Abstract
Functional brain imaging techniques depend on the relationship between regional hemodynamic responses and neural activity. The positive hemodynamic response (PHR) has widely been discussed and has generally been associated with an increase in neuronal signals. In contrast, the negative hemodynamic response (NHR) has not been investigated extensively, and its underlying nature is highly controversial. In the present study, we employed an optical imaging (OI) technique and microelectrode array (MEA) recordings in the rat cortex to examine the NHR to hindlimb electrical stimulation; we primarily focused on the NHR adjacent to a PHR region. We determined that the dynamics of the total blood volume signal in the NHR regions lagged slightly behind those in the PHR areas. Additionally, the deoxyhemoglobin signal in the PHR areas increased immediately after stimulation and the deoxyhemoglobin signal in the NHR regions remained unchanged or increased. Consistent with the change in the deoxyhemoglobin signal, the MEA recordings demonstrated that neural activity in the PHR regions was elevated and that activity in the NHR areas was unchanged or increased during stimulation, implying that the NHR occurred in the absence of neural deactivation. These results suggest that the NHR may be explained by purely hemodynamic contributions, specifically “blood stealing” or increased neural activity, and indicate that caution should be exercised when interpreting the NHR as a decrease in neural activity, especially when the NHR is adjacent to a PHR.
Keywords: microelectrode array, negative hemodynamic response, optical imaging
functional imaging techniques, which have introduced a new era in neuroscience by providing insights into the investigation of brain functions, rely on the quantitative relationship between localized neural and hemodynamic signals (Boorman et al. 2010). Most studies evaluate the positive hemodynamic response (PHR) and associate it with an increase in underlying neuronal activity, specifically synaptic potentials (Huang et al. 2014b; Logothetis et al. 2001; Viswanathan and Freeman 2007). Whereas the underlying nature of the PHR is generally agreed upon, the negative hemodynamic response (NHR) is sparsely reported, and its origin is highly debatable (Hayes and Huxtable 2012; Moraschi et al. 2012).
The most popular theory states that the NHR can be interpreted in terms of neuronal deactivation. An important study by Shmuel et al. (2006) involving simultaneous electrophysiological recording and functional magnetic resonance imaging (fMRI) proposed a tight coupling between the negative blood oxygenation level-dependent (BOLD) response and decreased neuronal activity in the monkey striate cortex. Subsequent investigations using multiunit activity (MUA) recording have supported this explanation (Boorman et al. 2010; Yin et al. 2011). Furthermore, several studies have shown that the NHR was induced by a reduction in cerebral blood flow (CBF) due to suppressed neural activity and that this reduction in CBF was accompanied by a smaller reduction in the cerebral metabolic rate of oxygen consumption (CMRO2) (Mullinger et al. 2014; Shmuel et al. 2002). It has been suggested that a neural component accounts for at least 60% of the NHR (Pasley et al. 2007).
However, the NHR does not always correspond to decreases in neural activity and may occasionally be related to nonneural hemodynamic contributions or increased neural signaling, especially when the NHR occurs in cortical areas that are adjacent to regions that exhibit a PHR. Schridde et al. (2008) first reported that negative BOLD responses in the hippocampus may be associated with increased neuronal signals during seizures in rats and proposed that the NHR did not necessarily indicate a decrease in neural activity. These authors suggested that the NHR could be produced by an increase in neuronal activity and CMRO2 without concomitant compensation by an increase in CBF. Ekstrom et al. (2009) subsequently found no significant correlation between hippocampal BOLD decreases and electrophysiological signals during spatial navigation in humans. Another study also observed the NHR in conjunction with increased neural activity and discussed the influence of endogenous neurotransmission on interpretation of negative fMRI data (Shih et al. 2009). Harel et al. (2002) reported negative cerebral blood volume (CBV) and BOLD signals in high-order visual areas where neural activation, rather than deactivation, is expected to occur during moving grating stimulation (in contrast with positive CBV and BOLD changes in the nearby primary visual cortex). They suggested that the CBF demand elicited by increased neural activity could be met by the blood supply of neighboring regions. This “blood stealing” notion has since been proposed by others (Devor et al. 2005; Kannurpatti and Biswal 2004). Mishra et al. (2011) found that fMRI, CBV, and CBF decreases in the caudate-putamen were accompanied by increases in local field potentials (LFPs) and MUA signals. They inferred that, in the absence of a corresponding reduction in neural activity, the NHR may arise from the blood stealing effect or from local vasoconstriction. Additionally, the NHR can be generated in regions that are devoid of neuronal tissue, such as large cerebral veins (Bianciardi et al. 2011). The NHR may provide important insights into the anatomical and functional organization of inhibitory or suppressive circuits throughout the cerebral cortex if it can be used to identify deactivated neuronal populations (Hlushchuk and Hari 2006; Pasley et al. 2007). Nevertheless, if the NHR can arise independent of neural activity and can originate from purely hemodynamic factors or if it is closely associated with increased neural activity, current interpretations of neuroimaging data in brain research that assume that the NHR represents the neurophysiological inverse of the PHR may be called into question.
To date, most NHR studies have been based on sustained negative BOLD signals recorded with fMRI (Liu et al. 2011; Moraschi et al. 2012). Few studies have examined the NHR with optical imaging (OI) technology, which can produce percent-change maps for the oxyhemoglobin (HbO) and deoxyhemoglobin (Hbr) levels and the total blood volume (Hbt) (Boorman et al. 2010; Devor et al. 2005, 2007; Yin et al. 2011). Additionally, these OI investigations do not reach a consensus regarding the origin of the NHR. In the present study, we employed the OI technique and microelectrode array (MEA) recordings in the rat cortex to examine the NHR to hindlimb electrical stimulation. We focused on the NHR adjacent to a PHR because the origin of this type of NHR is the most controversial. The dynamics of the blood volume signal in the NHR regions lagged slightly behind those in the PHR areas. The Hbr levels in the PHR areas promptly increased after the stimulus, but the Hbr levels in the NHR regions did not decrease (unchanged in 12 rats and increased in 5 rats). Furthermore, the electrophysiological recordings via MEA demonstrated that neural activity in the PHR regions was increased and the activity in the NHR areas was unchanged or increased during stimulation (consistent with the change in Hbr levels). This observation implies that the NHR occurred in the absence of neural deactivation. These findings indicate that caution should be exercised in the interpretation of the NHR as a decrease in neural activity, especially when the NHR is adjacent to a PHR.
METHODS
Experimental data were obtained from anesthetized animals bred in the facilities of the Neurophysiology Department at Xiangya Medical College of Center South University (Permit No. SCXK 2009-0012). All experiments were performed in full accordance with the American Physiological Society's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training. The protocol was specifically approved by the Ethics Committee of Xiangya Medical College of Center South University. The procedures for anesthesia and surgery have previously been described in detail (Huang et al. 2014a; Yin et al. 2011;). We briefly outline these procedures below.
Animal preparation.
Healthy adult male Sprague-Dawley rats (n = 17, 200–310 g) were used. The animals were initially anesthetized via injection of urethane (1.2 g/kg) into the celiac plexus, and additional doses were administered if necessary. The area of the skull overlying the somatosensory cortex (Paxinos and Watson 2007) was exposed and then thinned to translucency with a saline-cooled dental drill. The animals were constrained in a standard stereotaxic head frame such that the thinned region was positioned in a horizontal plane. Dental cement was used to construct a well that was filled with silicone oil to reduce specularity.
Body temperature was maintained at ∼37°C with a feedback-controlled heating pad. Respiration, heartbeat, blood pressure, and blood gases were monitored with a physiological recording system (MP150, BIOPAC Systems, Goleta, CA). After data acquisition, the animals were euthanized immediately via an overdose of urethane (4 g/kg).
Stimulation paradigm.
The stimulation consisted of an electrical pulse sequence (5 Hz, 0.4–1.6 mA, 5-ms duration) applied to one of the hindlimbs through two thin needle electrodes inserted into the footpad. We regulated the stimulus intensity by observing digit twitching (Shih et al. 2009). Each experiment consisted of 32 trials lasting 25 s per trial, with an interstimulation interval of 50 s to avoid adaptation. In each trial, the electrical stimulation was triggered at 5 s and lasted for 2 s. For each experiment, individual trials were averaged to obtain a mean trial result, which was analyzed with the methods described below.
Optical imaging.
OI sequences of the targeted cortical area were captured with an Imager 3001 system (Optical Imaging, Germantown, NY) at a spatial resolution of ∼8 μm/pixel. The sampling rate of the camera was set to 10 frames/s. Filtered light with green (546 ± 10 nm) and red (630 ± 10 nm) wavelengths was utilized to illuminate the cortex. According to the hemoglobin absorption curves shown in Fig. 1 (Prahl 1998), green illumination reflects the Hbt, as HbO and Hbr have the same absorption coefficient. Red illumination reflects the Hbr level, as the absorption coefficient of Hbr is substantially larger than that of HbO (Pouratian et al. 2002; Wang et al. 2011).
Fig. 1.

Absorption characteristics of oxyhemoglobin (HbO) and deoxyhemoglobin (Hbr). Two vertical dashed lines illustrate the 2 wavelengths employed in our experiments (λ = 546 and 630 nm).
The time course of each pixel was defined as the fractional change in the absorbance at the given pixel: (R0 − Ri)/R0, where Ri is the gray level of the pixel for the ith image and R0 is the baseline, which is defined as the average value of R in the 2-s period prior to stimulation (i.e., 3–5 s into each trial) (Yin et al. 2011). We created the time series using the relative change in the original pixel intensity sequence to adjust for uneven illumination (Toga and Mazziotta 2002).
Analysis of optical imaging data.
The blood volume signal for each pixel calculated with the above method was then used to determine the spatial areas of the PHR and the NHR induced by electrical stimulation of the hindlimb. A pixel was incorporated into the PHR region if the blood volume was significantly increased after stimulation. Similarly, a pixel was incorporated into the NHR region if the blood volume was significantly decreased after stimulation. Specifically, the differences in blood volume between before (3–5 s) and after (7–9 s) stimulation were compared with a two-tailed t-test with a confidence level of 99%. The period of 7–9 s was selected because the hemodynamic response reached its peak ∼3 s after stimulus onset in the present and previous studies (Toga and Mazziotta 2002). The PHR and NHR cortical regions were delimited based on the calculation of each pixel; the average blood volume signals in the PHR and NHR regions were then obtained.
To compare the differences in the response amplitudes and the temporal dynamics between the NHR and the PHR elicited by hindlimb electrical stimulation, the peak amplitude, the time-to-peak (tmax), and the onset time (ton, the time to reach 10% of the peak amplitude) were computed after fitting of the average blood volume signals in the PHR and NHR regions to smoothing spline functions (Kannurpatti and Biswal 2004; Liu et al. 2011). The differences in these parameters of the blood volume signal between the PHR and NHR regions were examined via a one-way analysis of variance (ANOVA) followed by a Tukey-Kramer post hoc test using a 99.5% confidence level (Harel et al. 2002). In addition, cross-correlation analysis was employed to examine the time lag between the NHR and the PHR. Specifically, the Pearson correlation coefficients between the NHR and the PHR were computed using varying NHR lags from −15 s to 15 s (in 0.1-s increments).
To examine whether the Hbr level was altered in the PHR and NHR areas after hindlimb electrical stimulation, the differences in the Hbr level between before (3–5 s) and after (during a 2-s window spanning the next peak time after 5 s) stimulation were determined based on the average Hbr signals in the PHR and NHR areas with a two-tailed t-test with a 99% confidence level. The peak time after stimulation was determined using the spline-fitted average Hbr signal when the signal-to-noise ratio of the original mean Hbr signal was low. The activated region under red illumination was also derived with the above method, delimiting the PHR and NHR regions but utilizing the Hbr signal of each pixel.
Electrophysiological recording.
To examine the relationship between the NHR and neural activity, a 16-channel tungsten MEA (2 × 8 electrodes, 1-MΩ impedance, 250-μm interelectrode spacing) was implanted into the NHR or PHR region as previously determined based on the green illumination signal. We focused on recording neurophysiological signals in the NHR area because the PHR has been addressed extensively. Electrodes were inserted into the NHR region in a total of eight animals; electrodes were inserted into the PHR region in the other two cases. The MEA was implanted into the targeted area after removal of the overlying skull and dura mater and was advanced with a microdrive in 2-μm steps. When advancing the MEA, the electrodes were guided to the targeted region by visual inspection. The tips of the MEA were placed ∼500 μm below the cortical surface (Huang et al. 2014b; Yin et al. 2011). Neural activity from three other depths, including approximately 300, 650, and 800 μm, was also recorded in the NHR region (in the same 8 animals) to compare the results from different cortical layers.
The neural signals were obtained with a Cerebus system (Cyberkinetics Neurotechnology Systems) connected to the MEA (with a 30-kHz sampling rate). The electrical stimulus interference was removed via off-line filtering (Logothetis et al. 2001; Yin et al. 2011). The MUA and the LFP were estimated with power in the ranges of 300-3,000 Hz and 10–130 Hz, respectively (Logothetis et al. 2001); both measures were subsequently downsampled to 10 Hz to generate data that were consistent with the OI data. Next, the MUA and the LFP were transformed into fractional change signals using a baseline of 3–5 s. Finally, the MUA and the LFP were averaged across MEA channels, which were located in the targeted area, specifically either the NHR or the PHR region. Occasionally, some channels were not inserted into the target area to avoid damaging large blood vessels. To test whether the neural activity (MUA and LFP) changed in the PHR and NHR regions during stimulation, the differences in neural activity between before (3–5 s) and during (5–7 s) stimulation were determined with a two-tailed t-test with a 99% confidence level. A stimulus window of 5–7 s was employed because the neural response was instantaneous (Logothetis et al. 2001). In this study, all data analyses were performed in the MATLAB environment (MathWorks, Natick, MA). All results are presented as means and SDs.
RESULTS
Optical observation of NHR.
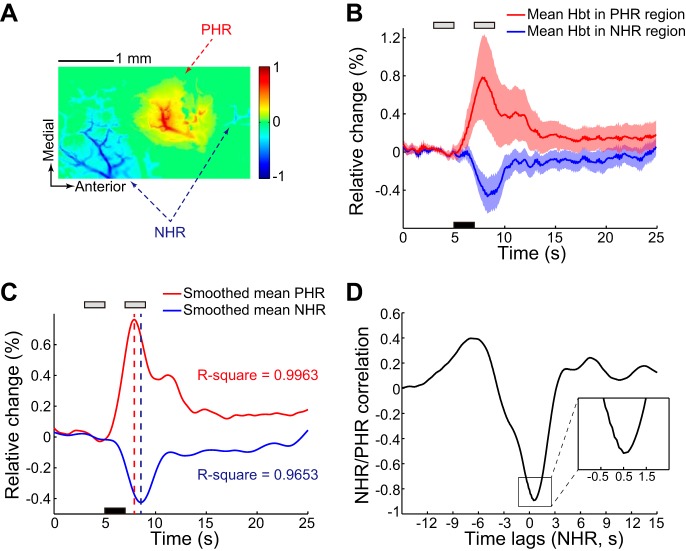
As described in methods, the PHR and NHR regions were determined based on pixel-by-pixel analysis of the Hbt signal obtained under 546-nm illumination. Figure 2 shows a representative experiment investigating the NHR regions, which were located proximal to a positively activated region. The mean blood volume signals for all pixels in the PHR and NHR regions are shown in Fig. 2B. Smoothed curves are also displayed to clearly demonstrate the peak amplitude and the temporal dynamics of the mean PHR and NHR (Fig. 2C). We found that the temporal dynamics of the NHR lagged slightly behind those of the PHR and that the amplitude of the NHR was smaller than that of the PHR. Figure 2D shows the time-lagged correlation between the NHR and PHR. The NHR lagged by 0.5 s relative to the PHR. This issue is discussed in detail below.
Fig. 2.
Hemodynamic response to hindlimb electrical stimulation under green illumination in a representative experiment. A: positive hemodynamic response (PHR) and negative hemodynamic response (NHR) regions were determined based on total blood volume (Hbt) as described in methods. Colorized scale bar represents the normalized signal change from baseline after stimulation. B: average Hbt across pixels in PHR and NHR regions (17,798 pixels in PHR region and 14,633 pixels in NHR region). Shading surrounding the mean time series represents the SD. Black bar denotes the 2-s stimulation, which was initiated at 5 s. Gray bars denote the periods before and after stimulation in which data were collected for analysis. C: spline-fitted average Hbt signals in the PHR and NHR regions. Corresponding R2 values (coefficients of determination) are shown. Dashed red and blue lines indicate the peak times of the mean PHR and NHR, respectively. D: time-lagged correlation between NHR and PHR. Magnified version of the curve in inset illustrates the peak time lag.
The mean blood volume signals for the PHR and NHR regions across all 17 animals are shown in Fig. 3. The mean positive/negative blood volume signals for all animals were computed by averaging the mean blood volume signal in the PHR or NHR region of each animal. A PHR to electrical stimulation was repeatedly observed in the contralateral primary somatosensory hindlimb (S1HL) area (approximately −0.6–3.5 mm posterior to bregma and 1.2–4.1 mm lateral to the midsagittal plane) as reported in our previous study (Yin et al. 2011), which also discussed the spatial distribution of the NHR. Here we focused on the NHR regions adjacent to a PHR. Because the vascular architecture of the cortex is diverse and the location of the NHR varies across animals, normalizing the spatial distribution across different animals was avoided (Kannurpatti and Biswal 2004).
Fig. 3.
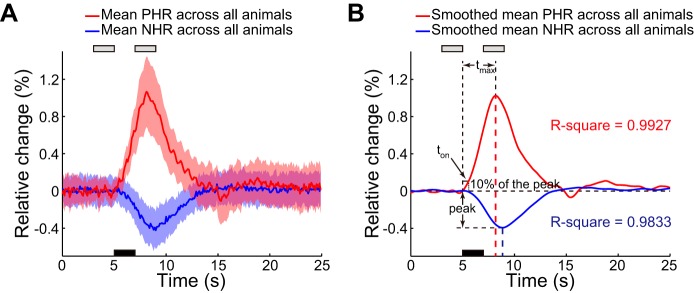
Mean PHR and NHR across all animals. A: mean Hbt values and corresponding SD in the PHR and NHR regions for 17 animals. Black bar denotes the 2-s stimulation, which was initiated at 5 s. Gray bars denote the periods before and after stimulation in which data were collected for analysis. B: corresponding spline-fitted mean Hbt signals. The characteristics of the peak response amplitude and of 2 parameters of temporal dynamics, time to peak (tmax) and onset time (ton), are shown. Dashed red and blue lines indicate peak times of mean PHR and NHR, respectively.
Relationship between PHR and NHR.
To quantify the peak amplitude and the temporal dynamics of the PHR and the NHR, the peak amplitude, tmax, and ton were estimated from the spline-fitted blood volume signal. The characteristics of these three parameters are illustrated in Fig. 3B, which shows the spline-fitted mean blood volume signals in the PHR and NHR regions for all animals.
The statistical results are summarized in Table 1. The mean peak amplitude of the PHR was 1.03 ± 0.47%, and this peak occurred ∼3.18 s after stimulus onset. The mean NHR peak amplitude (0.40 ± 0.21%) was approximately one-third of the PHR [1-way ANOVA, F(1,32) = 25.43, P < 10−4], and the NHR peak (tmax = 3.85 ± 0.51 s) was slightly delayed compared with the PHR peak [1-way ANOVA, F(1,32) = 13.14, P < 10−3]. The ton of the NHR (1.02 ± 0.36 s) was significantly longer than that of the PHR (0.58 ± 0.31 s) in all animals [1-way ANOVA, F(1,32) = 14.10, P < 10−3]. Furthermore, the NHR was found to lag relative to the PHR based on cross-correlation analysis. The mean lag time for all animals was 0.67 ± 0.27 s. Briefly, the NHR displayed the same waveform shape as the PHR, but the NHR exhibited a smaller peak amplitude and slower temporal dynamics than the PHR (Fig. 3B).
Table 1.
Mean response amplitude and temporal dynamics of Hbt across all animals
| Peak Amplitude, % | ton, s | tmax, s | |
|---|---|---|---|
| Hbt in PHR region | 1.03 ± 0.47 | 0.58 ± 0.31 | 3.18 ± 0.57 |
| Hbt in NHR region | 0.40 ± 0.21** | 1.02 ± 0.36* | 3.85 ± 0.51* |
Mean ± SD mean peak amplitude, time to reach 10 % of the peak amplitude (ton), and time to peak (tmax) of total blood volume (Hbt) in the positive hemodynamic response (PHR) and negative hemodynamic response (NHR) regions across all animals (n = 17) are presented.
P < 10−3,
P < 10−4 compared with the corresponding parameters of Hbt in the PHR region.
Deoxyhemoglobin signal changes in PHR and NHR regions.
In each experiment, the identical cortical area was illuminated with a 630-nm light to obtain the Hbr signal after collecting the blood volume data under 546-nm illumination. The changes in the Hbr levels in the PHR and NHR regions (determined via 546-nm illumination) were examined. Figure 4 shows the hemodynamic response of the PHR and NHR regions in the same experiment as that shown in Fig. 2 but under 630-nm illumination. The Hbr signal in the PHR region was significantly increased after stimulation [2-tailed t-test, t(38) = 37.84, P < 10−30], but that in the NHR region was unchanged after stimulation [2-tailed t-test, t(38) = 0.08, P = 0.939]. The 6.7–8.7 s and 5.8–7.8 s time windows were used to determine the changes in the Hbr levels in the PHR and NHR regions because the subsequent peak times after stimulation in these two regions were 7.7 and 6.8 s, respectively. Figure 4C shows the activated region under red illumination. The red and blue borders denote the PHR and NHR regions displayed in Fig. 2, respectively. The activated region under red light was located in a position similar to the PHR region. No reduction in the Hbr signal was observed in the NHR region after stimulation.
Fig. 4.
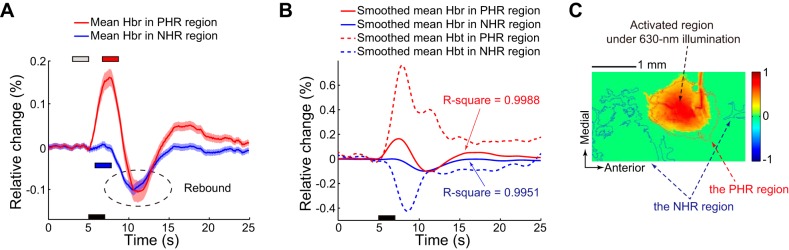
Hemodynamic response to electrical stimulation of the hindlimb under 630-nm illumination in the experiment described in Fig. 2. A: mean Hbr signals across pixels in the PHR and NHR regions determined based on the Hbt signals (obtained under 546-nm illumination). Shading surrounding the mean time series represents the SD. Black bar denotes the 2-s stimulation, which was initiated at 5 s. Gray bar denotes the baseline period. Red and blue bars denote the periods after stimulation used to determine the changes in the Hbr level in the PHR and NHR regions, respectively. Dashed ellipse indicates the rebound of the Hbr level. B: spline-fitted average Hbr level in PHR and NHR regions. Corresponding R2 values are shown. Spline-fitted average Hbt signals in the PHR and NHR regions are also shown to compare the temporal dynamics between the Hbr and Hbt signals. C: activated region under 630-nm illumination. Red and blue borders denote PHR and NHR regions, respectively, which were determined based on the Hbt data, which were obtained under 546-nm illumination.
In summary, the Hbr level in the PHR area was increased immediately after stimulation in each rat. The Hbr level in the NHR region was unchanged in 12 rats and increased in 5 rats; i.e., no decrease in the Hbr level was found in the NHR region after stimulation. The statistical analyses of the changes in the Hbr signal in the PHR and NHR regions after stimulation are shown in Fig. 5. All Hbr levels prior to stimulation were set to zero because the Hbr signal was calculated as the relative change compared with baseline (see methods); thus the mean Hbr level prior to stimulation (i.e., the mean of the baseline) was zero. The Hbr level in the PHR region after stimulation was significantly increased to 0.215 ± 0.138% [n = 17; 2-tailed t-test, t(32) = 6.40, P < 10−6]. Based on a confidence level of 99%, the NHR region was stratified according to whether the Hbr level was increased or unchanged. In the increased Hbr group, the Hbr level was increased to 0.121 ± 0.045% after stimulation [n = 5; 2-tailed t-test, t(8) = 6.03, P < 0.001]. In the unchanged Hbr group, the Hbr level was nonsignificantly increased to 0.004 ± 0.024% after stimulation [n = 12; 2-tailed t-test, t(22) = 0.62, P = 0.542]. Additionally, the increase in the Hbr level was not significantly different between the PHR regions and the NHR regions in the increased Hbr group [2-tailed t-test, t(20) = 1.47, P = 0.157].
Fig. 5.
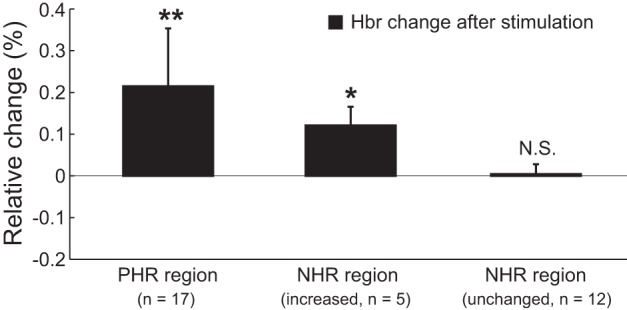
Statistical analysis of Hbr signal changes in PHR and NHR regions after stimulation in all animals. Data are presented as means ± SD. *P < 0.001, **P < 10−6 compared with Hbr prior to stimulation (zero) based on the 2-tailed t-test. N.S., not significant.
Notably, the Hbr signal rebounded after the initial period following stimulation not only in the PHR region but also in the NHR region (e.g., the area marked with the dashed ellipse in Fig. 4A). In the NHR region, the Hbr signal rebounded to a level below baseline in all animals (2-tailed t-test; P < 0.01). The issue of rebounding of the Hbr level in the NHR region is discussed below.
Neural activity changes in PHR and NHR regions.
To explore the changes in neural activity induced by electrical stimulation, the LFP and the MUA in the PHR and NHR regions were measured with an MEA. In total, neural activity was recorded in the NHR region of eight animals and in the PHR region of two animals. Figure 6 illustrates the LFP and MUA changes in the PHR and NHR regions from three representative experiments. Neural activity in the PHR area was markedly increased [2-tailed t-test, t(38) = 8.92, P < 10−10 for LFP; t(38) = 10.40, P < 10−11 for MUA] (Fig. 6A). The LFP and the MUA were averaged across the 12 MEA channels implanted in the PHR region. Figure 6B shows unchanged neural signals in the NHR region. The LFP and the MUA, which were averaged across eight electrodes located in the NHR region, were not significantly altered after stimulation [2-tailed t-test; t(38) = −0.49, P = 0.628; t(38) = 0.37, P = 0.715, respectively]. The alternative scenario that occurred in the NHR region, in which neural activity was increased after stimulation [2-tailed t-test, t(38) = 6.96, P < 10−7 for LFP; t(38) = 9.98, P < 10−11 for MUA], is shown in Fig. 6C. These data were averaged from six channels located in the NHR area. The spontaneous oscillations that occurred during neural activity have been discussed in our previous studies (Huang et al. 2014b), and these oscillations did not influence our analysis. In summary, neural activity in the PHR region was increased (in both animals examined) and that in the NHR area was either unchanged (in 6 animals) or increased (in 2 animals) during stimulation.
Fig. 6.
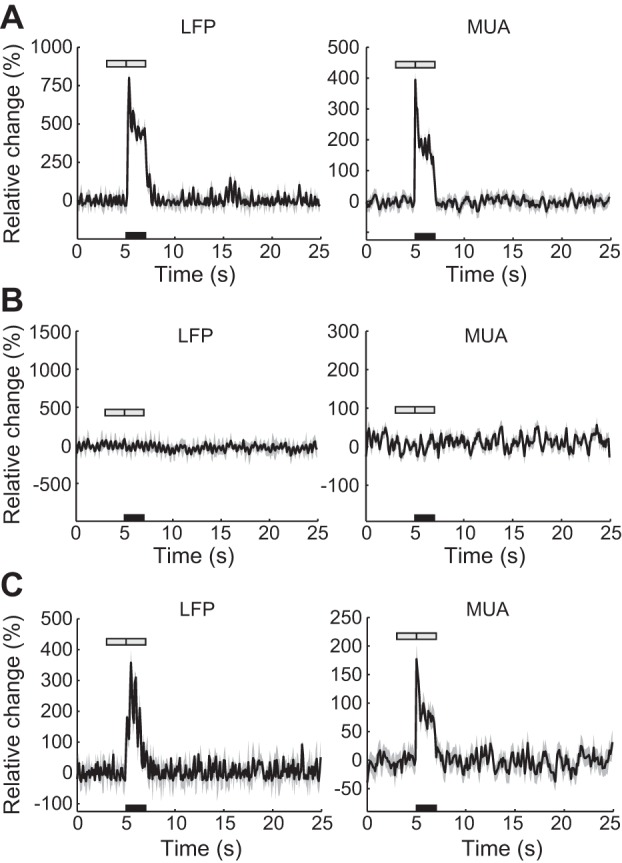
Neural activity changes in PHR and NHR regions. A: mean local field potential (LFP) and multiunit activity (MUA) recorded from a typical experiment. Data were averaged from 12 microelectrode array (MEA) channels located in the PHR region. Shading surrounding the mean time series represents the SD. Black bar denotes the 2-s stimulation, which was initiated at 5 s. Gray bars denote the periods before and after stimulation in which data were collected for analysis. B and C: same measurement as in A, except that the mean LFP and MUA were obtained from 2 representative experiments examining the NHR area. B and C show unchanged and increased neural activity, respectively; the data were averaged from 8 and 6 electrodes inserted into the NHR region, respectively.
Figure 7 shows the statistical analysis of the neural activity changes in the PHR and NHR regions in all 10 animals. For both the PHR regions and the NHR regions in the increased neural activity group, data were generated from two animals, and the statistical significance of these results was not computed (although neural activity in each animal of these 2 groups was increased based on the 2-tailed t-test). In the NHR regions in the unchanged neural activity group, no significant stimulation-induced change was found in the LFP [n = 6; 2-tailed t-test, t(10) = 0.747, P = 0.472] or the MUA [n = 6; t(10) = −0.856, P = 0.412]. Table 2 shows the Hbr signal and neural activity changes elicited by stimulation in different animals. We observed that the change in neural activity was analogous to that in the Hbr signal, either unchanged or increased in the NHR region, suggesting that the NHR occurred in the absence of neural deactivation. To further examine the correlation between neural activity and the NHR, we compared the t-statistic of the blood volume with the t-statistic of the LFP and the MUA in the NHR region. t-Statistic values were employed because they can suitably represent the changes in variables (Ekstrom et al. 2009). The correlation plot is shown in Fig. 8. All blood volume t-statistic values were below zero because the blood volume was decreased after stimulation in the NHR region. The slopes for the regressions of Hbt vs. LFP and Hbt vs. MUA were −0.039 and −0.098, respectively. No significant correlation was found between the blood volume and either the LFP changes (P = 0.929) or the MUA changes (P = 0.764).
Fig. 7.
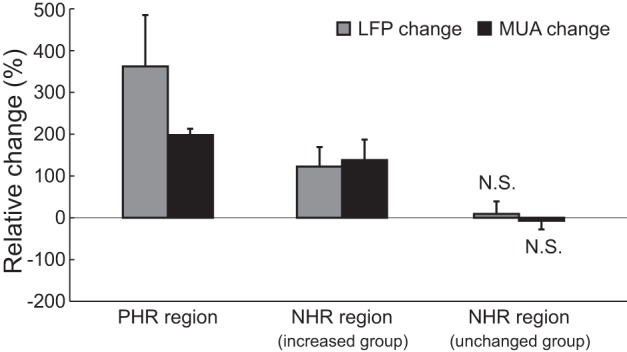
Statistical analysis of stimulation-elicited neural activity changes in the PHR and NHR regions. Data are presented as means ± SD. N.S., not significant based on 2-tailed t-test.
Table 2.
Hbr signal and neural activity changes in all animals
| Animal |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| PHR region | |||||||||||||||||
| Hbr | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| LFP and MUA | ↑ | ↑ | |||||||||||||||
| NHR region | |||||||||||||||||
| Hbr | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | ↑ | ↑ | ↑ | ↑ |
| LFP and MUA | – | – | – | – | – | – | ↑ | ↑ | |||||||||
Hbr, deoxyhemoglobin; LFP, local field potential; MUA, multiunit activity; ↑, increased in response to stimulation; –, unchanged in response to stimulation.
Fig. 8.
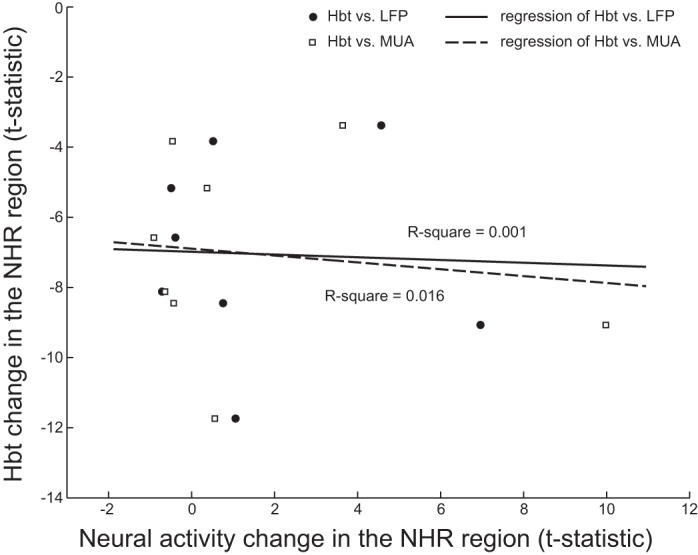
Correlation plot of Hbt changes (t-statistic values) against neural activity changes in the NHR region. Corresponding R2 values (coefficients of determination) are shown.
In addition to the neural activity at a depth of ∼500 μm, neural activity was recorded at three other cortical depths, approximately 300, 650, and 800 μm, in the NHR region (in 8 animals) to compare the results from different layers. No significant differences in neural activity changes were found between different cortical layers. Based on a study by Boorman et al. (2010), the NHR was confined to deeper cortical layers (predominantly in layers V–VI) of the barrel region. However, Devor et al. (2005) reported that the NHR can also occur in superficial layers (layer II/III). These results may depend on the stimulus paradigm and the observation method. Examination of the NHR at various cortical layers will require further specialized research.
DISCUSSION
In this study, we investigated the NHR to hindlimb electrical stimulation in the rat cortex and focused on the NHR regions adjacent to a PHR region. Based on their temporal dynamics, the NHR occurred shortly after the PHR. Additionally, the Hbr signal in the PHR areas was increased immediately after stimulation in all of the animals but was unchanged or increased in the NHR regions. The electrophysiological recordings using a MEA suggested that no neural deactivation occurred in the NHR areas. These results imply that blood stealing and increased neural activity are possible origins of the NHR and that caution should be applied when interpreting the NHR as neural deactivation, especially when the NHR is adjacent to a PHR.
Origin of the NHR.
Currently, the NHR is attributed to two major factors: the reduction or suppression of neural activity and the hemodynamic blood stealing effect (Harel et al. 2002; Shih et al. 2009). Our results from the 12 animals displaying unchanged Hbr levels, 6 of which exhibited unaltered neural activity in the NHR region, support the blood stealing effect. The blood stealing hypothesis proposes that an increase in blood flow in the activated region causes a decrease in blood flow in an adjoining area. If this phenomenon is present, the dynamics of the adjoining area will be temporally slower than or equal to those of the “stealing” area (Harel et al. 2002; Kannurpatti and Biswal 2004). In the present study, the NHR occurred after the PHR; specifically, ton and tmax were ∼0.44 s and ∼0.67 s longer for the NHR than for the PHR (Table 1). Additionally, a lag of ∼0.67 s was observed based on cross-correlation analysis, providing support for the blood stealing concept. This delay may have been generated by the transportation of fresh blood from the NHR region to the PHR region. Our calculations revealed the mean center distance between the PHR and NHR regions (1.09 ± 0.46 mm, n = 17). According to the time delay mentioned above, this distance corresponds well with the observation that the capillary blood flow velocities range from 1 to 2.5 mm/s (Ivanov et al. 1981).
Notably, the Hbr level rebounded after the initial period after stimulation in both the PHR and NHR regions (e.g., Fig. 4A). In the PHR area, it is relatively simple for us to conclude that the rebound was elicited by the increase in neural activity and the oversupply of oxygenated blood, which was detected with a common fMRI technique. In the NHR region, the Hbr level also rebounded to a level below baseline. One possible explanation for this result is that the NHR area transported blood to the PHR region and that the Hbr level decreased together with the decline in Hbt. In fact, during the rebound period there was no change in the LFP or the MUA (e.g., Fig. 6). Generally, fMRI maps the rebound signal of Hbr, thus facilitating the consideration of the NHR and PHR regions as equivalent. From this perspective, the blood volume signal may display a higher resolution than the Hbr signal, as proposed by previous reports (Moon et al. 2013; Sirotin et al. 2009; Zhao et al. 2006). Moreover, the ratio of the peak PHR amplitudes to the peak NHR amplitudes was ∼2.6:1, which is comparable to the results of previous studies (Boorman et al. 2010; Kannurpatti and Biswal 2004; Yin et al. 2011). This similar amplitude ratio implies that the NHR can be reliably recorded across different modalities and stimulus paradigms, at least in the rat somatosensory cortex.
The data from the five animals displaying increased Hbr levels, two of which exhibited increased neural activity in the NHR region, suggest that the NHR may be produced during increased neural activity. This hypothesis has been proposed in recent years based on the proposal that the NHR may reflect a mismatch between increased neural activity and insufficient blood supply (Moraschi et al. 2012; Mullinger et al. 2014; Schridde et al. 2008). Importantly, the present study revealed no reduction in neural activity, and these results provide a compelling argument against the neuronal suppression theory. Nevertheless, it should be emphasized that our study does not refute the deactivation model regarding the NHR. We simply conclude in this discussion that neural deactivation cannot be adaptive in all cases, especially when the NHR is adjacent to a PHR, and that other mechanisms such as blood stealing and increased neural activity represent viable explanations for the NHR.
Comparisons with previous studies.
To date, studies investigating the mechanisms of the NHR are sparse, and those performing electrophysiological recordings are even less common. For instance, two pioneering investigations exploring the origin of the NHR have proposed the blood stealing theory (Harel et al. 2002; Kannurpatti and Biswal 2004). Both studies lacked electrophysiological recordings but suggested that the measurement of neural activity, especially the LFP, was required to precisely elucidate the source of the NHR. Smith et al. (2004) ruled out the blood stealing effect in their study because the negative BOLD signal change was observed in the hemisphere contralateral to the positive BOLD signal change rather than in a local area; they also suggested that direct observation of neuronal activity was necessary.
A few studies have recorded comparable decreases in the MUA corresponding to the NHR; these studies supported the neural deactivation model of the NHR (Boorman et al. 2010; Shmuel et al. 2006; Yin et al. 2011). Nevertheless, the change in the LFP, which is the most important parameter regarding the generation of the fMRI signal (Ekstrom 2010; Huang et al. 2014b; Logothetis et al. 2001), was not fully consistent between these studies. Shmuel et al. (2006) explained analogous decreases in the LFP to those in the MUA, whereas Boorman et al. (2010) showed significant increases in the LFP and Yin et al. (2011) reported no change in the LFP during stimulation. In a recent study employing EEG, Mullinger et al. (2014) proposed that the NHR was neurogenic but that it may not simply originate from the neurophysiological inverse of the PHR. Schridde et al. (2008) observed increased MUA and LFP in conjunction with negative BOLD signal changes in the rat hippocampus during seizures and proposed that the NHR might be generated by an increase in neural activity. Nevertheless, seizures are different from normal task performance or resting states, and seizures may affect the relationship between hemodynamic and neural activity. Ekstrom et al. (2009) further investigated how the human hippocampal BOLD responses related to neural activity changes during virtual navigation and reported that the correlation between theta-band LFP and BOLD signal changes was significant in the parahippocampal cortex but not in the hippocampus. The authors speculated that the lack of a significant correlation in the hippocampus may have occurred because the hippocampus theta-band LFP did not correspond to decreases in BOLD. They also indicated that their results may have been influenced by different regions and by the tasks selected and that firm conclusions would require additional experiments. Devor et al. (2005) demonstrated no change in the LFP or the MUA of the NHR region surrounding the area of hemodynamic activation. The present study revealed both increased and unchanged neural activity in the NHR area not only in the MUA but also in the LFP. Table 3 lists representative studies that have explored the mechanisms of the NHR.
Table 3.
Representative studies that investigated mechanisms of the NHR
| Study | Model | Stimulus | Recording Method | Neural Activity Changes in NHR Region | Potential Mechanism |
|---|---|---|---|---|---|
| Harel et al. 2002 | Cat cortex | Visual/10 s | fMRI | Blood stealing | |
| Kannurpatti and Biswal 2004 | Rat cortex | Whisker/2 s | LDF | Blood stealing | |
| Smith et al. 2004 | Human cortex | Visual/15 s | fMRI | Blood stealing was ruled out | |
| Devor et al. 2005 | Rat cortex | Whisker/transient | OI/MEA | MUA – | No neural deactivation |
| LFP – | |||||
| Shmuel et al. 2006 | Monkey cortex | Visual/20 s | fMRI/electrode | MUA ↓ | Neural deactivation |
| LFP ↓ | |||||
| Schridde et al. 2008 | Rat hippocampus | During seizures | fMRI/LDF/MEA | MUA ↑ | Increased neural activity |
| LFP ↑ | |||||
| Ekstrom et al. 2009 | Human hippocampus | Virtual navigation | fMRI/depth electrode | No correlation with NHR | No neural deactivation |
| Boorman et al. 2010 | Rat cortex | Whisker/16 s | OI/fMRI/LDF/MEA | MUA ↓ | Neural deactivation |
| LFP ↑ | |||||
| Yin et al. 2011 | Rat cortex | Hindlimb/10 s | OI/MEA | MUA ↓ | Neural deactivation |
| LFP – | |||||
| Mullinger et al. 2014 | Human cortex | Median nerve/10 s | fMRI/EEG | Correlated with NHR | Not simply neural deactivation |
| Present study | Rat cortex | Hindlimb/2 s | OI/MEA | MUA–or ↑ | No neural deactivation |
| LFP–or ↑ |
fMRI, functional magnetic resonance imaging; LDF, laser Doppler flowmetry; OI, optical imaging; MEA, microelectrode array; ↑, neural activity was increased in response to stimulation; –, neural activity was unchanged in response to stimulation; ↓, neural activity was decreased in response to stimulation.
Notably, different origins of the NHR are not mutually exclusive. They may occur in different cases and may even be concomitant in a scenario consisting of an identical stimulation paradigm, as shown in this study. In fact, we have reported a neuronal suppression origin of the NHR by employing a different hindlimb stimulus paradigm in a previous study (Yin et al. 2011), in which neural activity was recorded from the relatively remote visual cortex rather than the somatosensory cortex. In this study, we used a progressive MEA and obtained neural activity from the PHR regions and the NHR areas adjacent to a PHR region. In this respect, we believe that the present study enriches the current knowledge and perspectives regarding the NHR in the cerebral cortex. The presence and the characteristics of the NHR may be affected by various factors, including species, brain region, physiological state (including anesthesia), stimulus paradigm, and cerebral vascular structure (Ekstrom et al. 2009; Yin et al. 2011). For example, although there is no immediate evidence confirming this effect, the neural deactivation-related NHR tended to appear during prolonged stimulation (Boorman et al. 2010; Shmuel et al. 2006), whereas blood stealing-related NHR was more likely to be induced by brief stimulation (Devor et al. 2005; Kannurpatti and Biswal 2004). The relationship between the stimulus duration and the NHR modality merits further investigation; this topic will be addressed in our future research. Furthermore, the mechanism underlying the NHR may be anesthesia dependent. Harel et al. (2002), who used a ketamine and xylazine cocktail, and Kannurpatti and Biswal (2004), who used urethane, have proposed the blood stealing mechanism. Boorman et al. (2010) also employed urethane but supported the neural deactivation mechanism. Devor et al. (2005) and Schridde et al. (2008) employed halothane and found no neural deactivation and increased neural activity in the NHR region, respectively. Currently, it is difficult to compare the influence of different anesthetics on the NHR because the presence and the characteristics of the NHR may be affected by other factors, such as those mentioned above. In the future, we could employ various anesthetics and fix other variables to investigate the effect of anesthetics on the NHR (Magnuson et al. 2014).
Several previous studies have reported that positive blood volume regions corresponded to negative Hbr maps and vice versa (Boorman et al. 2010; Devor et al. 2005). Alternatively, as discussed above, we found that the Hbr signal was increased in the PHR region after stimulation. The reason for this discrepancy may be that the previous studies interpreted the change in the Hbr signal over relatively long periods (tens of seconds after a stimulus) but we focused on the Hbr signal change immediately after stimulation. Indeed, in the PHR area, the Hbr signal was also decreased after the initial period following stimulation in this study (see Fig. 4). Conversely, in previous reports and in this study, the Hbr level was increased in the positive blood volume regions immediately after a stimulus [e.g., Fig. 1a of Devor et al. (2005)]; an initial increase in the Hbr level was also recorded in these studies but was not the main focus.
Limitations.
Our definition of the NHR was different from that used in some other studies considering the negative BOLD response, in which the NHR predominantly reflected the local changes in oxygenation. In this study, the NHR was defined as a reduction in the Hbt (i.e., CBV) signal induced by the stimulus, as defined in previous studies (Harel et al. 2002; Yin et al. 2011). The blood volume and the BOLD signals were found to be covariant, and the negative blood volume served as an excellent substitute for the negative BOLD response in several studies (Boorman et al. 2010; Harel et al. 2002). Nonetheless, the BOLD signal contrast depends on complex interactions between various parameters, including CBF, CBV, and CMRO2 (Ogawa et al. 1990), all of which may be related to neural activity (Schridde et al. 2008). Furthermore, Goense et al. (2012) have reported opposing changes between CBF and CBV in negative BOLD signal regions in the monkey cortex (upon application of prolonged visual stimuli) and have proposed a novel mechanism underlying NHR generation; a decrease in cortical CBF in the upper layers was accompanied by an increase in CBV in the middle layers (Mullinger et al. 2014). In addition, the OI technique captures the signals on the surface of the brain, whereas the fMRI obtains those in deeper layers. It is unclear whether the PHR or the NHR at the surface, where the larger vessels are located, is truly representative of the activity within the cortex. Therefore, the results from this study cannot be directly compared with those of fMRI studies and can only provide implications regarding fMRI results. Similar multimodal investigations involving other components, such as CBF (Kannurpatti and Biswal 2004), are recommended to investigate the discrepancy between the negative signals delineated using different hemodynamic parameters. CBF may provide the most direct evidence of the blood stealing mechanism and may contribute to the discrepancies between CBV and the Hbr signals.
One limitation of this study is that the hemodynamic responses and neural activity were not observed simultaneously, as performed in several previous studies (Ekstrom et al. 2009; Shih et al. 2009). The electrophysiological measurements were performed in the same animals after the hemodynamic recordings using the identical experimental paradigm. During optical recording, the measurements were performed through a region of thinned translucent skull in anesthetized rats. Alternatively, for the observation of neural activity, the skull and dura were removed and electrodes were subsequently inserted into the cortex. Moreover, the blood volume and the Hbr signal were not measured simultaneously. We obtained the Hbr signal under 630-nm illumination after collecting the blood volume under 546-nm illumination in the same animals with the identical stimulus paradigm. These asynchronous operational procedures may have affected our findings regarding the NHR origin. In addition, the penetration depths of 546-nm and 630-nm light are different (∼0.58 and 0.74 mm, respectively) (Yaroslavsky et al. 2002). However, we recorded neural activity at different depths and found no significant differences in the neural activity changes between different cortical layers. Thus penetration depth may affect the comparison of blood volume with the Hbr level but not the comparison of the hemodynamic (blood volume and Hbr level) responses with neural activity in this study. However, alternative approaches in which hemodynamics and neural activity are simultaneously recorded would help to thoroughly address the controversies regarding the mechanism(s) involved in the NHR (Hayes and Huxtable 2012).
Conclusions.
In the present study, the NHR adjacent to a PHR region was induced by hindlimb electrical stimulation and was observed via OI and electrophysiological recording. The dynamics of the blood volume in the NHR regions lagged slightly behind those in the PHR areas, and the Hbr levels and neural activity in the NHR regions were unchanged or were increased immediately after stimulation. These results imply that no neural deactivation occurred during the NHR. Furthermore, these findings suggest that blood stealing and increased neural activity are possible origins of the NHR and that caution should be taken when interpreting the NHR as a decrease in neural activity, especially when the NHR is adjacent to a PHR. This study, which provides evidence against the neural deactivation theory, may enhance our understanding of the NHR in the cerebral cortex.
GRANTS
This work was supported by the National Basic Research Program of China (Grant 2011CB707802) and the National Natural Science Foundation of China (Grants 91420302 and 61420106001).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.H. and L.H. conception and design of research; D.H. and L.H. interpreted results of experiments; D.H. and L.H. drafted manuscript; D.H. and L.H. edited and revised manuscript; D.H. and L.H. approved final version of manuscript; L.H. performed experiments; L.H. analyzed data; L.H. prepared figures.
REFERENCES
- Bianciardi M, Fukunaga M, Van Gelderen P, De Zwart JA, Duyn JH. Negative BOLD-fMRI signals in large cerebral veins. J Cereb Blood Flow Metab 31: 401–412, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, Berwick J. Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci 30: 4285–4294, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci 27: 4452–4459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA 102: 3822–3827, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev 62: 233–244, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S. Correlation between BOLD fMRI and theta-band local field potentials in the human hippocampal area. J Neurophysiol 101: 2668–2678, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense J, Merkle H, Logothetis NK. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron 76: 629–639, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab 22: 908–917, 2002. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Huxtable AG. Interpreting deactivations in neuroimaging. Front Psychol 3: 27, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci 26: 5819–5824, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Liu Y, Gui J, Li M, Hu D. Stimulus-dependent modulation of spontaneous low-frequency oscillations in the rat visual cortex. Neuroreport 25: 823–828, 2014a. [DOI] [PubMed] [Google Scholar]
- Huang L, Liu Y, Li M, Hu D. Hemodynamic and electrophysiological spontaneous low-frequency oscillations in the cortex: directional influences revealed by Granger causality. Neuroimage 85: 810–822, 2014b. [DOI] [PubMed] [Google Scholar]
- Ivanov KP, Kalinina MK, Levkovich YI. Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc Res 22: 143–155, 1981. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Negative functional response to sensory stimulation and its origins. J Cereb Blood Flow Metab 24: 703–712, 2004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shen H, Zhou Z, Hu D. Sustained negative BOLD response in human fMRI finger tapping task. PLoS One 6: e23839, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001. [DOI] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD. Time-dependent effects of isoflurane and dexmedetomidine on functional connectivity, spectral characteristics, and spatial distribution of spontaneous BOLD fluctuations. NMR Biomed 27: 291–303, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, Desalvo MN, Enev M, Sanganahalli BG, Hyder F, Blumenfeld H. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci 31: 15053–15064, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon CH, Fukuda M, Kim SG. Spatiotemporal characteristics and vascular sources of neural-specific and -nonspecific fMRI signals at submillimeter columnar resolution. Neuroimage 64: 91–103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraschi M, DiNuzzo M, Giove F. On the origin of sustained negative BOLD response. J Neurophysiol 108: 2339–2342, 2012. [DOI] [PubMed] [Google Scholar]
- Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG-BOLD-CBF study in humans. Neuroimage 94: 263–274, 2014. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage 36: 269–276, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). Zürich, Switzerland: Academic, 2007. [Google Scholar]
- Pouratian N, Cannestra AF, Martin NA, Toga AW. Intraoperative optical intrinsic signal imaging: a clinical tool for functional brain mapping. Neurosurg Focus 13: e1, 2002. [DOI] [PubMed] [Google Scholar]
- Prahl S. Tabulated Molar Extinction Coefficient for Hemoglobin in Water [EB/OL]. http://omlc.ogi.edu/spectra/hemoglobin/summary.html, 1998. [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex 18: 1814–1827, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci 29: 3036–3044, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9: 569–577, 2006. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36: 1195–1210, 2002. [DOI] [PubMed] [Google Scholar]
- Sirotin YB, Hillman EM, Bordier C, Das A. Spatiotemporal precision and hemodynamic mechanism of optical point spreads in alert primates. Proc Natl Acad Sci USA 106: 18390–18395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AT, Williams AL, Singh KD. Negative BOLD in the visual cortex: evidence against blood stealing. Hum Brain Mapp 21: 213–220, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga A, Mazziotta JC. Brain Mapping: The Methods. Zürich, Switzerland: Academic, 2002. [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci 10: 1308–1312, 2007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hu D, Liu Y, Li M. Cerebral artery-vein separation using 0.1-Hz oscillation in dual-wavelength optical imaging. IEEE Trans Med Imaging 30: 2030–2042, 2011. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky AN, Schulze PC, Yaroslavsky IV, Schober R, Ulrich F, Schwarzmaier HJ. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys Med Biol 47: 2059–2073, 2002. [DOI] [PubMed] [Google Scholar]
- Yin H, Liu Y, Li M, Hu D. Hemodynamic observation and spike recording explain the neuronal deactivation origin of negative response in rat. Brain Res Bull 84: 157–162, 2011. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K, Kim SG. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. Neuroimage 30: 1149–1160, 2006. [DOI] [PubMed] [Google Scholar]