation, extraluminal findings (abscesses, fistulas) |
| Horsthuis 2010 [26] | MRI | EGD, ICC | Patient | <=14 | Colon, TI and duodenum | None, mild, moderate, severe (subjective) | None, mild, moderate, severe (subjective) | Bowel wall thickness, T1 enhancement, stenosis and prestenotic dilatation. |
| Girometti 2008 [25] | MRI | B | Patient | NS | TI | None, mild, moderate/severe | None, mild, moderate/severe | Bowel wall thickness, T1 enhancement, mucosal abnormalities, inflammatory mass, mesenteric involvement, motility, stenosis and extraluminal findings (lymph nodes, fistulas) |
| Horsthuis 2006 [24] | MRI | ICC | Patient | 1–48 | Colon and TI | None, mild, moderate, severe (subjective) | None, mild, moderate, severe (subjective) | Bowel wall thickness, T1 enhancement, ulceration, length of diseased segment, cobblestoning, extraluminal findings (lymph nodes, abscesses, fistulas, comb sign and creeping fat) |
| Florie 2005 [21] | MRI | ICC | Patient | <=14 | Colon and TI | None, mild, moderate, severe (subjective) | None, mild, moderate, severe (subjective) | Bowel wall thickness, T1 enhancement, stenosis, target sign, cobblestoning |
| Shoenut 1994 [20] | MRI | B | Patient | <=3 | Colon and TI | Mild, moderate, severe | Mild, moderate, severe (subjective) | Bowel wall thickness, T1 enhancement, length of diseased segment |
| Shoenut 1993 [19] | MRI | CC, SS | Patient | <=7 | Colon and TI | Mild, moderate, severe | Mild, moderate, severe | Bowel wall thickness, T1 enhancement, length of diseased segment |
| Schreyer 2005 [22] | MRI | ICC | Segment | <=7 | Colon and TI | 0–2 | 0–2 | Bowel wall thickness, T1 enhancement, stenosis, lymph nodes, local injection for inflammation assessment |
| Schreyer 2005 [23] | MRI | ICC | Segment | 1 | Colon and TI | 0–2 | 0–2 | Bowel wall thickness, T1 enhancement, lymph nodes, mesenteric injection |
| Drews 2009 [32] | US | B | Patient | <=5 | Colon and (neo-) terminal ileum | 0–4 | 0–4 | Vascularization and thickness of the bowel wall, preservation of five-layer structure, length of diseased segment |
| Neye 2004 [31] | US | ICC | Segment | <=3 | Colon and TI | 0–3 | 0–3 | Vascularization and thickness of the bowel wall |
| Bozkurt 1996 [30] | US | B | Segment | NS | Colon | 0–2 | 0–2 (subjective) | Bowel wall thickness, echogenicity of the bowel wall, smoothness of boundaries, visibility of individual bowel wall layers |
| Biancone 1997 [34] | SG | B | Patient | <=14 | Neoterminal ileum | 0–3 | 0–3 (subjective) | Uptake of tracer compared to bone marrow and liver |
| Sciarretta 1998 [33] | SG | B | Segment | <=7 | Colon and TI | 0–3 | 0–3 (subjective) | Uptake of tracer compared to bone marrow and liver |
B biopsies, CC colonoscopy, CT computed tomography, EGD esophagogastroduodenoscopy, ICC ileocolonoscopy, MRI magnetic resonance imaging, NS not specified, SES-CD simple endoscopic score for Crohn’s disease, SG scintigraphy, SS surgical specimens, TI terminal ileum, US ultrasound
aTime interval was not specified for patients undergoing surgery
Imaging and reference test interpretation
Thirteen of the studies used an interval of less than one month between imaging and reference test [17, 19–23, 26, 28, 29, 31–34]. The imaging features most commonly used for evaluation were bowel wall thickness and post-contrast enhancement (or tracer uptake for scintigraphy), which were both used in 17 studies (Table 7). The reference test and imaging criteria for each study are presented in Tables 8 and 9.
Table 10.
Comparison table with results for imaging tests from the 3 × 3 data analysis and corresponding P values
| Accurate grading | Over-grading | Under-grading | |
|---|---|---|---|
| Per-patient (13 datasets) | |||
| CT (n = 2) vs MRI (n = 9) | 0.86 vs 0.84 (P = 0.8) | 0.10 vs 0.09 (P = 0.8) | 0.03 vs 0.06 (P = 0.5) |
| CT (n = 2) vs US (n = 1) | 0.86 vs 0.44 (P = 0.0001) | 0.10 vs 0.25 (P = 0.07) | 0.03 vs 0.31 (P = 0.002) |
| CT (n = 2) vs SG (n = 1) | 0.86 vs 0.40 (P = 0.003) | 0.10 vs 0.10 (P = 1.0) | 0.03 vs 0.50 (P = 0.0005) |
| MRI (n = 9) vs US (n = 1) | 0.84 vs 0.44 (P = 0.001) | 0.09 vs 0.25 (P = 0.03) | 0.06 vs 0.31 (P = 0.003) |
| MRI (n = 9) vs SG (n = 1) | 0.84 vs 0.40 (P = 0.01) | 0.09 vs 0.10 (P = 0.9) | 0.06 vs 0.50 (P = 0.001) |
| US (n = 1) vs SG (n = 1) | 0.44 vs 0.40 (P = 0.8) | 0.25 vs 0.10 (P = 0.3) | 0.31 vs 0.50 (P = 0.3) |
| Per-segment (3 datasets) a | |||
| CT (n = 1) vs SG (n = 2) | 0.87 vs 0.86 (P = 0.8) | 0.00 vs 0.04 (P = 0.2) | 0.13 vs 0.10 (P = 0.5) |
aData on MRI and US were not pooled and included in the comparison, as the data were too heterogeneous (I2 > 75 %)
Table 8.
Original reference test criteria and categorization for this study
| Study | None | Mild | Severe | ||
|---|---|---|---|---|---|
| Mao et al. histological score (Rutgeerts score) [17] | i0: No lesions | i1: Less than 5 aphthous lesions | i2: More than 5 aphthous lesions with normal mucosa between the lesions or skip areas of larger lesions or lesions confined to ileocolonic anastomosis | i3: Diffuse aphthous ileitis with diffusely inflamed mucosa | i4: Diffuse inflammation with already large ulcers, nodules, and/or narrowing |
| Mohamed et al. histological score (subjective) [16] | - | Mild | Moderate | Severe | |
| Kolkman et al histological score [18] | 0: No abnormalities, or plain fibrosis | 1: Some infiltration of polymorphonuclear leukocytes, no ulceration | 2: Moderate infiltration of polymorphonuclear leukocytes, some ulceration present | 3: Severely ulcerated with massive infiltration of polymorphonuclear leukocytes | |
| Schill et al. surgical score (based on Montreal classification) [29] | - | B1: Non-stricturing and non-penetrating | B2: Stricturing | B3: Penetrating | |
| Gallego et al. endoscopic score (SES-CD) a [28] | 0–2 points: Inactive | 3–6 points: Mild disease | ≥7 points: Moderate/severe disease | ||
| Koilakou et al. histological score (Rutgeerts score) [18] | i0: No lesions | i1: Less than 5 aphthous lesions | i2: More than 5 aphthous lesions with normal mucosa between the lesions or skip areas of larger lesions or lesions confined to ileocolonic anastomosis | i3: Diffuse aphthous ileitis with diffusely inflamed mucosa | i4: Diffuse inflammation with already large ulcers, nodules, and/or narrowing |
| Horsthuis et al. endoscopic score (subjective) [26] | No disease | Mild disease | Moderate disease | Severe disease | |
| Girometti et al. histological score (subjective) [25] | No disease (or chronic, quiescent disease) | Mild disease | Moderate-to-severe disease | ||
| Horsthuis et al. endoscopic score (subjective) [24] | No disease | Mild disease | Moderate disease | Severe disease | |
| Florie et al. endoscopic score (subjective) [21] | No disease | Mild disease | Moderate disease | Severe disease | |
| Shoenut et al. endoscopic score [20] | - | Mild: Mucosal erythema, friability and granularity | Moderate: Marked edema, linear or patchy ulceration | Severe: Coalescing ulceration, exudative colitis | |
| Shoenut et al. histological score (subjective) [19] | - | Mild disease | Moderate disease | Severe disease | |
| Schreyer et al. endoscopic score [22] | 0: No findings | 1: Erythema, decreased or absent vascular pattern, friability of the mucosa, single or multiple aphthous lesions, and small ulcers | 2: Presence of large ulcerous lesions, nodules, and/or narrowing | ||
| Schreyer et al. endoscopic score [23] | 0: No findings | 1: Erythema, decreased or absent vascular pattern, friability of the mucosa, single or multiple aphthous lesions, and small ulcers | 2: Presence of spontaneous bleeding, and large ulcerous lesions, nodules, and/or narrowing | ||
| Drews et al. histological score [32] | 0: No inflammation | 1: Chronic non-active inflammation | 2: Mild active inflammation | 3: Moderate active inflammation | 4: Severe active inflammation |
| Neye et al. endoscopic score [31] | 0: No lesions | 1: Aphtes | 2: Aphtes and ulcers < 50 % | 3: Aphtes and ulcers > 50 % | |
| Bozkurt et al. histological score (subjective) [30] | 0 | 1 | 2 | ||
| Biancone et al. histological score (subjective) [34] | 0 | 1 | 2 | 3 | |
| Sciarretta et al. histological score (subjective) [33] | 0 | 1 | 2 | ||
a0–3 points are given for the following are given to following features: size of ulcers (0: none, 1: aphthous ulcers (0.1–0.5 cm), 2: large ulcers (0.5–2 cm), 3: very large ulcers (>2 cm)), ulcerated surface (0: none, 1: <10 %, 2: 10–30 %, 3: >30 %), affected surface (0: none, 1: <50 %, 2: 50–75 %, 3: >75 %) and presence of narrowing (0: none, 1: single, can be passed, 2: multiple, can be passed, 3: cannot be passed)
Publication bias
Linear regression analysis on MRI per-patient data showed a regression coefficient of 0.4 (95 % CI: −0.9 to 0.9), with no significant relationship between accurate grading and 1/√ESS (P = 0.09). Data in other groups were deemed insufficient for performing linear regression analyses.
Data analysis
Results from our data analysis are presented in Table 10. Three-by-three contingency tables for each study can be found in the supplementary materials (Appendix E3).
Table 9.
Original imaging criteria and categorization for this study
| Study | None | Mild | Severe | ||
|---|---|---|---|---|---|
| Mao et al. CT score [17] | 0: No findings | 1: Minor mucosal irregularities with slight wall thickening and mural contrast enhancement | 2: Mucosal hyperdensity with distinct bowel wall thickening, no stenosis, or stenosis without prestenotic dilatation | 3: Major mucosal abnormalities, distinct bowel wall thickening with target sign and extravisceral signs such as perienteric stranding, comb sign, fibrofatty proliferation, stenosis with prestenotic dilatation and/or the presence of complications | |
| Mohamed et al. CT score [16] | - | Mild: Mucosal hyperenhancement | Moderate: Abnormal mucosal enhancement and wall thickening (>3 mm) | Severe: Abnormal mucosal enhancement, wall thickening (>3 mm) and one or more extra-enteric manifestations (edema of the mesenteric fat, engorged vasa recta, lymphadenopathy, fistula, abscess) | |
| Kolkman et al CT score [18] | 0: No thickening of the bowel wall, normal mesentery | 1: Thickened bowel wall, homogenous aspect, no enhancement with intravenous contrast, no double-halo sign | 2: Thickened bowel wall, enhancement with intravenous contrast or double-halo sign, ulceration, or mesenteric fibrofatty proliferation | 3: Thickened bowel wall, enhancement with intravenous contrast, ulceration, and mesenteric fibrovascular strands | |
| Kolkman et al scintigraphic score [18] | 0: No activity | 1: Uptake less than bone marrow | 2: Uptake equal to bone marrow | 3: Uptake higher than bone marrow, but less than liver | 4: Uptake equal or higher than liver |
| Schill et al. MRI score (based on Montreal classification) [29] | - | B1: Non-stricturing and non-penetrating | B2: Stricturing | B3: Penetrating | |
| Gallego et al. MRI scorea [28] | 0–1 points: No disease | 2–6 points: Mild disease | ≥7 points: Moderate/severe disease | ||
| Koilakou et al. MRI score [18] | 0: | 1: | 2: | 3: | |
| Horsthuis et al. MRI score (subjective) [26] | No disease | Mild disease | Moderate disease | Severe disease | |
| Girometti et al. MRI scoreb [25] | 0–1 points: No disease | 2–6 points: Mild disease | ≥7 points: Moderate/severe disease | ||
| Horsthuis et al. MRI score (subjective) [24] | No disease | Mild disease | Moderate disease | Severe disease | |
| Florie et al. MRI score (subjective) [21] | No disease | Mild disease | Moderate disease | Severe disease | |
| Shoenut et al. MRI score [20] | - | Mild: ≤70 % contrast-enhancement in the most diseased segment (by wall thickness and length) | Moderate: 71–119 % contrast-enhancement in the most diseased segment (by wall thickness and length) | Severe: ≥120 % contrast-enhancement in the most diseased segment (by wall thickness and length) | |
| Shoenut et al. MRI score [19] | - | Mild: Length of diseased segment < 5 cm, bowel wall thickness < 5 mm, contrast-enhancement < 50 % | Moderate: Length of diseased segment > 5 cm, bowel wall thickness 0.5-1 cm, contrast-enhancement < 100 % | Severe: Length of diseased segment > 5 cm, bowel wall thickness > 1 cm, contrast-enhancement > 100 % | |
| Schreyer et al. MRI score [22] | 0: No criteria | 1: One of the following criteria: bowel wall thickening, bowel stenosis, increased contrast media uptake, enlarged local lymph nodes and local injection for inflammation assessment | 2: Two or more of the following criteria: bowel wall thickening, bowel stenosis, increased contrast media uptake, enlarged local lymph nodes and local injection for inflammation assessment | ||
| Schreyer et al. MRI score [23] | 0: No criteria | 1: One of the following criteria: bowel wall thickening with contrast enhancement enlarged local lymph nodes and mesenteric injection | 2: Two or more of the following criteria: bowel wall thickening with contrast enhancement enlarged local lymph nodes and mesenteric injection | ||
| Drews et al. US score [32] | 0: Bowel wall thickness 3–4 mm with preserved five-layer structure, no increased vascularity | 1: Bowel wall thickness > 4 mm, no increased vascularity | 2: Grade 1 plus short stretches of increased vascularity | 3: Grade 2 plus longer stretches of increased vascularity | 4: Grade 3 plus vascularity extending into surrounding mesentery |
| Neye et al. US score [31] | 1: 0 vessels/cm2 and bowel wall thickness < 5 mm | 2: 0 vessels/cm2 + bowel wall thickness > 5 mm or 1-2 vessels/cm2 + bowel wall thickness < 5 mm | 3: 1–2 vessels/cm2 + bowel wall thickness > 5 mm or > 2 vessels/cm2 + bowel wall thickness < 5 mm | 4: > 2 vessels/cm2 + bowel wall thickness > 5 mm | |
| Bozkurt et al. US score [30] | 0: Normal bowel wall with an echo-poor layer of ≤4 mm with a smooth boundary. Only the ventral wall visualizable to gaseous distention | 1: Bowel wall thickness > 4 mm with individual layers visible | 2: Bowel wall thickness with poorly defined individual layers and decreased echogenicity | ||
| Biancone et al. scintigraphic score [34] | 0: No labeling | 1: Less than bone marrow | 2: More than bone marrow, less than liver | 3: Equal or more than liver | |
| Sciarretta et al. histological score (subjective) [33] | 0: No uptake | 1: Less than bone marrow | 2: More than bone marrow, less than liver | 3: Equal or more than liver | |
a0–2 points are given for the following are given to following features: bowel wall thickness (0: < 3 mm, 1: 3–4 mm, 2: > 4 mm), relative enhancement (0: <70 %, 1: 70–100 %, 2: >100 %), motility (0: normal, 1: reduced, 2: absent), percentage stenosis (0: ≤60 %, 1: >60 %), bowel wall edema (0: absent, 1: present), mucosal abnormalities (0: absent, 1: present), lymph nodes (0: absent, 1: present), fistulae or sinus tracts (0: absent, 1: present), inflammatory masses (0: absent, 1: present)
b0–2 points are given for the following are given to following features: bowel wall thickness (0: < 3 mm, 1: 3–4 mm, 2: > 4 mm), wall-contrast enhancement (0: <70 %, 1: 70–100 %, 2: >100 %), percentage stenosis (0: <50 %, 1: 50–80 %, 2: >80 %), mucosal abnormalities (0: absent, 1: present), layered wall enhancement (0: absent, 1: present), peristalsis (0: present, 1: absent), distensibility (0: present, 1: absent), mesenteric involvement (0: absent, 1: present), pathologic lymph nodes (n > 3) (0: absent, 1: present), fistulae or sinus tracts (0: absent, 1: present), inflammatory masses (0: absent, 1: present)
Per-patient
Data was provided on a per-patient basis in 13 studies (evaluating CT in 2, MRI in 9, US in 1 and scintigraphy in 1) (Fig. 4). I2 values for overall grading accuracy for groups with more than one dataset were as follows: 67.7 % (95 % CI: 42.6–81.8 %) for CT, and 73.9 % (95 % CI: 56.2 − 84.4 %) for MRI.
Fig. 4.

Accurate grading, over- and under-grading per study on a per-patient and per-segment basis
CT and MRI data were pooled for each modality (I2 < 75 %). US and scintigraphy were not pooled, as only one dataset was available for each modality. CT, MRI, US and scintigraphy showed accurate grading estimates of 86 % (95 % CI: 75–93 %), 84 % (95 % CI: 67–93 %), 44 % (95 % CI: 28–61 %) and 40 % (95 % CI: 16–70 %), respectively. CT and MRI showed similar overall grading accuracy (P = 0.8), both higher than US (P = 0.0001 and P = 0.001, respectively) and scintigraphy (P = 0.003 and P = 0.01, respectively). CT and MRI showed similar over-grading (P = 0.8) and under-grading (P = 0.5). Both showed less under-grading than US (P = 0.002 and P = 0.003, respectively) and scintigraphy (P = 0.0005 and P = 0.001, respectively).
Per-segment
Data were provided on a per-segment basis in seven articles, of which one evaluated both CT and scintigraphy, two evaluated MRI, two evaluated US, and two evaluated scintigraphy, respectively (Fig. 4). I2 values were 86.3 % (95 % CI: 66.4–94.4 %) for MRI, 91.5 % (95 % CI: 79.1–96.6 %) for US, and 0 % for scintigraphy. MRI and US data were not pooled, as data were too heterogeneous (I2 ≥ 75 %). Data on CT were also not pooled, as only one dataset was available. The overall grading accuracy was 87 % (95 % CI: 77–93 %) for CT and 86 % (95 % CI: 80–91 %) for scintigraphy. CT and scintigraphy showed similar overall grading accuracy (P = 0.8), over-grading (P = 0.2) and under-grading (P = 0.5). Accuracy for MRI and US ranged from 67 to 82 % and 56 to 75 %, respectively.
Discussion
In this study, we have shown that MRI and CT are highly accurate for grading Crohn’s disease activity. These findings are important, as cross-sectional imaging plays an increasing role in the assessment of Crohn’s disease activity, and there has been ongoing debate regarding the modality that should be the preferred choice [35–37]. Several studies have compared two or more modalities in the same patient group [38–41], but they have had relatively small sample sizes or only evaluated the terminal ileum.
CT and MRI showed similar accuracy in grading Crohn’s disease activity (86 % and 84 % on a per-patient basis, respectively), and no significant differences in accuracy were seen between these two modalities. Data on over- and under-grading showed similar results for CT and MRI, further strengthening our conclusion of their comparability. Scintigraphy showed high accuracy of 86 % and 86 % for the studies using per-segment data, while accuracy of 40 % was reported in per-patient data. However, per-patient data for scintigraphy was reported in only one study, and with a small sample size (n = 10) [34]. Furthermore, scintigraphy had the least number of included patients (n = 58) in our meta-analysis. US showed low accuracy of 44 % in the per-patient data and 75 % and 56 % for studies in the per-segment data. However, a relatively small number of patients (n = 86) were included. In addition, no eligible studies evaluated luminal or intravenous contrast medium for US. The use of intravenous contrast appears to be a particularly promising technique, and may increase the accuracy of US. However, no robust reference standard or appropriate grading scale were used in these studies. We considered the possibility of performing subgroup and covariate analyses on the differences in technique, imaging criteria, reference methods and methodological criteria, but the results of these analyses would not be meaningful given the limited amount of available data. We examined MRI imaging features in three studies with the highest accuracy values. The following MRI features were used by at least two of these studies: bowel wall thickness, T1 enhancement and pattern, T2 mural signal intensity, mucosal abnormalities, presence of inflammatory mass, stenosis (with pre-stenotic dilatation), lymph nodes, abscesses, and fistulas [25, 27, 29].
The observed heterogeneity of the grading criteria for the index and reference tests in the studies that we included, our adjustment to construct 3 × 3 tables, and the differences in available data between imaging modalities were the major limitations of this meta-analysis. Although the grading criteria for index and reference tests differed by study, and different imaging features were used, the studies included showed considerable overlap in the use of imaging features and grading criteria. No generally accepted scoring systems exist for imaging of Crohn’s disease. To construct 3 × 3 tables from original 4 × 4 data, we merged moderate and severe disease into one group. Our decision to merge these grades was based on five articles [22, 23, 25, 28, 30] that had originally used 3 × 3 tables; two of these studies explicitly stated that their highest grade represented moderate and severe disease combined [25, 28]. The remaining three studies [22, 23, 30] used similar grading criteria. Another limitation was the heterogeneity of grading results, which we examined using I2 statistics. Following those results, some of the datasets could not be pooled. In our conclusions, we took into account the greater availability of data for MRI compared to CT, US and scintigraphy. Furthermore, US and scintigraphy studies showed varying results, hampering our ability to arrive at a firm conclusion. There was only one head-to-head comparison study, which compared CT and scintigraphy in 17 patients [18].
We selected three reference standards for this meta-analysis [35]. Intraoperative findings served as the gold standard for assessing Crohn’s disease. We also included endoscopy and endoscopic biopsies as reference standards, although they are not ideal, as they are incapable of assessing proximal ileum, jejunum and extraluminal disease, which could have led to incorrect estimation of disease activity. On the other hand, surgery is performed only in select patients, whereas endoscopy is applied across a wider spectrum. For our analysis, we gave precedence to results from biopsies over endoscopic results, but we recognize that this was a controversial choice, as there is no widespread consensus on which is the better reference standard. The number of studies included could have been increased if VCE and/or double-balloon enteroscopy (DBE) were also used as a reference standard. We chose not to include these studies because interpretation of VCE and DBE has not yet been standardized, and so this would further increase heterogeneity in our study. A growing number of studies are using correlative statistics to examine quantitative scoring systems [42]. Because we used an ordinal outcome measure, we could not include these studies. Nevertheless, a meta-analysis focused on this type of data would be very useful. Finally, only patients with suspected IBD or known Crohn’s disease were included, possibly introducing observer bias, leading to over-grading of disease activity.
Assessment of study quality using the QUADAS tool showed overall moderate quality of the studies included in this meta-analysis. The domains of reference test and patient flow showed the highest risk of bias, while patient selection and index test domains showed the lowest. Concern about the applicability of patient selection and index and reference tests was generally low.
Recently, Vermeire et al. stated that MR enterography had become the reference standard for assessing small and large bowel disease activity [43]. Based on our results, we can agree with this statement. Considering the radiation exposure from CT, it is not appropriate for repeated examinations, even with present-day reduced ionizing radiation exposure per examination, although it still has an important role in the acute setting [44]. Compared to endoscopy, MRI is non-invasive and able to investigate trans- and extramural disease, making it possible to evaluate both the small bowel and colon in one examination. Steps are being taken to come to a more uniform evaluation of MRI in Crohn’s disease, which may improve accuracy [42, 45]. Furthermore, the versatility of MRI may be advantageous with new sequences being studied.
In conclusion, CT and MRI can both be used to grade disease activity in Crohn’s disease, while no conclusions can be made on US and scintigraphy due to the limited and inconsistent data.
Electronic supplementary material
(DOCX 105 kb)
Acknowledgements
The scientific guarantor of this publication is Jaap Stoker. The authors of this manuscript declare relationships with the following companies: Jaap Stoker is a consultant for Robarts.
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding through a research grant from the European Union’s Seventh Framework Program (project number 270379). The European Union was not involved in designing and conducting this study, did not have access to the data, and was not involved in data analysis or preparation of this manuscript. One of the authors (Shandra Bipat) has significant statistical expertise in systematic reviews and meta-analyses. Institutional review board approval was not required because this is a literature study.
References
- 1.Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247:64–79. doi: 10.1148/radiol.2471070611. [DOI] [PubMed] [Google Scholar]
- 2.Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125–145. doi: 10.1111/j.1365-2036.2011.04710.x. [DOI] [PubMed] [Google Scholar]
- 3.Travis SP, Stange EF, Lemann M, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut. 2006;55:i16–i35. doi: 10.1136/gut.2005.081950b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hommes DW, van Deventer SJ. Endoscopy in inflammatory bowel diseases. Gastroenterology. 2004;126:1561–1573. doi: 10.1053/j.gastro.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher JG, Fidler JL, Bruining DH, Huprich JE. New concepts in intestinal imaging for inflammatory bowel diseases. Gastroenterology. 2011;140:1795–1806. doi: 10.1053/j.gastro.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Rimola J, Ordas I, Rodriguez S, Ricart E, Panes J. Imaging indexes of activity and severity for Crohn’s disease: current status and future trends. Abdom Imaging. 2012;37:958–966. doi: 10.1007/s00261-011-9820-z. [DOI] [PubMed] [Google Scholar]
- 7.Horsthuis K, Bipat S, Stokkers PC, Stoker J. Magnetic resonance imaging for evaluation of disease activity in Crohn’s disease: a systematic review. Eur Radiol. 2009;19:1450–1460. doi: 10.1007/s00330-008-1287-0. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 10.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 14.Bipat S, Zwinderman AH, Bossuyt PM, Stoker J. Multivariate random-effects approach: for meta-analysis of cancer staging studies. Acad Radiol. 2007;14:974–984. doi: 10.1016/j.acra.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PMM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/S0895-4356(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed AM, Amin SK, El-Shinnawy MA, Elfouly A, Baki AH. Role of CT enterography in assessment of Crohn’s disease activity: correlation with histopathologic diagnosis. Egypt J Radiol Nucl Med. 2012;43:353–359. doi: 10.1016/j.ejrnm.2012.05.005. [DOI] [Google Scholar]
- 17.Mao R, Gao X, Zhu ZH, et al. CT enterography in evaluating postoperative recurrence of Crohn’s disease after ileocolic resection: complementary role to endoscopy. Inflamm Bowel Dis. 2013;19:977–982. doi: 10.1097/MIB.0b013e318280758c. [DOI] [PubMed] [Google Scholar]
- 18.Kolkman JJ, Falke TH, Roos JC, et al. Computed tomography and granulocyte scintigraphy in active inflammatory bowel disease. Comparison with endoscopy and operative findings. Dig Dis Sci. 1996;41:641–650. doi: 10.1007/BF02213118. [DOI] [PubMed] [Google Scholar]
- 19.Shoenut JP, Semelka RC, Silverman R, Yaffe CS, Micflikier AB. Magnetic resonance imaging in inflammatory bowel disease. J Clin Gastroenterol. 1993;17:73–78. doi: 10.1097/00004836-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Shoenut JP, Semelka RC, Magro CM, Silverman R, Yaffe CS, Micflikier AB. Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. J Clin Gastroenterol. 1994;19:31–35. doi: 10.1097/00004836-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Florie J, Horsthuis K, Hommes DW, et al. Magnetic resonance imaging compared with ileocolonoscopy in evaluating disease severity in Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:1221–1228. doi: 10.1016/S1542-3565(05)00853-0. [DOI] [PubMed] [Google Scholar]
- 22.Schreyer AG, Golder S, Scheibl K, et al. Dark lumen magnetic resonance enteroclysis in combination with MRI colonography for whole bowel assessment in patients with Crohn’s disease: first clinical experience. Inflamm Bowel Dis. 2005;11:388–394. doi: 10.1097/01.MIB.0000164022.72729.06. [DOI] [PubMed] [Google Scholar]
- 23.Schreyer AG, Rath HC, Kikinis R, et al. Comparison of magnetic resonance imaging colonography with conventional colonoscopy for the assessment of intestinal inflammation in patients with inflammatory bowel disease: a feasibility study. Gut. 2005;54:250–256. doi: 10.1136/gut.2003.037390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gemert-Horsthuis K, Florie J, Hommes DW, et al. Feasibility of evaluating Crohn’s disease activity at 3.0 Tesla. J Magn Reson Imaging. 2006;24:340–348. doi: 10.1002/jmri.20650. [DOI] [PubMed] [Google Scholar]
- 25.Girometti R, Zuiani C, Toso F, et al. MRI scoring system including dynamic motility evaluation in assessing the activity of Crohn’s disease of the terminal ileum. Acad Radiol. 2008;15:153–164. doi: 10.1016/j.acra.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Horsthuis K, de Ridder L, Smets AM, et al. Magnetic resonance enterography for suspected inflammatory bowel disease in a pediatric population. J Pediatr Gastroenterol Nutr. 2010;51:603–609. doi: 10.1097/MPG.0b013e3181dee5bd. [DOI] [PubMed] [Google Scholar]
- 27.Koilakou S, Sailer J, Peloschek P, et al. Endoscopy and MR enteroclysis: equivalent tools in predicting clinical recurrence in patients with Crohn’s disease after ileocolic resection. Inflamm Bowel Dis. 2010;16:198–203. doi: 10.1002/ibd.21003. [DOI] [PubMed] [Google Scholar]
- 28.Gallego JC, Echarri AI, Porta A, Ollero V. Ileal Crohn’s disease: MRI with endoscopic correlation. Eur J Radiol. 2011;80:e8–e12. doi: 10.1016/j.ejrad.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Schill G, Iesalnieks I, Haimerl M, et al. Assessment of disease behavior in patients with Crohn’s disease by MR enterography. Inflamm Bowel Dis. 2013;19:983–990. doi: 10.1097/MIB.0b013e31828029dd. [DOI] [PubMed] [Google Scholar]
- 30.Bozkurt T, Rommel T, Stabenow-Lohbauer U, Langer M, Schmiegelow P, Lux G. Sonographic bowel wall morphology correlates with clinical and endoscopic activity in Crohn’s disease and ulcerative colitis. Eur J Ultrasound. 1996;4:27–33. doi: 10.1016/0929-8266(95)00169-7. [DOI] [Google Scholar]
- 31.Neye H, Voderholzer W, Rickes S, Weber J, Wermke W, Lochs H. Evaluation of criteria for the activity of Crohn’s disease by power Doppler sonography. Dig Dis. 2004;22:67–72. doi: 10.1159/000078737. [DOI] [PubMed] [Google Scholar]
- 32.Drews BH, Barth TF, Hanle MM, et al. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn’s disease. Eur Radiol. 2009;19:1379–1386. doi: 10.1007/s00330-008-1290-5. [DOI] [PubMed] [Google Scholar]
- 33.Sciarretta G, Furno A, Mazzoni M, Basile C, Malaguti P. Technetium-99 m hexamethyl propylene amine oxime granulocyte scintigraphy in Crohn’s disease: diagnostic and clinical relevance. Gut. 1993;34:1364–1369. doi: 10.1136/gut.34.10.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biancone L, Scopinaro F, Ierardi M, et al. 99mTc-HMPAO granulocyte scintigraphy in the early detection of postoperative asymptomatic recurrence in Crohn’s disease. Dig Dis Sci. 1997;42:1549–1556. doi: 10.1023/A:1018843516651. [DOI] [PubMed] [Google Scholar]
- 35.Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Masselli G, Gualdi G. CT and MR enterography in evaluating small bowel diseases: when to use which modality? Abdom Imaging. 2013;38:249–259. doi: 10.1007/s00261-012-9961-8. [DOI] [PubMed] [Google Scholar]
- 37.Grand DJ, Harris A, Loftus EV., Jr Imaging for luminal disease and complications: CT enterography, MR enterography, small-bowel follow-through, and ultrasound. Gastroenterol Clin N Am. 2012;41:497–512. doi: 10.1016/j.gtc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113–121. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 39.Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–761. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 40.Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124–129. doi: 10.1016/j.cgh.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MD, Kjeldsen J, Rafaelsen SR, Nathan T. Diagnostic accuracies of MR enterography and CT enterography in symptomatic Crohn’s disease. Scand J Gastroenterol. 2011;46:1449–1457. doi: 10.3109/00365521.2011.613947. [DOI] [PubMed] [Google Scholar]
- 42.Rimola J, Rodriguez S, Garcia-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–1120. doi: 10.1136/gut.2008.167957. [DOI] [PubMed] [Google Scholar]
- 43.Vermeire S, Ferrante M, Rutgeerts P. Recent advances: personalised use of current Crohn’s disease therapeutic options. Gut. 2013;62:1511–1515. doi: 10.1136/gutjnl-2012-303958. [DOI] [PubMed] [Google Scholar]
- 44.Peloquin JM, Pardi DS, Sandborn WJ, et al. Diagnostic ionizing radiation exposure in a population-based cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:2015–2022. doi: 10.1111/j.1572-0241.2008.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81:2080–2088. doi: 10.1016/j.ejrad.2011.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 105 kb)