Abstract
The ability to differentially alter specific brain functions via deep brain stimulation (DBS) represents a monumental advance in clinical neuroscience, as well as within medicine as a whole. Despite the efficacy of DBS in the treatment of movement disorders, for which it is often the gold-standard therapy when medical management becomes inadequate, the mechanisms through which DBS in various brain targets produces therapeutic effects is still not well understood. This limited knowledge is a barrier to improving efficacy and reducing side effects in clinical brain stimulation. A field of study related to assessing the network effects of DBS is gradually emerging that promises to reveal aspects of the underlying pathophysiology of various brain disorders and their response to DBS that will be critical to advancing the field. This review summarizes the nascent literature related to network effects of DBS measured by cerebral blood flow and metabolic imaging, functional imaging, and electrophysiology (scalp and intracranial electroencephalography and magnetoencephalography) in order to establish a framework for future studies.
Keywords: deep brain stimulation, electrocorticography, magnetoencephalography
deep brain stimulation (DBS) provides a unique opportunity for further understanding of healthy and aberrant human brain function. DBS is the only paradigm in which specific deep brain regions may be manipulated through focal electrical stimulation while simultaneously recording brain activity. An emerging field of study has begun to elucidate how different DBS targets modulate network activity in connected brain regions.
The field of DBS has experienced substantial growth since its reestablishment in the modern era by Benabid nearly 30 years ago (Benabid et al. 1987). A wealth of clinical evidence has established a role for DBS in the management of movement disorders, including Parkinson's disease (PD) (Limousin et al. 1998b), essential tremor (ET) (Miocinovic et al. 2013), and dystonia (Coubes et al. 2004). More recently, the use of DBS has expanded into the field of neuropsychiatry, with investigators exploring the potential of DBS to produce clinical improvement for patients with major depressive disorder (MDD) (Holtzheimer and Mayberg 2012), obsessive-compulsive disorder (OCD) (Mallet et al. 2008), Tourette syndrome (Ackermans et al. 2011), neuropathic pain (Pereira and Aziz 2014), and addiction (Alba-Ferrara et al. 2014). Epilepsy also is an important emerging indication for DBS (Bergey et al. 2015; Salanova et al. 2015). In parallel, the technology itself is improving, with closed-loop systems and novel electrode arrays currently under development that are expected to improve the efficacy of DBS (McIntyre et al. 2015).
Despite the fact that DBS has been investigated for over 20 different indications, with targets in nearly 40 distinct brain regions (Hariz et al. 2013), the mechanisms through which DBS modulates brain networks and the effects of focal stimulation on both local and distributed brain functions are not yet clear. Several critical questions remain to be answered in the context of each target and therapeutic application: 1) What are the most therapeutically effective target structures, 2) What are the relevant neurophysiological effects of DBS on those structures, and 3) How do these effects modulate the activation of diffuse functional networks to produce desired or undesired behavioral changes? Brain regions targeted by DBS are variably composed of neuronal somata making up the gray matter in the vicinity of the stimulated electrode contact, axonal projections both through and adjacent to the target, or primarily white matter tracts themselves. The complexity of determining the neurobiological mechanisms of DBS therapy is compounded by the fact that target structures act as relay nodes in larger networks, which are perturbed by the reverberating effects of stimulation (McIntyre et al. 2004; McIntyre and Hahn 2010; Vitek 2002). An initial explanation for the effects of high-frequency DBS was that stimulation inhibits the targeted structure, thereby producing a functional lesion during (Filali et al. 2004) and after (Beurrier et al. 2001) stimulation. Alternate hypotheses have proposed that activation of the target or induction of long-term synaptic plasticity alters excitability (Kombian et al. 2000). Furthermore, given the oscillatory nature of the train of stimulation pulses employed in DBS, its effects can alter the rhythmic interaction of targeted networks, effectively altering information flow without clearly inhibiting or activating neural tissue (Chiken and Nambu 2014). For instance, axonal and synaptic failures induced by short-term depression following axonal excitation by DBS have been hypothesized to suppress information transfer (Rosenbaum et al. 2014). Finally, DBS may induce a regular rhythm driven by high-frequency stimulation that overrides the pathological rhythm present in the target area (Garcia et al. 2005).
This review describes the cortical effects of DBS primarily in networks linked with the 1) subthalamic nucleus (STN), 2) globus pallidus internus (GPi), 3) thalamus, and 4) nucleus accumbens (NAc) (Fig. 1). We have integrated reports from the functional magnetic resonance imaging (fMRI), positron emission tomography (PET), electroencephalography (EEG), electrocorticography (ECoG), and magnetoencephalography (MEG) literature. Each of these techniques offers different advantages and has unique limitations, which must be appreciated in order to interpret the findings. For instance, functional and metabolic imaging offer spatial resolution superior to scalp EEG, allow for measuring activity in subcortical structures, and are not affected by the electrical artifact of stimulation, but the temporal resolution of network activity is limited and static. MEG and ECoG can demonstrate spectral changes at the cortical level with high temporal and spatial resolution but require that the effect of electrical stimulation is filtered out. These data are reviewed in the context of current hypotheses related to the mechanisms of action of DBS on motor, cognitive, and neuropsychiatric functions.
Fig. 1.
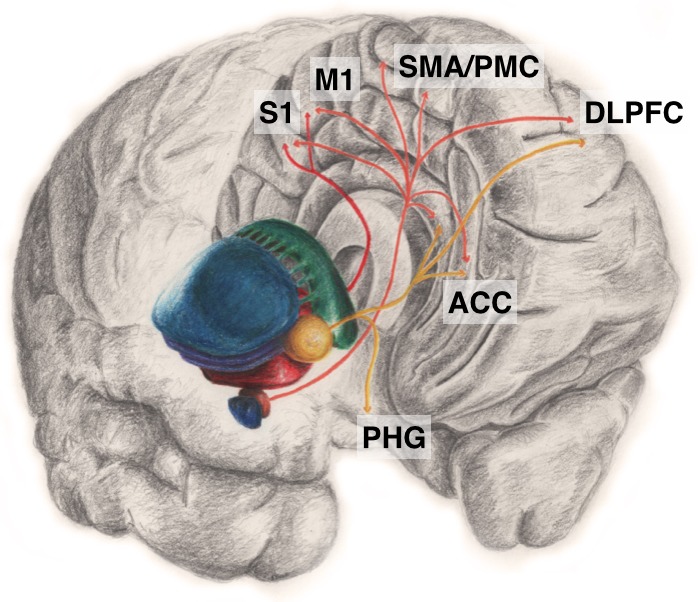
Schematic of network modulation by deep brain stimulations (DBS) in 3 major targets. Brain regions modulated by DBS of the subthalamic nucleus (STN; orange), thalamus (red), and nucleus accumbens (yellow) are indicated (in the contralateral hemisphere for visual clarity). S1, primary sensory cortex; M1, primary motor cortex; SMA, supplementary motor area; PMC, premotor cortex; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; PHG, parahippocampal gyrus.
Network Effects of Subthalamic Nucleus Stimulation
Although over 100,000 patients have undergone DBS implantation for various indications, the majority of our experience with recording network effects during DBS comes from STN stimulation for the treatment of PD. Unless otherwise stated, the studies reviewed in the STN sections below involve DBS of the sensorimotor territory of the STN for PD. A current hypothesis for the therapeutic effects of STN DBS in PD is that STN stimulation decreases pathological synchronization in the beta frequency band between STN and primary motor cortex (PMC). This pathological synchronization is disrupted by both STN stimulation and dopaminergic therapies and has been correlated with clinical improvement (de Hemptinne et al. 2013; Oswal et al. 2013). However, various associations with disinhibition in cognition and mood have also been documented in patients after surgery (Castrioto et al. 2014). These changes indicate stimulation effects extending beyond local targets in sensorimotor STN. These effects can be explained anatomically given the known involvement of the STN in multiple circuits connecting the basal ganglia to the cortical regions that regulate motor, cognitive, and emotional behavior (Alexander et al. 1986). Furthermore, these diverse effects can be attributed to the systematic and random procedural errors leading to minor location inaccuracies but with substantial neurophysiological effects (Tsai et al. 2007). The mechanisms by which stimulation modulates these brain networks remain controversial and are the subject of active investigation.
STN studies using PET.
A substantial amount of work has been accomplished with metabolic imaging using both 18-FDG and [15O]H2O PET (Akatsubo and Akabayashi 2009; Asanuma et al. 2006; Ceballos-Baumann et al. 1999; Chul et al. 2007; Devos et al. 2004; Geday et al. 2009; Haegelen et al. 2010; Haslinger et al. 2005; Hilker et al. 2002, 2004; Karimi et al. 2008; Limousin et al. 1997; Mure et al. 2011; Sestini et al. 2002, 2007; Sidtis et al. 2012; Strafella et al. 2003; Trost et al. 2006), the former measuring glucose metabolism and the latter measuring regional changes in cerebral blood flow (rCBF). The main aim of these studies in STN DBS was to assess motor system function in PD and its relation to treatment by examining cortical metabolic changes induced by DBS during both resting state and simple motor tasks. Considering studies with a minimum of 15 subjects, testing patients off medications and without previous surgeries, a general pattern emerges in a motor network including the PMC, lateral premotor cortex, dorsolateral prefrontal cortex (DLPFC), supplementary motor area (SMA), and anterior cingulate cortex (ACC). Compared with off-stimulation, there is decreased activation in this network at rest during STN DBS. In contrast to the effect of DBS at rest, during self-initiated movement STN DBS is associated with increased metabolism in rostral SMA, ACC, and DLPFC. A prospective study in which 40 PD patients and age-matched control subjects were scanned at rest demonstrated that clinical improvement was associated with perfusion decrements in primary motor and premotor cortices. Cerebellar activation increased during stimulation, in conjunction with evidence of modulation of a cerebello-thalamo-cortical loop functionally connected to the cortico-basal ganglia motor loop (Cilia et al. 2009). Thus STN DBS appears to differentially affect the resting-state network compared with the functional motor network. Part of the discrepancy found among studies recording during movement, however, is also likely attributable to the type of task employed.
Metabolic imaging while subjects perform cognitive tasks has shed some light on the neural bases of cognitive changes reported in DBS. Verbal fluency performance during stimulation has been shown to correlate with activation of the left inferior frontal gyrus, left inferior temporal gyrus, left DLPFC, and ACC (Cilia et al. 2007; Kalbe et al. 2009; Schroeder et al. 2003). STN DBS effects on the DLPFC and ACC have also been investigated in relation to conflict monitoring using the Stroop task (Schroeder et al. 2002), random number generation (Thobois et al. 2007), and working memory and response inhibition (Campbell et al. 2008). In all of these studies, a decrease in regional activity of those regions induced by DBS correlated with the impairments in the corresponding cognitive function being assayed in the former two studies, while the opposite effect was observed in the latter study. Notably, a specific pattern of activation at rest, characterized by metabolic reductions in frontal and parietal association areas and relative increases in the cerebellar vermis and dentate nuclei, has separately been shown to predict memory performance, visuospatial function, and perceptual motor speed but was not significantly altered by antiparkinsonian medications or DBS (Huang et al. 2007). These findings may suggest a discrepancy between DBS affects during task-related epochs compared with epochs in which a subject is not engaged in a task.
Modulation of key parts of the limbic circuit has been demonstrated in a large series of patients; however, no resultant cognitive improvement was detected on neuropsychological testing except modest improvements in a card-sorting task (Le Jeune et al. 2010). This result is potentially confounded because patients were tested who were still on medication in addition to DBS. In a study of apathy following DBS, positive correlations were observed between apathy scores and increases in glucose metabolism, especially in the right frontal middle gyrus and inferior frontal gyrus (Le Jeune et al. 2009). Finally, DBS-induced deactivation in the fusiform gyrus has been correlated with the impairment of facial expression recognition (Geday et al. 2006).
STN DBS has also been used as a treatment for OCD, with electrode placement in the limbic territory of the STN. A PET study showed that this therapy resulted in a decrease in metabolism of the left cingulate and medial gyrus of the left frontal lobe. Clinical improvement was correlated with a decrease of metabolic activity in the orbitofrontal cortex (OFC)-ventral medial prefrontal region in ON vs. OFF conditions (Le Jeune et al. 2010).
STN studies using functional magnetic resonance imaging.
The application of fMRI to investigation of DBS has been hindered by concerns regarding safety (Finelli et al. 2002; Georgi et al. 2004; Shrivastava et al. 2012) and by significant artifacts in the acquired images, although there is evidence that its use under strict guidelines may be safe (Carmichael et al. 2007). Because of these safety concerns, this imaging modality remains underutilized, with few studies published using fMRI during active stimulation and limited to the use of 1.5-T scanners (Arantes et al. 2006; Hesselmann et al. 2004; Jech et al. 2001; Kahan et al. 2012, 2014; Stefurak et al. 2003) except for Phillips et al. (2006), where a 3-T scanner was used. Each of these patient cohorts did not exceed five patients, outside of the work by Kahan and colleagues. The reader also is referred to Boertien and colleagues (Boertien et al. 2011) for a recent extensive review of functional imaging in STN DBS.
Nonetheless, initial fMRI studies of DBS effects at rest essentially have corroborated earlier metabolic imaging findings. In addition, Kahan and colleagues studied the effects of DBS on the motor network directly by using dynamic causal modeling in 10 subjects (Kahan et al. 2014). Their findings suggest that the therapeutic effects of DBS arise from strengthening the cortico-striatal, direct, and thalamocortical pathways while disrupting all afferent and efferent inputs to the STN. Disruption or weakening of incoming and outgoing connectivity with STN DBS is beneficial when it involves the overly connected motor network. However, this interference with other networks also may explain side effects related to cognitive functions for which the STN is an important network node. While it offers potential new insight into DBS effects, this model lacks some elements of the actual human network (like the globus pallidus) and still requires validation on a larger data set.
STN studies using EEG and ECoG.
During rest, STN DBS has been shown via scalp EEG to induce a broadband decrease in spectral power in frontocentral electrodes (Jech et al. 2006) and a widespread decrease of coherence predominantly in the beta frequency band (Hotton et al. 2005). During movement preparation, STN stimulation was shown to partially restore normal patterns of cortical activation by decreasing abnormal desynchronization prior to movement onset over the bilateral frontocentral regions (Devos et al. 2004), suggesting increased activation of the premotor cortex by DBS.
In addition to motor effects, STN stimulation can have effects on the processing of sensory information. Cortical encoding of sensory stimuli has often been analyzed with event-related potentials (ERPs), which represent time-locked cortical neuronal population activity in response to a certain stimulus. The synchronization of this activity depends on several factors including the intrinsic membrane properties of the neurons and the state of their local and global networks (Pfurtscheller and Lopes da Silva 1999). The downstream effects of stimulation on these factors can be seen in the generation of ERPs and possibly extrapolated to the cognitive processes related to the stimulus used. This paradigm was used in several studies in the PD population listed in Table 1. Cortical sensory ERPs typically were reduced or unchanged by stimulation. Thus stimulation may interfere with neuronal synchronization, potentially disrupting the phase reorganization needed for the generation of an ERP (Sayers et al. 1974), but this effect on sensory processing, when present, appears to be weak. Stimulation generally has not demonstrated an effect on ERP latency. Only one study (Selzler et al. 2013) has reported a slowing in latency to match that of the control group, although task performance did not change. The lack of correlation between task performance and ERP generation suggests that more sophisticated measures are required to assess the effects of DBS, a point directly demonstrated by Swann et al. (2011), where time-frequency plots showed greater sensitivity to the effects of DBS whereas ERPs were insensitive to these effects. This implies that DBS affects oscillatory dynamics not well captured by ERPs. This concern, in addition to the difficulty associated with removing the stimulation artifact in EEG, has led some to turn to MEG to study the cortical effects of DBS.
Table 1.
Studies reporting ERPs measured by scalp EEG after STN stimulation
| Study | Sensory Stimulus Used | Cognitive Task Used | ERP Recorded and Stimulation-Induced Amplitude Changes |
|---|---|---|---|
| Pierantozzi (1999) | Somatosensory EPs | None | N20/P25, unchanged |
| N30 increased | |||
| Priori et al. (2001) | Somatosensory EPs and visual EPs | None | N20/P100, decreased |
| Insola et al. (2005) | Somatosensory EPs | None | N9/P14, unchanged |
| N20/N30, increased | |||
| Jech et al. (2006) | Visual EPs | None | N70/P100, decreased |
| Conte et al. (2010) | Somatosensory EPs | None | N20/P25 and P25/N33, decreased |
| Gulberti et al. (2014) | Auditory EPs | None | P1/N1, unchanged |
| Gerschlager et al. (2001) | None | Auditory oddball | Not reported |
| Kovacs et al. (2008) | None | Auditory oddball | P300/N200, unchanged |
| Klostermann et al. (2010) | None | Choice response, oddball | P300, unchanged |
| Swann et al. (2011) | None | Stop signal | N2/P3, unchanged |
| Selzler et al. (2013) | None | Working memory | N200, decreased |
ERP, event-related potential; STN, subthalamic nucleus; EP, evoked potential.
Electrical stimulation has a predilection for activating axons in long tracts compared with cell bodies near the source (Nowak and Bullier 1998). This can be captured in subjects as evoked potentials (EPs) time-locked to the stimulation train. The resultant EP demonstrated synchronization of cortical activity with stimulation, where the different parts of the EP were thought to represent different modes of synaptic transmission. Both a short latency (<8 ms) and a long latency (>18 ms) were observed (Ashby et al. 2001; Baker et al. 2002; Eusebio et al. 2009; Kuriakose et al. 2010; Limousin et al. 1998a; MacKinnon et al. 2005; Walker et al. 2012b). The topography of these EPs encompassed frontal and central areas. The timing of the short-latency components is more consistent with antidromic conduction through the corticosubthalamic projections. The longer latency might represent orthodromic polysynaptic conduction through the basothalamocortical circuit. Of note, Walker and colleagues went further to explore the short-latency component by summating two sets of recordings with reversed anode/cathode pairs in order to suppress the stimulation artifact. This uncovered a component at a latency of 1 ms, suggesting that the short-latency components seen in previous studies might represent the tail of the waveform that was obscured by the stimulation artifact. However, a number of these studies only use low-frequency stimulation (5–30 Hz), making interpretation of these results to explain clinical high-frequency stimulation difficult.
Patients with PD often exhibit cognitive dysfunction in addition to classical motor symptoms. An important question is how pathology in brain networks contributes to this dysfunction and how DBS affects these networks. Even in early, untreated PD patients, widespread increases in the amplitude of theta and low alpha rhythms have been observed, with increased alpha power in the centroparietal region associated with abnormal perseveration (Stoffers et al. 2007). Separately, STN DBS has been shown to result in a decrease in spectral power in the alpha band (Jech et al. 2006), but whether this normalization of low-frequency activity contributes to cognitive improvement is unknown.
On the other hand, impulsivity is a consequence of STN stimulation not seen in patients receiving l-DOPA therapy (Frank et al. 2007; Hälbig et al. 2009). The leading explanation is that stimulation interferes with normal STN inhibition that withholds actions until a decision threshold is reached, especially during conflict. For instance, during conflict monitoring, DBS has been shown to disrupt the relationship between the level of theta in medial prefrontal cortex and the decision threshold during conflict leading to response speeding (Cavanagh et al. 2011). Swann et al. (2011), however, showed that DBS improved response inhibition and linked this improvement to modulating the beta response seen in the frontal cortex to comparable levels seen in the control group. The difference in DBS effects on response inhibition might be explained by the task employed. For example, improvement was seen with exclusively motor decision making tasks (Mirabella et al. 2012; Swann et al. 2011; van den Wildenberg et al. 2006), while cognitive decision making tasks were associated with a decline in performance (Cavanagh et al. 2011; Frank et al. 2007). These findings suggest that disrupting the STN locally may affect functionally connected cortical regions via differential effects on two different circuits converging in the STN (Wylie et al. 2010). Likewise, a recent study of the effects of STN stimulation on go-only and countermanding tasks also indicated that both inhibitory control and the selection of the most appropriate motor strategy for a given context may be regulated by two different circuits that both include the STN (Mirabella et al. 2013).
The use of ECoG offers better localization and higher signal-to-noise ratio compared with EEG, both of which remain hurdles to the use of EEG during active stimulation. The use of ECoG, by temporarily sliding a strip electrode through the burr hole, during DBS surgery was introduced by Starr's group at the University of California San Francisco. For instance, de Hemptinne and colleagues explored the relationship between the pathological beta rhythm in PD and gamma activity in M1. Intraoperative EcoG recordings from PD, cervical dystonia, and epilepsy patients showed exaggerated phase-amplitude coupling (PAC) between the beta rhythm and broadband gamma activity in PD, with the phase of the beta rhythm in the STN modulating the amplitude of broadband gamma activity in M1 (de Hemptinne et al. 2013). This coupling was decreased in the recording after therapeutic stimulation, suggesting that pathological STN-M1 synchronization is disrupted by DBS. In addition, these authors proposed that broadband cortical gamma activity might maintain this abnormal coupling by feedback via the hyperdirect pathway from cortex to STN. Separately, Whitmer and colleagues targeted the hyperdirect pathway of the M1 cortex for ECoG, based on diffusion tractography imaging. They compared the effects of DBS on the signal from that region to nonspecific regions. In the two patients that had accurate targeting, they demonstrated a selective decrease in neural synchrony over M1 during stimulation in the frequency band between 5 and 35 Hz (Whitmer et al. 2012). However, the findings of Whitmer and colleagues were not reproducible across a larger patient sample, and in more extensive work de Hemptinne and colleagues showed that DBS reversibly reduced an exaggerated coupling between the phase of the beta rhythm and the amplitude of broadband activity in motor cortex (de Hemptinne et al. 2015). This effect was seen across different stages of movement and regardless of the effect on cortical beta frequency power changes. These findings strongly argue for DBS disrupting abnormal cortical synchronization as a mechanism for its therapeutic effects.
STN studies using MEG.
MEG offers superior spatial resolution compared with EEG and superior temporal resolution compared with fMRI. In addition, the relatively large number of sensors used in MEG (most modern MEG systems have 200 or more sensors) greatly improves researchers' ability to remove the stimulation artifact from the signal relative to EEG. Specifically, the introduction of temporal signal space separation (tSSS) (Taulu and Simola 2006) and null beamforming (Mohseni et al. 2012) has been shown to successfully minimize the effects of the stimulator artifacts, facilitating studies examining the acute effects of DBS by comparing cortical activity during the DBS-on state to the DBS-off state. As a validity study, Park et al. (2009) showed increased corticomuscular coherence in the beta band during STN stimulation. Another report supporting MEG feasibility during stimulation comes from the same group that developed the tSSS method for stimulator artifact removal (Airaksinen et al. 2011). In a group of PD patients, these authors showed that the recording of somatosensory ERPs and auditory ERPs was feasible during stimulation.
In an effort to study DBS-induced modulation of cortical activity, Airaksinen et al. (2012) demonstrated that stimulation decreased the amplitude of the alpha peak, corroborating the findings of Jech et al. (2006) from EEG. They also showed correlation between the amplitudes of pericentral alpha and low beta to the Unified Parkinson Disease Rating Scale (UPDRS) rigidity scores with stimulation turned on. A similar study by Cao et al. (2015) described DBS effects detectable by MEG emerging 1 yr after stimulation rather than after 1 wk. These phenomena consisted of acceleration in the average cortical frequency in multiple regions, a widespread decrease in theta power, and an increase in low beta (14–18 Hz) power. Finally, they reproduced (Airaksinen et al. 2012) results describing correlations with UPDRS scores and cortical activity. The work related to beta oscillatory reduction is important since excessive beta synchronization of the cortico-basal ganglia loop is considered a pathological hallmark of PD. This excessive synchronization impairs the ability of the basal ganglion system to receive and process new information, thus hindering the initiation of new actions (Brittain et al. 2014). Nevertheless, the importance of changes in other oscillatory frequencies remains to be defined.
Conclusions regarding STN stimulation.
STN stimulation effects across measurement modalities fall into a number of different categories. During movement, PMC activation appears to be enhanced with increased involvement of frontal regions during movement execution. A global effect of stimulation appears to be disruption of neuronal synchronization, particularly in the alpha and low beta frequency ranges. While this is shown to improve the motor symptoms of PD, cognitive function may be negatively affected in some cases after DBS, particularly if DLPFC and ACC activity are significantly affected (Pinto et al. 2014).
Network Effects of Globus Pallidus Internus Stimulation
The GPi is the convergence point of the classically described indirect, direct, and hyperdirect basal ganglion pathways. This structure provides the most important inhibitory outflow from the basal ganglia to the thalamus and is hypothesized to play a role in action selection, among other functions (Mink 1996). Stimulation of the GPi provides therapeutic suppression of both primary and secondary dystonia (Katsakiori et al. 2009; Kupsch et al. 2006; Vercueil et al. 2001). In patients with PD, stimulation of the GPi is an attractive alternative to STN stimulation (Follett et al. 2010), especially when dopaminergic therapies are complicated by dystonia (Vitek 2002). Stimulation of the GPi has been studied with metabolic imaging and EEG. These studies have largely demonstrated that GPi stimulation aids in reducing overactivity in cortical areas associated with dystonia and in desynchronizing oscillatory activity in downstream sensorimotor cortices.
GPi studies using PET.
An early FDG-PET study found that high-frequency GPi stimulation increased glucose metabolism in ipsilateral premotor cortex and bilateral cerebellum that correlated with improvement in UPDRS scores (Fukuda et al. 2001). A subsequent H215O PET study found that DBS of GPi in six patients with severe primary generalized dystonia decreased abnormal overactivity in the DLPFC, gyrus frontalis medialis, superior frontal gyrus, fronto-orbital cortex, and thalamus that is characteristic of dystonia; this effect accompanied significant improvement in dystonic symptoms during a motor task (Detante et al. 2004). These studies suggest that patients with dystonia have characteristic overactivity in numerous cortical regions notably including premotor cortex. Furthermore, this overactivity can be suppressed by pallidal stimulation, and suppression is associated with improved motor symptoms.
GPi studies using EEG.
An early study using EEG during a movement task in patients with PD similarly found that GPi stimulation aided desynchronization over premotor cortex during movement planning and movement-related desynchronization over PMC (Devos et al. 2002). A study of EEG during low-frequency (10 Hz) GPi stimulation in subjects with dystonia (Tisch et al. 2008) found EPs that were maximal in the central contacts but absent on a patient-side with previous thalamotomy. The timing was consistent with pallidothalamocortical pathway activation of sensorimotor cortex that has also been documented after STN stimulation (MacKinnon et al. 2005).
Conclusions regarding GPi stimulation.
Although sparse data exist related to stimulation of GPi, EEG findings are supported by observations from functional imaging studies that the downstream effects of pallidal stimulation are expressed in premotor cortex. Furthermore, EEG studies suggest that motor improvement from GPi DBS may be at least partially due to stimulation-aided desynchronization of low-frequency oscillations (mu rhythms) in premotor cortex that have been associated with movement suppression.
Effects of Thalamic Stimulation
The thalamus is a complex structure composed of many nuclei, segregated by motor and associative functions, that offer both accepted and proposed DBS targets for various disease states affecting thalamocortical networks. The heterogeneity of patient populations and specific thalamic targets has generated compelling yet somewhat contradictory results related to network effects of thalamic DBS.
Thalamic studies using PET.
A H215O PET study in ET patients (Ceballos-Baumann et al. 2001) described increases in rCBF of the ipsilateral PMC with DBS of the ventral intermediate nucleus (Vim). Later, they went on to show linear and nonlinear relationships between cortical activity and increases in stimulation frequency and amplitude suggesting that DBS recruits different parts of a larger circuit gradually with varying stimulation parameters (Haslinger et al. 2003). Perlmutter and colleagues (using PET; Perlmutter et al. 2002) and Rezai and colleagues (using 1.5-T fMRI; Rezai et al. 1999) both failed to reproduce these findings, instead showing SMA and primary somatosensory activation, respectively. These heterogeneous effects might be attributed to the small number of subjects enrolled in each of the studies (n = 2–10), or to the statistical stringency each group employed. Importantly, all these studies were performed in the resting state, and none examined treatment effects on the action tremor of ET.
A different picture is seen in Vim stimulation for PD compared with Vim stimulation in ET patients. Multiple PET studies have shown results of decreased activation in the primary sensorimotor areas, SMA, and contralateral cerebellum (Deiber et al. 1993; Fukuda et al. 2004; Wielepp et al. 2001). This is contrary to what is seen in ET and probably relates to the nature of the resting tremor of PD, compared with the action tremor of ET. Mure and colleagues (Mure et al. 2011) later used the data set from Fukuda et al. (2004) to delineate a tremor-related metabolic network in PD, referred to as a PD tremor pattern (PDTP), comprised of the cerebellum, PMC, and to a lesser extent the striatum. This pattern was validated on a different subset of patients and correlated with tremor amplitude. Previous work characterized a network responsible for the akinesia and rigidity in PD, a PD-related pattern (PDRP), shown as a relative decrease in metabolic activity of the premotor, SMA, and parietal association regions (Eidelberg 2009). While STN stimulation modulated the PDRP network and improved rigidity, it had little effect on the PDTP network (Mure et al. 2011). This justifies the clinical use of Vim stimulation for tremor management (Rehncrona et al. 2003) while STN stimulation is used for rigidity management (Katz et al. 2015).
Although stimulation of the somatosensory thalamus also has been used for more than two decades to treat chronic pain, the mechanisms mediating stimulation-produced therapeutic analgesia are not understood. A PET study of rCBF in five patients who received successful long-term relief of chronic pain with somatosensory thalamic stimulation demonstrated activation of the insular cortex ipsilateral to the thalamic electrodes (Duncan et al. 1998). A subsequent case report demonstrated that blood flow significantly increased in the amygdala and anterior insular cortex during successful stimulation in a patient with chronic facial pain (Kupers et al. 2000). Thus activation of the ipsilateral insula may be important for relief of chronic pain treated by thalamic DBS.
Thalamic studies using EEG.
The technique of using ECoG to record intraoperatively was also applied by Starr and colleagues in ET patients (Air et al. 2012). They showed that Vim stimulation resulted in desynchronization of the alpha activity in primary motor and theta in primary somatosensory cortical areas. The relation to pathology remains unclear. In another arm of the same study, the authors found that thalamotomy had the opposite effect on these oscillations, despite the same level of observed tremor control. Although the relationship between Vim stimulation and M1 activation prior to movement execution was not elaborated upon in that study, it is possible that Vim stimulation activates M1 in preparation for movement execution. Clearly more work is needed to understand these findings.
Walker et al. (2012a) demonstrated short-latency cortical effects of Vim DBS recorded with scalp EEG that were a part of a complex EP. Effective Vim thalamic stimulation appeared to activate the cortex at ∼1 ms after the stimulus pulse, leading the authors to suggest that DBS may improve tremor by synchronizing the precise timing of discharges through the thalamocortical network to the stimulation frequency or one of its subharmonics.
A small case series using scalp EEG and low-resolution electromagnetic tomography (LORETA) investigated effects of anterior nucleus of the thalamus (ANT) stimulation on cortical synchronization in epilepsy (Zumsteg et al. 2006b). Similar evoked cortical potentials following low-frequency stimulation were observed in the frontal and temporal contacts on EEG for stimulation of the anterior and dorsomedial nuclei. With LORETA, the underlying sequential activation of the different ipsilateral cortical areas responsible for these responses was demonstrated. Despite the fact that the low number of subjects studied precludes drawing definitive conclusions about the functional relationship between the sources of cortical responses and these thalamic nuclei, this study also was valuable in demonstrating the need to overcome the limitations of standard EEG analyses. These authors subsequently reported that stimulation caused EEG synchronization (Zumsteg et al. 2006a), indicating downstream activation of thalamocortical pathways, but synchronization was not demonstrated with objective metrics. In a separate case report, evoked responses were recorded from bilateral hippocampal depth electrodes, simultaneous with scalp EEG, in response to acute ANT stimulation (Kim et al. 2014). The authors used this technique during repositioning of the DBS electrode in the ANT to confirm electrode placement in a patient with refractory bilateral temporal epilepsy, suggesting that recording synchronous cortical and mesial temporal network activity may be useful in guiding DBS placement for this indication.
Conclusions regarding thalamic stimulation.
The literature on thalamic stimulation is diffuse because of the wide number of disease states treated and the number of nuclei targeted within the thalamus itself. More work is needed to define network effects from stimulation of individual thalamic nuclei. However, evidence collected regarding Vim stimulation suggests it is involved in a network that is separate from the network related to akinesia in PD.
Network Effects of Nucleus Accumbens Stimulation
Several studies have shown that stimulation of the NAc holds promise for treating neuropsychiatric diseases, including OCD (Denys et al. 2010; Greenberg et al. 2006), major depression (Malone et al. 2009), Tourette syndrome (Neuner et al. 2009), and addiction (Kuhn et al. 2007). Overall success rates have been low in these experimental therapies, however, including the recent report of a failed pivotal trial for treatment-resistant depression (Denys et al. 2010). It is possible that NAc DBS may have different mechanisms of therapeutic action within different patient populations, and that the modest symptom control achieved in these studies is not a result of normalizing the fundamental etiology of the respective disorders. However, the NAc is a basal ganglion structure whose function has been heavily implicated in goal-directed behavior, reward processing, and addiction. Importantly, this nucleus receives major inputs from the prefrontal cortex and amygdala and dopaminergic innervation from the ventral tegmental area, and it projects to the ventral pallidum as part of the limbic basal ganglion circuit (Kopell and Greenberg 2008). Furthermore, the NAc is situated immediately adjacent to the anterior limb of the internal capsule of the basal ganglia, whose white matter tracts connect limbic and orbitofrontal structures (Tass et al. 2003). Thus, in addition to altering local activity, NAc stimulation can modulate the function of a number of distal targets that are implicated in psychiatric disorders. Indeed, both imaging and electrophysiological studies support a broad network mechanism for the effects of NAc DBS.
NAc studies using PET.
One of the consistent metabolic correlates of OCD is hyperactivity in the striatum and OFC at rest (Baxter et al. 1988; Swedo et al. 1989) and after symptom provocation (Breiter et al. 1996; McGuire et al. 1994; Rauch et al. 1994). Importantly, successful pharmacological and behavioral interventions, as well as basal ganglion capsulotomy have been shown to normalize this activity, particularly in the OFC. These findings suggest that the abnormal hyperactivation the OFC-NAc pathway underlies the pathophysiology of this disorder and implicates the OFC as a potential distal functional target of NAc DBS for OCD. Indeed, PET studies using stimulation of the NAc and the internal capsule have shown that symptom control correlates with metabolic decreases in prefrontal areas (Abelson et al. 2005; Van Laere et al. 2006; Nuttin et al. 2003).
An FDG PET imaging study in a series of four patients with anorexia nervosa (AN) found that DBS modulated the activity of frontal and hippocampal regions that were discovered as abnormal compared with the control group (Zhang et al. 2013). This was hypothesized by the authors to reflect interference of the abnormal limbic circuit and the impaired reward network. Another case series involving three patients receiving acute NAc stimulation for major depression (Schlaepfer et al. 2008) showed modulation of the prefrontal cortex, cingulate, amygdala, and parts of the basal ganglia with FDG-PET. The authors argued that the changes seen in the reward circuit are related to the reversal of anhedonia and increased pleasure response.
NAc studies using EEG.
One group studying NAc stimulation for OCD (Figee et al. 2013) showed that stimulation in the region of the NAc modulated abnormal prefrontal connectivity and decreased the low-frequency oscillation response seen for symptom-provoking stimuli, which correlated with clinical improvement. Low-frequency oscillations in the range of 2–5 Hz over the prefrontal cortex were linked to the severity of symptoms in OCD (Pogarell et al. 2006). Although the role of frontal low-frequency oscillations in the pathophysiology of OCD is not clear, frontal theta is hypothesized to correlate with the cognitive control and working memory load (Cavanagh and Frank 2014; Meltzer et al. 2008). Interpretation of the study by Pogarell and colleagues is limited, however, because of the small number of patients, the presence of comorbidities, and most importantly the use of psychoactive medications by a subset of subjects. In this setting, synaptic plastic changes induced by chronic stimulation (Fenoy et al. 2014) might be interrupted by the use of medication. Therefore, the true extent of the network modulated in this setting might not have been fully defined. In a follow-up report Smolders et al. (2013) showed that DBS reduced phase stability of the theta oscillations recorded from frontal regions. This finding may explain how high-frequency DBS decreases the power of low-frequency oscillations by interfering with their synchronization.
Conclusions regarding NAc stimulation.
Cross-modality evidence implicates the frontal cortex and particularly the prefrontal cortex as the main area of effect for NAc stimulation. Possible electrophysiological markers have been demonstrated for clinical effects, but they await validation in future studies.
Other Brain Targets
The network effects of various other brain regions investigated as targets for DBS have been sporadically assessed.
Targets for pain.
A few studies have examined the cortical effects of hypothalamic stimulation used to treat cluster headaches. Results from PET imaging have demonstrated modulation of somatosensory cortex, precuneus, middle temporal gyrus, inferior temporal gyrus, insula, and anterior and posterior cingulate (May et al. 2006); however, proper washout between scans was not employed. Therefore, these findings might represent plasticity induced from chronic stimulation instead of modulation of the circuitry from DBS. Additionally, corresponding MEG studies have shown cortical activation in the mid-anterior OFC, but only in one case study (Ray et al. 2007).
In single case reports regarding chronic pain, MEG has been used to demonstrate correlations between pain relief and target activity following stimulation in periventricular gray (Ray et al. 2009) and ACC (Mohseni et al. 2012). These studies were important for beginning to demonstrate the feasibility of performing MEG recordings with implanted DBS electrodes, but further assessment of the effects of DBS in these regions will require the study of greater numbers of subjects.
Pedunculopontine nucleus.
Pedunculopontine nucleus (PPN) stimulation for the treatment of movement disorders initially showed promise for improvement of gait disturbances in PD (Strafella et al. 2008). However, subsequent studies suggest greater utility as a compassionate option in patients with PD, as it may improve sleep, memory, and executive function (Alessandro et al. 2010). PPN stimulation increased glucose uptake in multiple prefrontal cortices and left ventral striatum (Alessandro et al. 2010; Ceravolo et al. 2011; Stefani et al. 2010) and caused increased blood flow to multiple structures in the cerebello-thalamo-cortical circuit (Ballanger et al. 2009). What these results mean in the context of mixed clinical results with PPN stimulation for PD is not clear.
Subcallosal cingulate.
Stimulation of the subcallosal cingulate white matter (SCCWM) has shown positive results for the treatment of MDD (Holtzheimer et al. 2012). Metabolic imaging with PET showed a decrease in metabolism of subgenual cingulate, OFC, and medial frontal and insular cortex and increases in DLPFC and dorsal cingulate (Mayberg et al. 2005). These results were corroborated by Lozano and colleagues, who additionally showed a metabolic increase in the posterior and anterior midcingulate gyri (Lozano et al. 2008). Acute stimulation also decreased activation in the cingulate gyrus (Martín-Blanco et al. 2015). Further studies investigated potential biomarkers of long-term antidepressant DBS response (Broadway et al. 2012). Theta cordance is an EEG measure thought to correlate with regional blood perfusion. Frontal theta cordance decreases were previously shown to correlate with antidepressant medication effects. Increasing frontal theta cordance over the course of DBS (24 mo), however, was found to predict greater decreases in depression severity scores in patients undergoing SCCWM stimulation (Broadway et al. 2012). These opposite results between pharmacological and DBS effects on theta cordance may reflect technical differences in EEG measurements but nonetheless indicate the need for multiple studies in which more than one recording modality and a larger cohort of patients are used to fully develop a feasible pathologic-therapeutic network model. Although a tractography study has suggested that simultaneous electrical activation of a volume of tissue containing fibers connected to medial frontal cortex, rostral and dorsal cingulate cortex, and subcortical nuclei is necessary for antidepressant effects in SCCWM DBS, neurophysiological data supporting modulation of these connected regions have not been reported.
Discussion
In this state-of-the-science review, we have attempted to summarize the network effects of DBS on cortical activity in the human brain. These studies suggest that DBS modulates network activity through a combination of effects. Direct cortical activation evidenced by stimulation-induced responses with variable timing suggests that multiple transmission pathways may be involved. Most importantly, DBS seems to prevent abnormal synchronization in distant cortical regions by disrupting afferent or efferent communication with the stimulation target. This effect was observed across modalities. In EEG studies this was seen in the widespread amplitude decrease of ERPs, decreased coherence across the cortical regions, and disruption of frontal theta. ECoG has been used to demonstrate that therapeutic STN DBS in PD normalizes PAC within the cortical motor system (de Hemptinne et al. 2015). Functional imaging models suggest that differential and specific modulation of a target's connections best predicts therapeutic response, in line with results from optogenetic studies in parkinsonian rodents that demonstrate that direct selective stimulation of afferent axons projecting to the STN is responsible for therapeutic effects (Gradinaru et al. 2009). This antidromic activation has been demonstrated across multiple studies as EPs and associated with facilitation of motor responses (Kuriakose et al. 2010). This antidromic activation was hypothesized to disrupt the timing of orthodromic discharges (Walker et al. 2012b).
Neuronal oscillations, rhythmic changes in neuronal excitability that result from the activity of synchronized groups of neurons and are thought to promote multineuronal task coordination, are now commonly recorded in a variety of clinical scenarios. The result has been an increased focus on elucidating their potential role in the regulation of local stimulus processing and long-range information transfer in the human brain. One mechanism for synchronizing neuronal activity is PAC, the involvement of which has been implicated in multiple aspects of cognition (Canolty and Knight 2010). The combined results of Smolders et al. related to DBS effects on phase stability and de Hemptinne et al. showing a DBS-related decrease in PAC at rest and during movement (de Hemptinne et al. 2015) suggest that modulation of PAC is a possible mechanism causing network desynchronization. Network desynchronization by disruption of phase relationships with coordinated reset stimulation is a concept that has been pioneered by Tass and colleagues, who showed remarkable efficacy of this approach in a study of three parkinsonian monkeys (Tass et al. 2012). How this desynchronization leads to clinical improvement is still not clear, and the details are likely different across disorders and DBS targets. In PD, one hypothesis is that DBS normalizes aberrant network synchrony by disrupting the prevalent pathological beta frequency activity arising in the basal ganglia (Oswal et al. 2013). Another possibility is that DBS enhances compensatory networks at the expense of disruptions in baseline pathological network activity. This possibility is suggested by findings from functional imaging studies showing typically inactive areas coming online only during DBS, while areas typically active during performance in the absence of DBS are suppressed.
Common confounders were present in a number of studies, some of which are unavoidable because of the nature of the disease process being studied. First, the subjects being studied have underlying pathologies that have altered the normal brain circuitry and have possibly led to the emergence of new compensatory alterations in communication pathways through surviving connections (Mikell et al. 2015). Second, the inherent variance in the surgical placement of electrodes leads to minor differences in the substructure being stimulated within a given target, evidenced by the typical variability of responses between and within subjects to standard stimulation parameters. Third, a large portion of these studies were performed retrospectively, leading to wide ranges in disease duration and time from surgery to assessment. It is difficult to account for the progressive nature of many DBS indications, as well as the different levels of network plasticity that may be induced by stimulation over different time periods in the setting of variable levels of integrity of remaining network connections. Also, chronic changes induced by the medications patients receive, even with a decrease in dosage after some surgeries, cannot be excluded. In some of the reported studies, acute drug effects cannot be excluded either, because medications were not withdrawn or otherwise standardized. Fourth, the decrease in signal-to-noise ratio caused by stimulation artifact likely leads to missing subtle changes induced by modulation when performing electrophysiological recording. Finally, most of these studies were performed in a small number of subjects during a resting state. Therefore, inferring the impact of the modulation found in different cortical regions becomes difficult without assaying a particular function through a specific task or neuropsychological test.
In conclusion, understanding how DBS affects multiple cortical regions downstream of each specific target should allow important fundamental advances in the use of this therapeutic modality. Greater knowledge of the network effects of DBS will permit the tailoring of treatment based on biomarkers related to these effects in order to increase therapeutic efficacy and avoid undesirable side effects. Further advancement in the field is anticipated from the development of closed-loop stimulation systems that allow stimulation parameters to be adjusted based on recorded activity. Reaching this goal, however, will require more neurophysiology studies in DBS patients that go beyond simple enumeration of activation changes and that are designed to test specific hypotheses regarding motor or cognitive functions, while controlling for as many experimental confounders as possible.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A., M.M.M., and R.M.R. conception and design of research; A.A., M.M.M., M.J.R., T.A.W., E.D.K., W.J.L., J.F.K., A.S.G., and R.M.R. interpreted results of experiments; A.A., M.M.M., M.J.R., T.A.W., E.D.K., W.J.L., S.B., J.F.K., A.S.G., and R.M.R. drafted manuscript; A.A., M.M.M., M.J.R., T.A.W., E.D.K., W.J.L., S.B., J.F.K., A.S.G., and R.M.R. edited and revised manuscript; A.A., M.M.M., M.J.R., T.A.W., E.D.K., W.J.L., S.B., J.F.K., A.S.G., and R.M.R. approved final version of manuscript; M.J.R. performed experiments; M.J.R. and R.M.R. analyzed data; M.J.R., S.B., and R.M.R. prepared figures.
REFERENCES
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, Martis B, Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry 57: 510–516, 2005. [DOI] [PubMed] [Google Scholar]
- Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, Kleijer M, Nederveen P, Bruggeman R, Tromp S, van Kranen-Mastenbroek V, Kingma H, Cath D, Visser-Vandewalle V. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain 134: 832–844, 2011. [DOI] [PubMed] [Google Scholar]
- Air EL, Ryapolova-Webb E, de Hemptinne C, Ostrem JL, Galifianakis NB, Larson PS, Chang EF, Starr PA. Acute effects of thalamic deep brain stimulation and thalamotomy on sensorimotor cortex local field potentials in essential tremor. Clin Neurophysiol 123: 2232–2238, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen K, Butorina A, Pekkonen E, Nurminen J, Taulu S, Ahonen A, Schnitzler A, Mäkelä JP. Somatomotor mu rhythm amplitude correlates with rigidity during deep brain stimulation in Parkinsonian patients. Clin Neurophysiol 123: 2010–2017, 2012. [DOI] [PubMed] [Google Scholar]
- Airaksinen K, Mäkelä JP, Taulu S, Ahonen A, Nurminen J, Schnitzler A, Pekkonen E. Effects of DBS on auditory and somatosensory processing in Parkinson's disease. Hum Brain Mapp 32: 1091–1099, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akatsubo DN, Akabayashi TW. Changes in regional blood flow induced by unilateral subthalamic nucleus stimulation in patients with Parkinson's disease. Neurol Med Chir (Tokyo) 49: 507–513, 2009. [DOI] [PubMed] [Google Scholar]
- Alba-Ferrara LM, Fernandez F, de Erausquin GA. The use of neuromodulation in the treatment of cocaine dependence. Addict Disord Their Treat 13: 1–7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, Placidi F, Romigi A, Iani C, Marzetti F, Peppe A. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci 289: 44–48, 2010. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381, 1986. [DOI] [PubMed] [Google Scholar]
- Arantes PR, Cardoso EF, Barreiros MA, Teixeira MJ, Gonçalves MR, Barbosa ER, Sukwinder SS, Leite CC, Amaro E. Performing functional magnetic resonance imaging in patients with Parkinson's disease treated with deep brain stimulation. Mov Disord 21: 1154–1162, 2006. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson's disease. Brain 129: 2667–2678, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P, Paradiso G, Saint-Cyr JA, Chen R, Lang AE, Lozano AM. Potentials recorded at the scalp by stimulation near the human subthalamic nucleus. Clin Neurophysiol 112: 431–437, 2001. [DOI] [PubMed] [Google Scholar]
- Baker KB, Montgomery EB, Rezai AR, Burgess R, Lüders HO. Subthalamic nucleus deep brain stimulus evoked potentials: physiological and therapeutic implications. Mov Disord 17: 969–983, 2002. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, Cilia R, Houle S, Poon YY, Lang AE, Strafella AP. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: a [15O]H2O PET study. Hum Brain Mapp 30: 3901–3909, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am J Psychiatry 145: 1560–1563, 1988. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol 50: 344–346, 1987. [DOI] [PubMed] [Google Scholar]
- Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O'Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, Seale CG. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84: 810–817, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85: 1351–1356, 2001. [DOI] [PubMed] [Google Scholar]
- Boertien T, Zrinzo L, Kahan J, Jahanshahi M, Hariz M, Mancini L, Limousin P, Foltynie T. Functional imaging of subthalamic nucleus deep brain stimulation in Parkinson's disease. Mov Disord 26: 1835–1843, 2011. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, Stern CE, Belliveau JW, Baer L, O'Sullivan RL, Savage CR, Jenike MA, Rosen BR. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry 53: 595–606, 1996. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Sharott A, Brown P. The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci 39: 1951–1959, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadway JM, Holtzheimer PE, Hilimire MR, Parks NA, Devylder JE, Mayberg HS, Corballis PM. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology 37: 1764–1772, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, Karimi M, Weaver PM, Wu J, Perantie DC, Golchin NA, Tabbal SD, Perlmutter JS, Hershey T. Neural correlates of STN DBS-induced cognitive variability in Parkinson disease. Neuropsychologia 46: 3162–3169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci 14: 506–515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Li D, Jiang T, Ince NF, Zhan S, Zhang J, Sha Z, Sun B. Resting state cortical oscillations of patients with Parkinson disease and with and without subthalamic deep brain stimulation. J Clin Neurophysiol 32: 109–118, 2015. [DOI] [PubMed] [Google Scholar]
- Carmichael DW, Pinto S, Limousin-Dowsey P, Thobois S, Allen PJ, Lemieux L, Yousry T, Thornton JS. Functional MRI with active, fully implanted, deep brain stimulation systems: safety and experimental confounds. Neuroimage 37: 508–517, 2007. [DOI] [PubMed] [Google Scholar]
- Castrioto A, Lhommée E, Moro E, Krack P. Mood and behavioural effects of subthalamic stimulation in Parkinson's disease. Lancet Neurol 13: 287–305, 2014. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 18: 414–421, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 14: 1462–1467, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, Moringlane JR, Alesch F. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol 56: 997–1003, 1999. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Fogel W, Alesch F, Bartenstein P, Conrad B, Diederich N, von Falkenhayn I, Moringlane JR, Schwaiger M, Tronnier VM. Thalamic stimulation for essential tremor activates motor and deactivates vestibular cortex. Neurology 56: 1347–1354, 2001. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Brusa L, Galati S, Volterrani D, Peppe A, Siciliano G, Pierantozzi M, Moschella V, Bonuccelli U, Stanzione P, Stefani A. Low frequency stimulation of the nucleus tegmenti pedunculopontini increases cortical metabolism in parkinsonian patients. Eur J Neurol 18: 842–849, 2011. [DOI] [PubMed] [Google Scholar]
- Chiken S, Nambu A. Disrupting neuronal transmission: mechanism of DBS? Front Syst Neurosci 8: 33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chul HL, Aalto S, Rinne JO, Ki OL, Seung HO, Jin WC, Myung SL. Different cerebral cortical areas influence the effect of subthalamic nucleus stimulation on Parkinsonian motor deficits and freezing of gait. Mov Disord 22: 2176–2182, 2007. [DOI] [PubMed] [Google Scholar]
- Cilia R, Marotta G, Landi A, Isaias IU, Mariani CB, Vergani F, Benti R, Sganzerla E, Pezzoli G, Antonini A. Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson's disease: a prospective case-control study. Clin Neurol Neurosurg 111: 140–146, 2009. [DOI] [PubMed] [Google Scholar]
- Cilia R, Siri C, Marotta G, De Gaspari D, Landi A, Mariani CB, Benti R, Isaias IU, Vergani F, Pezzoli G, Antonini A. Brain networks underlining verbal fluency decline during STN-DBS in Parkinson's disease: an ECD-SPECT study. Parkinsonism Relat Disord 13: 290–294, 2007. [DOI] [PubMed] [Google Scholar]
- Conte A, Modugno N, Lena F, Dispenza S, Gandolfi B, Iezzi E, Fabbrini G, Berardelli A. Subthalamic nucleus stimulation and somatosensory temporal discrimination in Parkinson's disease. Brain 133: 2656–2663, 2010. [DOI] [PubMed] [Google Scholar]
- Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, Picot MC, Tuffery S, Claustres M, Echenne B, Frerebeau P. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg 101: 189–194, 2004. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Pollak P, Passingham R, Landais P, Gervason C, Cinotti L, Friston K, Frackowiak R, Mauguière F, Benabid AL. Thalamic stimulation and suppression of parkinsonian tremor. Evidence of a cerebellar deactivation using positron emission tomography. Brain 116: 267–279, 1993. [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, Bosch A, Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 67: 1061–1068, 2010. [DOI] [PubMed] [Google Scholar]
- Detante O, Vercueil L, Thobois S, Broussolle E, Costes N, Lavenne F, Chabardes S, Lebars D, Vidailhet M, Benabid AL, Pollak P. Globus pallidus internus stimulation in primary generalized dystonia: a H215O PET study. Brain 127: 1899–1908, 2004. [DOI] [PubMed] [Google Scholar]
- Devos D, Derambure P, Bourriez JL, Cassim DF, Blond S, Guieu JD. Influence of internal globus pallidus stimulation on motor cortex activation pattern in Parkinson's disease. Clin Neurophysiol 113: 1110–1120, 2002. [DOI] [PubMed] [Google Scholar]
- Devos D, Labyt E, Derambure P, Bourriez JL, Cassim F, Reyns N, Blond S, Guieu JD, Destée A, Defebvre L. Subthalamic nucleus stimulation modulates motor cortex oscillatory activity in Parkinson's disease. Brain 127: 408–419, 2004. [DOI] [PubMed] [Google Scholar]
- Duncan GH, Kupers RC, Marchand S, Villemure JG, Gybels JM, Bushnell MC. Stimulation of human thalamus for pain relief: possible modulatory circuits revealed by positron emission tomography. J Neurophysiol 80: 3326–3330, 1998. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci 32: 548–557, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Pogosyan A, Wang S, Averbeck B, Gaynor LD, Cantiniaux S, Witjas T, Limousin P, Azulay JP, Brown P. Resonance in subthalamo-cortical circuits in Parkinson's disease. Brain 132: 2139–2150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoy AJ, Goetz L, Chabardès S, Xia Y. Deep brain stimulation: are astrocytes a key driver behind the scene? CNS Neurosci Ther 20: 191–201, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, de Koning P, Vulink N, Levar N, Droge L, van den Munckhof P, Schuurman PR, Nederveen A, van den Brink W, Mazaheri A, Vink M, Denys D. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 16: 386–387, 2013. [DOI] [PubMed] [Google Scholar]
- Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res 156: 274–281, 2004. [DOI] [PubMed] [Google Scholar]
- Finelli DA, Rezai AR, Ruggieri PM, Tkach JA, Nyenhuis JA, Hrdlicka G, Sharan A, Gonzalez-Martinez J, Stypulkowski PH, Shellock FG. MR imaging-related heating of deep brain stimulation electrodes: in vitro study. Am J Neuroradiol 23: 1795–1802, 2002. [PMC free article] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 362: 2077–2091, 2010. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318: 1309–1312, 2007. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, Giladi N, Fodstad H, Ma Y, Eidelberg D. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage 21: 608–615, 2004. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Mentis MJ, Ma Y, Dhawan V, Antonini A, Lang AE, Lozano AM, Hammerstad J, Lyons K, Koller WC, Moeller JR, Eidelberg D. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson's disease: a PET study of resting-state glucose metabolism. Brain 124: 1601–1609, 2001. [DOI] [PubMed] [Google Scholar]
- Garcia L, D'Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson's disease: more or less? Trends Neurosci 28: 209–216, 2005. [DOI] [PubMed] [Google Scholar]
- Geday J, Ostergaard K, Gjedde A. Stimulation of subthalamic nucleus inhibits emotional activation of fusiform gyrus. Neuroimage 33: 706–714, 2006. [DOI] [PubMed] [Google Scholar]
- Geday J, Østergaard K, Johnsen E, Gjedde A. STN-stimulation in Parkinson's disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp 30: 112–121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi JC, Stippich C, Tronnier VM, Heiland S. Active deep brain stimulation during MRI: a feasibility study. Magn Reson Med 51: 380–388, 2004. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Bloem BR, Alesch F, Lang W, Deecke L, Cunnington R. Bilateral subthalamic nucleus stimulation does not improve prolonged P300 latencies in Parkinson's disease. J Neurol 248: 285–289, 2001. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 31: 2384–2393, 2006. [DOI] [PubMed] [Google Scholar]
- Gulberti A, Hamel W, Buhmann C, Boelmans K, Zittel S, Gerloff C, Westphal M, Engel AK, Schneider TR, Moll CK. Subthalamic deep brain stimulation improves auditory sensory gating deficit in Parkinson's disease. Clin Neurophysiol 126: 565–574, 2014. [DOI] [PubMed] [Google Scholar]
- Haegelen C, García-Lorenzo D, Le Jeune F, Péron J, Gibaud B, Riffaud L, Brassier G, Barillot C, Vérin M, Morandi X. SPECT and PET analysis of subthalamic stimulation in Parkinson's disease: analysis using a manual segmentation. J Neurol 257: 375–382, 2010. [DOI] [PubMed] [Google Scholar]
- Hälbig TD, Tse W, Frisina PG, Baker BR, Hollander E, Shapiro H, Tagliati M, Koller WC, Olanow CW. Subthalamic deep brain stimulation and impulse control in Parkinson's disease. Eur J Neurol 16: 493–497, 2009. [DOI] [PubMed] [Google Scholar]
- Hariz M, Blomstedt P, Zrinzo L. Future of brain stimulation: new targets, new indications, new technology. Mov Disord 28: 1784–1792, 2013. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Boecker H, Büchel C, Vesper J, Tronnier V, Pfister R, Alesch F, Moringlane J, Krauss J, Conrad B, Schwaiger M, Ceballos-Baumann A. Differential modulation of subcortical target and cortex during deep brain stimulation. Neuroimage 18: 517–524, 2003. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Kalteis K, Boecker H, Alesch F, Ceballos-Baumann AO. Frequency-correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson's disease. Neuroimage 28: 598–606, 2005. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci USA 110: 4780–4785, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci 18: 779–886, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann V, Sorger B, Girnus R, Lasek K, Maarouf M, Wedekind C, Bunke J, Schulte O, Krug B, Lackner K, Sturm V. Intraoperative functional MRI as a new approach to monitor deep brain stimulation in Parkinson's disease. Eur Radiol 14: 686–690, 2004. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Thiel A, Ghaemi M, Herholz K, Sturm V, Heiss WD. Deep brain stimulation of the subthalamic nucleus versus levodopa challenge in Parkinson's disease: measuring the on- and off-conditions with FDG-PET. J Neural Transm 109: 1257–1264, 2002. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, Lehrke R, Koulousakis A, Herholz K, Sturm V, Heiss WD. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab 24: 7–16, 2004. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69: 150–158, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. Neuromodulation for treatment-resistant depression. F1000 Med Rep 4: 22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotton G, Tisch S, Silberstein P, Pogosyan A, Ku AA, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P. Cortico-cortical coupling in Parkinson's disease and its modulation by therapy. Brain 128: 1227–1291, 2005. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage 34: 714–723, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insola A, Mazzone P, Valeriani M. Somatosensory evoked potential and clinical changes after electrode implant in basal ganglia of parkinsonian patients. Muscle Nerve 32: 791–797, 2005. [DOI] [PubMed] [Google Scholar]
- Jech R, Ruzicka E, Urgosik D, Serranova T, Volfova M, Novakova O, Roth J, Dusek P, Mecir P. Deep brain stimulation of the subthalamic nucleus affects resting EEG and visual evoked potentials in Parkinson's disease. Clin Neurophysiol 117: 1017–1028, 2006. [DOI] [PubMed] [Google Scholar]
- Jech R, Urgosík D, Tintera J, Nebuzelský A, Krásenský J, Liscák R, Roth J, Růzicka E. Functional magnetic resonance imaging during deep brain stimulation: a pilot study in four patients with Parkinson's disease. Mov Disord 16: 1126–1132, 2001. [DOI] [PubMed] [Google Scholar]
- Le Jeune F, Drapier D, Bourguignon A, Péron J, Mesbah H, Drapier S, Sauleau P, Haegelen C, Travers D, Garin E, Malbert CH, Millet B, Vérin M. Subthalamic nucleus stimulation in Parkinson disease induces apathy: a PET study. Neurology 73: 1746–1751, 2009. [DOI] [PubMed] [Google Scholar]
- Le Jeune F, Vérin M, N'Diaye K, Drapier D, Leray E, Du Montcel ST, Baup N, Pelissolo A, Polosan M, Mallet L, Yelnik J, Devaux B, Fontaine D, Chereau I, Bourguignon A, Peron J, Sauleau P, Raoul S, Garin E, Krebs MO, Jaafari N, Millet B. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry 68: 1016–1022, 2010. [DOI] [PubMed] [Google Scholar]
- Kahan J, Mancini L, Urner M, Friston K, Hariz M, Holl E, White M, Ruge D, Jahanshahi M, Boertien T, Yousry T, Thornton JS, Limousin P, Zrinzo L, Foltynie T. Therapeutic subthalamic nucleus deep brain stimulation reverses cortico-thalamic coupling during voluntary movements in Parkinson's disease. PLoS One 7: e50270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J, Urner M, Moran R, Flandin G, Marreiros A, Mancini L, White M, Thornton J, Yousry T, Zrinzo L, Hariz M, Limousin P, Friston K, Foltynie T. Resting state functional MRI in Parkinson's disease: the impact of deep brain stimulation on “effective” connectivity. Brain 137: 1130–1144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E, Voges J, Weber T, Haarer M, Baudrexel S, Klein JC, Kessler J, Sturm V, Heiss WD, Hilker R. Frontal FDG-PET activity correlates with cognitive outcome after STN-DBS in Parkinson disease. Neurology 72: 42–49, 2009. [DOI] [PubMed] [Google Scholar]
- Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, Usche JW, Revilla FJ, Hartlein JM, Wernle AR, Mink JW, Perlmutter JS. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain 131: 2710–2719, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsakiori PF, Kefalopoulou Z, Markaki E, Paschali A, Ellul J, Kagadis GC, Chroni E, Constantoyannis C. Deep brain stimulation for secondary dystonia: results in 8 patients. Acta Neurochir (Wien) 151: 473–478, 2009. [DOI] [PubMed] [Google Scholar]
- Katz M, Luciano MS, Carlson K, Luo P, Marks WJ, Larson PS, Starr PA, Follett KA, Weaver FM, Stern MB, Reda DJ, Ostrem JL. The differential effects of DBS target on motor subtypes in Parkinson's disease. Ann Neurol 77: 710–719, 2015. [DOI] [PubMed] [Google Scholar]
- Kim SH, Son BC, Lim S, Kim W, Bae DW, Shon YM. EEG driving response during low-frequency stimulation of anterior thalamic nucleus: is it a good predictor of the correct location of DBS electrode? Clin Neurophysiol 125: 1065–1066, 2014. [DOI] [PubMed] [Google Scholar]
- Klostermann F, Wahl M, Marzinzik F, Vesper J, Sommer W, Curio G. Speed effects of deep brain stimulation for Parkinson's disease. Mov Disord 25: 2762–2768, 2010. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Chen X, Pittman QJ. Short-term potentiation of miniature excitatory synaptic currents causes excitation of supraoptic neurons. J Neurophysiol 83: 2542–2553, 2000. [DOI] [PubMed] [Google Scholar]
- Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev 32: 408–422, 2008. [DOI] [PubMed] [Google Scholar]
- Kovacs N, Balas I, Kellenyi L, Janszky J, Feldmann A, Llumiguano C, Doczi TP, Ajtay Z, Nagy F. The impact of bilateral subthalamic deep brain stimulation on long-latency event-related potentials. Parkinsonism Related Disord 14: 476–480, 2008. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Huff W, Lee SH, Koulousakis A, Klosterkoetter J, Sturm V. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry 78: 1152–1153, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers RC, Gybels JM, Gjedde A. Positron emission tomography study of a chronic pain patient successfully treated with somatosensory thalamic stimulation. Pain 87: 295–302, 2000. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Müller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355: 1978–1990, 2006. [DOI] [PubMed] [Google Scholar]
- Kuriakose R, Saha U, Castillo G, Udupa K, Ni Z, Gunraj C, Mazzella F, Hamani C, Lang AE, Moro E, Lozano AM, Hodaie M, Chen R. The nature and time course of cortical activation following subthalamic stimulation in Parkinson's disease. Cereb Cortex 20: 1926–1936, 2010. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, Cosyns P. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med 47: 740–747, 2006. [PubMed] [Google Scholar]
- Limousin P, Brown P, Marsden J, Defebvre L, Rothwell J. Evoked potentials from subthalamic nucleus, internal pallidum and thalamic stimulation in parkinsonian and postural tremor patients. J Physiol 509P: 176–177, 1998a. [Google Scholar]
- Limousin P, Greene J, Pollak P. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson's disease. Ann Neurol 42: 283–291, 1997. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 339: 1105–1111, 1998b. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 64: 461–467, 2008. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Webb RM, Silberstein P, Tisch S, Asselman P, Limousin P, Rothwell JC. Stimulation through electrodes implanted near the subthalamic nucleus activates projections to motor areas of cerebral cortex in patients with Parkinson's disease. Eur J Neurosci 21: 1394–1402, 2005. [DOI] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardès S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Vérin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B, Pelissolo A. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 359: 2121–2134, 2008. [DOI] [PubMed] [Google Scholar]
- Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 65: 267–275, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Blanco A, Serra-Blasco M, Pérez-Egea R, de Diego-Adeliño J, Carceller-Sindreu M, Puigdemont D, Molet J, Álvarez E, Pérez V, Portella MJ. Immediate cerebral metabolic changes induced by discontinuation of deep brain stimulation of subcallosal cingulate gyrus in treatment-resistant depression. J Affect Disord 173: 159–162, 2015. [DOI] [PubMed] [Google Scholar]
- May A, Leone M, Boecker H, Sprenger T, Juergens T, Bussone G, Tolle TR. Hypothalamic deep brain stimulation in positron emission tomography. J Neurosci 26: 3589–3593, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660, 2005. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry 164: 459–468, 1994. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Chaturvedi A, Shamir RR, Lempka SF. Engineering the next generation of clinical deep brain stimulation technology. Brain Stimul 8: 21–26, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis 38: 329–337, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol 115: 589–595, 2004. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, Spencer DD, Constable RT. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex 18: 1843–1855, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikell CB, Banks GP, Frey HP, Youngerman BE, Nelp TB, Karas PJ, Chan AK, Voss HU, Connolly ES, Claassen J. Frontal networks associated with command following after hemorrhagic stroke. Stroke 46: 49–57, 2015. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 70: 163–171, 2013. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Modugno N, Giannini G, Lena F, Cantore G. Stimulation of subthalamic nuclei restores a near normal planning strategy in Parkinson's patients. PLoS One 8: e62793, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Romanelli P, Modugno N, Lena F, Manfredi M, Cantore G. Deep brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson's patients. Cereb Cortex 22: 1124–1132, 2012. [DOI] [PubMed] [Google Scholar]
- Mohseni HR, Smith PP, Parsons CE, Young KS, Hyam AJ, Stein A, Stein JF, Green AL, Aziz TZ, Kringelbach ML. MEG can map short and long-term changes in brain activity following deep brain stimulation for chronic pain. PLoS One 7: e37993, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure H, Hirano S, Tang CC, Isaias IU, Antonini A, Ma Y, Dhawan V, Eidelberg D. Parkinson's disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 54: 1244–1253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I, Podoll K, Lenartz D, Sturm V, Schneider F. Deep brain stimulation in the nucleus accumbens for intractable Tourette's syndrome: follow-up report of 36 months. Biol Psychiatry 65: e5–e6, 2009. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. Exp Brain Res 118: 489–500, 1998. [DOI] [PubMed] [Google Scholar]
- Nuttin BJ, Gabriëls LA, Cosyns PR, Meyerson BA, Andréewitch S, Sunaert SG, Maes AF, Dupont PJ, Gybels JM, Gielen F, Demeulemeester HG. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery 52: 1263–1272, 2003. [DOI] [PubMed] [Google Scholar]
- Oswal A, Brown P, Litvak V. Synchronized neural oscillations and the pathophysiology of Parkinson's disease. Curr Opin Neurol 26: 662–670, 2013. [DOI] [PubMed] [Google Scholar]
- Park H, Kim JS, Paek SH, Jeon BS, Lee JY, Chung CK. Cortico-muscular coherence increases with tremor improvement after deep brain stimulation in Parkinson's disease. Neuroreport 20: 1444–1449, 2009. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Aziz TZ. Neuropathic pain and deep brain stimulation. Neurotherapeutics 11: 496–507, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW, Bastian AJ, Zackowski K, Hershey T, Miyawaki E, Koller W, Videen TO. Blood flow responses to deep brain stimulation of thalamus. Neurology 58: 1388–1394, 2002. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857, 1999. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Baker KB, Lowe MJ, Tkach JA, Cooper SE, Kopell BH, Rezai AR. Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus—initial experience. Radiology 239: 209–216, 2006. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M. The effect of deep brain stimulation on the frontal N30 component of somatosensory evoked potentials in advanced Parkinson's disease patients. Clin Neurophysiol 110: 1700–1707, 1999. [DOI] [PubMed] [Google Scholar]
- Pinto S, Ferraye M, Espesser R, Fraix V, Maillet A, Guirchoum J, Layani-Zemour D, Ghio A, Chabardès S, Pollak P, Debû B. Stimulation of the pedunculopontine nucleus area in Parkinson's disease: effects on speech and intelligibility. Brain 137: 2759–2772, 2014. [DOI] [PubMed] [Google Scholar]
- Pogarell O, Juckel G, Mavrogiorgou P, Mulert C, Folkerts M, Hauke W, Zaudig M, Möller HJ, Hegerl U. Symptom-specific EEG power correlations in patients with obsessive-compulsive disorder. Int J Psychophysiol 62: 87–92, 2006. [DOI] [PubMed] [Google Scholar]
- Priori A, Cinnante C, Genitrini S, Pesenti A, Tortora G, Bencini C, Barelli MV, Buonamici V, Carella F, Girotti F, Soliveri P, Magrini F, Morganti A, Albanese A, Broggi S, Scarlato G, Barbieri S. Non-motor effects of deep brain stimulation of the subthalamic nucleus in Parkinson's disease: preliminary physiological results. Neurol Sci 22: 85–86, 2001. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 51: 62–70, 1994. [DOI] [PubMed] [Google Scholar]
- Ray N, Kringelbach M, Jenkinson N, Owen S, Davies P, Wang S, De Pennington N, Hansen P, Stein J, Aziz T. Using magnetoencephalography to investigate brain activity during high frequency deep brain stimulation in a cluster headache patient. Biomed Imaging Interv J 3: e25, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, Bain PG, Yousif N, Green A, Stein JS, Aziz TZ. The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson's disease. Neuropsychologia 47: 2828–2834, 2009. [DOI] [PubMed] [Google Scholar]
- Rehncrona S, Johnels B, Widner H, Törnqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord 18: 163–170, 2003. [DOI] [PubMed] [Google Scholar]
- Rezai AR, Lozano AM, Crawley AP, Joy ML, Davis KD, Kwan CL, Dostrovsky JO, Tasker RR, Mikulis DJ. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. J Neurosurg 90: 583–590, 1999. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R, Zimnik A, Zheng F, Turner RS, Alzheimer C, Doiron B, Rubin JE. Axonal and synaptic failure suppress the transfer of firing rate oscillations, synchrony and information during high frequency deep brain stimulation. Neurobiol Dis 62: 86–99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, Labar D, Sperling MR, Sharan A, Sandok E, Handforth A, Stern JM, Chung S, Henderson JM, French J, Baltuch G, Rosenfeld WE, Garcia P, Barbaro NM, Fountain NB, Elias WJ, Goodman RR, Pollard JR, Troster AI, Irwin CP, Lambrecht K, Graves N, Fisher R. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 84: 1017–1025, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers BM, Beagley AH, Henshall WR. The mechansim of auditory evoked EEG responses. Nature 247: 481–483, 1974. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33: 368–377, 2008. [DOI] [PubMed] [Google Scholar]
- Schroeder U, Kuehler A, Haslinger B, Erhard P, Fogel W, Tronnier VM, Lange KW, Boecker H, Ceballos-Baumann AO. Subthalamic nucleus stimulation affects striato-anterior cingulate cortex circuit in a response conflict task: a PET study. Brain 125: 1995–2004, 2002. [DOI] [PubMed] [Google Scholar]
- Schroeder U, Kuehler A, Lange KW, Haslinger B, Tronnier VM, Krause M, Pfister R, Boecker H, Ceballos-Baumann AO. Subthalamic nucleus stimulation affects a frontotemporal network: a PET study. Ann Neurol 54: 445–450, 2003. [DOI] [PubMed] [Google Scholar]
- Selzler K, Burack M, Bender R, Mapstone M. Neurophysiological correlates of motor and working memory performance following subthalamic nucleus stimulation. J Cogn Neurosci 25: 37–48, 2013. [DOI] [PubMed] [Google Scholar]
- Sestini S, Pupi A, Ammannati F, Silvia R, Sorbi S, Castagnoli A. Are there adaptive changes in the human brain of patients with Parkinson's disease treated with long-term deep brain stimulation of the subthalamic nucleus? A 4-year follow-up study with regional cerebral blood flow SPECT. Eur J Nucl Med Mol Imaging 34: 1646–1657, 2007. [DOI] [PubMed] [Google Scholar]
- Sestini S, Scotto de Luzio A, Ammannati F, De Cristofaro MT, Passeri A, Martini S, Pupi A. Changes in regional cerebral blood flow caused by deep-brain stimulation of the subthalamic nucleus in Parkinson's disease. J Nucl Med 43: 725–732, 2002. [PubMed] [Google Scholar]
- Shrivastava D, Abosch A, Hughes J, Goerke U, DelaBarre L, Visaria R, Harel N, Vaughan JT. Heating induced near deep brain stimulation lead electrodes during magnetic resonance imaging with a 3 T transceive volume head coil. Phys Med Biol 57: 5651–5665, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ, Tagliati M, Alterman R, Sidtis D, Dhawan V, Eidelberg D. Therapeutic high-frequency stimulation of the subthalamic nucleus in Parkinson's disease produces global increases in cerebral blood flow. J Cereb Blood Flow Metab 32: 41–49, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders R, Mazaheri A, van Wingen G, Figee M, de Koning PP, Denys D. Deep brain stimulation targeted at the nucleus accumbens decreases the potential for pathologic network communication. Biol Psychiatry 74: e27–e28, 2013. [DOI] [PubMed] [Google Scholar]
- Stefani A, Pierantozzi M, Ceravolo R, Brusa L, Galati S, Stanzione P. Deep brain stimulation of pedunculopontine tegmental nucleus (PPTg) promotes cognitive and metabolic changes: a target-specific effect or response to a low-frequency pattern of stimulation? Clin EEG Neurosci 41: 82–86, 2010. [DOI] [PubMed] [Google Scholar]
- Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, Saint-Cyr J, Lozano A. Deep brain stimulation for Parkinson's disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord 18: 1508–1516, 2003. [DOI] [PubMed] [Google Scholar]
- Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Berendse HW, Stam CJ. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain 130: 1847–1860, 2007. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Dagher A, Sadikot AF. Cerebral blood flow changes induced by subthalamic stimulation in Parkinson's disease. Neurology 60: 1039–1042, 2003. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Lozano AM, Ballanger B, Poon YY, Lang AE, Moro E. rCBF changes associated with PPN stimulation in a patient with Parkinson's disease: a PET study. Mov Disord 23: 1051–1054, 2008. [DOI] [PubMed] [Google Scholar]
- Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. J Neurosci 31: 5721–5729, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry 46: 518–23, 1989. [DOI] [PubMed] [Google Scholar]
- Tass PA, Klosterkötter J, Schneider F, Lenartz D, Koulousakis A, Sturm V. Obsessive-compulsive disorder: development of demand-controlled deep brain stimulation with methods from stochastic phase resetting. Neuropsychopharmacology 28, Suppl 1: S27–S34, 2003. [DOI] [PubMed] [Google Scholar]
- Tass PA, Qin L, Hauptmann C, Dovero S, Bezard E, Boraud T, Meissner WG. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol 72: 816–820, 2012. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51: 1759–1768, 2006. [DOI] [PubMed] [Google Scholar]
- Thobois S, Hotton GR, Pinto S, Wilkinson L, Limousin-Dowsey P, Brooks DJ, Jahanshahi M. STN stimulation alters pallidal-frontal coupling during response selection under competition. J Cereb Blood Flow Metab 27: 1173–1184, 2007. [DOI] [PubMed] [Google Scholar]
- Tisch S, Rothwell JC, Zrinzo L, Bhatia KP, Hariz M, Limousin P. Cortical evoked potentials from pallidal stimulation in patients with primary generalized dystonia. Mov Disord 23: 265–273, 2008. [DOI] [PubMed] [Google Scholar]
- Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, Ma Y, Eidelberg D. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. Neuroimage 31: 301–307, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai ST, Lin SH, Lin SZ, Chen JY, Lee CW, Chen SY. Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson's disease. Neurosurgery 61: E1024–E1029, 2007. [DOI] [PubMed] [Google Scholar]
- Vercueil L, Pollak P, Fraix V, Caputo E, Moro E, Benazzouz A, Xie J, Koudsie A, Benabid AL. Deep brain stimulation in the treatment of severe dystonia. J Neurol 248: 695–700, 2001. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Deep brain stimulation for Parkinson's disease. A critical re-evaluation of STN versus GPi DBS. Stereotact Funct Neurosurg 78: 119–131, 2002. [DOI] [PubMed] [Google Scholar]
- Walker HC, Huang H, Gonzalez CL, Bryant JE, Killen J, Knowlton RC, Montgomery EB, Cutter GC, Yildirim A, Guthrie BL, Watts RL. Short latency activation of cortex by clinically effective thalamic brain stimulation for tremor. Mov Disord 27: 1404–1412, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker HC, Huang H, Gonzalez CL, Killen J, Cutter GC, Knowlton RC, Erwin B, Guthrie BL, Watts RL. Short latency activation of cortex during clinically effective subthalamic DBS for Parkinson disease. Mov Disord 27: 864–873, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease. Front Hum Neurosci 6: 155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielepp JP, Burgunder JM, Pohle T, Ritter EP, Kinser JA, Krauss JK. Deactivation of thalamocortical activity is responsible for suppression of parkinsonian tremor by thalamic stimulation: a 99mTc-ECD SPECT study. Clin Neurol Neurosurg 103: 228–231, 2001. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson's disease. J Cogn Neurosci 18: 626–636, 2006. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WP. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson's disease. Brain 133: 3611–3624, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HW, Li DY, Zhao J, Guan YH, Sun BM, Zuo CT. Metabolic imaging of deep brain stimulation in anorexia nervosa: a 18F-FDG PET/CT study. Clin Nucl Med 38: 943–948, 2013. [DOI] [PubMed] [Google Scholar]
- Zumsteg D, Lozano AM, Wennberg RA. Rhythmic cortical EEG synchronization with low frequency stimulation of the anterior and medial thalamus for epilepsy. Clin Neurophysiol 117: 2272–2278, 2006a. [DOI] [PubMed] [Google Scholar]
- Zumsteg D, Lozano AM, Wieser HG, Wennberg RA. Cortical activation with deep brain stimulation of the anterior thalamus for epilepsy. Clin Neurophysiol 117: 192–207, 2006b. [DOI] [PubMed] [Google Scholar]


