Abstract
Background
The outcome of hematopoietic stem cell transplantation (HSCT) is poor in patients with secondary iron overload (SIO). We evaluated the relationship between SIO and veno-occlusive disease (VOD) in an animal model with radiation for HSCT.
Methods
We used a 6-week-old female BDF1 (H-2b/d) and a male C57/BL6 (H-2b) as recipient and donor, respectively. Recipient mice were injected intraperitoneally with 10 mg of iron dextran (cumulative doses of 50 mg, 100 mg, and 200 mg). All mice received total body irradiation for HSCT. We obtained peripheral blood for alanine transaminase (ALT) and liver for pathologic findings, lipid hyperoxide (LH) as reactive oxygen species (ROS), and liver iron content (LIC) on post-HSCT day 1 and day 7. The VOD score was assessed by pathologic findings.
Results
ALT levels increased depending on cumulative iron dose, with significant differences between days 1 and 7 for mice loaded with 200 mg of iron (P<0.01). LH levels significantly increased in mice loaded with 200 mg of iron compared to those in other groups (P<0.01). For mice loaded with 100 mg of iron, the LH level depended on the radiation dose (P<0.01). There was a statistically significant relationship among ALT, LH, and LIC parameters (P<0.05). Pathologic scores for VOD correlated with LIC (P<0.01).
Conclusion
Livers with SIO showed high ROS levels depending on cumulative iron dose, and correlations with elevated liver enzyme and LIC. The pathologic score for VOD was associated with the LIC. Our results suggest that SIO may induce VOD after HSCT with irradiation.
Keywords: Iron overload, Hepatic veno-occlusive disease, Radiation, Reactive oxygen species
INTRODUCTION
Patients with hematologic malignancies or hemoglobinopathies may receive multiple red blood cell transfusions during treatment. As a result, these patients may have secondary iron overload (SIO) [1]. SIO leads to high infection rates, and increased morbidity from hepatic, cardiac, or endocrinologic diseases [2]. Patients with transfusion dependency should be monitored for SIO status using markers including liver biopsy findings, MRI findings, or serum ferritin level [1,3,4].
Hematopoietic stem cell transplantation (HSCT) is a curative treatment for hematologic malignancies or hemoglobinopathies. In patients with transfusion dependency, the mortality and morbidity after HSCT were related to the iron burden [5]. HSCT in patients with SIO resulted in high infection rates, acute graft-versus-host disease (GVHD), and hepatic veno-occlusive disease (VOD) in the early post-trans plant period, and high infection, cardiac fibrosis, and abnormal liver function in the late post-transplant period [6,7,8,9]. High level of serum ferritin as a surrogate marker of SIO was related to risk of acute GVHD and VOD [7,10]. VOD is one of the life threatening complications after HSCT [10,11]. VOD develops in about 11-31% in HSCT recipients, with a mortality rate up to 50% [11,12,13]. Hepatic VOD after HSCT is associated with hepatotoxic conditioning regimen and pretransplant liver damage [11,12,14].
Iron accumulation in the liver results in the generation of reactive oxygen species (ROS) via the Fenton and Haber-Weiss reaction, and oxidant damage of cellular structures and molecules. In the animal model, livers containing excessive iron stimulated hepatic stellate cells to produce collagen protein, and subsequently induced hepatic fibrosis in the periportal area [14,15]. In HSCT, radiation, as part of the conditioning regimen, injured the sinusoidal and central vein endothelium, and initiated activation of the coagulation cascade [16]. This pro-coagulant status induced fibrin deposit and clot formation, and eventually occluded the vessel [8,16]. Hence, both hepatic iron excess and pre-transplant irradiation might be associated with the development of post-transplant VOD, possibly in an additive manner. We evaluated the relationship between an iron excessive state and the development of VOD in a SIO animal model treated with irradiation-based HSCT.
MATERIALS AND METHODS
Animals
We used 6-week-old female BDF1 (H-2b/d) mice as recipients, and C57B/6 (H-2b) mice as donor for HSCT. All mice were acclimatized for 1 week before commencing the experiments, and were housed in temperature- and humidity-controlled rooms with light-dark cycles. All mice were given irradiated food and sterile water ad libitum. The Institutional Animal Care and Use Committee, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea approved these experiments. All mice were purchased from OrientBio (Gapyung, Gyeonggi do, Korea).
Establishment of secondary iron overload
We used the SIO animal model, as previously described [17]. Recipient mice were injected intraperitoneally with 300 µL (10 mg) of iron dextran (Sigma-Aldrich, St. Louis, MO) in PBS (phosphate buffer solution) for five consecutive days per week according to the cumulative iron dose. The experimental design was for cumulative iron dose of 50 mg, 100 mg, and 200 mg, respectively. The placebo control group received the same volume of PBS. The mice were observed for 2 weeks in order to monitor their viability after iron loading until the next experiment. All experimental groups used at least six mice according to study design.
HSCT
All recipient mice received total body irradiation using the linear accelerator (Linac, Varion, CA) with 75 cGy/min of dose rate according to different doses. Mice were transplanted with donor bone marrow mononuclear cells and splenocytes, 1×107 and 5×106 respectively, per mouse, by tail vein within 4 hours after irradiation. Under 1,100 cGy with the same dose rate, mice were transplanted with bone marrow and splenocytes, according to the cumulative iron dose (N=6). Mice with 100 mg iron overload received 500 cGy, 700 cGy, 900 cGy, and 1,100 cGy under the same dose rate (N=6). HSCT was done as both syngeneic and allogeneic type (N=6). We assessed the mice daily for survival and clinical manifestations of graft-versus-host disease.
Blood sampling and analysis
Murine peripheral blood was collected from the retro-orbital sinus using blood collection capillary tubes under anesthesia. Blood sampling was done at post-HSCT day 1 and 7. The collected peripheral blood was transferred to 1.5-mL Eppendorf tubes and centrifuged. The obtained plasma was stored in a -80℃ freezer until the measurement of alanine transaminase (ALT) and creatinine (Cr). An automatic biochemical analyzer (Hitachi 7600-110, Hitachi, Tokyo, Japan) measured plasma ALT (IU/L) and Cr (mg/dL).
Liver iron content (LIC) and lipid hyperoxide (LH)
We obtained liver specimens just before death at around post-HSCT day 10. The hepatic iron concentration (mg/dry 1 g of liver) was analyzed by atomic absorbance spectrophotometer. We assessed lipid hyperoxide (LH) as a surrogate marker of reactive oxygen species (ROS) in fresh liver by the BCA protein assay kit (Thermoscientific Co., Rockford, IL). The analysis of LH was performed as follows. The liver (0.05-0.1 g) was homogenized with 1 mL of sucrose buffer composed of 210 mM mannitol, 70 mM sucrose, 1 mM EDTA, and 10 mM Hepes solution. Lysate from the liver was quantified for protein. Lysate (100 µg) was added with 100 µL of distilled water. We prepared standard H2O2 according to dilution, from 100 µM to 0 µM. The sample was mixed with standard H2O2 and subsequently added to a FOX solution, which included 1 mM xylenol orange, 25 mM ferrous ammonium sulfate, 1 mM sorbitol, and 0.25 M H2SO4. The sample (200 µL) was incubated at room temperature for 30 minutes and analyzed by spectrophotometer under 560 nm.
Pathology of the liver
The liver was fixed in 10% formalin and stained by the hematoxylin-eosin and trichrome staining method. We evaluated the pathologic score using eight parameters including endothelial damage of central venule (CV), coagulation necrosis of hepatocytes, subendothelial hemorrhage, sinusoidal hemorrhage, subendothelial fibrosis of CV, adventitial fibrosis of CV, inflammation of the CV, and lobular inflammation [18]. Each parameter was graded on a 4-point system.
Statistical analysis
We analyzed the values of the LH, cumulative iron amount, and ALT according to the radiation dose, one-way ANOVA, and experimental design by repeated measures ANOVA. We identified correlations among the ALT, LH, LIC, and the pathologic score, respectively. The statistical significance was set at a P-value below 0.05.
RESULTS
Elevation of liver enzyme in mice with different cumulative iron doses after allogeneic HSCT with irradiation
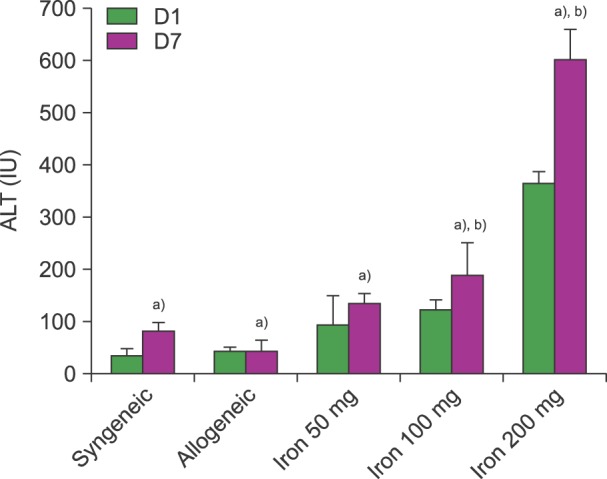
The ALT was not significantly different between post-HSCT days 1 and 7 in the allogeneic group without iron and the syngeneic group. The level of ALT at post-HSCT days 1 and 7 in mice with iron were dependent on cumulative iron dose after 1,100 cGy irradiation (P<0.01). The level of ALT in mice that have been loaded with 200 mg of iron was highest among the experimental groups (P<0.01). Although there was a trend towards higher ALT levels on post-HSCT day 7 than on post-HSCT day 1 in the other iron loaded groups (P>0.05), the ALT on post-HSCT day 7 was higher than that on day 1 (P<0.05) in mice with 200 mg of iron (Fig. 1). The creatinine levels in all experimental groups were within normal limits.
Fig. 1. A comparison of the levels of alanine transaminase (ALT) at post-HSCT days 1 and 7. ALT (IU/L) increased according to the cumulative iron dose. The ALT in mice that have been loaded with 200 mg of iron was highest among all the other groups at post-HSCT day 7 (P<0.01). All mice received 1,100 cGy (N=6). a)P<0.01 in t-test, b)P<0.01 in ANOVA.
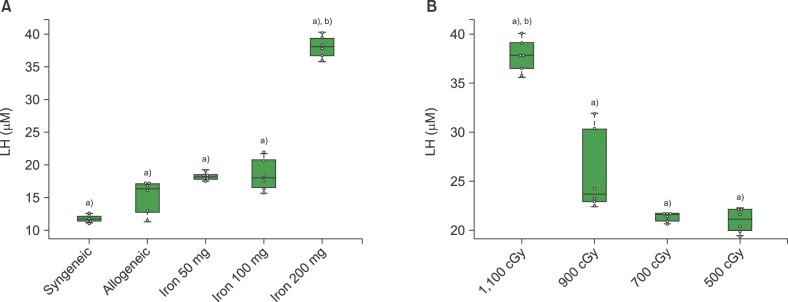
Elevation of lipid hyperoxide levels in mice with different cumulative iron and radiation doses
We obtained liver tissue in order to check the LH levels at post-HSCT day 7. In mice that received 1,100 cGy, the LH levels in iron-loaded mice were higher than in those without iron, including syngeneic transplantation (P<0.05). LH levels were highest among mice that have been loaded with 200 mg of iron (P<0.01) (Fig. 2A). For analysis of the radiation effects, we evaluated LH levels in the liver in mice with 100 mg of iron, according to the radiation dose. The LH levels in mice that received 1,100 cGy were highest among the radiation dose groups (P<0.01). There were rapid increments of LH levels in mice that received radiation doses of over 900 cGy compared to those that received less than 700 cGy (P<0.01). LH levels were not significantly different between iron-loaded mice, which received doses of 700 and 500 cGy, respectively (P>0.05) (Fig. 2B).
Fig. 2. The lipid hyperoxide (LH) levels according to the cumulative iron dose (A) and radiation dose (B). (A) LH (µM) was highest in the mice that received 200 mg of iron at post-HSCT day 7 (P=0.01). All mice (N=6) treated with allogeneic HSCT received 1,100 cGy. (B) LH was highest in the recipients of 1,100 cGy (P<0.01) of radiation. All mice (N=6) treated with allogeneic HSCT had cumulative iron levels of 100 mg. Beeswarm boxplots show the median values and the quartiles, and single measurements are shown as open circles. The middle bar in the box denotes mean value. a)P<0.01 in t-test, b)P<0.01 in ANOVA.
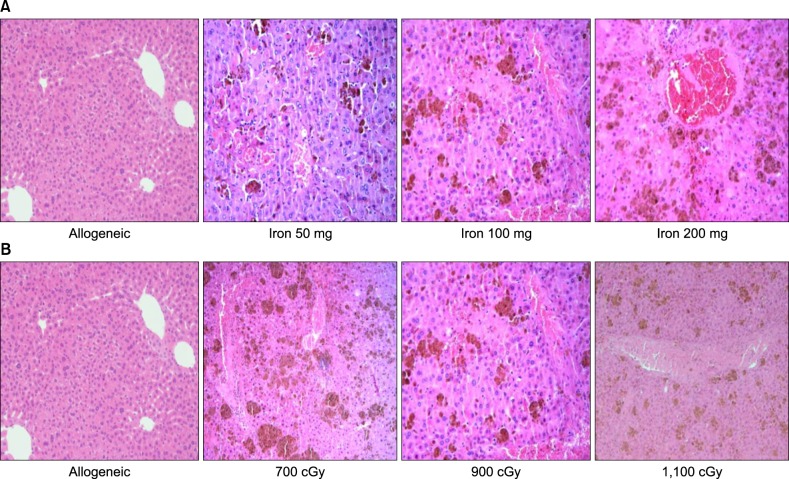
Pathologic findings and scores in the liver
There was sinusoidal hemorrhage and endothelial damage in all mice with iron load (Fig. 3). These pathologic findings in mice were of greater severity and showed a higher score according to a greater cumulative iron dose (Fig. 3A). However, pathologic findings and scores were not significantly different in mice that have been loaded with 100 mg of iron with different irradiation doses (data was not shown) (Fig. 3B). These results suggest that the severity of pathologic findings may be related to the iron-loading dose and not to the radiation dose.
Fig. 3. The pathologic findings in the liver (H-E stain; ×200). (A) The findings show the hepatic pathology according to cumulative iron dose. There is no evidence of veno-occlusion in allogeneic transplantation without iron load. However, there are iron deposits, sinusoidal hemorrhages, hepatic necrosis, and endothelial damage in the iron loaded group. (B) The hepatic findings in mice that received 100 mg of iron are shown according to radiation dose. Damage to hepatocytes and sinusoidal hemorrhages are not significantly different according to radiation dose.
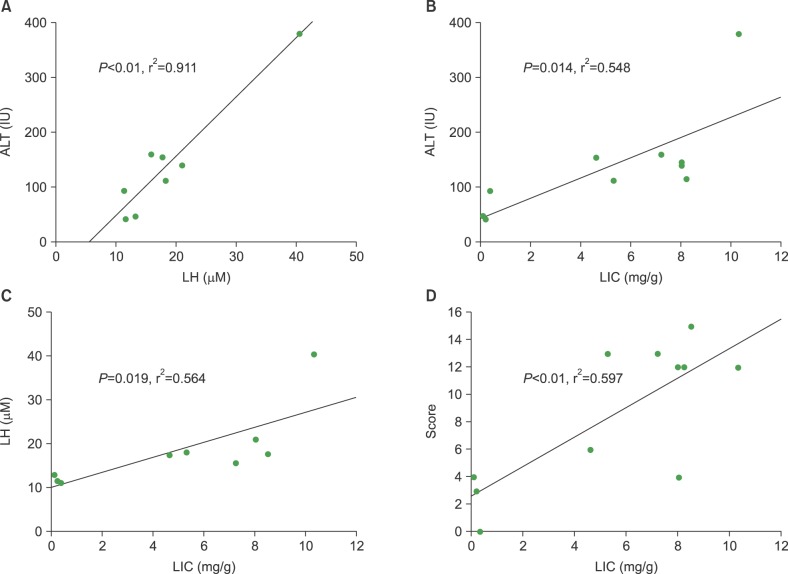
The correlation among lipid hyperoxide, liver enzyme, liver iron content, and pathologic scores
We analyzed correlation between the laboratory and pathologic findings for risk of VOD in iron-loaded mice that received allogeneic HSCT. The levels of ALT were correlated with LH (Fig. 4A, P<0.01, r2=0.911), and LIC (Fig. 4B, P=0.014, r2=0.548). The LH levels were significantly correlated with the LIC levels (Fig. 4C, P=0.019, r2=0.564). We evaluated the relationship among the pathologic findings and other parameters. Significant correlation was only found between the pathologic scores and LIC (Fig. 4D, P<0.01, r2=0.597). The other correlations were not significant.
Fig. 4. A comparison of the individual correlations between lipid hyperoxide (LH), alanine transaminase (ALT), liver iron content (LIC) levels, and pathologic score, respectively. (A) Correlation between LH and ALT levels (P<0.01, r2=0.911). (B) Correlation between ALT and LIC (mg/gm) (P=0.014, r2=0.548). (C) Correlation between LIC and LH (P=0.019, r2=0.564), and (D) pathologic score (P<0.01, r2=0.0597), respectively.
DISCUSSION
VOD may be an obstacle to successful outcomes in patients who receive HSCT [11]. Many patients who have been treated with HSCT received multiple transfusions before transplantation [4]. SIO due to repeated transfusions might lead to several problems including infection, acute GVHD, and hepatotoxicity during and after HSCT [7,8]. The hepatotoxic effects of HSCT are associated with the use of radiation as part of the conditioning regimen, and may worsen with an abnormal liver state, including iron overload before transplant [7,8,10,16]. In our study, the development of pathology was closely related to LIC and radiation. The excessive hepatic iron induced high levels of LH as a ROS and the elevation of ALT levels. LH increased according to radiation dose. Higher amounts of ROS due to iron overload and radiation may induce hepatic inflammation, and the development of VOD.
In our results, the ALT levels in SIO mice were higher than those in non-iron loaded mice (syngeneic and allogeneic), and increased according to a cumulative iron dose. The SIO contributed to liver damage through ROS. Mice with chronic dietary iron overload showed increased ALT and hepatocyte apoptosis [19]. Excess intracellular iron leads to oxidative injury to the liver by ROS [1,15,20]. In this study, the level of ALT after radiation increased according to cumulative iron dose, although ALT showed no significant differences between recipients of 50 mg iron and non-iron loaded mice. Radiation induces hepatic damage and serious injury to hepatic sinusoidal and central vein endothelium [16,21]. The ALT levels in mice treated with HSCT and radiation have been reported to be higher than normal, with high levels persisting until 30 days post-transplant [22]. Therefore, the ALT levels in SIO increased according to the cumulative iron dose due to oxidative damage by radiation and cumulative iron.
We evaluated the change of LH as a surrogate marker for ROS according to doses of cumulative iron and radiation. In this study, the level of LH in recipients of 200 mg iron and 1,100 cGy was highest among the groups. The level of LH was similar in the allogeneic HSCT group with different cumulative iron dose under the same radiation exposure, except for recipients of 200 mg iron. There were no significant differences between the syngeneic and allogeneic groups. These results may represent high ROS production due to the accumulation of iron and exposure to high doses of radiation [2,5,16]. The ROS play a role in hepatic damage by oxidative stress through iron overload and radiation [2,15,16,19,20]. Ionizing radiation leads to the generation of ROS and free radicals, and results in oxidative injury to normal tissue [23].
The pathologic scores for VOD, including sinusoidal hemorrhage and endothelial damage, were higher in SIO mice than in non-iron loaded mice (Fig. 3). Pathologic findings were particularly significantly related to the cumulative iron dose regardless of radiation dose. This result may be very important for hepatic damage in SIO mice that has been treated with HSCT. Our data suggested that radiation played a role in the production of ROS. However, pathologic findings suggested that cumulative iron dose mainly contributed to liver damage in SIO with HSCT. Higher pretransplant serum ferritin levels as well as mortality rates during transplant in HSCT patients have been reported with VOD than without VOD [10]. Jastanish et al suggested that VOD after HSCT was associated with liver inflammation and elevated LIC [5]. Pre-transplant risk factors for developing VOD included elevations of the liver enzyme and HLA disparity including performance status, more intensive alkylator use, and prior abdominal radiation [12]. Iron chelation therapy has been previously recommended for successful outcomes in HSCT [7,8,10,24]. A prospective cohort study reported that iron overload was not associated with HSCT outcome including VOD and mortality. However, this data has limitations in the evaluation of iron content because of the use of R2-MRI for measurement of liver iron content [25].
We investigated for common factors between VOD and SIO. Correlations were found among ALT, LH, LIC, and the pathologic score, respectively. The LIC showed correlations with ALT, LH, and the pathologic score, respectively. The pathologic score may represent the severity of hepatic VOD [18,26]. These results suggest that the level of LIC may be strongly associated with severity of hepatic VOD in HSCT, including radiation. Hepatic iron overload induced liver damage by ROS [1,2,9,19,23], and radiation in HSCT contributed to hepatic injury caused by ROS and free radicals [16,21,23]. In HSCT, iron accumulation in the liver plays a major role in the development of hepatic VOD, while radiation has also been associated with VOD [6,7,8]. Our study has some limitations in terms of the evaluation for long-term biological effects between iron overload and hepatic injury in SIO mice after allogeneic HSCT. As previously mentioned, we need to study the relationship between iron overload and graft-versus-host disease, and whether iron overload induces inflammation and aggravates GVHD in allogeneic HSCT [6,7].
In this study, SIO in the liver induced ROS production, according to the cumulative iron dose. ROS also showed correlations with elevated liver enzyme and LIC. The pathologic findings of hepatic VOD were strongly associated with LIC. We suggest that SIO may induce hepatic VOD after HSCT with irradiation, and iron chelation therapy should be considered for improvement of HSCT outcomes in patients with SIO due to prior multiple transfusions [8].
ACKNOWLEDGMENTS
The authors are grateful to Hee Chul Lee and Eun Sun Kang, Department of Clinical Research Laboratory, Incheon St. Mary's Hospital, Incheon, South Korea, for technical their support and assistance.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009;97:185–197. doi: 10.1111/j.1423-0410.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 2.Porter JB, Garbowski M. The pathophysiology of transfusional iron overload. Hematol Oncol Clin North Am. 2014;28:683–701. doi: 10.1016/j.hoc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Shander A, Sazama K. Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion. 2010;50:1144–1155. doi: 10.1111/j.1537-2995.2009.02551.x. [DOI] [PubMed] [Google Scholar]
- 4.Jensen PD. Evaluation of iron overload. Br J Haematol. 2004;124:697–711. doi: 10.1111/j.1365-2141.2004.04838.x. [DOI] [PubMed] [Google Scholar]
- 5.Jastaniah W, Harmatz P, Pakbaz Z, Fischer R, Vichinsky E, Walters MC. Transfusional iron burden and liver toxicity after bone marrow transplantation for acute myelogenous leukemia and hemoglobinopathies. Pediatr Blood Cancer. 2008;50:319–324. doi: 10.1002/pbc.21260. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ, Spaulding E, Shulman HM. Iron overload, hematopoietic cell transplantation, and graft-versus-host disease. Leuk Lymphoma. 2009;50:1566–1572. doi: 10.1080/10428190903144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda J, Kawabata H, Chao NJ. Iron overload and allogeneic hematopoietic stem-cell transplantation. Expert Rev Hematol. 2011;4:71–80. doi: 10.1586/ehm.10.81. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008;41:997–1003. doi: 10.1038/bmt.2008.99. [DOI] [PubMed] [Google Scholar]
- 9.Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease) J Clin Exp Hepatol. 2014;4:332–346. doi: 10.1016/j.jceh.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SH, Yoo KH, Sung KW, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant. 2010;45:1287–1293. doi: 10.1038/bmt.2009.349. [DOI] [PubMed] [Google Scholar]
- 11.Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–168. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10:451–462. doi: 10.1097/00062752-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 13.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther. 2006;23:11–25. doi: 10.1111/j.1365-2036.2006.02742.x. [DOI] [PubMed] [Google Scholar]
- 15.Ramm GA, Ruddell RG. Hepatotoxicity of iron overload: mechanisms of iron-induced hepatic fibrogenesis. Semin Liver Dis. 2005;25:433–449. doi: 10.1055/s-2005-923315. [DOI] [PubMed] [Google Scholar]
- 16.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21:256–263. doi: 10.1016/j.semradonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon SN, Han JW, Hwang HS, et al. Establishment of secondary iron overloaded mouse model: evaluation of cardiac function and analysis according to iron concentration. Pediatr Cardiol. 2011;32:947–952. doi: 10.1007/s00246-011-0019-4. [DOI] [PubMed] [Google Scholar]
- 18.DeLeve LD, McCuskey RS, Wang X, et al. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999;29:1779–1791. doi: 10.1002/hep.510290615. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, He H, Yin D, et al. Mechanism of chronic dietary iron overload-induced liver damage in mice. Mol Med Rep. 2013;7:1173–1179. doi: 10.3892/mmr.2013.1316. [DOI] [PubMed] [Google Scholar]
- 20.Fujita N, Miyachi H, Tanaka H, et al. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. 2009;18:424–432. doi: 10.1158/1055-9965.EPI-08-0725. [DOI] [PubMed] [Google Scholar]
- 21.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation Hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 22.Qi K, Li H, An L, et al. The correlation between platelet activation and liver injury by conditioning and bone marrow transplantation. Transplant Proc. 2014;46:1523–1530. doi: 10.1016/j.transproceed.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 24.McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–1460. doi: 10.1002/hep.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trottier BJ, Burns LJ, DeFor TE, Cooley S, Majhail NS. Association of iron overload with allogeneic hematopoietic cell transplantation outcomes: a prospective cohort study using R2-MRI-measured liver iron content. Blood. 2013;122:1678–1684. doi: 10.1182/blood-2013-04-499772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Huo JR, Yang L, Zhu HY. Effect of ligustrazine on mice model of hepatic veno-occlusive disease induced by Gynura segetum. J Gastroenterol Hepatol. 2011;26:1016–1021. doi: 10.1111/j.1440-1746.2011.06661.x. [DOI] [PubMed] [Google Scholar]