Abstract
Serine palmitoyltransferase (SPT) is a key enzyme in the first step of sphingolipid biosynthesis. Mutations in the SPTLC1 gene that encodes for SPT subunits cause hereditary sensory neuropathy type 1. However, little is understood about how mutant SPT regulates mechanisms of sensory neuron and axonal growth. Using transgenic mice overexpressing the C133W SPT mutant, we found that mutant dorsal root ganglia (DRG) during growth in vitro exhibit increased neurite length and branching, coinciding with elevated expression of actin-cross-linking proteins at the neuronal growth cone, namely phosphorylated Ezrin/Radixin/Moesin. In addition, inhibition of SPT was able to reverse the mutant phenotype. Because mutant SPT preferentially uses l-alanine over its canonical substrate l-serine, we also investigated the effects of substrate availability on DRG neurons. Supplementation with l-serine or removal of l-alanine independently restored normal growth patterns in mutant SPTLC1C133W DRG. Therefore, we report that substrate availability and selectivity of SPT influence the regulation of neurite growth in DRG neurons.
SIGNIFICANCE STATEMENT Hereditary sensory neuropathy type 1 is an autosomal-dominant disorder that leads to a sensory neuropathy due to mutations in the serine palmitoyltransferase (SPT) enzyme. We investigated how mutant SPT and substrate levels regulate neurite growth. Because SPT is an important enzyme in the synthesis of sphingolipids, our data are of broader significance to other peripheral and metabolic disorders.
Keywords: dorsal root ganglia, Ezrin/Radixin/Moesin, l-alanine, l-serine, neurite development, sensory neuropathy
Introduction
Hereditary sensory neuropathy type 1 (HSN-1) is a disorder of the peripheral nervous system (PNS), leading to progressive axonal degeneration and sensory loss accompanied by symptoms of lancinating/shooting pain. The SPTLC1 gene, which encodes for subunits of the enzyme serine palmitoyltransferase (SPT), is mutated in HSN-1 (Bejaoui et al., 2001).
Normally, SPT initiates the de novo synthesis of sphingolipids, specifically the condensation of palmitoyl-CoA with l-serine. In HSN-1, however, mutant SPT loses enzymatic selectivity and incorporates l-alanine as an alternative substrate (Gable et al., 2010). The enzymatic promiscuity of mutant SPT is suggested to be the cause of pathology (Eichler et al., 2009; Penno et al., 2010). Despite the known deleterious impact of mutant SPT in HSN-1, how it regulates the mechanisms of axonal growth in sensory neurons remains poorly understood.
Here, we isolated dorsal root ganglia (DRG) neurons of transgenic SPTLC1C133W mice, which overexpress the C133W SPT mutant (McCampbell et al., 2005). Neurite growth in vitro was assessed by analyzing length, branching, and the expression of p-ERM actin cross-linking proteins at the neuronal growth cone. In neurons, p-ERM is localized at neurites and growth cones, links the cytoskeleton to plasma membrane proteins, and is important for growth and axon guidance via modulation of the actin cytoskeleton during normal and regenerative growth (Gonzalez-Agosti and Solomon, 1996; Haas et al., 2004; Khan et al., 2013). We confirmed the effects of mutant SPT using myriocin, a potent SPT inhibitor (Wadsworth et al., 2013). Further, we manipulated the availability of SPT substrates to determine how they influence SPTLC1C133W DRG growth. Because previous in vivo studies found that varying l-serine or l-alanine levels can influence disease severity in HSN-1 (Garofalo et al., 2011), we examined their role in DRG growth in vitro.
Materials and Methods
Transgenic mice.
Generation of transgenic mice has been described previously (McCampbell et al., 2005). Overexpression of WT and C133W mutant copy of SPTLC1 was driven by the chicken β-actin promoter. Mice were generated in the BL6/C57 background. SPTLC1C133W mice were HSN-1 models and WT and SPTLC1WT mice were controls.
Neuronal culture.
Mice were anesthetized and killed at 6 months of age. Experiments were conducted with DRG from three male animals per genotype. DRG were extracted, digested with 0.05% trypsin (Life Technologies), and collagenase-IV/dispase (1 mg/ml and 0.25 mg/ml; Worthington Biochemical); resuspended in DMEM (Life Technologies) with 10% fetal bovine serum (Atlanta Biologicals) and DNase-I (Sigma-Aldrich); and triturated with heat-polished Pasteur pipettes. On chamber slides precoated with poly-d-lysine and laminin (Sigma-Aldrich), cells were plated in neurobasal or l-alanine-free medium (AFM) supplemented with 2% B27 (Life Technologies). Neurobasal medium is modified DMEM with optimized concentrations of components (Brewer et al., 1993). However, because neurobasal medium contains l-alanine, we developed AFM for use in our experiments. AFM was prepared using DMEM with optimized formulation of certain components (0.4 mm l-asparagine, 0.26 mm l-cysteine, 0.5 mm l-glutamate, 0.06 mm l-proline, 5 × 10−6 mm vitamin B12, and 26.1 mm sodium bicarbonate).
Amino acid supplementation.
After plating, cells were supplemented with l-serine or l-alanine at a final concentration of 10 mm (Sigma-Aldrich) and grown for 2 d in vitro (DIV).
SPT inhibition.
After 1 DIV, cells were treated with myriocin (Santa Cruz Biotechnology) at a final concentration of 10 or 20 μm.
Immunofluorescence.
Cells were fixed in 4% paraformaldehyde (Affymetrix). Primary antibodies were added in blocking solution containing 2% normal goat serum (Vector Labs) and 0.1% Triton X-100 (Sigma-Aldrich) overnight. Cells were washed and incubated with secondary antibodies for 1 h. Antibodies were against phosphorylated-ERM (rabbit anti-phospho-ezrin/radixin/moesin, 1:700; Cell Signaling Technology), neurofilament-heavy-chain (mouse-monoclonal SMI-32R, 1:700; Covance), goat anti-rabbit Alexa Fluor 488, and goat anti-mouse Alexa Fluor 555 (1:200; Life Technologies).
Western blotting.
DRG were collected in RIPA buffer (Sigma-Aldrich) with Complete Protease Inhibitor Cocktail (Roche). Samples were separated on NuPAGE 4–12% Bis-tris gels (Invitrogen), transferred onto PVDF membranes, blocked with 5% milk in PBS containing 0.05% Tween 20, and probed with antibody against phosphorylated-ERM (1:1000) and β-actin (rabbit anti-β-actin, 1:5000; Santa Cruz Biotechnology). Membranes were developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) after incubation with HRP-conjugated secondary antibodies (Abcam).
Microscopy and analysis.
Images were captured using Zeiss LSM510 confocal microscope and Zen 2009 software, keeping exposure/gain settings constant. Analyses were performed on ImageJ software by a blinded experimenter, with minimum 18 neurons analyzed per mouse. Using the NeuronJ plug-in, the longest neurite length of a DRG was measured, the average value calculated for the neurons of each mouse, and the mean neurite length derived from the three average values per genotype (subsequent mean values were determined similarly). Neurites were traced manually from the soma outward, excluding those <10 μm. For branching, the Sholl analysis plug-in was used to quantify branch intersections. Neurons with considerable overlap were excluded to avoid overestimation. The expression of p-ERM was measured as the mean fluorescence intensity (MFI) in neuronal growth cones. The MFI were background subtracted and averaged. Soma size was quantified by manually tracing the area around the neuron. All quantitative data were normalized to the WT control.
Statistics.
Statistical analyses were performed on GraphPad Prism 6. Genotype comparisons among SPTLC1C133W, SPTLC1WT, and WT were analyzed via one-way ANOVA. Comparisons after treatment (myriocin, l-serine, l-alanine, and AFM) were analyzed via two-way ANOVA. Analyses were followed up by Bonferroni's post hoc tests. The criterion for significance was *p < 0.05. Error bars are expressed as ± SEM.
Results
Mutant DRG exhibit increased neurite growth in vitro
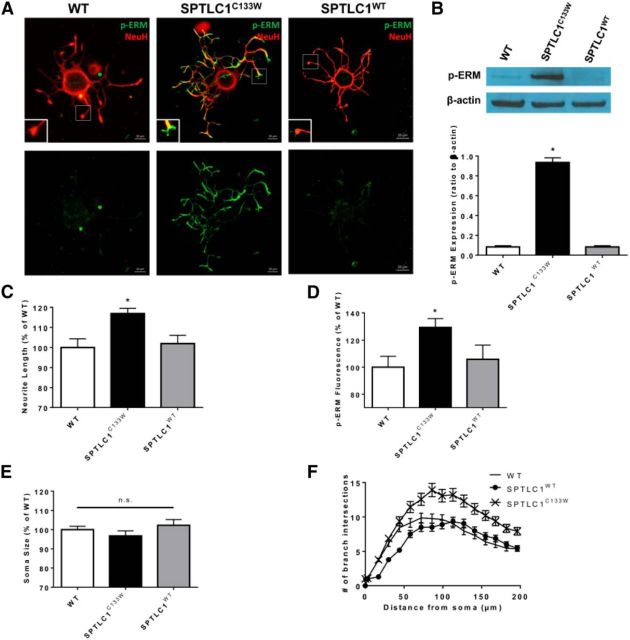
After extraction from the spinal cord, DRG began to grow neuronal processes in vitro. Initial characterization revealed morphology of mutant SPTLC1C133W DRG (Fig. 1A). To eliminate the possibility of the transgene overexpression having adverse effects, SPTLC1WT DRG were also used as controls. WT and SPTLC1WT DRG were not significantly different in any quantitative measures analyzed in this study. Compared with controls, SPTLC1C133W DRG exhibited a marked 29.2% increase (*p = 0.002, *p = 0.004) in p-ERM expression at neuronal growth cones, as detected by immunofluorescence and Western blotting (Fig. 1B,D). Mutant SPTLC1C133W DRG also exhibited a 16.9% increase (*p = 0.004, *p = 0.011) in neurite length and branching compared with controls (Fig. 1C,F), suggesting that the mutation is regulating axon elongation at neuronal growth cones. Quantification of soma size revealed no significant differences across all conditions (Fig. 1E) and we found no evidence of apoptosis (data not shown).
Figure 1.
Characterization of the mutant SPTLC1C133W DRG phenotype in in vitro. A, DRG from WT, SPTLC1C133W, and SPTLC1WT mice (n = 3) were stained with p-ERM and NeuH. Scale bar, 20 μm. B, Western blot revealing an increase in p-ERM in SPTLC1C133W, but not in WT or SPTLC1WT, DRG. C, SPTLC1C133W DRG had significantly longer neurite length compared with WT and SPTLC1WT. D, Expression of p-ERM at the neuronal growth cone is significantly elevated in SPTLC1C133W DRG compared with WT and SPTLC1WT. E, Soma size of DRG showed no significant difference across all groups. F, Sholl analysis revealed increased neuronal branching in SPTLC1C133W DRG compared with WT and SPTLC1WT.
SPT inhibition rescues the mutant phenotype
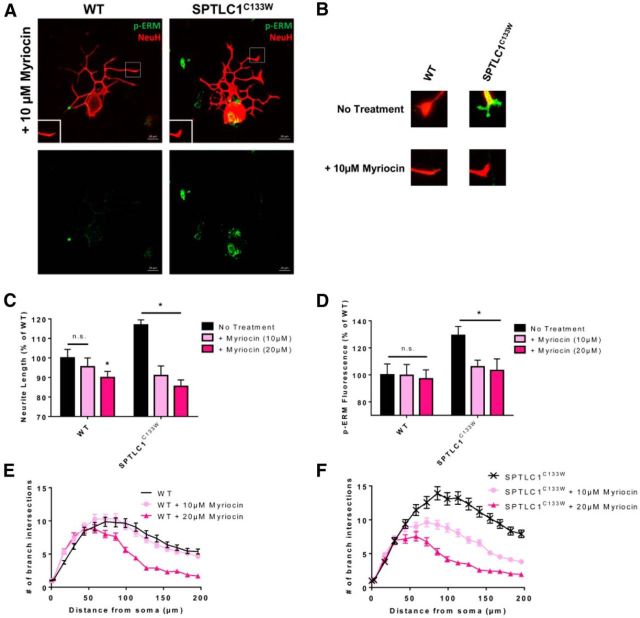
We confirmed whether the mutant SPTLC1C133W phenotype was linked to mutant enzymatic activity by using myriocin, a SPT inhibitor. WT and SPTLC1C133W DRG were treated with 10 or 20 μm myriocin (Fig. 2A). After treatment, we found that the expression of p-ERM at the neuronal growth cone was significantly decreased (*p = 0.003, *p = 0.004) in SPTLC1C133W DRG by 13.3% and 15.8% (Fig. 2B,D), which corresponded with 15.86% and 31.46% decreases in neurite length (*p = 0.003, *p < 0.001; Fig. 2C). However, myriocin had minimal effects on WT DRG, only reaching significance at the 20 μm concentration (*p = 0.021), which decreased neurite length by 15.8% (Fig. 2C,D). In terms of branching, myriocin led to a dose-dependent decrease in SPTLC1C133W DRG, but only showed a noticeable effect at 20 μm in WT DRG (Fig 2E,F). Overall, the mutant SPTLC1C133W condition, but not the WT, responds strongly to SPT inhibition.
Figure 2.
SPT inhibition via myriocin alleviates the mutant SPTLC1C133W condition. A, DRG from WT and SPTLC1C133W mice treated with 10 μm myriocin (n = 3) were stained with p-ERM and NeuH. Scale bar, 20 μm. B, Representative images of WT and SPTLC1C133W DRG growth cones before and after treatment. C, WT DRG exhibit significantly decreased neurite length when treated with 20 μm myriocin, but not at 10 μm. SPTLC1C133W DRG show significantly decreased neurite length after 10 and 20 μm myriocin treatments. D, There is no significant difference in p-ERM expression at the growth cones of WT DRG after treatment with 10 or 20 μm myriocin. In SPTLC1C133W DRG, p-ERM expression is significantly reduced after 10 and 20 μm myriocin treatments. E, Sholl analysis revealed no difference in branching of WT DRG after 10 μm myriocin, but a reduction at 20 μm. F, Branching of SPTLC1C133W DRG after 10 and 20 μm myriocin treatments is decreased in a dose-dependent manner.
Restorative effects of l-serine supplementation or l-alanine removal on mutant DRG
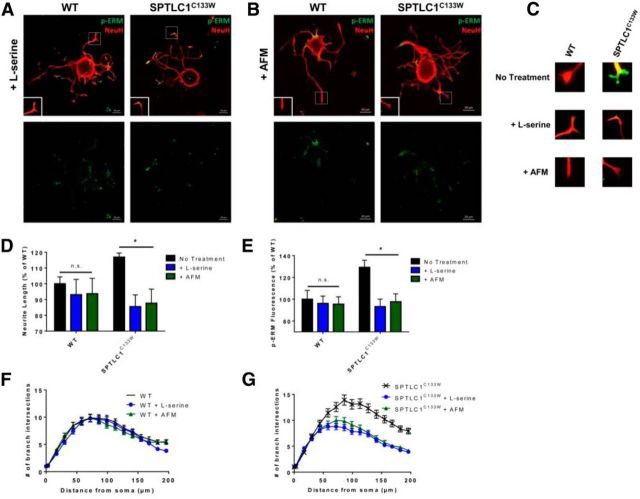
Supplementation with either l-serine or l-alanine has been shown to influence myelination, axon diameter, and sensory performance of HSN-1 mice (Garofalo et al., 2011). Specifically, excess l-serine ameliorates peripheral neuropathic symptoms, whereas excess l-alanine exacerbates those symptoms. Here, we found that the mutant condition was rescued when SPTLC1C133W DRG were treated with 10 mm l-serine (Fig. 3A). Not only did l-serine lead to a 31.36% decrease (*p = 0.001) in neurite length and branching of mutant SPTLC1C133W DRG (Fig. 3D,G), it also reduced the expression of p-ERM by 36% (*p < 0.001) to resemble the WT level (Fig. 3A,C). Conversely, l-serine had no significant effects on the WT neurite length or p-ERM expression (p = 0.155, p = 0.351; Fig. 3A,D–F).
Figure 3.
Supplementation with l-serine or removal of l-alanine rescues the mutant SPTLC1C133W condition. A, B, DRG from WT and SPTLC1C133W mice treated with 10 mm l-serine (A) or with AFM (B) (n = 3) were stained with p-ERM and NeuH. Scale bar, 20 μm. C, Representative images of WT and SPTLC1C133W DRG growth cones before and after treatment. D, SPTLC1C133W DRG treated with l-serine or AFM exhibit significantly decreased neurite length. WT DRG show no significant difference in neurite length after l-serine or AFM. E, Expression of p-ERM is significantly decreased in SPTLC1C133W DRG growth cones after treatment with l-serine or AFM. There is no significant difference in p-ERM expression in WT DRG after l-serine or AFM. F, Sholl analysis revealed no difference in branching of WT DRG after l-serine or AFM treatment. G, Branching of SPTLC1C133W DRG after l-serine or AFM treatment is reduced to levels similar to WT DRG.
Further, removal of l-alanine had similar restorative effects as l-serine supplementation on SPTLC1C133W DRG (Fig. 3B). When cultured in AFM, the mutant neurons exhibited a 29.2% decrease (*p = 0.012) in neurite length and branching (Fig. 3D,G) and a 31.58% reduction in p-ERM expression (*p = 0.001; Fig. 3C,E). The absence of l-alanine had no significant effects on the WT condition (p = 0.211, p = 0.304; Fig. 3B,D–F).
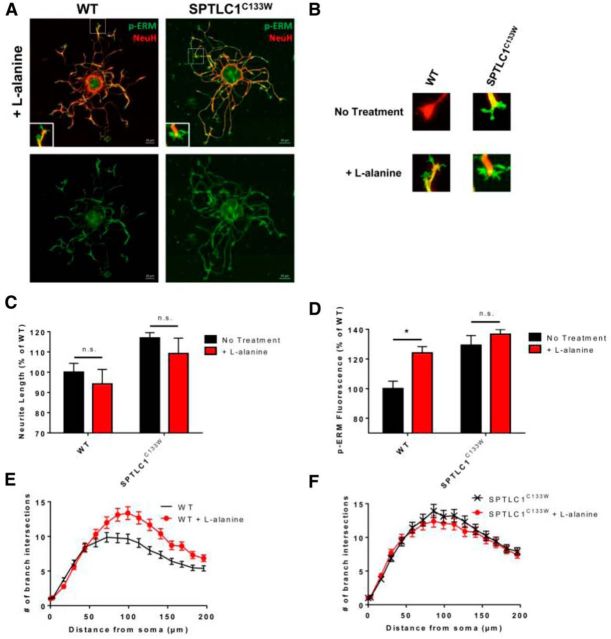
Excess l-alanine alters WT phenotype
Conversely, when treated with excess 10 mm l-alanine, mutant SPTLC1C133W DRG showed no significant differences in neurite length (p = 0.095), branching, or p-ERM expression (p = 0.131; Fig. 4A,C,D,F). However, WT DRG treated with excess l-alanine in part resembled the mutant phenotype (Fig. 4A): branching was elevated and p-ERM expression was increased by 24% (*p = 0.004) to resemble the mutant condition (Fig. 4B,D,E). Although we also expected an increase in WT neurite length, we found no significant difference (p = 0.116; Fig. 4C), suggesting that mechanisms of neuronal branching via growth cone proteins such as p-ERM is more susceptible to l-alanine availability than neurite outgrowth from the soma itself.
Figure 4.
Supplementation with l-alanine increases WT DRG branching and p-ERM expression. A, DRG from WT and SPTLC1C133W mice treated with 10 mm l-alanine (n = 3) were stained with p-ERM and NeuH. Scale bar, 20 μm. B, Representative images of WT and SPTLC1C133W DRG growth cones before and after treatment. C, Treatment with l-alanine has no significant effect on neurite length of WT or SPTLC1C133W DRG. D, Expression of p-ERM is significantly increased in WT DRG growth cones after l-alanine treatment. l-alanine treatment had no significant effect on p-ERM expression in SPTLC1C133W DRG. E, Sholl analysis revealed branching of WT DRG after l-alanine treatment is elevated to levels similar to mutant SPTLC1C133W DRG. F, Branching of SPTLC1C133W DRG is not altered after l-alanine treatment.
Discussion
Mutations in genes encoding subunits of SPT are one of the most common causes of hereditary sensory neuropathies (Dawkins et al., 2001; Rotthier et al., 2011, 2012). Although the progressive axonopathy and accumulation of atypical sphingolipid metabolites in HSN-1 suggests putative neurotoxicity (Penno et al., 2010), it remains poorly understood how the altered activity of mutant SPT leads to selective damage of the PNS.
Our current in vitro study provides evidence of increased neuronal growth and branching after extraction of DRG from HSN-1 mice and its dependence upon SPT substrate availability. We observed corresponding increases of p-ERM expression in the neuronal growth cones of SPTLC1C133W DRG, implicating these actin-cross-linking proteins as regulators of neurite growth. The increased neurite growth could be a result of elevated p-ERM in mutant DRG, which can aid axon elongation via attractive growth cone guidance or modulation of adhesion to the plasma membrane (Marsick et al., 2012). Further, the upregulation of growth cone proteins such as ERM has been linked to neuroregeneration after injury (Haas et al., 2004). However, the current in vitro model of neurite growth does not include a preconditioned in vivo injury, suggesting that the mutant morphology is indicative of compensatory rather than regenerative growth. Because progression of HSN-1 pathology is often exacerbated by peripheral injury in patients, a detailed investigation of the injury response in HSN-1 and how it affects the regulation of p-ERM and other relevant growth cone proteins may help to elucidate their role in the PNS.
Although it has been suggested that sphingolipid metabolites (Canals et al., 2010; Gandy et al., 2013) and various kinases (Ramesh 2004) can regulate p-ERM activation, how mutant SPT in HSN-1 leads to ERM phosphorylation is not known. We previously reported increased levels of TNF-α in SPTLC1C133W DRG and sciatic nerves (Eichler et al., 2009). This proinflammatory cytokine can act downstream via PKC or p38 MAPK to phosphorylate ERM proteins (Koss et al., 2006). Further, it is conceivable that canonical sphingolipid levels may be disrupted in HSN-1 due to altered activity of the mutant SPT enzyme. Although existing data on canonical sphingolipid levels in HSN-1 remain inconclusive (Dawkins et al., 2001; Dedov et al., 2004), the accumulation of neurotoxic deoxysphingolipids is well described (Eichler et al., 2009; Rotthier et al., 2011). Previous studies have reported that exogenous treatment of at least 1 μm deoxysphingolipids caused neurite loss and apoptosis (Cuadros et al., 2000; Penno et al., 2010). Endogenous levels are lower, however, ranging from 100 to 500 nm in plasma (Penno et al., 2010; Rotthier et al., 2011). We suggest that these metabolites may affect neuronal growth and viability indirectly via regulation of kinase activity (Hannun and Bell, 1987; Sánchez et al., 2008; Fyrst and Saba, 2010), alterations in sphingolipid metabolism (Dawkins et al., 2001; Zitomer et al., 2009), or disruption of cellular membranes (Jiménez-Rojo et al., 2014). A closer examination into how mutant SPT regulates these pathways and alters ERM activation will be important to help understand the molecular mechanisms of disease progression in HSN-1.
Because mutant SPT preferentially incorporates l-alanine over l-serine (Gable et al., 2010; Penno et al., 2010), we manipulated their availability in vitro. The beneficial effects of l-serine upon behavior and nerve pathology in HSN-1 mice have been described previously (Garofalo et al., 2011). We demonstrate rescue of the SPTLC1C133W condition, not only after l-serine supplementation, but also after removal of l-alanine, presumably due to restoration of canonical SPT activity. Despite its increased affinity for l-alanine, mutant SPT retains residual affinity for l-serine (Km = ∼1.4 mm and Vmax = ∼275 pmol/mg/min vs wild-type Km = ∼0.75 mm and Vmax = ∼1350 pmol/mg/min; Gable et al., 2010), with the result that mutant SPT can still incorporate l-serine, especially in the case of excess l-serine or lack of l-alanine.
Conversely, l-alanine supplementation elevated branching and p-ERM expression in WT DRG to resemble the mutant condition, which is not surprising because l-alanine exacerbates peripheral neuropathic symptoms in HSN-1 mice (Garofalo et al., 2011). However, because both l-serine and l-alanine are gluconeogenic amino acids involved in pathways regulating oxidative stress and neurotransmission (Rowsell et al., 1969; de Koning et al., 2003; Grosser et al., 2004; Fuchs et al., 2006), we cannot categorically rule out that the observed effects on DRG were due to their metabolic and neurotrophic properties.
Indeed, l-serine is crucial for lipid synthesis, neuronal survival, and function in the CNS (de Koning and Klomp, 2004; Hirabayashi and Furuya, 2008). In the absence of l-serine, neurons from the CNS do not grow as robustly as those treated with l-serine in vitro, because glia are the main suppliers of l-serine to neurons in vivo (Savoca et al., 1995; Furuya et al., 2000). After neuronal insult, the PNS receives glial support needed for regeneration (Scheib and Höke, 2013). This trophic activity is important for axons, especially those in the metabolically isolated PNS (Nave, 2010). Glia in the PNS tend to ensheath axons in a much lower ratio (1:1) than in the CNS (up to 60:1), emphasizing the importance of the neuron–glia interaction in peripheral nerves (Nave and Werner, 2014). Therefore, the distinct environment of the PNS may render peripheral axons susceptible to certain metabolic fluctuations. Our current in vitro data show contrasting effects of l-serine and l-alanine upon the mutant DRG phenotype, indicating a selective vulnerability to these amino acids, as suggested by studies of HSN-1 mice (Garofalo et al., 2011). Manipulating the l-serine/l-alanine ratio in HSN-1 may be of broader relevance to other peripheral nerve disorders and sphingolipidoses.
Here, we describe aberrant morphology of SPTLC1C133W DRG characterized by increased neurite growth, branching, and expression of p-ERM at neuronal growth cones. This phenotype indicates that neurite growth is being altered by the SPTLC1C133W mutation and is suggestive of compensatory growth in vitro. Because the eventual course of HSN-1 is distal axonal degeneration, it will be important to understand how the mutation in vivo leads to a length-dependent axonopathy. Further, because SPT inhibition and treatment with the canonical SPT substrate L-serine was able to rescue the mutant phenotype of elevated p-ERM, a detailed examination into how the mutation regulates the expression of important growth cone proteins will help ro identify therapeutic targets for HSN-1.
Footnotes
This work was supported by the National Institutes of Health (Grant R01 NS072446) and the Deater Foundation (F.S.E.).
The authors declare no competing financial interests.
References
- Bejaoui K, Wu C, Scheffler MD, Haan G, Becausehby P, Wu L, de Jong P, Brown RH., Jr SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet. 2001;27:261–262. doi: 10.1038/85817. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented neurobasalTM, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Canals D, Jenkins RW, Roddy P, Hernández-Corbacho MJ, Obeid LM, Hannun YA. Differential effects of ceramide and sphingosine-1-phosphate on ERM phosphorylation. J Biol Chem. 2010;285:32476–32485. doi: 10.1074/jbc.M110.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros R, Montejo de Garcini E, Wandosell F, Faircloth G, Fernández-Sousa JM, Avila J. The marine compound spisulosine, an inhibitor of cell proliferation, promotes the disassembly of actin stress fibers. Cancer Lett. 2000;152:23–29. doi: 10.1016/S0304-3835(99)00428-0. [DOI] [PubMed] [Google Scholar]
- Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- Dedov VN, Dedova IV, Merrill AH, Jr, Nicholson GA. Activity of partially inhibited serine palmitoyltransferase is sufficient for normal sphingolipid metabolism and viability of HSN1 patient cells. Biochem Biophys Acta. 2004;1688:168–175. doi: 10.1016/j.bbadis.2003.12.005. [DOI] [PubMed] [Google Scholar]
- de Koning TJ, Klomp LW. Serine deficiency syndromes. Curr Opin Neurol. 2004;17:197–204. doi: 10.1097/00019052-200404000-00019. [DOI] [PubMed] [Google Scholar]
- de Koning TJ, Snell K, Duran M, Berger R, Poll-The BT, Surtees R. L-Serine in disease and development. Biochem J. 2003;371:653–661. doi: 10.1042/bj20021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler FS, Hornemann T, McCampbell A, Kuljis D, Penno A, Vardeh D, Tamrazian E, Garofalo K, Lee HJ, Kini L, Selig M, Frosch M, Gable K, von Eckardstein A, Woolf CJ, Guan G, Harmon JM, Dunn TM, Brown RH., Jr Overexpression of the wild-type SPT1 subunit lowers deoxysphingolipid levels and rescues the phenotype of HSAN1. J Neurosci. 2009;29:14646–14651. doi: 10.1523/JNEUROSCI.2536-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SA, Dorland L, de Sain-vna der Velden MG, Hendriks M, Klomp LW, Berger R, de Koning TJ. D-serine in the developing human central nervous system. Ann Neurol. 2006;60:476–480. doi: 10.1002/ana.20977. [DOI] [PubMed] [Google Scholar]
- Furuya S, Tabata T, Mitoma J, Yamada K, Yamasaki M, Makino A, Yamamoto T, Watanabe M, Kano M, Hirabayashi Y. L-serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc Natl Acad Sci U S A. 2000;97:11528–11533. doi: 10.1073/pnas.200364497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable K, Han G, Monaghan E, Bacikova D, Natarajan M, Williams R, Dunn TM. Mutations in the yeast LCB1 and LCB2 genes, including those corresponding to the hereditary sensory neuropathy type I mutations, dominantly inactivate serine palmitoyltransferase. J Biol Chem. 2002;277:10194–10200. doi: 10.1074/jbc.M107873200. [DOI] [PubMed] [Google Scholar]
- Gable K, Gupta SD, Han G, Niranjanakumari S, Harmon JM, Dunn TM. A disease-causing mutation in the active site of serine palmitoyltransferase causes catalytic promiscuity. J Biol Chem. 2010;285:22846–22852. doi: 10.1074/jbc.M110.122259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy KA, Canals D, Adada M, Wada M, Roddy P, Snider AJ, Hannun YA, Obeid LM. Sphingosine-1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. Biochem J. 2013;449:661–672. doi: 10.1042/BJ20120213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo K, Penno A, Schmidt BP, Lee HJ, Frosch MP, von Eckardstein A, Brown RH, Hornemann T, Eichler FS. Oral L-serine supplementation reduces production of neurotoxic deoxyphingolipds in mice and humans with hereditary sensory autonomic neuropathy type 1. J Clin Invest. 2011;121:4735–4745. doi: 10.1172/JCI57549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Agosti C, Solomon F. Response of radixin to perturbations of growth cone morphology and motility in chick sympathetic neurons in vitro. Cell Motility Cytoskel. 1996;34:122–136. doi: 10.1002/(SICI)1097-0169(1996)34:2<122::AID-CM4>3.0.CO%3B2-D. [DOI] [PubMed] [Google Scholar]
- Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schröder H. Antioxidant action of L-alanine; heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Comm. 2004;314:351–355. doi: 10.1016/j.bbrc.2003.12.089. [DOI] [PubMed] [Google Scholar]
- Haas MA, Vickers JC, Dickson TC. Binding partners L1 cell adhesion molecule and the ERM proteins are involved in development and regenerative response to injury of hippocampal and cortical neurons. Eur J Neurosci. 2004;20:1436–1444. doi: 10.1111/j.1460-9568.2004.03620.x. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Bell RM. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987;235:670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Furuya S. Roles of L-serine and sphingolipid synthesis in brain development and neuronal survival. Prog Lipid Res. 2008;47:188–203. doi: 10.1016/j.plipres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Jiménez-Rojo N, Sot J, Busto JV, Shaw WA, Duan J, Merrill AH, Jr, Alonso A, Goñi FM. Biophysical properties of novel 1-deoxy-(dihydro)ceramides occuring in mammalian cells. Biophys J. 2014;107:2850–2859. doi: 10.1016/j.bpj.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, Buechner M, Hall DH, Gobel V. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat Cell Biol. 2013;15:143–156. doi: 10.1038/ncb2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss M, Pfeiffer GR, 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, Wang Q. Ezrin/Radixin/Moesin proteins are phosphorylated by TNF-α and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol. 2006;176:1218–1227. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- Marsick BM, San Miguel-Ruiz JE, Letourneau PC. Activation of Ezrin/Radixin/Moesin mediates attractive growth cone guidance through regulation of growth cone actin and adhesion receptors. J Neurosci. 2012;32:282–296. doi: 10.1523/JNEUROSCI.4794-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell A, Truong D, Broom DC, Allchorne A, Gable K, Cutler RG, Mattson MP, Woolf CJ, Frosch MP, Harmon JM, Dunn TM, Brown RH., Jr Mutant SPTLC1 dominantly inhibits serine palmitoyltransferase activity in vivo and confers an age-dependent neuropathy. Hum Mol Gen. 2005;14:3507–3521. doi: 10.1093/hmg/ddi380. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Penno A, Reilly MM, Houlden H, Laurá M, Rentsch K, Niederkofler V, Stoeckli ET, Nicholson G, Eichler F, Brown RH, Jr, von Eckardstein A, Hornemann T. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem. 2010;285:11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh V. Merlin and the ERM proteins in Schwann cells, neurons and growth cones. Nat Rev Neurosci. 2004;5:462–470. doi: 10.1038/nrn1407. [DOI] [PubMed] [Google Scholar]
- Rowsell EV, Snell K, Carnie JA, Al-Tai AH. Liver L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969;115:1071–1073. doi: 10.1042/bj1151071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotthier A, Penno A, Rautenstrauss B, Auer-Grumbach M, Stettner GM, Becauseselbergh B, Van Hoof K, Sticht H, Lévy N, Timmerman V, Hornemann T, Janssens K. Characterization of two mutations in the SPTLC1 subunit of serine palmitoyltransferase associated with hereditary sensory and autonomic neuropathy type 1. Hum Mutat. 2011;32:E2211–E2225. doi: 10.1002/humu.21481. [DOI] [PubMed] [Google Scholar]
- Rotthier A, Baets J, Timmerman V, Janssens K. Mechanisms of disease in hereditary sensory and autonomic neuropathies. Nat Rev Neurosci. 2012;8:73–85. doi: 10.1038/nrneurol.2011.227. [DOI] [PubMed] [Google Scholar]
- Sánchez AM, Malagarie-Cazenave S, Olea N, Vara D, Cuevas C, Díaz-Laviada I. Spisulosine (ES-285) induces prostate tumor PC-3 and LNCaP cell death by de novo synthesis of ceramide and PKCζ activation. Eur J Pharmacol. 2008;584:237–245. doi: 10.1016/j.ejphar.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Savoca R, Ziegler U, Sonderegger P. Effects of L-serine on neurons in vitro. J Neurosci Methods. 1995;61:159–167. doi: 10.1016/0165-0270(95)00038-V. [DOI] [PubMed] [Google Scholar]
- Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- Wadsworth JM, Clarke DJ, McMahon SA, Lowther JP, Beattie AE, Langridge-Smith PR, Broughton HB, Dunn TM, Naismith JH, Campopiano DJ. The chemical basis of serine palmitoyltransferase inhibition by myriocin. J Am Chem Soc. 2013;135:14276–14285. doi: 10.1021/ja4059876. [DOI] [PubMed] [Google Scholar]
- Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Garnier-Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, Merrill AH, Jr, Riley RT. Ceramide synthase inhibition by Fumonisin B1 causes accumulation of 1-deoxysphinganine. J Biol Chem. 2009;284:4786–4795. doi: 10.1074/jbc.M808798200. [DOI] [PMC free article] [PubMed] [Google Scholar]