Abstract
A fundamental goal of systems neuroscience is to understand the neural mechanisms underlying decision making. The midbrain superior colliculus (SC) is known to be central to the selection of one among many potential spatial targets for movements, which represents an important form of decision making that is tractable to rigorous experimental investigation. In this review, we first discuss data from mammalian models—including primates, cats, and rodents—that inform our understanding of how neural activity in the SC underlies the selection of targets for movements. We then examine the anatomy and physiology of inputs to the SC from three key regions that are themselves implicated in motor decisions—the basal ganglia, parabrachial region, and neocortex—and discuss how they may influence SC activity related to target selection. Finally, we discuss the potential for methodological advances to further our understanding of the neural bases of target selection. Our overarching goal is to synthesize what is known about how the SC and its inputs act together to mediate the selection of targets for movements, to highlight open questions about this process, and to spur future studies addressing these questions.
Keywords: decision making, motor planning, substantia nigra, pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus
elucidating the neural mechanisms of decision making is a fundamental goal of neurobiology (Glimcher 2003; Gold and Shadlen 2007; Hikosaka et al. 2014; Sugrue et al. 2005). Among the many forms of common decisions, one that is both critical for survival and amenable to study across a range of model systems is the process of selecting a spatial target for movement from among several alternatives (Wolpert and Landy 2012). Targets—considered here as the spatial locations themselves—for movements are selected based on several sensory and nonsensory factors, including stimulus input, the learned value associated with each target (e.g., based on previous experience), and internally generated goals (Gold and Shadlen 2007). Understanding how and where these factors are integrated in the nervous system in order to select a target for movement is an active area of research.
Accumulating evidence points to the mammalian superior colliculus (SC) playing an important role in the selection of spatial targets for movement, particularly when cued by sensory stimuli (Krauzlis et al. 2004). While we focus here on the mammalian SC, the analogous structure in other vertebrates, the optic tectum, serves a similar function (Dutta and Gutfreund 2014; Mysore and Knudsen 2011; Nevin et al. 2010). The SC is a midbrain structure composed of seven distinct cytoarchitectonically defined layers and can be divided into superficial and deep functional subdivisions. While the superficial subdivision receives visual input, the deep subdivision of the SC—comprising the gray and white sublayers of the intermediate and deep layers of the SC—receives somatosensory, auditory, and visual input and is a source of commands to downstream reticular premotor nuclei that contribute to the production of coordinated orienting movements to spatial targets (King 2004; Sparks and Hartwich-Young 1989). Critically, the deep subdivision of the SC also receives input from a wide range of cortical and subcortical structures (Edwards et al. 1979; Fries 1984; Sparks and Hartwich-Young 1989). Therefore, the SC is well positioned to serve as a functional hub in the interconnected network of brain regions underlying the integration of multisensory and contextual information in order to select a target for orienting movements (Platt et al. 2004; Stein and Rowland 2011). Examining how this integration occurs is important in its own right for understanding goal-directed behavior, and can also serve as a model for studying how multiple brain regions can work together to process information and transform neural representations.
An exhaustive examination of every functional input to the SC would be beyond the scope of this review. Instead, after first describing intrinsic SC processing underlying target selection for movement, we focus on three inputs that have been examined in the context of their influence on intrinsic SC processing: GABAergic input from the substantia nigra pars reticulata (SNr), cholinergic input from the parabrachial region [primarily composed of the pedunculopontine tegmental nucleus (PPTg) and laterodorsal tegmental nucleus (LDTg)], and glutamatergic input from neocortical regions (Fig. 1A). These three projections converge anatomically in discrete zones in the deep subdivision of the SC (Fig. 1B; Illing and Graybiel 1985), suggesting that they may play complementary functional roles. We discuss below the data and theories bearing upon these roles. It is our hope that this review, by synthesizing data from a range of studies to provide a comprehensive description of what is currently known about how the SC and its inputs select targets for movements, will stimulate future study in this field.
Fig. 1.

Input to the superior colliculus (SC) from key regions underlying sensorimotor decision making that are the focus of this review. A: schematic of anatomical projections and information flow. While the SC receives input from numerous regions, we focus here on the glutamatergic input from the neocortex, cholinergic input from the pedunculopontine tegmental nucleus (PPTg) and laterodorsal tegmental nucleus (LDTg), and GABAergic input from the substantia nigra pars reticulata (SNr). This input influences intrinsic processing in the SC that may underlie the selection of targets for movements, which are then coordinated and initiated via SC output to premotor nuclei in the brain stem and spinal cord. B: schematic of synaptic inputs to the deep subdivision of the SC from the above regions. Notably, axons projecting from regions that may “drive” SC activity (e.g., neocortex) are thicker and synapse more proximal to the soma than axons projecting from regions that may “modulate” SC activity (e.g., SNr, PPTg/LDTg).
Activity in Superior Colliculus Underlying Selection of Targets for Movements
While early recordings in head-fixed primates established a clear relationship between activity in specific regions of the deep subdivision of the SC and the initiation of saccadic eye movements to specific locations in the contralateral visual field (Goldberg and Wurtz 1972a, 1972b; Lee et al. 1988; Wurtz and Goldberg 1972), subsequent work demonstrated that SC activity was not particularly related to saccades but to orienting movements in general (Sparks 1999). Specifically, the SC seems to similarly encode spatial targets for multistep saccades (Bergeron et al. 2003) and smooth eye movements (Carello and Krauzlis 2004; Krauzlis 2004; Missal et al. 1996). In addition, SC activity represents orienting movements of the head and body (Corneil et al. 2002; Felsen and Mainen 2008; Freedman et al. 1996; Gandhi and Katnani 2011; Guillaume and Pélisson 2001; Walton et al. 2007) as well as of the limbs (i.e., reaching movements; Courjon et al. 2004; Philipp and Hoffmann 2014; Werner et al. 1997), although the topographic organization within the SC of targets for these forms of movements may be less precise than it is for eye movements (Gandhi and Katnani 2011; Philipp and Hoffmann 2014; Walton et al. 2007). While the SC has been most intensively studied in the context of visuomotor transformations, it has been shown to mediate responses to nonvisual inputs as well (Aronoff et al. 2010; Bajo et al. 2010; Felsen and Mainen 2008; Groh and Sparks 1996; Jay and Sparks 1987). Together, this body of work demonstrates that the SC is critical for initiating coordinated orienting movements to targets, independent of the specific motor groups involved and independent of sensory modality.
This role is consistent with traditional cognitive theories positing that separate brain regions mediate the selection and initiation of actions in a serial fashion (Miller et al. 1960). Under this view, input to the SC would specify the target for movement and the SC would contribute to the coordination of the desired movement via its well-described downstream motor projections (Dean et al. 1989; Gandhi and Katnani 2011). However, much experimental and theoretical work has suggested that, in organisms engaged in continually sensing and acting on their environment, these processes may be mediated in parallel by overlapping sets of brain regions (Cisek 2007; Cisek and Kalaska 2010; Gold and Shadlen 2000; Hernandez et al. 2002). Consistent with this view, and extending it to subcortical structures, many studies have suggested that the SC is involved in selecting spatial targets for movements as well as initiating such movements (Krauzlis et al. 2004; Mays and Sparks 1980).
Early work addressing target selection in the SC extended the classic head-fixed primate paradigm to focus on activity during an enforced delay period preceding saccade initiation, during which the direction of upcoming movement is known and can be prepared (Glimcher and Sparks 1992). Such “prelude” activity, displayed by “buildup” neurons, typically exhibits as a modest increase during the delay period, leading to a burst of activity at the time of contraversive saccade initiation (Fig. 2B; Dorris et al. 1997; Mays and Sparks 1980; Munoz and Wurtz 1995). The fact that the prelude activity occurs well before the saccade is initiated and predicts its direction provided a first indication that it may be playing a role beyond movement initiation. Indeed, manipulation of SC activity has been shown to predictably bias target selection even in the absence of an effect on overt movements (Carello and Krauzlis 2004; Glimcher and Sparks 1993; Song et al. 2011), consistent with this idea.
Fig. 2.

Examples of recordings from the SC of rodents performing behavioral tasks requiring the selection of a target for movement. A: generalized schematic of the events in the task during which recordings in B and in Fig. 4 were taken. The rodent enters the central port, receives an odor associated with reward at one side, waits for a go signal, and orients to a port for a water reward. B: smoothed peristimulus time histograms (mean ± SE) for 2 SC neurons recorded in a rat performing the task shown in A. Black, activity associated with selecting contralateral target; gray, activity associated with selecting ipsilateral target. Stimulus presentation is shown as the gradient along the x-axis, and go signal is shown as a vertical dotted line. Some neurons exhibit higher activity preceding movement to contraversive target (as at top), and some exhibit higher activity preceding movement to ipsiversive target (as at bottom). Data from Felsen and Mainen (2012). For comparable data recorded in primates in a similar experimental paradigm, see Horwitz and Newsome (2001a).
Subsequent studies examined prelude activity in the SC using behavioral tasks in which the difficulty of selecting the correct target for movement varied across trials. For example, one set of studies systematically varied the number of potential targets, and therefore the probability that a particular target would be selected. These studies found that the level of prelude activity reflected the probability of selecting the target in the response field of that neuron (Basso and Wurtz 1997, 1998; Dorris and Munoz 1998; Krauzlis et al. 2004; McPeek and Keller 2002a). Other studies varied the strength of the stimulus that cued target selection and found that prelude activity more reliably predicted which target would be selected for stronger stimuli (i.e., easier trials) than weaker stimuli (i.e., more difficult trials). Interestingly, this increased reliability was largely due to a dependence of activity on difficulty for movements away from, but not toward, the preferred target of the neuron. Specifically, for movements away from the preferred target, weaker stimuli elicited relatively high firing rates (Felsen and Mainen 2012; Horwitz and Newsome 2001a; Ratcliff et al. 2007; Thevarajah et al. 2009). Together, these data suggest that prelude activity represents targets for movements that are considered but are not ultimately selected, providing further support for such selection occurring in the SC itself or via its influence on premotor nuclei, as opposed to upstream of the SC.
Given that multiple potential targets for movement are represented in SC activity, what is the mechanism by which only one is ultimately selected? Early studies proposed that the selected target corresponded to a population average weighted by the activity of all active SC neurons, as proposed in other motor systems (Georgopoulos et al. 1982; Sparks et al. 1976). This hypothesis was corroborated by the results of experiments in which inhibiting one population of SC neurons biased the resulting saccade away from the region of space it represented (Lee et al. 1988). However, when spatially segregated targets are considered, distinct populations of SC neurons representing these targets are active, and a population code would predict that an average location between the targets would be selected. Instead, one of the represented targets can be selected, which implies a competition between circuits representing distinct spatial targets (how this competition might be realized neurobiologically is described below). This framework would predict that subtly manipulating the activity of SC neurons representing one target may have a greater influence on target selection as trial difficulty increases, in which activity is increasingly balanced between the competing circuits. Indeed, studies that have manipulated the activity of one group of SC neurons—presumably corresponding to one of the targets—have indeed observed a larger effect on difficult than on easy trials (Fig. 3; McPeek and Keller 2004; Song et al. 2011; Stubblefield et al. 2013; Thevarajah et al. 2009). Other studies have taken advantage of the known topographic representation of target locations in the SC to demonstrate that inactivating neurons representing one location biases target selection away from the inactivated locus (McPeek and Keller 2004; Nummela and Krauzlis 2010, 2011). This idea also applies to the decision to either move to a target or remain at the current location (Krauzlis et al. 2004): Inactivating neurons in the rostral SC, which represent low-amplitude movements or no movement (i.e., maintaining fixation) (Dorris et al. 1997; Dorris and Munoz 1998; Hafed et al. 2009; Munoz and Wurtz 1992, 1993a), disrupt fixation and result in irrepressible saccades to distal targets (Goffart et al. 2012; Munoz and Wurtz 1993b). In addition, this framework would predict that on more difficult trials activity in competing circuits would be more balanced, leading to a higher likelihood of selecting the incorrect target. This idea was tested by a study that simultaneously recorded activity in four SC neurons in primates performing an oddball detection task in which one neuron represented the location of the correct target while the other three represented the location of the distractors. Behavioral performance was found to correlate with the difference in prelude activity between the “target” neuron and the “distractor” neurons rather than with the magnitude of activity in either population (Kim and Basso 2008). Together, these inactivation and recording studies establish a clear relationship between representations of spatial targets in the SC and the selection of a spatial target for an orienting movement.
Fig. 3.
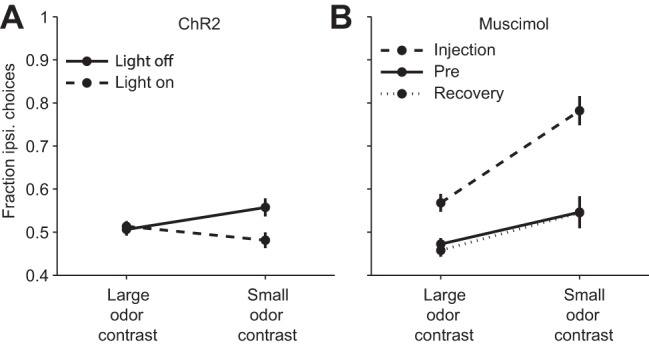
Difficulty dependence of the behavioral effect of manipulating SC activity. A: unilateral channelrhodopsin-2 (ChR2)-mediated optical activation in the SC has a greater effect (contraversive shift) on hard trials (small odor mixture contrasts) than easy trials (large odor contrast). Data from Stubblefield et al. (2013). B: unilateral inhibition via muscimol infusion into the SC has a greater effect (ipsiversive shift) on hard trials than easy trials. Saline was delivered during Pre and Recovery sessions. Data from Felsen and Mainen (2008). For comparable data collected in primates in a similar experimental paradigm, see McPeek and Keller (2004).
How is this competition mediated? In particular, do competing populations of neurons actively inhibit one another (Dorris et al. 2007; Lo and Wang 2006; Munoz and Fecteau 2002; Munoz and Istvan 1998; Munoz and Wurtz 1993a; Trappenberg et al. 2001) or does their activity “race,” independently, to a fixed threshold (Paré and Hanes 2003; Ratcliff et al. 2007, 2011)? Addressing this question requires closer examination of the role of intrinsic inhibition in the SC in target selection. Presumably, GABAergic SC neurons, which comprise up to 30% of neurons in the SC (Mize 1988, 1992), play a critical role. Anatomical and slice studies have found that these neurons project within and between SC layers and from one SC to the other, and the majority are spontaneously active and fast spiking (Behan and Appell 1990; Sooksawate et al. 2011). Thus these neurons have been thought to potentially provide mutually inhibitory input between regions of the deep subdivision of the SC representing distinct targets. Consistent with this possibility, functional inhibition has been demonstrated in slices (Bayguinov et al. 2015; Lee and Hall 2006; Meredith and Ramoa 1998; Phongphanphanee et al. 2014; Sooksawate et al. 2011), and an in vivo study in primates found that SC neurons representing saccade targets were inhibited shortly after electrical stimulation of either SC (Munoz and Istvan 1998). However, other studies have found no evidence for functional inhibition between competing populations of SC neurons (Ratcliff et al. 2011), and inhibitory influence is less spatially extensive than excitatory influences (Phongphanphanee et al. 2014). This restricted spatial extent of inhibition is not consistent with the requirement for long-range inhibition in winner-take-all models (Trappenberg et al. 2001; White and Munoz 2011); instead the pattern of excitation and inhibition suggests a role in integrating information across the SC. Critically, the function of these GABAergic neurons has not been directly examined in behaving animals, because of methodological obstacles in targeting these neurons for study (although the effect of pharmacologically manipulating GABAergic signaling in the SC has been studied, as described above). Future studies that directly record or manipulate these neurons in vivo—for example, with cell type-specific tools such as optogenetics (Cardin 2012) or chemogenetics (Urban and Roth 2015)—have the potential to determine the functional role of intrinsic inhibition in the SC and advance our understanding of how targets are selected for movement.
Thus questions remain about how intrinsic SC activity underlies the selection of targets for movements. It is even possible that SC activity reflects an abstract “priority map” for target selection rather than the competition, discussed above, between circuits representing distinct targets (McPeek and Keller 2002b; Song et al. 2011). In addition, the SC receives input from numerous brain regions, and little is known about how this input influences processing within the SC. In the following sections, we examine how three of these regions—the SNr, the parabrachial region, and the neocortex (Fig. 1)—contribute to target selection via their input to the SC.
GABAergic Input to Superior Colliculus from Substantia Nigra Pars Reticulata
In general, the basal ganglia are thought to control movement initiation via phasic release from tonic inhibition of downstream motor structures (Gulley et al. 2002; Mink 1996; Mink and Thach 1993); we refer to this idea as the “disinhibition model.” The GABAergic projection from the SNr to the SC mediates basal ganglia control of orienting movements to spatial targets (Hikosaka et al. 2006; Utter and Basso 2008). Accordingly, some of the first studies of SNr activity, SC activity, and orienting movements were consistent with the disinhibition model: In tonically active nigrotectal neurons in primates and cats, a decrease in activity corresponded to a burst of activity in the ipsilateral SC and the initiation of a contraversive orienting movement (Hikosaka and Wurtz 1983; Joseph and Boussaoud 1985). A similar relationship between the activity of SNr and tectospinal SC motor output neurons was observed in rats (Chevalier et al. 1985). Projections of the nigrotectal pathway are segregated in a manner similar to the organization of the output channels from the striatum to the SNr (Deniau and Chevalier 1992; Redgrave et al. 1992; Williams and Faull 1985). These studies suggest a simple serial process by which specific striatonigral neurons inhibit specific nigrotectal neurons, which control the initiation of orienting movements to spatial targets (Hikosaka et al. 2000). However, several pieces of evidence complicate this view and suggest that SNr input to the SC may play a broader role in motor control, perhaps including the selection of targets for orienting movements (Basso and Sommer 2011; Deniau et al. 2007; Shires et al. 2010).
One set of studies focused on the relationship between SNr activity and orienting movements. These studies showed that the activity of many SNr neurons is modulated well before movement initiation (Handel and Glimcher 1999), depends on the magnitude of reward associated with the movement (Bryden et al. 2011; Handel and Glimcher 2000; Sato and Hikosaka 2002), and depends on the number of potential targets for movements (Basso and Wurtz 2002). Notably, SNr activity was often found to increase preceding (and even during) contralateral movement initiation (Fig. 4A; Gulley et al. 2002; Handel and Glimcher 1999; Lintz and Felsen 2014; Sato and Hikosaka 2002). In addition, microstimulating SNr neurons had a greater effect on saccades to remembered targets than on visually guided saccades (Basso and Liu 2007), particularly when the stimulation occurred during the delay period preceding saccade initiation (Mahamed et al. 2014). Thus the relationship between SNr activity and contraversive orienting movements is more complicated than the disinhibition model would predict.
Fig. 4.
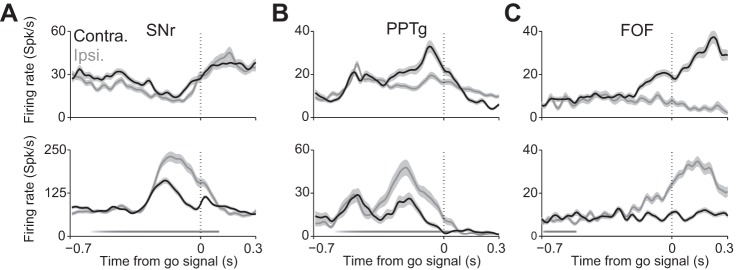
Examples of neural recordings from rodents performing behavioral tasks requiring the selection of a target for movement. A: smoothed peristimulus time histograms (mean ± SE) for 2 SNr neurons recorded in a mouse performing the task shown in Fig. 2A. Black, activity associated with selecting contralateral target; gray, activity associated with selecting ipsilateral target. Stimulus presentation is shown as the gradient along the x-axis, and go signal is shown as a vertical dotted line. Some neurons exhibit higher activity preceding movement to contraversive target (as at top), and some exhibit higher activity preceding movement to ipsiversive target (as at bottom). Data from Lintz and Felsen (2014). B: as in A, for 2 mouse PPTg neurons. Data from Thompson and Felsen (2013). C: as in A, for 2 rat frontal orienting field (FOF) neurons. Rat FOF is thought to be homologous to primate frontal eye field (FEF). In this task, target selection was cued by an auditory rather than olfactory stimulus. Data from Erlich et al. (2011). For comparable data recorded in primates in a similar experimental paradigm, see Handel and Glimcher (1999) (SNr), Okada and Kobayashi (2009) (PPTg), and Sommer and Wurtz (2000) (FEF).
A second set of studies suggesting a broader role for SNr input to the SC examined the anatomy and physiology of this input. First, while the disinhibition model is based on ipsilaterally projecting (i.e., “uncrossed”) nigrotectal neurons, some (“crossed”) nigrotectal neurons project contralaterally (Beckstead et al. 1981; Chevalier et al. 1981; Deniau et al. 1977; Gerfen et al. 1982; Jayaraman et al. 1977; Liu and Basso 2008) and a small number project to both SCs (Cebrián et al. 2005). Given that crossed nigrotectal projections are also inhibitory, they would appear to promote movements ipsiversive to the phasically inactivated SNr, inconsistent with the disinhibition model. Second, in addition to the influence of SNr activity on bursting SC neurons that initiate movements (Hikosaka and Wurtz 1983), SNr input modulates the activity of buildup SC neurons (Liu and Basso 2008) thought to subserve the selection of targets for movement (but see Corneil et al. 2007). Finally, uncrossed nigrotectal neurons project to GABAergic SC neurons—which, as discussed above, may mediate the inhibition between SC circuits representing competing movements—as often as they project to glutamatergic SC neurons (Kaneda et al. 2008), further suggesting a broader role for the SNr than simple disinhibition.
Given these findings, how might input from the SNr modulate SC activity underlying the selection of orienting movements? Can these findings be reconciled with the idea that the SNr controls motor output via phasic release from tonic inhibition? One possibility is that the diversity of SNr activity preceding movement can be explained by the diversity of downstream targets of SNr neurons. SNr neurons project to several motor-related regions besides the SC, including the thalamus and the PPTg (Saitoh et al. 2003; Utter and Basso 2008), as well as nonmotor regions such as the substantia nigra pars compacta (Pan et al. 2013; Saitoh et al. 2004; Tepper et al. 1995). The range of activity profiles observed in SNr may reflect the distinct roles of these downstream pathways. Even within the nigrotectal projection, the targeted SC (ipsilateral or contralateral to the source of the projection) may explain why the activity of some SNr neurons increases, while the activity of others decreases, preceding identical movements. Specifically, a crossed nigrotectal neuron exhibiting an increase in activity, and an uncrossed nigrotectal neuron exhibiting a decrease in activity, would promote the common goal of contraversive movement (relative to the side of the SNr). This idea is supported by a study in cats that found that the activity of crossed nigrotectal neurons increases, while the activity of uncrossed neurons decreases, in response to visual stimuli (Jiang et al. 2003). Similarly, it is possible that the activity profile relates to the type of neurons (e.g., glutamatergic or GABAergic) to which the SNr neuron projects. Given that some neurons have complex activity profiles that change during the course of the trial (Handel and Glimcher 1999), it may be the case that individual neurons contribute to multiple aspects of motor control, including selecting the target, planning the movement, and initiating the movement (Basso and Sommer 2011). Future experiments can begin to test these ideas by targeting recordings to specific types of SNr neurons—for example, based on whether they project to the ipsilateral or contralateral SC—with techniques like antidromic stimulation (Hikosaka and Wurtz 1983) or optogenetics (Gradinaru et al. 2010; Lima et al. 2009).
Although many details remain to be determined, it is clear that the SNr plays a broader role in modulating SC activity than via simple disinhibition. As discussed below, one idea is that the SNr provides value-related information to the target selection process by biasing activity in SC circuits such that spatial targets associated with the highest value are most likely to be selected (Hikosaka et al. 2006).
Cholinergic Input to Superior Colliculus from Parabrachial Region
The SC receives cholinergic innervation via two distinct pathways that independently innervate either the superficial or deep subdivision (Hall et al. 1989). Experiments in rodents, cats, and primates have mapped the immunohistochemical profile and the afferent topography of the SC and have demonstrated that the cholinergic innervation of the superficial subdivision originates from the brain stem's parabigeminal nucleus (Feig et al. 1992; Graybiel 1978; Hall et al. 1989; Mufson et al. 1986; Roldán et al. 1983) whereas the deep subdivision innervation arises from the parabrachial region, primarily composed of the PPTg and the LDTg (Beninato and Spencer 1986; Graybiel 1978; Hall et al. 1989; Harting and Van Lieshout 1991; Illing and Graybiel 1985; Jones and Webster 1988; Ma et al. 1991; Stubblefield et al. 2015; Wallace and Fredens 1988; Woolf and Butcher 1986). We focus on this latter innervation because the deep subdivision of the SC is directly involved in selecting targets for movements, although the superficial cholinergic input could in turn modulate intralaminar processing within the SC (Ghitani et al. 2014; Isa et al. 1998; Lee et al. 1997). Notably, the cholinergic input to the deep subdivision appears to overlap with that of the SNr input described above (Harting and Van Lieshout 1991; Illing and Graybiel 1985).
Electrophysiological studies in slices, anesthetized animals, and awake animals have found that the overall effect of acetylcholine (ACh) in the SC is excitatory (Bezdudnaya and Castro-Alamancos 2014; Isa and Hall 2009; Li et al. 2004; Sooksawate et al. 2008, 2011; Sooksawate and Isa 2006; Stubblefield et al. 2015). Stimulation of endogenous release of ACh in the SC in an in vivo preparation demonstrated variability in the magnitude and timescale of this excitatory influence, consistent with both monosynaptic and polysynaptic cholinergic input (Stubblefield et al. 2015). These studies suggest that endogenous cholinergic input serves to promote SC-dependent motor output. Consistent with this idea, infusing nicotine into the intermediate layers of one SC induces rotational behavior in rats (Weldon et al. 1983) and decreases reaction time in a saccade task in primates (Aizawa et al. 1999; Watanabe et al. 2005).
Although the PPTg and LDTg have not been as extensively examined in behavioral contexts as have the SC and the SNr, electrophysiological studies in multiple animal models and various behavioral paradigms demonstrate a role for these regions in goal-directed motor control (Martinez-Gonzalez et al. 2011; Mena-Segovia et al. 2004; Winn 2006, 2008). Specifically, the activity of PPTg neurons correlates with orienting movements of the eyes, body, and limbs (Dormont et al. 1998; Kobayashi et al. 2002; Matsumura et al. 1997; Norton et al. 2011; Okada and Kobayashi 2009; Thompson and Felsen 2013; see example neurons recorded in freely moving mice shown in Fig. 4B), and LDTg activity shows task-dependent modulation preceding and during movements in rats navigating a radial arm maze (Redila et al. 2015). Given the similar timing of activity exhibited by neurons in these areas and SC neurons (Felsen and Mainen 2012; Thompson and Felsen 2013; see examples in Fig. 2B and Fig. 4B) as well as the anatomical and physiological data discussed above, the PPTg and LDTg are likely to modulate movement-related activity in the SC (Kobayashi and Isa 2002). Separate studies using similar behavioral paradigms have shown that the firing rates of most (but not all; see Fig. 2B and Fig. 4B) PPTg and SC neurons prior to movement initiation are higher for contraversive than ipsiversive movements (Felsen and Mainen 2012; Horwitz and Newsome 2001a; Okada and Kobayashi 2009; Thompson and Felsen 2013). These studies, along with anatomical studies showing stronger input from the ipsilateral than the contralateral PPTg (Beninato and Spencer 1986; Hall et al. 1989; Krauthamer et al. 1995; Li et al. 2004), suggest that the uncrossed PPTg-SC projection plays a primary role in motor output. However, the PPTg and LDTg both project widely to several other motor regions (Jenkinson et al. 2009; Oh et al. 1991), and to our knowledge no studies have specifically targeted SC-projecting PPTg or LDTg neurons for recording. Thus the specific functional role of the cholinergic input to the SC, and whether it relates to the selection of targets for movement, remains unknown.
One possibility, however, is that cholinergic input to the SC serves to modulate the competition between circuits representing targets for movement, similar to how other brain stem sources of cholinergic input modulate sensory representations in target structures (Castro-Alamancos 2004; Froemke et al. 2007; Goard and Dan 2009; Kilgard and Merzenich 1998; Ma and Luo 2012; Rothermel et al. 2014). Indeed, cholinergic input to brain stem nuclei has been proposed to modulate motor-related functions, including locomotor activity (Steckler et al. 1994) and muscle control (Kelland and Asdourian 1989). Given that the activity of many PPTg neurons increases preceding movement (Thompson and Felsen 2013), this excitatory cholinergic input could serve to bias the selection of particular targets for movement, providing a counterbalance to the spatially overlapping inhibitory input from the SNr (Harting and Van Lieshout 1991; Illing and Graybiel 1985) that has also been proposed to bias target selection (Hikosaka et al. 2006; Kobayashi and Isa 2002). Thus the SC may integrate antagonistic influences from two major neural systems—inhibition from the basal ganglia and excitation from the mesencephalic cholinergic system—that may serve to modulate its intrinsic processing underlying the selection of targets for movement, as well as the influence of the converging cortical inputs discussed below.
Glutamatergic Input to Superior Colliculus from Neocortex
In an early landmark study demonstrating the importance of cortical influence on the SC, hemianopsia due to removal of the contralateral occipitotemporal cortex was shown to be mediated by inter-SC interaction: subsequent removal of the SC contralateral to the lesioned cortex reversed the deficit (Sprague 1966). A series of subsequent studies found that excitatory cortical input to the deep subdivision of the SC arrives from a wide range of neocortical regions (Fries 1984), including the frontal eye field (FEF), supplementary eye field (SEF), lateral intraparietal area (LIP), and dorsolateral prefrontal cortex (DLPFC), as well as from parietal networks important for reaching behavior (Borra et al. 2014; Distler and Hoffmann 2015; Lock et al. 2003; Munoz and Everling 2004; Paré and Wurtz 2001; Sommer and Wurtz 2001; Sparks 1986). The majority of these inputs originate in ipsilateral cortical regions, but SEF, FEF, premotor cortex, and the supplementary motor area have been shown to project contralaterally as well (Distel and Fries 1982; Shook et al. 1990). While the roles of many of these regions in decision making have been extensively studied in their own right, we focus here on how they may directly influence target selection processes in the SC. Although many of these cortical regions project directly to brain stem premotor circuits (Schnyder et al. 1985; Segraves 1992; Shook et al. 1990; Stanton et al. 2004), inactivation and lesion studies, at least in FEF, show that the indirect projection to the brain stem via the SC is critical for normal motor output (Hanes and Wurtz 2001; Schiller et al. 1980).
Several studies have recorded from identified SC-projecting cortical neurons in primates performing visually guided and memory-guided saccade tasks. In general, the activity of corticotectal neurons—irrespective of their cortical region of origin—appears to resemble the activity of SC neurons themselves: Overlapping populations of neurons signal visual stimuli and upcoming movement (Fig. 4C; Paré and Wurtz 2001; Segraves and Goldberg 1987; Sommer and Wurtz 2000, 2001; Wurtz et al. 2001). However, more cortical neurons tend to represent the stimulus than the motor response, and more SC neurons tend to represent the motor response than the stimulus, providing further evidence that the SC functions along with its cortical inputs to transform stimulus representations to motor representations (Wurtz et al. 2001). Particularly for nonvisual stimuli, such cortical stimulus representations may be an important source of sensory input to the SC. Another study found that the distribution of activity profiles of FEF-recipient SC neurons during visuomotor tasks was similar to that of the SC population as a whole (e.g., some FEF-recipient SC neurons exhibit buildup and/or burst activity), although the activity of SC neurons with purely visual responses (Horwitz and Newsome 2001b) does not appear to be modulated by FEF stimulation (Helminski and Segraves 2003).
During an antisaccade task requiring that some saccades be made to the direction opposite the visual cue, in which the cortex is thought to be involved in suppressing the reflexive behavior to orient to the stimulus (Munoz and Everling 2004), interesting differences emerged between SC-projecting neurons in the FEF and DLPFC. Specifically, activity in FEF neurons increased more preceding prosaccades (i.e., to the visual cue) than antisaccades (i.e., away from the visual cue) (Everling and Munoz 2000), while activity in DLPFC neurons showed the opposite effect (Johnston and Everling 2006). These results suggest that FEF and DLPFC may be playing distinct roles in influencing the selection of targets for movement in the SC—one possibility is that FEF functions to categorize continuous evidence into a binary choice (Erlich et al. 2015; Hanks et al. 2015)—although the mechanism underlying these opposing downstream effects is not clear (Everling and Johnston 2013).
Some corticotectal input spatially overlaps with inputs from the SNr and PPTg (Fig. 1B; Illing and Graybiel 1985). What is the functional relevance of this overlap? As discussed above, the GABAergic and cholinergic input may modulate intrinsic SC activity related to target selection. The cortical input, in contrast, could serve as the primary source of information to the SC. This “driver-modulator” framework (Lee and Sherman 2010) would predict that a range of morphologically distinct axon terminals would be observed in the SC, suggesting a diversity of forms of synaptic transmission, which is indeed the case (Norita 1980). In addition, the modulator pathways would be expected to give rise to thin axons and project to distal dendrites, which is again consistent with ultrastructural data (Fig. 1B; Behan et al. 1987; Jeon et al. 1993). This framework may therefore prove useful in thinking about information integration in the SC, as it has in sensory systems (Lee and Sherman 2010). Below, we discuss what complementary information each of these inputs may provide to the SC in order to influence the selection of targets for movements.
Discussion
We have reviewed a wealth of data on how the SC selects targets for movements. The emerging framework posits target selection as the outcome of a competition between SC circuits representing specific targets, although the precise nature of this competition—i.e., whether SC circuits directly inhibit each other—is unclear. Notably, SC activity appears to represent the target abstractly, independent of whether the movement will be achieved by the eyes, head, body, or limbs. Indeed, this activity may even reflect the selection of a target to attend to, without any overt motor component at all (Carello and Krauzlis 2004; Corneil and Munoz 2014; Krauzlis et al. 2013; Lovejoy and Krauzlis 2010). Instead, the specific combination of motor output required to achieve the goal of orienting to the target appears to be represented in the activity of brain stem premotor nuclei coordinated, in part, by the SC (Gandhi and Katnani 2011). Thus the SC provides a convenient hub at which several neural systems can influence the selection of a target for movement, before specifying the particular manner in which that movement is achieved (Song et al. 2011; Song and McPeek 2015).
We then discussed how several of these systems may influence the competition in the SC underlying target selection for movement. Briefly, the basal ganglia exert GABA-mediated inhibitory influence via the SNr, the parabrachial region exerts ACh-mediated excitatory influence via the PPTg and LDTg, and cortex exerts glutamate-mediated excitatory influence via several interconnected regions (Fig. 1). What might be the functional relevance of the input received from all of these regions? One possibility is that each of these systems conveys complementary information critical to target selection beyond the relevant sensory input, perhaps related to internally generated behavioral goals, as suggested by microstimulation experiments and modeling work (Dorris et al. 2007; Trappenberg et al. 2001).
For example, animals generally act to maximize reward, and it is therefore important to select a target based in part on the likelihood that moving to that target will lead to a reward. Notably, the SC, PPTg, and SNr all provide direct input to dopaminergic neurons in the ventral tegmental area and substantia nigra pars compacta that are responsible for signaling a reward prediction error (Kobayashi and Okada 2007; Norton et al. 2011; Okada et al. 2009; Redgrave et al. 2010; Redgrave and Gurney 2006; Schultz et al. 1997; Watabe-Uchida et al. 2012). The basal ganglia, in particular, have been proposed to provide information about the value associated with targets for movements (Hikosaka et al. 2006); for example, the activity of movement-related caudate and SNr neurons is modulated by whether an upcoming movement will be rewarded (Lauwereyns et al. 2002; Sato and Hikosaka 2002). These data led to the idea that the SNr biases the pattern of activity in SC circuits during target selection such that, upon cortical input, rewarded movements are facilitated and unrewarded movements are suppressed (Hikosaka et al. 2006). The cholinergic input from the parabrachial region may provide complementary contextual information relevant to selecting a target for movement, perhaps in the form of “motor attention” (Goldberg and Segraves 1987). In addition to its dependence on the direction of upcoming orienting movements as described above, the activity of PPTg neurons has been shown to depend on reward (Kobayashi and Okada 2007; Norton et al. 2011; Okada et al. 2009; Thompson and Felsen 2013), and the direction dependence often long outlasts the choice (Thompson and Felsen 2013). These findings raise the possibility that PPTg activity could reflect movement choices and the resulting rewards in the recent past, which could be informative for selecting upcoming targets for movement (Lau and Glimcher 2008). Together, the complementary GABAergic and cholinergic input may function to tune the map of spatial targets represented in the SC according to their relative values, in advance of upcoming movement. This modulation would serve to bias the likelihood that, upon receiving parallel cortical input driving competing targets for movements, the highest-valued target will be selected. Indeed, the results of an analysis comparing several leading models for reading out SC activity to select a target found that a probabilistic model, which is well suited to integrating precisely these types of prior probabilities, most accurately predicted which targets were selected by the behaving animal (Kim and Basso 2010; Wolpert and Landy 2012).
These ideas are akin to those in models proposed by Hikosaka and colleagues (2006) and Lo and Wang (2006) to explain how cortical and basal ganglia input are integrated in the SC during visuomotor tasks, as well as to a model proposed by Kobayashi and Isa (2002), which also incorporated PPTg input. While these models can explain some neural and behavioral data (Aizawa et al. 1999; Lauwereyns et al. 2002), they do not capture the full range of connectivity and neural activity observed. For example, they incorporate ipsilateral, but not contralateral, input to the SC and burst, but not buildup, neurons. An open question is therefore how to extend and refine these models in order to synthesize the heterogeneous anatomical and physiological data observed (note that the example neurons shown in Fig. 2 and Fig. 4 exhibit diversity within each region).
Along these lines, the interactions in the network model that we have described here (Fig. 1A) are necessarily simplified. Most importantly, we have only discussed descending input to the SC, while the SC also provides ascending output that may mediate several functions, including attention (Krauzlis et al. 2014) and corollary discharge (Sommer and Wurtz 2008). In fact, activity in the SC and basal ganglia may be a primary driver of orienting attention, and while the mechanisms underlying attention in the SC are not fully understood, they may be similar to those we have described here (Krauzlis et al. 2013). In addition, we have not discussed how orienting movements to targets relate to other motor output in which the SC has been implicated, such as escape, avoidance, and freezing behaviors (Dean et al. 1986, 1989; Sahibzada et al. 1986). Finally, many of the input regions discussed are themselves interconnected. Thus there is the opportunity for multiple parallel and serial pathways for motor control through the SC, as well as recurrent loops. The fact that the timing of premovement activity across populations of neurons within these regions is heterogeneous (Felsen and Mainen 2012; Thompson and Felsen 2013) supports the idea that the flow of information may not be unidirectional. As discussed below, methods to examine cell type-specific functions could help illuminate these more complex processing features.
Although we have limited the scope of our discussion to cortical, SNr, and parabrachial input to the SC, the SC receives a host of other inputs (Edwards et al. 1979; Sparks and Hartwich-Young 1989). For example, cerebellar nuclei provide excitatory input to the same SC neurons that receive inhibition from the SNr (Westby et al. 1994), and inhibition of the cerebellar caudal fastigial nucleus alters gaze direction during fixation (Guerrasio et al. 2010) and disrupts gaze fixation in the presence of a competing target (Fukushima et al. 2011). These findings demonstrate a cerebellar contribution to SC-mediated fixation and its release (Goffart et al. 2012; Quinet and Goffart 2015). As the functional roles of this input, and others, are more thoroughly examined, they can be incorporated into the integrative framework presented here. Despite the wealth of data collected in the studies we describe, an obstacle to further progress is that our methods for examining the neural bases of selecting targets for movement are insufficient for obtaining a detailed circuit-level description of the process. While we predict that different types of neurons in the SC (e.g., excitatory vs. inhibitory, projection vs. local) play different functional roles in selecting targets for movements, these predictions have not been tested because it is difficult to identify specific neuron types in in vivo studies. For example, to our knowledge no in vivo studies in the SC have recorded from identified GABAergic neurons, despite their critical role in SC processing and despite some understanding of their anatomy and physiology from slice studies (Isa and Hall 2009; Sooksawate et al. 2011, 2012). New methods are beginning to allow for such targeted recordings. For example, restricting expression of ChR2 to GABAergic neurons would allow them to be identified during awake behaving recordings (Cardin 2012; Cardin et al. 2010; Lima et al. 2009; Roux et al. 2014). These experiments are currently feasible in mouse models because of the availability of transgenic mouse lines allowing for cell type-specific expression of optogenetic proteins (Taniguchi et al. 2011; Zhao et al. 2011) and technology to photostimulate and record neural activity in behaving animals (Anikeeva et al. 2011). While this particular approach requires transgenic animals and is thus less well suited for use in primates, it is worth noting that other optogenetic tools can be applied to primates (Diester et al. 2011; Jazayeri et al. 2012), including in the SC (Cavanaugh et al. 2012). For example, targeting neurons on the basis of their projection pattern (Gradinaru et al. 2010) would be feasible in the primate model.
While primates share the most homology with humans and remain an essential model for studying SC function, the potential afforded by new molecular tools suggests that we consider the complementary utility of rodent model systems. The control of head and body movements in rodents is not identical to the control of eye movements in primates, but the homology between these systems appears to be sufficient to make findings applicable across species (Takakusaki et al. 2004), and recordings in the regions discussed here appear fairly similar in rodents and primates (compare data shown in Fig. 2 and Fig. 4 with those in the papers referred to in their legends). In addition, rodents have been more commonly utilized in slice studies (Isa and Hall 2009). In vivo work in rodents therefore allows us to bridge the gap between in vivo and ex vivo paradigms: For example, complementary in vivo and slice experiments can be performed in the same transgenic mouse line to look at systems and cellular components of SC function. In addition, rodent models provide other useful features, beyond the ease of optogenetic applications noted above, for examining the neural bases of decision making (Carandini and Churchland 2013). First, rodents are more convenient than primates for studying SC control of freely moving behavior (Felsen and Mainen 2008; Hirokawa et al. 2011). Second, the applicability to mouse models of genetically encoded voltage and calcium indicators allows the activity of populations of genetically specified neurons to be imaged (Broussard et al. 2014; Tian et al. 2012); this technology was recently applied to the SC (Feinberg and Meister 2015). Such imaging combined with novel head-mounted microscopes will allow for imaging of population activity during freely moving behavioral tasks (Barretto et al. 2011; Chen et al. 2013; Flusberg et al. 2008; Ghosh et al. 2011; Wilt et al. 2009). Thus rodents are likely to provide a complementary model to primates in future studies addressing open questions about how the SC and its inputs mediate the selection of targets for movements.
Concluding remarks.
The results from a range of studies, across several species and motor effector systems, suggest a framework in which activity in the deep subdivision of the SC contributes to the selection of targets for orienting movements. Other studies have provided insight into how inputs to the SC, from the basal ganglia, parabrachial region, and neocortex, may influence SC activity in order to tune the probability of selecting a particular target consistent with internally generated goals. However, despite much work, several open questions remain about how the SC and these—as well as other—input regions mediate the selection of targets for movements. These questions can be addressed by future experiments identifying the functional roles of specific cell types in these circuits, and by theoretical models incorporating the richness of anatomical and physiological data observed.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS-079518 and R01 NS-079518-01A1S1 and the University of Colorado Medical Scientist Training Program (National Institute of General Medical Sciences 5T32 GM-008497).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B.W., M.J.L., J.A.T., E.A.S., and G.F. drafted manuscript; A.B.W., M.J.L., J.A.T., E.A.S., and G.F. edited and revised manuscript; A.B.W., M.J.L., J.D.C., J.A.T., E.A.S., and G.F. approved final version of manuscript; J.D.C. analyzed data; J.D.C. and G.F. interpreted results of experiments; J.D.C. prepared figures.
ACKNOWLEDGMENTS
We thank Richard Krauzlis and Jeffrey Erlich for helpful discussions and Jaclyn Essig, the editor, and four anonymous reviewers for insightful comments on the manuscript.
REFERENCES
- Aizawa H, Kobayashi Y, Yamamoto M, Isa T. Injection of nicotine into the superior colliculus facilitates occurrence of express saccades in monkeys. J Neurophysiol 82: 1642–1646, 1999. [DOI] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci 15: 163–170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci 31: 2221–2233, 2010. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, King AJ. The non-lemniscal auditory cortex in ferrets: convergence of corticotectal inputs in the superior colliculus. Front Neuroanat 21: 18, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan PF, Ferrera VP. Effects of gaze shifts on maintenance of spatial memory in macaque frontal eye field. J Neurosci 23: 5446–5454, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto RP, Ko TH, Jung JC, Wang TJ, Capps G, Waters AC, Ziv Y, Attardo A, Recht L, Schnitzer MJ. Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nat Med 17: 223–228, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Liu P. Context-dependent effects of substantia nigra stimulation on eye movements. J Neurophysiol 97: 4129–4142, 2007. [DOI] [PubMed] [Google Scholar]
- Basso MA, Sommer MA. Exploring the role of the substantia nigra pars reticulata in eye movements. Neuroscience 198: 205–212, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature 389: 66–69, 1997. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Neuronal activity in substantia nigra pars reticulata during target selection. J Neurosci 22: 1883–1894, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayguinov PO, Ghitani N, Jackson MB, Basso MA. A hard-wired priority map in the superior colliculus shaped by asymmetric inhibitory circuitry. J Neurophysiol 114: 662–676, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Edwards SB, Frankfurter A. A comparison of the intranigral distribution of nigrotectal neurons labeled with horseradish peroxidase in the monkey, cat, and rat. J Neurosci 1: 121–125, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Appell P. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol 302: 143–158, 1990. [DOI] [PubMed] [Google Scholar]
- Behan M, Lin CS, Hall WC. The nigrotectal projection in the cat: an electron microscope autoradiographic study. Neuroscience 21: 529–539, 1987. [DOI] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol 253: 525–538, 1986. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Matsuo S, Guitton D. Superior colliculus encodes distance to target, not saccade amplitude, in multi-step gaze shifts. Nat Neurosci 6: 404–413, 2003. [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA. Neuromodulation of whisking related neural activity in superior colliculus. J Neurosci 34: 7683–7695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra E, Gerbella M, Rozzi S, Tonelli S, Luppino G. Projections to the superior colliculus from inferior parietal, ventral premotor, and ventrolateral prefrontal areas involved in controlling goal-directed hand actions in the macaque. Cereb Cortex 24: 1054–1065, 2014. [DOI] [PubMed] [Google Scholar]
- Broussard GJ, Liang R, Tian L. Monitoring activity in neural circuits with genetically encoded indicators. Front Mol Neurosci 7: 97, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Johnson EE, Diao X, Roesch MR. Impact of expected value on neural activity in rat substantia nigra pars reticulata. Eur J Neurosci 33: 2308–2317, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Churchland AK. Probing perceptual decisions in rodents. Nat Neurosci 16: 824–831, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA. Dissecting local circuits in vivo: integrated optogenetic and electrophysiology approaches for exploring inhibitory regulation of cortical activity. J Physiol (Paris) 106: 104–111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc 5: 247–254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron 43: 575–583, 2004. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol 74: 213–247, 2004. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V, Wang KH, Boyden ES, Wurtz RH. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron 76: 901–907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrián C, Parent A, Prensa L. Patterns of axonal branching of neurons of the substantia nigra pars reticulata and pars lateralis in the rat. J Comp Neurol 492: 349–369, 2005. [DOI] [PubMed] [Google Scholar]
- Chen JL, Andermann ML, Keck T, Xu NL, Ziv Y. Imaging neuronal populations in behaving rodents: paradigms for studying neural circuits underlying behavior in the mammalian cortex. J Neurosci 33: 17631–17640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM, Thierry AM, Feger J. The nigro-tectal pathway. An electrophysiological reinvestigation in the rat. Brain Res 213: 253–263, 1981. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Vacher S, Deniau JM, Desban M. Disinhibition as a basic process in the expression of striatal functions. I. The striato-nigral influence on tecto-spinal/tecto-diencephalic neurons. Brain Res 334: 215–226, 1985. [DOI] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci 362: 1585–1599, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298, 2010. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP. Overt responses during covert orienting. Neuron 82: 1230–1243, 2014. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP, Olivier E. Priming of head premotor circuits during oculomotor preparation. J Neurophysiol 97: 701–714, 2007. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J Neurophysiol 88: 2000–2018, 2002. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Olivier E, Pélisson D. Direct evidence for the contribution of the superior colliculus in the control of visually guided reaching movements in the cat. J Physiol 556: 675–681, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Sahibzada N, Tsuji K. Head and body movements produced by electrical stimulation of superior colliculus in rats: effects of interruption of crossed tectoreticulospinal pathway. Neuroscience 19: 367–380, 1986. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby G. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci 12: 137–147, 1989. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G. The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience 46: 361–377, 1992. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Hammond-Le Guyader C, Feger J, McKenzie JS. Bilateral projection of nigro-collicular neurons: an electrophysiological analysis in the rat. Neurosci Lett 5: 45–50, 1977. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: a window to basal ganglia output. Prog Brain Res 160: 151–172, 2007. [DOI] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci 14: 387–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel H, Fries W. Contralateral cortical projections to the superior colliculus in the macaque monkey. Exp Brain Res 48: 157–162, 1982. [DOI] [PubMed] [Google Scholar]
- Distler C, Hoffmann KP. Direct projections from the dorsal premotor cortex to the superior colliculus in the macaque (Macaca mulatta). J Comp Neurol (April 28, 2015). doi: 10.1002/cne.23794. [DOI] [PubMed] [Google Scholar]
- Dormont JF, Condé H, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res 121: 401–410, 1998. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci 18: 7015–7026, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci 27: 5053–5062, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Gutfreund Y. Saliency mapping in the optic tectum and its relationship to habituation. Front Integr Neurosci 8: 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol 184: 309–329, 1979. [DOI] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron 72: 330–343, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Brunton BW, Duan CA, Hanks TD, Brody CD. Distinct behavioral effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. eLife 4: e05457, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Johnston K. Control of the superior colliculus by the lateral prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 368: 20130068, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20: 387–400, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig S, Van Lieshout DP, Harting JK. Ultrastructural studies of retinal, visual cortical (area 17), and parabigeminal terminals within the superior colliculus of Galago crassicaudatus. J Comp Neurol 319: 85–99, 1992. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Meister M. Orientation columns in the mouse superior colliculus. Nature 519: 229–232, 2015. [DOI] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron 60: 137–148, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Midbrain contributions to sensorimotor decision making. J Neurophysiol 108: 135–147, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RP, Ko TH, Burns LD, Jung JC, Schnitzer MJ. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat Methods 5: 935–938, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–951, 1996. [DOI] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol 230: 55–76, 1984. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature 450: 425–429, 2007. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Fukushima J, Kaneko CR, Belton T, Ito N, Olley PM, Warabi T. Memory-based smooth pursuit: neuronal mechanisms and preliminary results of clinical application. Ann NY Acad Sci 1233: 117–126, 2011. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci 2: 1527–1537, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Staines WA, Arbuthnott GW, Fibiger HC. Crossed connections of the substantia nigra in the rat. J Comp Neurol 207: 283–303, 1982. [DOI] [PubMed] [Google Scholar]
- Ghitani N, Bayguinov PO, Vokoun CR, McMahon S, Jackson MB, Basso MA. Excitatory synaptic feedback from the motor layer to the sensory layers of the superior colliculus. J Neurosci 34: 6822–6833, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nat Methods 8: 871–878, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW. The neurobiology of visual-saccadic decision making. Annu Rev Neurosci 26: 133–179, 2003. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature 355: 542–545, 1992. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Effects of low-frequency stimulation of the superior colliculus on spontaneous and visually guided saccades. J Neurophysiol 69: 953–964, 1993. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci 12: 1444–1449, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart L, Hafed ZM, Krauzlis RJ. Visual fixation as equilibrium: evidence from superior colliculus inactivation. J Neurosci 32: 10627–10636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature 404: 390–394, 2000. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Segraves MA. Visuospatial and motor attention in the monkey. Neuropsychologia 25: 107–118, 1987. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. J Neurophysiol 35: 542–559, 1972a. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol 35: 560–574, 1972b. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141: 154–165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. A stereometric pattern of distribution of acetylthiocholinesterase in the deep layers of the superior colliculus. Nature 272: 539–541, 1978. [DOI] [PubMed] [Google Scholar]
- Groh JM, Sparks DL. Saccades to somatosensory targets. II. Motor convergence in the primate superior colliculus. J Neurophysiol 75: 428–438, 1996. [DOI] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Büttner U, Goffart L. Fastigial oculomotor region and the control of foveation during fixation. J Neurophysiol 103: 1988–2001, 2010. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Pélisson D. Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. I. Effect of the locus and of the parameters of stimulation. Eur J Neurosci 14: 1331–1344, 2001. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Kosobud AE, Rebec GV. Behavior-related modulation of substantia nigra pars reticulata neurons in rats performing a conditioned reinforcement task. Neuroscience 111: 337–349, 2002. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323: 940–943, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol 287: 495–514, 1989. [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol 82: 3458–3475, 1999. [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Contextual modulation of substantia nigra pars reticulata neurons. J Neurophysiol 83: 3042–3048, 2000. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Wurtz RH. Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophysiol 85: 804–815, 2001. [DOI] [PubMed] [Google Scholar]
- Hanks TD, Kopec CD, Brunton BW, Duan CA, Erlich JC, Brody CD. Distinct relationships of parietal and prefrontal cortices to evidence accumulation. Nature 520: 220–223, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK, Van Lieshout DP. Spatial relationships of axons arising from the substantia nigra, spinal trigeminal nucleus, and pedunculopontine tegmental nucleus within the intermediate gray of the cat superior colliculus. J Comp Neurol 305: 543–558, 1991. [DOI] [PubMed] [Google Scholar]
- Helminski JO, Segraves MA. Macaque frontal eye field input to saccade-related neurons in the superior colliculus. J Neurophysiol 90: 1046–1062, 2003. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Zainos A, Romo R. Temporal evolution of a decision-making process in medial premotor cortex. Neuron 33: 959–972, 2002. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Basal ganglia circuits for reward value-guided behavior. Annu Rev Neurosci 37: 289–306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol 95: 567–584, 2006. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol 49: 1285–1301, 1983. [DOI] [PubMed] [Google Scholar]
- Hirokawa J, Sadakane O, Sakata S, Bosch M, Sakurai Y, Yamamori T. Multisensory information facilitates reaction speed by enlarging activity difference between superior colliculus hemispheres in rats. PLoS One 6: e25283, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task affecting pursuit target selection. J Neurophysiol 86: 2543–2558, 2001a. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: direction-selective visual responses in the superior colliculus. J Neurophysiol 86: 2527–2542, 2001b. [DOI] [PubMed] [Google Scholar]
- Illing RB, Graybiel AM. Convergence of afferents from frontal cortex and substantia nigra onto acetylcholinesterase-rich patches of the cat's superior colliculus. Neuroscience 14: 455–482, 1985. [DOI] [PubMed] [Google Scholar]
- Isa T, Endo T, Saito Y. The visuo-motor pathway in the local circuit of the rat superior colliculus. J Neurosci 18: 8496–8504, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol 102: 2581–2593, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. I. Motor convergence. J Neurophysiol 57: 22–34, 1987. [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Batton RR, Carpenter MB. Nigrotectal projections in the monkey: an autoradiographic study. Brain Res 135: 147–152, 1977. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat Neurosci 15: 1368–1370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord 24: 319–328, 2009. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Spencer RF, Mize RR. Organization and synaptic connections of cholinergic fibers in the cat superior colliculus. J Comp Neurol 333: 360–374, 1993. [DOI] [PubMed] [Google Scholar]
- Jiang H, Stein BE, McHaffie JG. Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature 423: 982–986, 2003. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J Neurosci 26: 12471–12478, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Webster HH. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain. Brain Res 451: 13–32, 1988. [DOI] [PubMed] [Google Scholar]
- Joseph JP, Boussaoud D. Role of the cat substantia nigra pars reticulata in eye and head movements. I. Neural activity. Exp Brain Res 57: 286–296, 1985. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci 28: 11071–11078, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland MD, Asdourian D. Pedunculopontine tegmental nucleus-induced inhibition of muscle activity in the rat. Behav Brain Res 34: 213–234, 1989. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science 279: 1714–1718, 1998. [DOI] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28: 2991–3007, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci 30: 2340–2355, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol 14: R335–R338, 2004. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Inoue Y, Yamamoto M, Isa T, Aizawa H. Contribution of pedunculopontine tegmental nucleus neurons to performance of visually guided saccade tasks in monkeys. J Neurophysiol 88: 715–731, 2002. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Isa T. Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Netw 15: 731–741, 2002. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Okada KI. Reward prediction error computation in the pedunculopontine tegmental nucleus neurons. Ann NY Acad Sci 1104: 310–323, 2007. [DOI] [PubMed] [Google Scholar]
- Krauthamer GM, Grunwerg BS, Krein H. Putative cholinergic neurons of the pedunculopontine tegmental nucleus projecting to the superior colliculus consist of sensory responsive and unresponsive populations which are functionally distinct from other mesopontine neurons. Neuroscience 69: 507–517, 1995. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Bollimunta A, Arcizet F, Wang L. Attention as an effect not a cause. Trends Cogn Sci 18: 457–464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: goals, choices and hypotheses. Vision Res 44: 1445–1451, 2004. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zénon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci 36: 165–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron 58: 451–463, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature 418: 413–417, 2002. [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332: 357–360, 1988. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Drivers and modulators in the central auditory pathways. Front Neurosci 4: 79–86, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Hall WC. An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. J Neurosci 26: 4763–4768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Helms MC, Augustine GJ, Hall WC. Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proc Natl Acad Sci USA 94: 13299–13304, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Endo T, Isa T. Presynaptic muscarinic acetylcholine receptors suppress GABAergic synaptic transmission in the intermediate grey layer of mouse superior colliculus. Eur J Neurosci 20: 2079–2088, 2004. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Hromádka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One 4: e6099, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintz MJ, Felsen G. Context-dependence of action selection in the basal ganglia. Soc Neurosci Abstr 2014: 442–20., 2014. [Google Scholar]
- Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol 100: 1098–1112, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci 9: 956–963, 2006. [DOI] [PubMed] [Google Scholar]
- Lock TM, Baizer JS, Bender DB. Distribution of corticotectal cells in macaque. Exp Brain Res 151: 455–470, 2003. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci 13: 261–266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci 32: 10105–10116, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TP, Graybiel AM, Wurtz RH. Location of saccade-related neurons in the macaque superior colliculus. Exp Brain Res 85: 21–35, 1991. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Garrison TJ, Shires J, Basso MA. Stimulation of the substantia nigra influences the specification of memory-guided saccades. J Neurophysiol 111: 804–816, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat 5: 22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Watanabe K, Ohye C. Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res 28: 155–165, 1997. [DOI] [PubMed] [Google Scholar]
- Mays E, Sparks DL. Dissociation of visual and saccade-related responses in superior colliculus neurons. J Neurophysiol 43: 207–232, 1980. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002a. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Superior colliculus activity related to concurrent processing of saccade goals in a visual search task. J Neurophysiol 87: 1805–1815, 2002b. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci 27: 585–588, 2004. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Ramoa AS. Intrinsic circuitry of the superior colliculus: pharmacophysiological identification of horizontally oriented inhibitory interneurons. J Neurophysiol 79: 1597–1602, 1998. [DOI] [PubMed] [Google Scholar]
- Miller G, Gallanter E, Pribram K. Plans and the Structure of Behavior. New York: Holt, 1960. [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia intrinsic circuits and their role in behavior. Curr Opin Neurobiol 3: 950–957, 1993. [DOI] [PubMed] [Google Scholar]
- Missal M, Lefevre P, Delinte A, Crommelinck M, Roucoux A. Smooth eye movements evoked by electrical stimulation of the cat's superior colliculus. Exp Brain Res 107: 382–390, 1996. [DOI] [PubMed] [Google Scholar]
- Mize R. Immunocytochemical localization of gamma-aminobutyric acid (GABA) in the cat superior colliculus. J Comp Neurol 276: 169–187, 1988. [DOI] [PubMed] [Google Scholar]
- Mize R. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res 90: 219–248, 1992. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Martin TL, Mash DC, Wainer BH, Mesulam MM. Cholinergic projections from the parabigeminal nucleus (Ch8) to the superior colliculus in the mouse: a combined analysis of horseradish peroxidase transport and choline acetyltransferase immunohistochemistry. Brain Res 370: 144–148, 1986. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218–228, 2004. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Fecteau JH. Vying for dominance: dynamic interactions control visual fixation and saccadic initiation in the superior colliculus. Prog Brain Res 140: 3–19, 2002. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79: 1193–1209, 1998. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Role of the rostral superior colliculus in active visual fixation and execution of express saccades. J Neurophysiol 67: 1000–1002, 1992. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70: 559–575, 1993a. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 70: 576–589, 1993b. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol 73: 2313–2333, 1995. [DOI] [PubMed] [Google Scholar]
- Mysore SP, Knudsen EI. The role of a midbrain network in competitive stimulus selection. Curr Opin Neurobiol 21: 653–660, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin LM, Robles E, Baier H, Scott EK. Focusing on optic tectum circuitry through the lens of genetics. BMC Biol 8: 126, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norita M. Neurons and synaptic patterns in the deep layers of the superior colliculus of the cat. A Golgi and electron microscopic study. J Comp Neurol 190: 29–48, 1980. [DOI] [PubMed] [Google Scholar]
- Norton AB, Jo YS, Clark EW, Taylor CA, Mizumori SJ. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci 33: 1885–1896, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummela SU, Krauzlis RJ. Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. J Neurophysiol 104: 1538–1548, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummela SU, Krauzlis RJ. Superior colliculus inactivation alters the weighted integration of visual stimuli. J Neurosci 31: 8059–8066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JD, Butcher LL, Woolf NJ. Thyroid hormone modulates the development of cholinergic terminal fields in the rat forebrain: relation to nerve growth factor receptor. Brain Res Dev Brain Res 59: 133–142, 1991. [DOI] [PubMed] [Google Scholar]
- Okada K, Kobayashi Y. Characterization of oculomotor and visual activities in the primate pedunculopontine tegmental nucleus during visually guided saccade tasks. Eur J Neurosci 30: 2211–2223, 2009. [DOI] [PubMed] [Google Scholar]
- Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y. Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci 29: 4858–4870, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Brown J, Dudman JT. Neural signals of extinction in the inhibitory microcircuit of the ventral midbrain. Nat Neurosci 16: 71–78, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci 23: 6480–6489, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré M, Wurtz RH. Progression in neuronal processing for saccadic eye movements from parietal cortex area lip to superior colliculus. J Neurophysiol 85: 2545–2562, 2001. [DOI] [PubMed] [Google Scholar]
- Philipp R, Hoffmann KP. Arm movements induced by electrical microstimulation in the superior colliculus of the macaque monkey. J Neurosci 34: 3350–3363, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phongphanphanee P, Marino RA, Kaneda K, Yanagawa Y, Munoz DP, Isa T. Distinct local circuit properties of the superficial and intermediate layers of the rodent superior colliculus. Eur J Neurosci 40: 2329–2343, 2014. [DOI] [PubMed] [Google Scholar]
- Platt ML, Lau B, Glimcher PW. Situating the superior colliculus within the gaze control network. In: The Superior Colliculus: New Approaches for Studying Sensorimotor Integration, edited by Hall WC, Moschovakis A. Boca Raton, FL: CRC, 2004. [Google Scholar]
- Quinet J, Goffart L. Cerebellar control of saccade dynamics: contribution of the fastigial oculomotor region. J Neurophysiol 113: 3323–3336, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Childers R, Smith PL, Segraves MA. Inhibition in superior colliculus neurons in a brightness discrimination task? Neural Comput 23: 1790–1820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Smith PL, Segraves MA. Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J Neurophysiol 97: 1756–1774, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Coizet V, Comoli E, McHaffie JG, Leriche M, Vautrelle N, Hayes LM, Overton P. Interactions between the midbrain superior colliculus and the basal ganglia. Front Neuroanat 4: 132, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci 7: 967–975, 2006. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Marrow L, Dean P. Topographical organization of the nigrotectal projection in rat: evidence for segregated channels. Neuroscience 50: 571–595, 1992. [DOI] [PubMed] [Google Scholar]
- Redila V, Kinzel C, Jo YS, Puryear CB, Mizumori SJ. A role for the lateral dorsal tegmentum in memory and decision neural circuitry. Neurobiol Learn Mem 117: 93–108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán M, Reinoso-Suárez F, Tortelly A. Parabigeminal projections to the superior colliculus in the cat. Brain Res 280: 1–13, 1983. [DOI] [PubMed] [Google Scholar]
- Rothermel M, Carey RM, Puche A, Shipley MT, Wachowiak M. Cholinergic inputs from basal forebrain add an excitatory bias to odor coding in the olfactory bulb. J Neurosci 34: 4654–4664, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, Stark E, Sjulson L, Buzsáki G. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr Opin Neurobiol 26: 88–95, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci 6: 723–733, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh K, Hattori S, Song WJ, Isa T, Takakusaki K. Nigral GABAergic inhibition upon cholinergic neurons in the rat pedunculopontine tegmental nucleus. Eur J Neurosci 18: 879–886, 2003. [DOI] [PubMed] [Google Scholar]
- Saitoh K, Isa T, Takakusaki K. Nigral GABAergic inhibition upon mesencephalic dopaminergic cell groups in rats. Eur J Neurosci 19: 2399–2409, 2004. [DOI] [PubMed] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 22: 2363–2373, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol 44: 1175–1189, 1980. [DOI] [PubMed] [Google Scholar]
- Schnyder H, Reisine H, Hepp K, Henn V. Frontal eye field projection to the paramedian pontine reticular formation traced with wheat germ agglutinin in the monkey. Brain Res 329: 151–160, 1985. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997. [DOI] [PubMed] [Google Scholar]
- Segraves MA. Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. J Neurophysiol 68: 1967–1985, 1992. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Functional properties of corticotectal neurons in the monkey's frontal eye field. J Neurophysiol 58: 1387–1419, 1987. [DOI] [PubMed] [Google Scholar]
- Shires J, Joshi S, Basso MA. Shedding new light on the role of the basal ganglia-superior colliculus pathway in eye movements. Curr Opin Neurobiol 20: 717–725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field. I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol 301: 618–642, 1990. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol 83: 1979–2001, 2000. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Frontal eye field sends delay activity related to movement, memory, and vision to the superior colliculus. J Neurophysiol 85: 1673–1685, 2001. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci 31: 317–338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, McPeek R. Neural correlates of target selection for reaching movements in superior colliculus. J Neurophysiol 113: 1414–1422, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Rafal RD, McPeek RM. Deficits in reach target selection during inactivation of the midbrain superior colliculus. Proc Natl Acad Sci USA 108: E1433–E1340, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooksawate T, Isa T. Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus. Eur J Neurosci 24: 3096–3108, 2006. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Isa K, Behan M, Yanagawa Y, Isa T. Organization of GABAergic inhibition in the motor output layer of the superior colliculus. Eur J Neurosci 33: 421–432, 2011. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Isa K, Isa T. Cholinergic responses in crossed tecto-reticular neurons of rat superior colliculus. J Neurophysiol 100: 2702–2711, 2008. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Yanagawa Y, Isa T. Cholinergic responses in GABAergic and non-GABAergic neurons in the intermediate gray layer of mouse superior colliculus. Eur J Neurosci 36: 2440–2451, 2012. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev 66: 118–171, 1986. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol 9: 698–707, 1999. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. In: The Neurobiology of Saccadic Eye Movements, edited by Wurtz RH, Goldberg ME. New York: Elsevier, 1989. [Google Scholar]
- Sparks DL, Holland R, Guthrie BL. Size and distribution of movement field in the monkey superior colliculus. Brain Res 113: 21–34, 1976. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 153: 1544–1547, 1966. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey. II. Topography of terminal fields in midbrain and pons. J Comp Neurol 271: 493–506, 1988. [DOI] [PubMed] [Google Scholar]
- Steckler T, Inglis W, Winn P, Sahgal A. The pedunculopontine tegmental nucleus: a role in cognitive processes? Brain Res Brain Res Rev 19: 298–318, 1994. [DOI] [PubMed] [Google Scholar]
- Stein BE, Rowland BA. Organization and plasticity in multisensory integration: early and late experience affects its governing principles. Prog Brain Res 191: 145–163, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield EA, Costabile JD, Felsen G. Optogenetic investigation of the role of the superior colliculus in orienting movements. Behav Brain Res 255: 55–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield EA, Thompson JA, Felsen G. Optogenetic cholinergic modulation of the mouse superior colliculus in vivo. J Neurophysiol 114: 978–988, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci 6: 363–375, 2005. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res 50: 137–151, 2004. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Kvitsani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71: 995–1013, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci 15: 3092–3103, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajah D, Mikulić A, Dorris MC. Role of the superior colliculus in choosing mixed-strategy saccades. J Neurosci 29: 1998–2008, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Felsen G. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol 110: 2817–2829, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Looger LL. Imaging neuronal activity with genetically encoded calcium indicators. Cold Spring Harb Protoc 2012: 647–656, 2012. [DOI] [PubMed] [Google Scholar]
- Trappenberg TP, Dorris MC, Munoz DP, Klein RM. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J Cogn Neurosci 13: 256–271, 2001. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol 55: 399–417, 2015. [DOI] [PubMed] [Google Scholar]
- Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev 32: 333–342, 2008. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Fredens K. Origin of high acetylcholinesterase activity in the mouse superior colliculus. Exp Brain Res 72: 335–346, 1988. [DOI] [PubMed] [Google Scholar]
- Walton MM, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol 98: 2022–2037, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74: 858–873, 2012. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kobayashi Y, Inoue Y, Isa T. Effects of local nicotinic activation of the superior colliculus on saccades in monkeys. J Neurophysiol 93: 519–534, 2005. [DOI] [PubMed] [Google Scholar]
- Weldon DA, Calabrese LC, Nicklaus KJ. Rotational behavior following cholinergic stimulation of the superior colliculus in rats. Pharmacol Biochem Behav 19: 813–820, 1983. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res 115: 191–205, 1997. [DOI] [PubMed] [Google Scholar]
- Westby GW, Collinson C, Redgrave P, Dean P. Opposing excitatory and inhibitory influences from the cerebellum and basal ganglia converge on the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci 6: 1335–1342, 1994. [DOI] [PubMed] [Google Scholar]
- White BJ, Munoz DP. The superior colliculus. In: The Oxford Handbook of Eye Movements, edited by Liversedge SP, Gilchrist I, Everling S. Oxford, UK: Oxford Univ. Press, 2011. [Google Scholar]
- Williams MN, Faull RL. The striatonigral projection and nigrotectal neurons in the rat. A correlated light and electron microscopic study demonstrating a monosynaptic striatal input to identified nigrotectal neurons using a combined degeneration and horseradish peroxidase procedure Neuroscience 14: 991–1010, 1985. [DOI] [PubMed] [Google Scholar]
- Wilt BA, Burns LD, Wei Ho ET, Ghosh KK, Mukamel EA, Schnitzer MJ. Advances in light microscopy for neuroscience. Annu Rev Neurosci 32: 435–506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci 248: 234–250, 2006. [DOI] [PubMed] [Google Scholar]
- Winn P. Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat Disord 14, Suppl 2: S194–S198, 2008. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Landy MS. Motor control is decision-making. Curr Opin Neurobiol 22: 996–1003, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain. III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull 16: 603–637, 1986. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. J Neurophysiol 35: 575–586, 1972. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA, Paré M, Ferraina S. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Res 41: 3399–3412, 2001. [DOI] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 8: 745–752, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


