Abstract
Perceptual thresholds are known to vary across the foot sole, despite a reported even distribution in cutaneous afferents. Skin mechanical properties have been proposed to account for these differences; however, a direct relationship between foot sole afferent firing, perceptual threshold, and skin mechanical properties has not been previously investigated. Using the technique of microneurography, we recorded the monofilament firing thresholds of cutaneous afferents and associated perceptual thresholds across the foot sole. In addition, receptive field hardness measurements were taken to investigate the influence of skin hardness on these threshold measures. Afferents were identified as fast adapting [FAI (n = 48) or FAII (n = 13)] or slowly adapting [SAI (n = 21) or SAII (n = 20)], and were grouped based on receptive field location (heel, arch, metatarsals, toes). Overall, perceptual thresholds were found to most closely align with firing thresholds of FA afferents. In contrast, SAI and SAII afferent firing thresholds were found to be significantly higher than perceptual thresholds and are not thought to mediate monofilament perceptual threshold across the foot sole. Perceptual thresholds and FAI afferent firing thresholds were significantly lower in the arch compared with other regions, and skin hardness was found to positively correlate with both FAI and FAII afferent firing and perceptual thresholds. These data support a perceptual influence of skin hardness, which is likely the result of elevated FA afferent firing threshold at harder foot sole sites. The close coupling between FA afferent firing and perceptual threshold across foot sole indicates that small changes in FA afferent firing can influence perceptual thresholds.
Keywords: microneurography, perception
it is well established that cutaneous feedback from the soles of the feet is fundamental in the control of upright stance. Previous work has shown foot sole cutaneous feedback to play a role in standing balance (Roll et al. 2002), gait (Eils et al. 2004; Perry et al. 2001), automatic postural adjustments (Inglis et al. 1994; Perry et al. 2000), as well as in the modulation of lower (Fallon et al. 2005) and upper limb (Bent and Lowrey 2013) muscle activity and vestibular reflexes (Muise et al. 2012). What remains unclear is the capacity of individual types of foot sole cutaneous afferent classes to transmit distinct tactile cues to the central nervous system and what impact this feedback has on balance control.
Tactile sensibility from the glabrous skin of the foot sole and hand arises from four classes of low-threshold cutaneous mechanoreceptors located in the dermal and epidermal layers of the skin. Each class is sensitive to unique features of tactile stimuli and demonstrate distinctive firing characteristics in response to indentation forces, skin stretches, textures, and vibrations (Aimonetti et al. 2007; Johansson et al. 1982; Johnson and Hsiao 1992). Cutaneous afferent firing characteristics and receptive field (RF) properties establish the classification of each subtype as fast adapting (FA) or slowly adapting (SA), and type I (small, distinct borders) or type II (large, undefined borders). The development of microneurography by Vallbo and Hagbarth in the 1960s allowed for the direct comparison between primary afferent activity and perceptual experience (Hagbarth and Vallbo 1967). Pioneering work in the hand found light touch perceptual threshold to most closely resemble the firing thresholds of FA afferents (Johansson and Vallbo 1979). In the most sensitive hand regions (fingers and lateral border), a small amount of activity from FAI afferents, even single spikes, was capable of evoking a percept. Further support for a one-to-one relationship between afferent firing has been demonstrated through the electrical microstimulation of individual cutaneous afferents. Using this technique, researchers have demonstrated that specific tactile sensations can be evoked from the activity of single cutaneous afferents, e.g., flutter (FAI), vibration (FAII), and pressure (SAI) (Macefield et al. 1990; Ochoa and Torebjörk 1983). These findings are in line with the lower envelope principle in that perception can be set by minimal activity in the most sensitive afferents (Parker and Newsome 1998).
Previous work that has investigated tactile perception has focused almost exclusively on cutaneous feedback from the hand. The fingers have been shown to have lower perceptual thresholds compared with the palm, despite similar afferent firing thresholds (AFTs) between these regions (Johansson and Vallbo 1979). This led the authors to postulate that cutaneous feedback is not weighted equally across the body, and that central mechanisms may integrate input from the fingertips with more fidelity than the palm of the hand. The higher density of afferents in the fingertips may increase the probability of activating highly sensitive afferents leading to the disparity in perception between these regions. However, Johansson and Vallbo (1979) argued this was not the case since subsensory stimuli at the palm still evoked firing in cutaneous afferents. Their investigation suggests that perceptual threshold can be set by the firing capacity of the most sensitive primary cutaneous afferents in some regions (e.g., in the fingers) while additional factors may raise perceptual threshold in less sensitive skin regions (e.g., in the palm).
The soles of the feet are not as sensitive as the hands, where in the feet, both perceptual thresholds (Hennig and Sterzing 2009) and cutaneous AFTs (Kennedy and Inglis 2002) are reportedly higher. Perceptual threshold differences have been reported across the foot sole (Hennig and Sterzing 2009; Kekoni et al. 1989; Strzalkowski et al. 2015) while mechanoreceptor density is thought to be evenly distributed (Kennedy and Inglis 2002). A direct comparison between foot sole cutaneous afferent firing and perceptual sensitivity has not been made at the foot sole, and the neural mechanisms underlying regional differences in perceptual threshold are not well understood.
Mechanical properties of the skin have been shown to differ across the sole of the foot (Strzalkowski et al. 2015) and between the foot sole and hand (Hoffmann et al. 1994). The ability of skin to deform and transmit force will presumably impact afferent firing, and differences in skin properties have been proposed to account for disparities between cutaneous afferent firing and perceptual thresholds between these regions (Kekoni et al. 1989; Kennedy and Inglis 2002; Kowalzik et al. 1996). While an attempt has been made to link mechanical properties with afferent firing in the glabrous skin of raccoons (Pubols and Pubols 1983), and with perceptual threshold in the foot (Strzalkowski et al. 2015), the influence of skin mechanics on the actual firing of foot sole cutaneous afferents has not been investigated.
The aim of the present study was to investigate the relationship between tactile perceptual threshold and cutaneous AFTs across the human foot sole. Skin hardness within each afferent's RF was also investigated to better understand the potential influence of skin mechanics on afferent firing and perceptual threshold. In following with previous work in the hand, FA afferents were expected to be more sensitive to light touch (i.e., fire at lower forces) compared with SA afferents, and have firing thresholds most similar to perceptual thresholds across the foot sole. AFTs are expected to increase with skin hardness and, at least partially, account for perceptual threshold differences across the foot sole.
MATERIALS AND METHODS
Subjects.
Fifty-nine recording sessions were performed on 21 healthy subjects (12 male, 9 female, mean age 24 yr, range 20–27 yr). None of the participants had any known neurological or musculoskeletal disorders. All subjects gave written informed consent to participate in the experiment. The protocol was approved by the University of Guelph research ethics board and complied with the Declaration of Helsinki.
Microneurography.
Microneurography was used to identify and record the firing patterns of single cutaneous afferents from the right tibial nerve. Subjects lay prone on an adjustable table with both legs extended, and supported with Versa Form positioning pillows. The path of the tibial nerve and microelectrode insertion sites was located at the level of the popliteal fossa using transdermal electrical stimulation (1-ms square wave pulse, 1 Hz, 0–10 mA, Grass S48, SIU-Isolation Unit; Grass Instruments). A low-impedance reference electrode (uninsulated, tungsten, 200 μm diameter; FHC, Bowdoinham, ME) was inserted percutaneously to a depth of 0.5 cm, 2 cm medial to the predetermined recording site. A recording electrode (insulated 10 MΩ, tungsten, 200 μm diameter, 1–2 μm recording tip, 55 mm length; FHC) was then inserted at the recording site and manipulated by hand to penetrate the nerve and to isolate single units. Electrode manipulations were guided by subject sensations and audio feedback of the neural activity initiated by mechanical activation (light tapping, stroking, and stretching) of the foot sole skin. Neural recordings were amplified and band-pass filtered (gain 104, bandwidth 300 Hz-3 kHz, model ISO-180; World Precision Instruments, Sarasota, FL), digitally sampled (40 kHz), and stored for analysis (CED 1401 and Spike2 version 6; Cambridge Electronic Design). Spike morphology was used to generate templates for the visual classification of single units. The sample of cutaneous afferents through microneurographic recordings is thought to be random, and the ratio of afferent classes and distribution of RFs in the present study are thought to reflect a representative sample of the cutaneous population in the foot sole.
Cutaneous mechanoreceptor identification.
Single afferents were classified as fast adapting type I (FAI) or II (FAII), and slowly adapting type I (SAI) or II (SAII) based on previously described criteria (Johansson 1978; Kennedy and Inglis 2002). Briefly, FA afferents adapt quickly to sustained indentations and are highly sensitive to dynamic events. In contrast, SA afferents respond throughout sustained indentations, and demonstrate a firing rate proportional to the magnitude of skin displacement. Type I afferents typically have small RFs with distinct borders and multiple hotspots, whereas type II afferents have large RFs with less well defined borders and a single hotspot.
Afferent firing and perceptual threshold testing.
After a single afferent was isolated, Semmes-Weinstein monofilaments (Touch Test; North Coast Medical, Gilroy, CA) were used to measure AFTs, perceptual threshold, and RF location and size. AFT was defined as the minimum monofilament force (mN) that reliably (100% confidence of unit identification) evoked an afferent discharge in at least three of four applications. AFT was determined at the most sensitive RF location (hotspot) for each identified cutaneous afferent. Perceptual threshold was also measured at each afferent's RF hotspot following single unit recordings. The AFT test site was marked with a pen to ensure perceptual threshold was measured at the same location. A modified 4-2-1 search method was employed (Dyck et al. 1993), and subjects were instructed that there would be multiple catch trials in which no monofilaments would be applied. Subjects were instructed to answer with a simple yes/no response when they were at least 90% confident that they perceived the tactile stimulus. Perceptual thresholds were determined to be the lowest monofilament force (mN) correctly perceived on at least 75% of applications. It is notable that perceptual threshold is the perception of force within an identified region (RF). Given the nature of microneurography, where we are recording from one single afferent, it may be possible for perception threshold to be lower than AFT when we are not recording from the most sensitive afferent.
RF characteristics.
Afferent RFs were measured with monofilaments that applied a force four to five times greater than AFT, and were drawn on the skin using a fine-tip pen (Fig. 1). RFs were always oval or circular in shape, and the major and minor axes were used to calculate RF area (mm2) (Table 1). Efforts were made to identify and map all isolated single afferents; however, searching was focused to the foot sole, and only afferents with their RF in the plantar surface were included in AFT and perceptual threshold analyses.
Fig. 1.
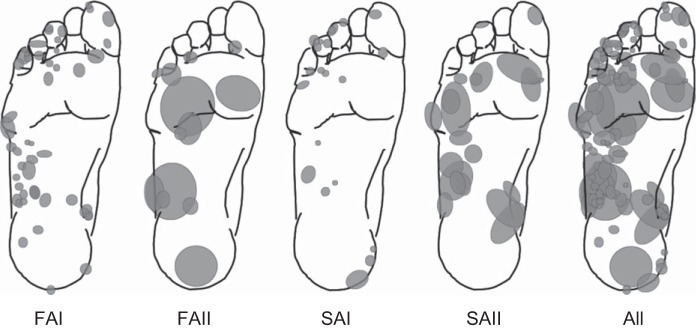
Afferent class receptive field distribution. Gray ovals indicate the relative size and location of cutaneous afferent receptive fields identified across the foot sole. FAI, fast adapting type I; FAII, fast adapting type II; SAI, slowly adapting type I; SAII, slowly adapting type II. These represent the receptive fields for all afferents included in the current study.
Table 1.
The number and percent of each afferent class identified and the monofilament threshold and receptive field area (mean and range)
| Threshold, mN |
Receptive Field Area, mm2 |
||||
|---|---|---|---|---|---|
| Afferent Class | Number (% of total) | Mean (median) | Range | Mean (median) | Range |
| FAI | 48 (47) | 13.2 (5.9) | 0.7–78.5 | 56.9 (47.1) | 11.8–226.2 |
| FAII | 13 (13) | 12.0 (3.9) | 0.4–98.1 | 701.4 (132.7) | 39.3–2686.1 |
| SAI | 21 (20) | 49.6 (39.2) | 3.9–255.0 | 61.3 (50.3) | 12.6–179.1 |
| SAII | 20 (20) | 222.5 (122.6) | 13.7–980.7 | 394.5 (249.0) | 88.0–1345.5 |
| Total | 102 | ||||
FAI, fast adapting type I; FAII, fast adapting type II; SAI, slowly adapting type I; SAII, slowly adapting type II.
Hardness measurements were taken at the RFs of each identified cutaneous afferent using a handheld durometer (type 1600-OO; Rex Gauge, Brampton, Ontario, Canada). The durometer had a 2-mm-diameter column-shaped indenter, which is ideally suited for skin measurements (Kissin et al. 2006). Durometers provide hardness measurements in arbitrary units between 1 (softest) and 100 (hardest), based on the penetration depth of the indenter. Two measurements of hardness were taken at each RF and averaged. Hardness measurements were not taken at some toe sites (10 of 30) due to the RF being too close to the nail, or an inability for the durometer to fit on the toe.
Data analysis.
The dependent variable assessed for both AFT and perceptual threshold was the applied monofilament force level (mN) necessary to evoke an afferent discharge or a percept, respectively. Analysis of variance (ANOVA) procedures were conducted on log-transformed AFT and perceptual threshold data to correct for violations of normality and homogeneity. A one-way ANOVA was used to determine if AFTs differed between afferent classes (FAI, FAII, SAI, SAII). Significant effects were followed up with a Gabriel post hoc analysis. Additionally, a mixed-design ANOVA was performed to determine if there were differences between afferent class firing threshold and associated perceptual thresholds (within factor), and if these differences were present at different foot sole locations (between factor). Significant effects were followed up with one-way ANOVAs and a Gabriel post hoc test.
Pearson's product-moment coefficients were calculated to measure the relationship between afferent class firing thresholds and associated perceptual thresholds. Relationships between RF hardness and AFT as well as RF hardness and perceptual threshold were also explored using Pearson's correlations.
The cumulative probabilities of afferent firing and the generation of a percept were calculated across monofilament force levels. These data demonstrate the proportion of afferents within each class that reached threshold, as well as the proportion of percepts evoked, at a given monofilament force application.
RESULTS
One hundred and two afferents were successfully identified with RFs in the plantar surface of the foot sole. These included 48 FAI (47%), 13 FAII (13%), 21 SAI (20%), and 20 SAII (20%) (Fig. 1). An additional 9 units were identified in the nail bed, dorsum, and back of the ankle (nail bed: 2 SAII; dorsum: 1 SAII; ankle: 1 FAI, 2 FAII, 1 SAI, 2 SAII); however, all nonfoot sole units were excluded from analysis. Cutaneous afferent class characteristics are presented in Table 1.
Afferent class firing threshold.
One-way repeated-measures ANOVA revealed that there was a significant difference in AFT between afferent classes (P < 0.001) (Fig. 2). Post hoc analysis indicated that AFT did not significantly differ between FAI (mean 13.2 mN) and FAII (mean 12.0 mN) afferents (P = 0.498), and that both FAI and FAII afferents had significantly lower thresholds compared with SAI (mean 49.6 mN) and SAII (222.5 mN) afferents (all P values <0.001). In addition, SAI AFT was significantly lower than SAII AFT (P = 0.001).
Fig. 2.
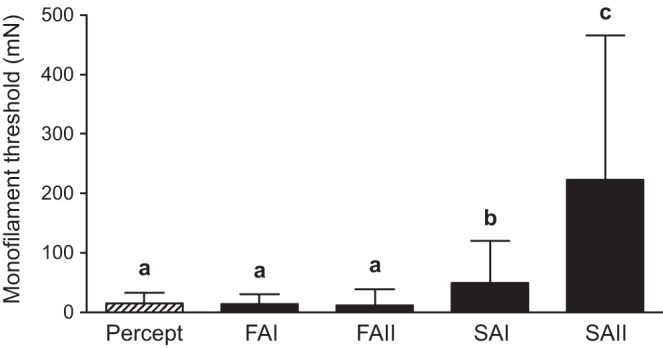
Mean (±SD) monofilament perceptual threshold (hatched bar) and afferent class firing thresholds (black bars). FAI and FAII afferent firing thresholds were significantly lower than SAI and SAII (all P values <0.001) but were not different from perceptual threshold (P > 0.05). SAI afferent firing threshold was significantly lower than SAII (P = 0.001), and both SAI and SAII afferent firing thresholds were significantly higher than perceptual threshold (SAI P = 0.004; SAII P < 0.001). The letters a, b, and c identify threshold categories that significantly differ from each other.
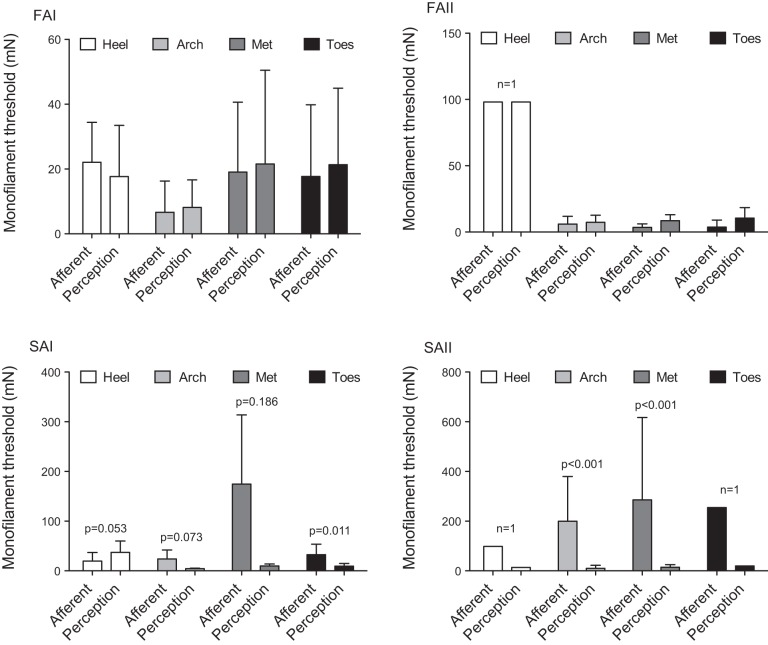
Across foot sole locations, FAI AFTs were found to be significantly different (P = 0.005), while location differences were not found for FAII (P = 0.174), SAI (P = 0.143), or SAII (P = 0.964) afferent classes (evaluated using 1-way repeated-measures ANOVAs). It should be noted that FAI afferents were the most abundant (n = 48); thus, the relatively lower sample size of the other classes may have contributed to the absence of observed differences in AFT across foot sole locations. Post hoc analysis revealed FAI AFTs to be significantly lower at the arch compared with the heel (P = 0.019) and toes (P = 0.043), and there was a trend toward a lower threshold at the arch compared with the metatarsals (Met, P = 0.073) (Fig. 3A).
Fig. 3.
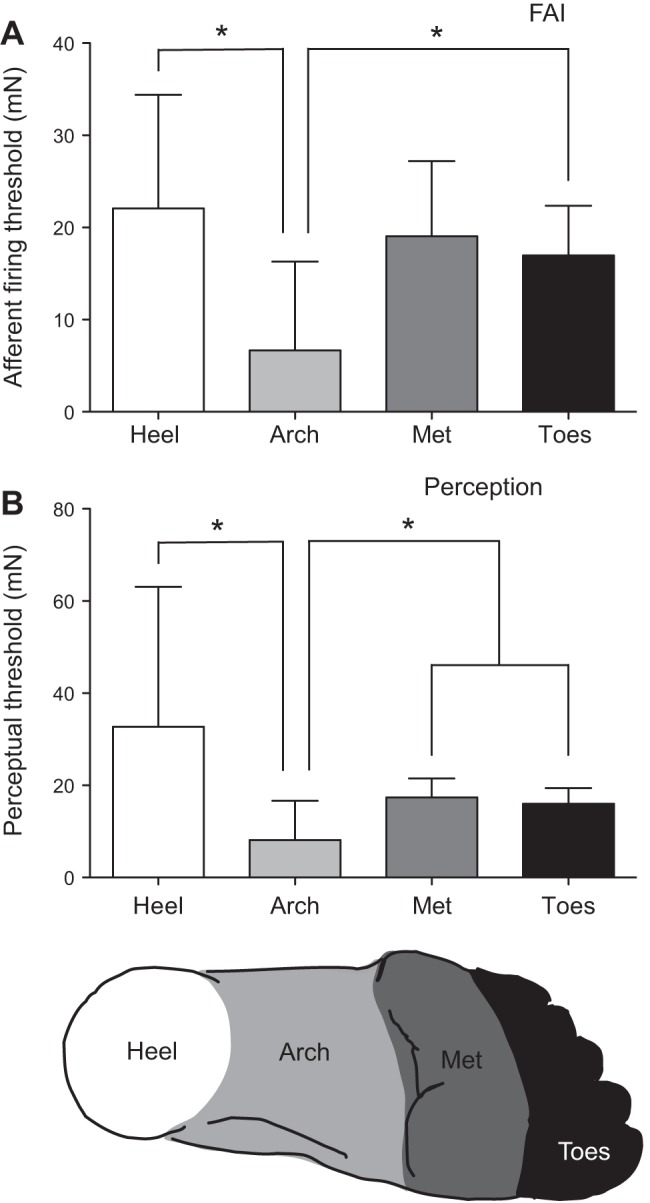
A: mean (±SD) FAI afferent firing thresholds (AFT) at the heel, arch, metatarsals (Met), and toes. FAI AFTs were significantly lower at the arch compared with the heel (P = 0.019) and toes (P = 0.043). B: mean (±SD) perceptual thresholds at each foot region. Perceptual thresholds were lowest in the arch compared with all other sites (*P < 0.05).
Perceptual thresholds.
Perceptual thresholds significantly differed across foot sole locations (P < 0.001; 1-way repeated-measures ANOVA). Similar to FAI AFT, the arch displayed the lowest perceptual thresholds; post hoc analysis revealed that perceptual threshold at the arch was significantly lower compared with the heel (P < 0.001), Met (P = 0.003), and toes (P = 0.007) (Fig. 3B).
Relationship between AFT and perceptual threshold.
Overall, perceptual threshold (mean 14.63 mN) was found to be most similar to both FAI (mean 13.2 mN) and FAII (mean 12.0 mN) AFTs (Fig. 2). Two-way mixed ANOVA results indicated that, across the foot sole, there were no significant differences between perceptual threshold and FAI or FAII AFT (P > 0.05) (Fig. 4). In contrast, SAI and SAII AFTs were found to be significantly higher than perceptual threshold (SAI P = 0.004, SAII P = 0.001) (Fig. 2). Post hoc analysis showed that SAI AFT at the toes was significantly higher compared with perceptual threshold (P = 0.011), with a similar trend at the arch (P = 0.073), and an opposite trend of lower SAI AFT compared with perceptual threshold at the heel (P = 0.053) (Fig. 4). SAII AFTs were significantly higher than perceptual threshold at the arch and Met (P < 0.001) (Fig. 4). Minimum, maximum, and median threshold values across foot sole sites are represented in Table 2. The small sample sizes at some locations (1 FAII and SAII at the heel, and 1 SAII at the toes) limited the comparisons that could be made.
Fig. 4.
Mean (±SD) afferent firing and perceptual threshold at the heel, arch, Met, and toes for each afferent class (FAI, FAII, SAI, SAII). There were no significant differences between FAI or FAII afferent firing (AFT) and perceptual threshold at any foot sole location. SAI AFTs were significantly higher than perceptual threshold at the toes (P = 0.011), and SAII AFTs were significantly higher than perceptual threshold at the arch and Met (P values <0.001).
Table 2.
Afferent firing threshold (AFT) values across foot sole locations
| Heel |
Arch |
Metatarsals |
Toes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | |
| FAI | 9.81 | 39.22 | 19.61 | 0.69 | 39.22 | 3.92 | 3.92 | 58.84 | 9.80 | 1.57 | 78.45 | 7.84 |
| FAII | 98.07 | 98.07 | 98.07 | 0.392 | 13.73 | 5.69 | 0.69 | 5.88 | 3.92 | 0.69 | 9.81 | 0.69 |
| SAI | 5.88 | 39.23 | 13.73 | 3.92 | 39.23 | 26.48 | 13.73 | 254.97 | 147.10 | 5.88 | 58.84 | 29.42 |
| SAII | 98.07 | 98.07 | 98.07 | 13.73 | 588.34 | 254.97 | 13.73 | 980.67 | 980.67 | 254.97 | 254.97 | 254.97 |
Data represented are minimum (min), maximum (max), and median values. Units are mN.
The cumulative probability of afferent firing and perceptual threshold across monofilament forces (mN) is presented in Fig. 5. These data demonstrate differences in the proportion of afferents recruited in each class across monofilament force levels. FAII afferents were shown to be the most sensitive, exhibiting a higher percentage of recruitment at lower forces compared with the other classes. By 1 mN of force, 40% of FAII afferents reached threshold, whereas 20% of FAI and 0% of SAI and SAII afferents were firing. The proportion of trials perceived increased with larger monofilament force and most closely related to the recruitment of FA afferents. Perceptual threshold was reached in 10% of trials before any SAI or SAII afferents reached firing threshold. At ∼6 mN of force, 50% of monofilament applications were perceived, whereas only 14% of SAI and 0% of SAII afferents reached threshold. In contrast 56% of FAI and 63% FAII were recruited by 6 mN of force. These data demonstrate that FAI and FAII AFTs are lower than perceptual threshold in some instances and that perception threshold is likely reached in the absence of SAI and SAII firing. Furthermore, a significant correlation was found between FAI AFT and perceptual threshold (r = 0.489, P = <0.001). In contrast, significant correlations were not found between perceptual threshold and AFT for any other afferent classes (Table 3).
Fig. 5.
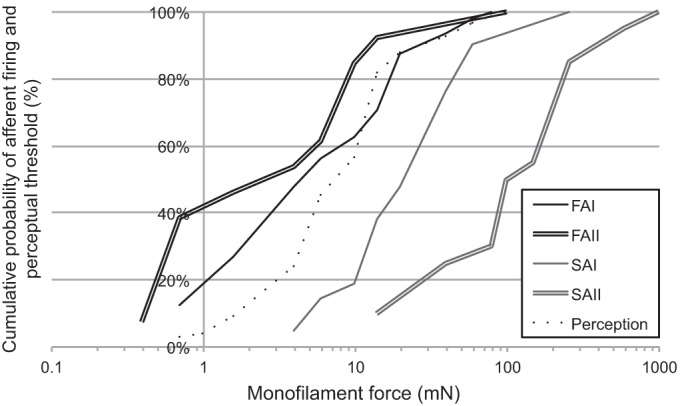
Cumulative probability of afferent class firing and perceptual threshold. This demonstrates the proportion of percepts evoked and AFTs reached at a given monofilament force level. Lines represent FAI (single black line), FAII (double black line), SAI (single gray line), SAII (double gray line), and perception (dotted line). These data demonstrate that some FAI and FAII AFTs were lower than perceptual threshold, and perceptual threshold was reached in 60% of trials in the absence of substantial SAI and SAII contributions.
Table 3.
Correlation data for comparisons between afferent firing threshold and perceptual threshold, afferent firing threshold, and receptive field hardness, and perceptual threshold and receptive field hardness
| Variables | n | r | P |
|---|---|---|---|
| FAI percept | 47 | 0.489* | <0.0001 |
| FAII percept | 13 | 0.350 | 0.241 |
| SAI percept | 20 | −0.066 | 0.781 |
| SAII percept | 19 | 0.297 | 0.218 |
| Percept hardness | 89 | 0.433* | <0.0001 |
| FAI hardness | 44 | 0.357* | 0.018 |
| FAII hardness | 11 | 0.758* | 0.007 |
| SAI hardness | 21 | −0.109 | 0.678 |
| SAII hardness | 20 | 0.422 | 0.064 |
Significant correlation.
RF hardness influences FA AFT and perceptual threshold.
RF hardness was found to significantly correlate with perceptual threshold (r = 0.433, P = <0.001). Similarly, RF hardness was found to significantly correlate with FAI AFT (r = 0.357, P = 0.018), and FAII AFT (r = 0.758, P = 0.007) (Table 3). No significant correlations were found between SAI or SAII RF hardness and AFT, although a trend was found for SAII afferents (SAI: r = −0.109, P = 0.678; SAII: r = 0.422, P = 0.064). These data suggest that the effects of skin hardness on perceptual threshold parallel the effects of skin hardness on FA afferent firing.
DISCUSSION
The present study examined the relationship between cutaneous AFTs and perceptual thresholds across the human foot sole. We have demonstrated that monofilament perceptual threshold is mediated by the activity of FA afferents, and, in turn, that both FA afferent firing and perceptual thresholds may be influenced by skin hardness. Across all foot sole locations, perceptual thresholds did not significantly differ from the firing thresholds of FAI and FAII afferents. The arch was perceptually the most sensitive region and also contained the most sensitive FAI afferents. In contrast, SAI and SAII afferents were significantly less sensitive than perceptual threshold across the foot sole and are thus not thought to mediate monofilament perceptual threshold.
Psychophysical detection.
Cutaneous afferents are the fundamental units that convey tactile feedback to the central nervous system. The lower envelope principle postulates that perceptual thresholds are set by the most sensitive afferents, and predicts that perceptual variability can be accounted for in the variability of individual afferent firing (Parker and Newsome 1998). Alternatively, afferent temporal or spatial summation may be required for tactile stimuli to have perceptual significance. In such pooling models, the relationship between perception and AFTs is expected to be small, since fluctuations in the activity of single neurons would have a minimal impact on whether cutaneous activity is perceived (Parker and Newsome 1998). Microneurography provides a tool to obtain single-unit recordings from awake human subjects, and thus permits the relationship between cutaneous afferent firing and perception to be directly examined. This is the first study to link the activity of single cutaneous afferents to perceptual threshold across the foot sole.
Afferent and perceptual thresholds across the foot sole.
Previous reports of foot sole cutaneous AFTs exhibit a range in median values that is similar to the threshold ranges measured in the present study. In all cases FAII afferents were found to have the lowest monofilament thresholds, with median values reported from 0.73 to 4 mN (3.9 mN in the present study). In most cases FAI afferents had the second lowest thresholds (3.84–11.8 mN, 5.9 mN present study), followed by SAI (4.08–35.6 mN, 39.2 mN present study) and SAII (1.42–115.3 mN, 122.6 mN present study) (Bent and Lowrey 2013; Fallon et al. 2005; Kennedy and Inglis 2002; Lowrey et al. 2013) afferents. Collectively, these median values demonstrate that large ranges in AFTs may exist within classes; however, these AFTs are averaged across the foot sole and not distinct by region. We made the link between afferent location and firing threshold because it is an important measure to identify factors contributing to both AFT and perceptual threshold. FAI AFT was found to be significantly lower at the arch compared with the heel and toes, whereas SA afferents did not show significant differences in threshold across the foot sole. Interestingly, perceptual threshold was also found to be lowest in the arch region compared with the heel, Met, and toes, which is in agreement with previous work (Eils et al. 2002; Hennig and Sterzing 2009; Nurse and Nigg 1999; Zhang and Li 2012). In general, we found that, across the foot sole, regional perceptual threshold differences closely mirrored the firing thresholds of FAI and FAII afferents; the force required to activate these FA afferents was not significantly different from those required to reach perceptual threshold. Although the relative perceptual contributions between FAI and FAII afferents cannot be determined from the present data, our results provide strong evidence that only FA, and not SA, afferent firing contributes to monofilament perceptual thresholds across the foot sole.
Regional differences: RF hardness.
The ability of cutaneous afferents to fire is set by the capacity of the skin and surrounding tissue to deform and transmit force to the mechanoreceptor endings. Mechanical property differences between the hands and feet and across the foot sole have been suggested to account for perceptual and afferent firing differences between these regions; however, this relationship has not been previously investigated (Kekoni et al. 1989; Kennedy and Inglis 2002; Trulsson 2001). Significant differences in hardness were found across the foot sole regions investigated in the current study. Additionally, we found significant correlations between both FAI and FAII AFT with RF hardness, which supports an influence of skin hardness on FA AFT. Perceptual thresholds were also found to correlate with RF hardness. As a whole, these correlational data suggest that, across the foot sole, higher FA AFTs may be the result of harder skin, and, as a consequence, perceptual thresholds are increased. These data cannot make this link unequivocally, but, when considered along with the significant regional differences observed in FAI AFT and perceptual thresholds, it appears that, indeed, RF hardness has an influence on these measures. These data suggest that regional differences in foot sole hardness may partially explain the consistent regional differences in foot sole monofilament thresholds reported in the literature.
Afferent characteristics between the hands and feet.
The hands and feet purportedly contain the same classes of mechanoreceptive afferents, despite serving distinct functional roles. Tactile feedback from the feet aids in the control of posture and upright stance by providing information about sway and weight distribution under the feet (Kavounoudias et al. 1998). In contrast, the hands are commonly used to manipulate objects and require high tactile acuity. It is therefore not surprising that firing thresholds of afferents in the hands are reported to be lower than those in the feet (Johansson et al. 1980; Kennedy and Inglis 2002). Median monofilament AFTs of FAI, FAII, SAI, and SAII afferents in the hand have been reported to be 0.58, 0.54, 1.3, and 7.5 mN, respectively (Johansson et al. 1980); these are 7–30 times more sensitive than the median afferent class thresholds found in the present study and in other studies examining cutaneous receptors in the feet (Bent and Lowrey 2013; Fallon et al. 2005; Kennedy and Inglis 2002; Lowrey et al. 2013). Elevated thresholds across the foot sole may reflect a peripheral adaptation of foot sole afferents that enables them to optimally function under loaded conditions. Despite these observations of overall elevated thresholds in the foot sole, the relative thresholds between afferent classes appear to be preserved in the feet; in both the hands and feet, FAII afferents are typically the most sensitive to perpendicular light touch followed by FAI and SAI afferents, whereas SAIIs characteristically are the least sensitive (Johansson et al. 1980; Kennedy and Inglis 2002).
Previous seminal work in the hand investigated the mechanisms behind the perception of light touch in the glabrous skin of the palm and fingers (Johansson and Vallbo 1979). These authors found FAI and FAII AFTs to mirror perceptual thresholds in the fingers and lateral border of the hand, which is similar to the relationship we found in the foot sole. However, while we found FA AFT and perceptual threshold to correlate across the entire foot sole, they found a discrepancy in the palm of the hand, where FA AFTs were considerably lower than perceptual thresholds. This disparity suggests that perception at the palm of the hand may be limited by noise or processing inefficiencies within the central nervous system. Such a discrepancy between AFT and perceptual threshold was not found in any regions of the foot sole. The alignment of FA AFTs with perceptual thresholds in the most sensitive regions of the hands (fingers and lateral boarder) is consistent with the lower envelope principle and with the present observations across the foot sole whereby minimal input from a few afferents is able to generate a percept.
Functional implications.
This study extends the large body of work that has investigated cutaneous afferent firing and sensory perception in the hand and the foot sole. Considering the importance of detailed tactile feedback from the fingers, it makes functional sense that minimal afferent input from the fingers would have a significant impact on perception (Johansson and Vallbo 1979). It may then be a surprise that a similar relationship, albeit at elevated thresholds, is present in the foot sole where high tactile discrimination may not be necessary for the control of standing balance. Research has identified a large proportion of FAI afferents in the foot sole, which highlights skin's important role in dynamic balance (Fallon et al. 2005; Kennedy and Inglis 2002). The transmission of FAI afferent information, with minimal firing and low signal noise, would ensure the fidelity of cutaneous dynamic input for balance and locomotor tasks. In the present study, the FA afferent-perceptual correspondence supports that small changes in FA AFTs can have a significant impact on perceptual threshold, and potentially on balance control. In support of this concept, low-amplitude white noise vibration applied to the foot sole has been shown to improve balance control in stroke and diabetic patients (Priplata et al. 2005). These vibrations are thought to increase the detection of weak cutaneous signals from the soles of the feet. Therefore, small changes in FA afferent firing are thought to impact both tactile perception, and balance control.
While the current study was conducted in a young healthy population, these data can help inform clinical assessments of tactile sensitivity. Diabetic neuropathy, which is present in 80% of both type 1 and 2 diabetics, is commonly diagnosed and assessed with monofilament testing (Collins et al. 2010; Valk et al. 1997). In these patients, the standard that is used to diagnose sensory neuropathy is a threshold of 10 g (98 mN) or higher, typically in the plantar surface of the great toe (Kumar et al. 1991; Lambert et al. 2009). The current data suggest that monofilament thresholds in the foot sole are mediated by the activity of FA afferents, and monofilament testing does not provide a measure of SA afferent function. Clinically, monofilaments remain a simple tool to assess tactile sensibility; however, other techniques, such as vibration, grating orientation tasks, and temperature thresholds, are needed to understand the function of the complete peripheral sensory system.
Limitations.
Microneurography is a powerful technique in that it provides a comparison between afferent activity and perception in human subjects. A limitation of studying single neurons is the inability to measure population behavior at different levels within the nervous system. The number of afferents responding to each monofilament application is unknown, but almost certainly includes more than the individual afferent being recorded. Consequently, the influence of spatial summation on these monofilament threshold outcomes remains unknown. Additionally, foot sole location and afferent class comparisons would be strengthened with large sample sizes; however, microneurography does not permit the selection of skin units based on class or foot sole location. Perceptual threshold is a relatively simple psychophysical measure, and may only be mediated by FA afferents. Understanding the perceptual contributions of SAI and SAII afferents could be achieved with different tactile stimuli and associated psychophysical tasks such as stimulus intensity threshold, location, and texture perception (Johnson and Hsiao 1992).
In conclusion, the current findings indicate that minimal FA afferent input from the foot sole can give rise to tactile percepts. These findings are in agreement with the lower envelope principle in that perception is set by the activity of the most sensitive FA afferents. SAI and SAII afferents were found to have elevated firing thresholds compared with FA afferents, and their firing did not contribute to foot sole light touch perceptual thresholds. Additionally, regional differences in RF hardness appear to relate to, and influence, the firing thresholds of FA afferents; this is thought to contribute to regional differences in perception across the foot sole.
GRANTS
This work was supported by funding from the Natural Science and Engineering Research Council (NSERC) of Canada Postsecondary Graduate Scholarship (Doctoral) to N. D. J. Strzalkowski, NSERC Canada Graduate Scholarship (Masters) to R. L. Mildren, and an NSERC Discovery Grant to L. R. Bent.
DISCLOSURES
The authors declare that there are no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Author contributions: N.D.J.S. and L.R.B. conception and design of research; N.D.J.S., R.L.M., and L.R.B. performed experiments; N.D.J.S. analyzed data; N.D.J.S., R.L.M., and L.R.B. interpreted results of experiments; N.D.J.S. prepared figures; N.D.J.S. drafted manuscript; N.D.J.S., R.L.M., and L.R.B. edited and revised manuscript; N.D.J.S., R.L.M., and L.R.B. approved final version of manuscript.
REFERENCES
- Aimonetti JMJ, Hospod VV, Roll JPJ, Ribot-Ciscar EE. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol (Lond) 580: 649–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent LR, Lowrey CR. Single low-threshold afferents innervating the skin of the human foot modulate ongoing muscle activity in the upper limbs. J Neurophysiol 109: 1614–1625, 2013. [DOI] [PubMed] [Google Scholar]
- Collins S, Visscher P, De Vet HC, Zuurmond WWA, Perez RSGM. Reliability of the Semmes Weinstein Monofilaments to measure coetaneous sensibility in the feet of healthy subjects. Disabil Rehabil 32: 2019–2027, 2010. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology 43: 1508–1512, 1993. [DOI] [PubMed] [Google Scholar]
- Eils E, Behrens S, Mers O, Thorwesten L, Völker K, Rosenbaum D. Reduced plantar sensation causes a cautious walking pattern. Gait Posture 20: 54–60, 2004. [DOI] [PubMed] [Google Scholar]
- Eils E, Nolte S, Tewes M, Thorwesten L, Völker K, Rosenbaum D. Modified pressure distribution patterns in walking following reduction of plantar sensation. J Biomech 35: 1307–1313, 2002. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Bent LR, McNulty PA, Macefield VG. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J Neurophysiol 94: 3795–3804, 2005. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AB. Mechanoreceptor Activity Recorded Percutaneously with Semi-Microelectrodes in Human Peripheral Nerves. Acta Physiol Scand 69: 121–122, 1967. [DOI] [PubMed] [Google Scholar]
- Hennig EM, Sterzing T. Sensitivity mapping of the human foot: thresholds at 30 skin locations. Foot Ankle Int 30: 986–991, 2009. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Stuücker M, Dirschka T, Goörtz S, Gammal El S, Dirting K, Hoffmann A, Altmeyer P. Twenty MHz B scan sonography for visualization and skin thickness measurement of human skin. J Eur Acad Dermatol Venereol 3: 302–313, 1994. [Google Scholar]
- Inglis JT, Horak F, Shupert C, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp Brain Res 101: 159–164, 1994. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Landstrom U, Lundstrom R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17–25, 1982. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. J Physiol (Lond) 297: 405–422, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. J Physiol (Lond) 281: 101, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RSR, Vallbo ABA, Westling GG. Thresholds of mechanosensitive afferents in the human hand as measured with von Frey hairs. Brain Res 184: 343–351, 1980. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annu Rev Neurosci 15: 227–250, 1992. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. The plantar sole is a “dynamometric map” for human balance control. NeuroReport 9: 3247–3252, 1998. [DOI] [PubMed] [Google Scholar]
- Kekoni J, Hämäläinen H, Rautio J, Tukeva T. Mechanical sensibility of the sole of the foot determined with vibratory stimuli of varying frequency. Exp Brain Res 78: 419–424, 1989. [DOI] [PubMed] [Google Scholar]
- Kennedy PM, Inglis JT. Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J Physiol (Lond) 538: 995–1002, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin EYE, Schiller AMA, Gelbard RBR, Anderson JJJ, Falanga VV, Simms RWR, Korn JHJ, Merkel PAP. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum 55: 603–609, 2006. [DOI] [PubMed] [Google Scholar]
- Kowalzik R, Hermann B, Biedermann H, Peiper U. Two-point discrimination of vibratory perception on the sole of the human foot. Foot Ankle Int 17: 629–634, 1996. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fernando DJS, Veves A, Knowles EA, Young MJ, Boulton AJM. Semmes-Weinstein monofilaments: a simple, effective and inexpensive screening device for identifying diabetic patients at risk of foot ulceration. Diabetes Res Clin Pract 13: 63–67, 1991. [DOI] [PubMed] [Google Scholar]
- Lambert GA, Mallos G, Zagami AS. Von Frey's hairs: a review of their technology and use: a novel automated von Frey device for improved testing for hyperalgesia. J Neurosci Methods 177: 420–426, 2009. [DOI] [PubMed] [Google Scholar]
- Lowrey CR, Strzalkowski NDJ, Bent LR. Cooling reduces the cutaneous afferent firing response to vibratory stimuli in glabrous skin of the human foot sole. J Neurophysiol 109: 839–850, 2013. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol (Lond) 429: 113–129, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muise SB, Lam CK, Bent LR. Reduced input from foot sole skin through cooling differentially modulates the short latency and medium latency vestibular reflex responses to galvanic vestibular stimulation. Exp Brain Res 218: 63–71, 2012. [DOI] [PubMed] [Google Scholar]
- Nurse MA, Nigg BM. Quantifying a relationship between tactile and vibration sensitivity of the human foot with plantar pressure distributions during gait. Clin Biomech (Bristol, Avon) 14: 667–672, 1999. [DOI] [PubMed] [Google Scholar]
- Ochoa JJ, Torebjörk EE. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol (Lond) 342: 633–654, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci 21: 227–277, 1998. [DOI] [PubMed] [Google Scholar]
- Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res 877: 401–406, 2000. [DOI] [PubMed] [Google Scholar]
- Perry SD, Santos LC, Patla AE. Contribution of vision and cutaneous sensation to the control of centre of mass (COM) during gait termination. Brain Res 913: 27–34, 2001. [DOI] [PubMed] [Google Scholar]
- Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol 59: 4–12, 2005. [DOI] [PubMed] [Google Scholar]
- Roll RR, Kavounoudias AA, Roll JPJ. Cutaneous afferents from human plantar sole contribute to body posture awareness. NeuroReport 13: 1957–1961, 2002. [DOI] [PubMed] [Google Scholar]
- Strzalkowski NDJ, Triano JJ, Lam CK, Templeton CA, Bent LR. Thresholds of skin sensitivity are partially influenced by mechanical properties of the skin on the foot sole. Physiol Rep 3: e12425–e12425, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulsson M. Mechanoreceptive afferents in the human sural nerve. Exp Brain Res 137: 111–116, 2001. [DOI] [PubMed] [Google Scholar]
- Valk GD, de Sonnaville JJ, van Houtum WH, Heine RJ, van Eijk JT, Bouter LM, Bertelsmann FW. The assessment of diabetic polyneuropathy in daily clinical practice: reproducibility and validity of Semmes Weinstein monofilaments examination and clinical neurological examination. Muscle Nerve 20: 116–118, 1997. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li L. The differential effects of foot sole sensory on plantar pressure distribution between balance and gait. Gait Posture 37: 532–535, 2013. [DOI] [PubMed] [Google Scholar]