Recently, successful cancer immunotherapy has aroused great interest in application of this approach in hematological malignancies. Immune responsiveness is a key clinical feature of hematological malignancies. Indeed, the efficacy of allogeneic hematopoietic stem cell transplantation (HSCT) largely derives from graft-versus-tumor effects that highlight the ability of the immune system to specifically and effectively eliminate tumors [1]. Here, we describe the status of cellular immunotherapy in hematological malignancies and discuss its future perspectives.
Dendritic cell (DC)-based cancer immunotherapy
DCs are professional antigen-presenting cells that serve as an essential link between innate and adaptive immune systems. For successful clinical application, factors such as choice of antigen source, DC vaccine formulation, delivery system, adjuvants, immunomodulation, and treatment schedule should be considered. Ex vivo-generated DCs should be optimally mature, migrate towards secondary lymphoid organs, and produce Th1-polarizing cytokines. Our group has previously developed potent DCs using toll-like receptors (TLRs) in combination with type I/II interferons or other proinflammatory cytokines, natural products, and natural killer (NK) cells. The ideal tumor antigens should be easily available, target specific and should enhance cross-presentation and minimize immune suppression. We tried to develop several kinds of tumor antigens using alternative sources, by ameliorating immunogenic cell death and enhancing immunogenicity using chemicals or nanoparticles. Our ongoing strategies involve development of tumor antigens as recombinant protein formulations to enhance cross presentation in DCs [2,3]. Recent clinical trials using DC vaccines are summarized in Table 1. In acute myeloid leukemia (AML), we applied clinical vaccination using monocyte-derived DCs pulsed with leukemic lysates to treat relapsing AML after autologous HSCT as a pilot trial [4]. Recent studies using DCs with autologous apoptotic leukemic cells or with WT1 peptide induced meaningful clinical responses in elderly patients with AML. Follicular and indolent B cell lymphomas were also successfully treated with DCs loaded with idiotype or autologous tumor cells, respectively. In multiple myeloma (MM), successful clinical trials were conducted using DCs loaded with idiotype, fused with tumor cells, pulsed with multi-peptides, and treated with α-galactosylceramide. Recently, we completed a phase I/IIa trial using potent DCs loaded with dying tumor cells against relapsed/refractory MM with immunological response induced in the most patients and some clinical response (unpublished data). More recently, we demonstrated a synergistic effect of DCs combined with lenalidomide in a murine myeloma model [5]. In the future, immunomodulatory drugs (IMiDs) and emerging immune checkpoint blockades for CTLA-4, PD-1, and PD-L1 can be good options to potentiate DC treatment efficacy in clinics.
Table 1. Recent clinical trials using cellular immunotherapy for hematological malignancies.
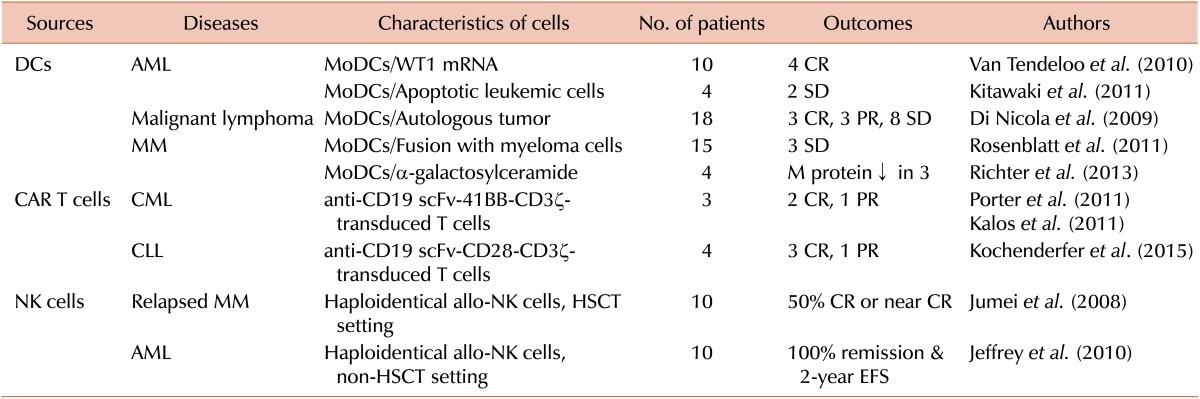
Abbreviations: DCs, dendritic cells; CAR, chimeric antigen receptor; NK, natural killer; AML, acute myeloid lymphoma; MM, multiple myeloma; CML, chronic myeloid leukemia; CLL, chronic lymphoid leukemia; MoDCs, monocyte-derived dendritic cells; WT1, Wilms tumor 1; CR, complete remission; PR, partial remission; SD, stable disease; allo, allogeneic; EFS, event-free survival; HSCT, hematopoietic stem cell transplantation.
Adoptive immunotherapy using genetically modified T cells bearing chimeric antigen receptor (CAR)
The key component of adoptive T cell therapy is generation of functional tumor-specific T cells without immune tolerance to self-antigens. This can be accomplished by genetic modification of patient T cells using genes encoding CARs. Recently, the third-generation of CAR T cells has been developed, which includes an additional co-stimulatory domain, such as CD28 plus 4-1BB or CD28 plus OX40 together with CD3zeta along with the specific target. Results from several clinical trials in different settings including CAR design, culture techniques, lymphodepleting strategies, and target diseases, have provided valuable insights, and CAR T cells are emerging as a powerful therapy in hematologic malignancies [6]. Most clinical successes of CAR T cell therapy are recorded in the setting of B-cell malignancies, by targeting CD19 (Table 1). Several B cell antigens, such as CD20, CD22, CD23, and CD38 are under evaluation as alternative CAR T cell targets in B-cell malignancies. However, targets that are expressed on majority of tumor cells but absent from normal cells and are thus more selective, should be developed to avoid on-target toxicity to normal cells following adoptive transfer of CAR T cells in patients. In MM, several CARs targeting BCMA, CD38, CD138, or CS-1 have shown preclinical efficacy. Currently, two clinical trials in Hodgkin's lymphoma are evaluating efficacy of CD30-directed CAR T cell therapy. Preclinical studies using anti-CD123 CAR and anti-CD44v6 CAR are under evaluation in AML with promising results. However, neither of these antigens is truly tumor-specific and significant hematopoietic or epithelial toxicity may be expected. "Bio-degradable" CAR T cells and other options regarding suicide mechanisms can be used to extinguish unwanted CAR T cell activity. Careful analysis of early-phase clinical trials using anti-CD19 targeting in B-cell malignancies has uncovered information regarding toxicity and continues to inform the next generation of CAR T cell trials. These observations will prove useful in extending this modality to other hematologic malignancies and solid tumors in the near future [7].
NK cell-based immunotherapy
NK cells are killer cells that have immune regulatory action against infected or malignant cells without prior sensitization. It is well known that NK cells can be long-lived, remember past exposures, and interact with MHC class I molecules to acquire full function. NK cell function is tightly regulated by signals from natural cytotoxicity receptors, CD16 receptor for antibody-dependent cellular cytotoxicity (ADCC), C-type lectins, and killer cell immunoglobulin-like receptors (KIR) [8]. The first NK cell immunotherapy in humans was performed with administration of IL-2 or adoptive transfer of IL-2-stimulated ex vivo activated autologous NK cells in the late 1990s. However, lymphokine-activated killer (LAK) cell therapy was discarded because of the significant toxicity and limited therapeutic efficacy. However, several groups have gradually overcome this in recent years. In an HSCT setting with T cell depletion, the Velardi group showed that haploidentical KIR-ligand mismatch NK cells were associated with remarkably improved outcomes in AML. In an autologous HSCT setting, the Rhee group showed that infusion of haploidentical KIR-ligand mismatched NK cells for relapsed MM resulted in complete remission in near 50% cases. In a non-HSCT setting, the Miller group first showed that haploidentical donor-related NK cell infusions expanded in vivo were safe and effective in patients with refractory AML. However, remission was not durable and the patients ultimately relapsed. The Rubnitz group also showed that low-dose immunosuppression followed by administration of donor-recipient inhibitory KIR-HLA mismatched NK cells is well tolerated in childhood AML and results in successful engraftment (Table 1) [9]. Recently, there have been advances in ex vivo techniques of NK cell activation and expansion to get adequate numbers of NK cells. Combination strategies with therapeutic monoclonal antibodies, radiation therapy, and IMiDs will improve NK cell-mediated cytotoxicity. The use of genetically modified NK cells or CAR-NK cell lines is also useful to enhance and redirect NK cell activity [10].
Future perspectives
Cancer immunotherapy using DCs, CAR T cells, and NK cells may be used in combination with other therapies including chemotherapy, radiation therapy, molecular gene targeting, adjuvants, or immune modulators such as checkpoint blockades and IMiDs, for changing the immunosuppressive nature of a tumor towards an immunitysupporting microenvironment. This will have high impact on enhancing therapeutic immunity in hematological malignancies by simultaneously enhancing potency of immune responses and offsetting immunosuppressive pathways.
Footnotes
This study was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science, and Technology (MEST) (2011-0030034), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI14C1898).
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15:201–215. doi: 10.1038/nrc3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoang MD, Jung SH, Lee HJ, et al. Dendritic cell-based cancer immunotherapy against multiple myeloma: from bench to clinic. Chonnam Med J. 2015;51:1–7. doi: 10.4068/cmj.2015.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen-Pham TN, Lee YK, Lee HJ, et al. Cellular immunotherapy using dendritic cells against multiple myeloma. Korean J Hematol. 2012;47:17–27. doi: 10.5045/kjh.2012.47.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JJ, Kook H, Park MS, et al. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J Clin Apher. 2004;19:66–70. doi: 10.1002/jca.10080. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen-Pham TN, Jung SH, Vo MC, et al. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine multiple myeloma. J Immunother. 2015;38:330–339. doi: 10.1097/CJI.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–2635. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho D, Kim SK, Carson WE., 3rd NK cell-based immunotherapy for treating cancer: will it be promising? Korean J Hematol. 2011;46:3–5. doi: 10.5045/kjh.2011.46.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chouaib S, Pittari G, Nanbakhsh A, et al. Improving the outcome of leukemia by natural killer cell-based immunotherapeutic strategies. Front Immunol. 2014;5:95. doi: 10.3389/fimmu.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]


